Watch a video presentation of this article
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- CI
confidence interval
- ESLD
end‐stage liver disease
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- MWA
microwave ablation
- RFA
radiofrequency ablation
- RR
relative risk
- SBRT
stereotactic body radiation therapy
- TACE
transarterial chemoembolization
- TARE
transarterial radioembolization
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer‐related deaths in the United States. With its current trend, HCC is projected to become the third leading cause of cancer‐related deaths in the United States by 2030.1 Currently, more than 55% of HCC cases are diagnosed beyond localized disease, and the majority do not receive definitive curative therapies, such as surgical resection or liver transplantation.1, 2 Curative therapies are mainly reserved for patients with localized disease or those within Milan criteria. However, many nonsurgical treatment options are still available to patients with unresectable, advanced stage HCC (Table 1). Although there are many options for locoregional or systemic therapies in the management of unresectable HCC, this review will focus specifically on transarterial radioembolization (TARE), radiofrequency (RFA)/microwave ablation (MWA), stereotactic body radiation therapy (SBRT), systemic therapy, and hospice/supportive medicine. It is important to note that the approach for HCC treatment is variable and dependent on many factors, such as medical expertise, performance status, tumor stage and location, and degree of liver dysfunction. Therefore, utilizing multidisciplinary teams may provide the best option for developing a treatment plan.
Table 1.
Curative Versus Noncurative Therapies for HCC
| Curative | Noncurative |
|---|---|
| Liver transplantation | TACE or TARE |
| Hepatic resection | Cryoablationa |
| RFA or MWA | Percutaneous ethanol injectiona |
| External‐beam radiation therapya | |
| Stereotactic body radiation therapy | |
| Sorafenib/regorafenib | |
| Hospice/palliative care |
Not discussed in this article.
Transarterial Radioembolization
There are two major modalities for intra‐arterial, catheter‐based therapies for unresectable HCC. Transarterial chemoembolization (TACE) relies on direct delivery of chemotherapeutic agents to tumor‐feeding hepatic arteries followed by embolization. TACE is currently the standard protocol for intermediate‐stage HCC as recommended by the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver (Fig. 1).3, 4 However, TACE is contraindicated in patients with main portal vein thrombosis. Although TACE has demonstrated survival advantage among those who receive treatment, not all patients are eligible and other treatment options must be pursued. Patients ineligible for TACE may be better candidates for TARE, a novel therapeutic option for unresectable HCC. TARE selectively delivers radioactive yttrium‐90 (Y90) to tumor tissues through tumor‐feeding hepatic arteries while potentially minimizing damage to surrounding healthy parenchyma.5 Lobo et al.5 recently performed a systematic review and meta‐analysis comparing TACE and TARE, which identified five studies with 553 patients and concluded no difference in survival or major posttreatment complications. However, patients who underwent TARE experienced less postprocedural pain (relative risk [RR], 0.51; 95% confidence interval [CI], 0.36‐0.72; p < 0.01) but greater subjective fatigue (RR, 1.68; 95% CI, 1.08‐2.62; p < 0.01).5 Unlike TACE, main portal vein thrombosis is not a contraindication for TARE. Additional research on the long‐term efficacy of TARE in the management of unresectable HCC is needed to better understand the role of TARE in relation to other curative and palliative therapies.
Figure 1.
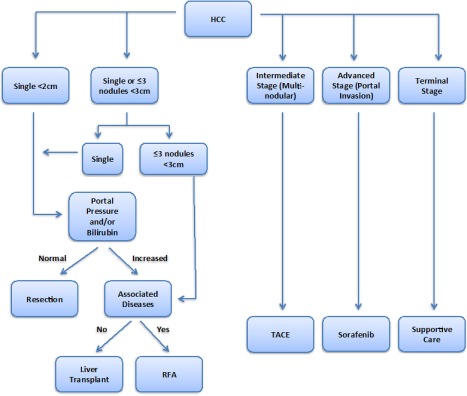
General approach for HCC treatment. Detailed indications and contradictions for treatment can be viewed in the 2017 AASLD guidelines for the treatment of HCC.
Radiofrequency/Microwave Ablation
RFA uses thermal energy produced from high radiofrequency to induce a spherical area of necrosis surrounding the tip of the needle electrode. Typically, RFA is most effective for tumors <3 cm in diameter and achieves a 1‐cm safety margin of healthy hepatic parenchyma neighboring the tumor. Among smaller, localized HCC, RFA can be considered a therapy with curative intent. However, larger tumors, including HCC >3 cm often require multiple needle deployments with the goal being control of tumor growth and not necessarily long‐term cure. In a recent study by Tateishi et al.,6 1000 cases of complete tumor ablation with RFA were evaluated. Increasing tumor size was associated with increased number of RFA treatment sessions to achieve tumor control: mean sessions 1.5 times for nodules <2 cm, 2.3 times for nodules 2.1 to 3.0 cm, 4.2 times for nodules 3.1 to 5.0 cm, and 11.7 times for nodules >11 cm.6 Moreover, a randomized control trial comparing RFA and hepatic resection in patients with HCC <4 cm and up to two nodules reported comparable 1‐, 2‐, and 3‐year overall survival rates; however, patients treated with RFA had higher rates of local recurrence from residual tumors that were primarily located underneath the liver capsule.7 Tumor location, such as subcapsular tumors or those involved with major hepatic vessels, is also a limiting factor for receipt of RFA caused by inadvertent damage to adjacent organs. Although RFA is currently standard care, alternative methods of producing thermal energy, such as MWA, are now emerging for the treatment of HCC. Potretzke et al.8 recently reported MWA to have a lower rate of local tumor progression (8.8%) compared with RFA (17.7%). Given similar survival benefits, procedure‐related complications, and decreased rates of local tumor progression, MWA can be seen as a viable alternative to RFA. Future prospective randomized control trials are needed to further evaluate and compare the two treatment modalities. Nevertheless, RFA or MWA remain favorable treatment options for patients with unresectable HCC given low procedural risk and complications and appreciable benefit.
Stereotactic Body Radiation Therapy
The role of SBRT in the management of HCC has traditionally been reserved for patients with expected poor outcomes because of moderate‐to‐advanced HCC who are not candidates for locoregional and surgical therapies. Modern radiotherapeutic advancements permit the delivery of an ablative dose of radiation without excessive exposure to noncancerous hepatocytes. Despite the lack of randomized evidence of SBRT compared with other standardized therapies for HCC, early‐phase trials and retrospective series have shown modest survival benefit from SBRT.9 Future studies are necessary to better determine the role of SBRT as either primary or neoadjuvant therapy in patients with inoperable HCC.
Systemic Therapy
In addition to surgical and locoregional therapies, oral systemic therapies also have a role in the management of patients with HCC, particularly those with unresectable HCC. Currently, sorafenib, a multikinase inhibitor that promotes antiangiogenic effects, inhibits tumor cell proliferation, and induces apoptosis, is the preferred first‐line systemic treatment. Llovet et al.10 conducted a multicenter, double‐blind, placebo‐controlled phase 3 trial and reported a modest increase in median overall survival with sorafenib (10.7 months) compared with placebo (7.9 months) (hazard ratio [HR] in sorafenib group, 0.69; 95% CI 0.55‐0.87; p < 0.007). Until recently, treatment options were unavailable to patients who did not respond positively or became refractory to sorafenib. A novel and more potent multikinase inhibitor, regorafenib, has emerged as a viable second‐line option for HCC patients who had unsuccessful sorafenib treatment. In the RESOURCE trial, Bruix et al.11 recently reported an increase in median overall survival with regorafenib (10.6 months; 95% CI, 9.1‐12.1) compared with placebo (7.8 months; 95% CI, 6.3‐8.8) (HR, 0.63; 95% CI, 0.50‐0.79; one‐sided p < 0.0001) among HCC patients who had developed disease progression despite sorafenib therapy. Despite improvements in systemic monotherapy for advanced HCC, additional studies are needed to explore the potential combination of systemic therapies to further improve overall survival.
Hospice/Supportive Medicine
The majority of patients with HCC are beyond localized disease at time of diagnosis, thus limiting eligibility for potentially curative therapies. Despite advancements in treatment options for HCC, the median overall 5‐year survival rate remains poor with overall 5‐year survival rate less than 30%.1 HCC patients with advanced unresectable tumors carry the bulk of this mortality burden, which is often exacerbated by serious comorbid conditions associated with HCC, such as cirrhosis and cirrhosis‐related complications (i.e., variceal bleeding, ascites, and hepatic encephalopathy). Although HCC treatment among these patients is often intended to be palliative, few have focused specifically on palliative care and hospice‐based management to improve outcomes. Temel et al.12 reported significant improvement in overall quality of life, mood, and even median survival in terminally ill patients with metastatic non‐small cell lung cancer who received early palliative care compared with standard oncological care. Notably, patients with HCC who received invasive procedures, such as surgery, ablation, and chemoembolization/radioembolization, were significantly less likely to use hospice therapy compared with untreated patients.13 Moreover, nonhospice recipients had greater rates of hospitalization, intensive care unit admission, and invasive procedures at the end of life, which culminated into higher costs of more than $12,000 compared with hospice beneficiaries.13, 14
Within the last decade, increased awareness in hospice care has led to a 7‐fold increase in hospice utilization among patients with end‐stage liver disease (ESLD); however, ethnic, socioeconomic, and geographic barriers limited access for hospice/palliative therapies for underrepresented communities.15 Furthermore, patients with ESLD may not use hospice as often because of misunderstanding of disease severity, focus on lifesaving interventions, and misperception of hospice as “death panels.”16 It is important for treating physicians to openly discuss realistic goals, prognosis, and treatment plans earlier in care rather than delaying end‐of‐life discussion until treatment options have been exhausted and the patient is near death.16, 17, 18 Multidisciplinary teams, including hospice care, are necessary to provide the best treatment options for patients with advanced HCC.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61:191‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ha J, Yan M, Aguilar M, Tana M, Liu B, Frenette CT, et al. Race/ethnicity‐specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol 2016;50:423‐430. [DOI] [PubMed] [Google Scholar]
- 3. European Association for Study of Liver ; European Organisation for Research and Treatment of Cancer . EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908‐943. [DOI] [PubMed] [Google Scholar]
- 4. Heimbach J, Kulik LM, Finn R, Sirlin CB, Abecassis M, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology; doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 5. Lobo L, Yakoub D, Picado O, Ripat C, Pendola F, Sharma R, et al. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta‐analysis. Cardiovasc Interv Radiol 2016;39:1580‐1588. [DOI] [PubMed] [Google Scholar]
- 6. Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201‐1209. [DOI] [PubMed] [Google Scholar]
- 7. Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794‐802. [DOI] [PubMed] [Google Scholar]
- 8. Potretzke TA, Ziemlewicz TJ, Hinshaw JL, Lubner MG, Wells SA, Brace CL, et al. Microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a comparison of efficacy at a single center. J Vasc Interv Radiol 2016;27:631‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murray LJ, Dawson LA. Advances in stereotactic body radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol 2017;27:247‐255. [DOI] [PubMed] [Google Scholar]
- 10. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378‐390. [DOI] [PubMed] [Google Scholar]
- 11. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2017;389:56‐66. [DOI] [PubMed] [Google Scholar]
- 12. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med 2010;363:733‐742. [DOI] [PubMed] [Google Scholar]
- 13. Sanoff HK, Chang Y, Reimers M, Lund JL. Hospice utilization and its effect on acute care needs at the end of life in medicare beneficiaries with hepatocellular carcinoma. J Oncol Pract 2017;13:e197‐e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor‐prognosis cancer. JAMA 2014;312:1888‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rush B, Walley KR, Celi LA, Rajoriya N, Brahmania M. Palliative care access for hospitalized patients with end stage liver disease across the United States. Hepatology; doi: 10.1002/hep.29297. [DOI] [PubMed] [Google Scholar]
- 16. Rakoski MO, Volk ML. Palliative care for patients with end‐stage liver disease: an overview. Clin Liver Dis 2015;6:19‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyd K, Kimbell B, Murray S, Iredale J. A “good death” with irreversible liver disease: talking with patients and families about deteriorating health and dying. Clin Liver Dis 2015;6:15‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly SG, Campbell TC, Hillman L, Said A, Lucey MR, Agarwal PD. The utilization of palliative care services in patients with cirrhosis who have been denied liver transplantation: a single center retrospective review. Ann Hepatol 2017;16:395‐401. [DOI] [PubMed] [Google Scholar]


