Watch the interview with the authors
Abbreviations.
BPR, boceprevir and pegylated interferon/ribavirin; BPR48, boceprevir and pegylated interferon/ribavirin for 48 weeks; DAA, direct‐acting antiviral; HCV, hepatitis C virus; IL‐28B, interleukin‐28B; PEG‐IFN, pegylated interferon; PR, pegylated interferon/ribavirin; RBV, ribavirin; RGT, response‐guided therapy; SPRINT‐2, Serine Protease Inhibitor Therapy 2; SVR, sustained virological response; T8PR, telaprevir for 8 weeks and pegylated interferon/ribavirin; T12PR, telaprevir for 12 weeks and pegylated interferon/ribavirin.
The recent approval of two protease inhibitors (telaprevir and boceprevir) and the resultant increase in the sustained virological response (SVR) rate when either of these drugs is added to pegylated interferon (PEG‐IFN) and ribavirin (RBV) have led to a flurry of patients with hepatitis C virus (HCV) infection seeking treatment. Some of these patients had been diagnosed with HCV for several years and had been hesitant to receive treatment with PEG‐IFN and RBV because of the low SVR rate, the high frequency of adverse events, and the need for injections. Others would have been suitable candidates for PEG‐IFN and RBV treatment but were fortunate to have early‐stage liver disease and were advised that they could afford to wait until a more efficacious and/or better tolerated treatment became available. Still others had gone through one or more courses of interferon (IFN)‐based therapy but had failed to achieve SVR or could not tolerate the side effects of PEG‐IFN and RBV. This article focuses on patients with an HCV genotype 1 infection because telaprevir and boceprevir have not been approved for other HCV genotypes. Patients with conditions for which telaprevir and boceprevir have not been approved are also not discussed (Table 1).
Table 1.
Conditions for Which the New Standard of HCV Therapy Has Not Been Approved
| Decompensated cirrhosis |
| Post‐transplant |
| Human immunodeficiency virus coinfection |
| Renal impairment |
| Non‐1 HCV genotype |
| Children |
Table 2 summarizes the factors that need to be considered in determining whether a patient should be started on HCV treatment now. Because the new standard of HCV therapy includes PEG‐IFN and RBV, patients with contraindications to these drugs should not be started on treatment now. A key factor to the success of triple therapy is the patient's ability to adhere to the complex treatment regimen. Telaprevir and boceprevir need to be administered every 8 hours with a snack, and in the case of telaprevir, the snack should contain 20 g of fat to facilitate absorption. Low trough concentrations of telaprevir have been shown to increase the risk of drug resistance.1 Therefore, treatment should be started only for patients who are motivated and are able to comply with the treatment regimen.
Table 2.
Factors to Be Considered in Determining Whether the New Standard of HCV Therapy Should Be Initiated
| Host factors: race, age, medical comorbidities, drug interactions, psychosocial circumstances, motivation, ability to adhere to a complex treatment regimen, and IL‐28B genotype |
| Disease factor: fibrosis stage |
| Treatment factor: IFN‐naive versus IFN‐experienced |
In comparison with Caucasians, African Americans have significantly lower SVR rates with PEG‐IFN and RBV dual therapy. The addition of a protease inhibitor to PEG‐IFN and RBV increases the SVR rate2, 3 (Fig. 1). Although the SVR rates for African Americans are still lower than those for Caucasians, the improved response to triple therapy has increased the enthusiasm of physicians and patients alike for pursuing HCV treatment.
Figure 1.
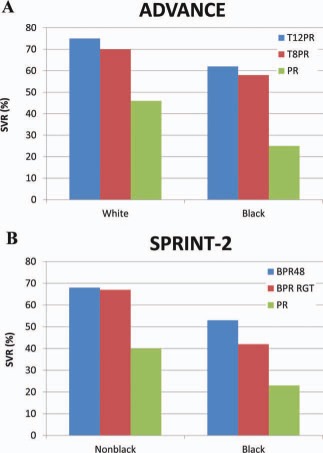
SVR rates (A) for blacks and whites in the ADVANCE trial2 and (B) for blacks and nonblacks in the SPRINT‐2 trial.3 Abbreviations: BPR, boceprevir and pegylated interferon/ribavirin; BPR48, boceprevir and pegylated interferon/ribavirin for 48 weeks; PR, pegylated interferon/ribavirin; RGT, response‐guided therapy; SPRINT‐2, Serine Protease Inhibitor Therapy 2; T8PR, telaprevir for 8 weeks and pegylated interferon/ribavirin; T12PR, telaprevir for 12 weeks and pegylated interferon/ribavirin.
In comparison with unfavorable genotypes (CT and TT), a favorable interleukin‐28B (IL‐28B) genotype (rs12979860 CC) is associated with a ≥2‐fold higher rate of SVR to treatment with PEG‐IFN and RBV.4 The addition of telaprevir or boceprevir to PEG‐IFN and RBV has a minimal impact on the SVR rate of patients with a favorable IL‐28B genotype, but there is a 2‐ to 3‐fold increase in the SVR rate of patients with unfavorable IL‐28B genotypes5, 6 (Fig. 2). Testing for the IL‐28B genotype has very little role in determining whether treatment should be recommended, but in countries with a high prevalence of favorable IL‐28B genotypes and limited resources, IL‐28B genotyping may help in identifying patients who would derive the greatest benefit from triple therapy.
Figure 2.
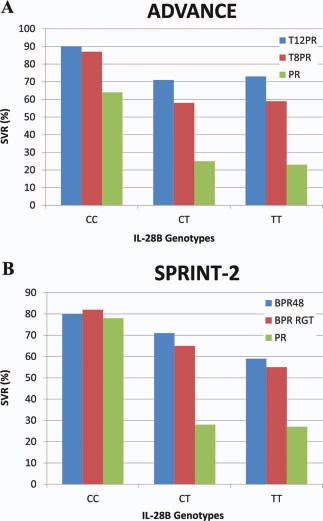
SVR rates for patients with IL‐28B genotypes CC, CT, and TT in (A) the ADVANCE trial5 and (B) the SPRINT‐2 trial.6 Abbreviations: BPR, boceprevir and pegylated interferon/ribavirin; BPR48, boceprevir and pegylated interferon/ribavirin for 48 weeks; PR, pegylated interferon/ribavirin; RGT, response‐guided therapy; SPRINT‐2, Serine Protease Inhibitor Therapy 2; T8PR, telaprevir for 8 weeks and pegylated interferon/ribavirin; T12PR, telaprevir for 12 weeks and pegylated interferon/ribavirin.
Not all patients with chronic HCV will progress to cirrhosis; therefore, previous guidelines recommended the initiation of treatment only in patients at risk of progressive liver disease. An assessment of the liver disease stage has been an integral part of the pretreatment evaluation. The improved SVR rate with triple therapy has raised the question whether HCV treatment should be recommended for all patients, including those with minimal or no fibrosis. Patients with minimal fibrosis are expected to have higher rates of SVR than those with advanced fibrosis, but some of these patients may never progress to cirrhosis, and most can afford to wait for second‐generation direct‐acting antiviral (DAA) agents or IFN‐free regimens. Patients with cirrhosis have the most urgent need for treatment, but they are also less likely to achieve SVR. Patients with bridging fibrosis or cirrhosis had lower rates of SVR than those with less advanced fibrosis in the phase 3 trials of boceprevir and telaprevir2, 3 (Fig. 3). Nonetheless, these response rates warrant the recommendation of triple therapy for patients with cirrhosis as long as some caveats are noted: the number of patients with cirrhosis included in the phase 3 trials was small, and all the patients had compensated cirrhosis with adequate cell counts. Patients with cirrhosis are more likely to experience adverse events. An inability to tolerate PEG‐IFN and RBV will compromise the response to triple therapy and increase the risk of antiviral drug resistance; therefore, caution must be exercised when triple therapy is being recommended for patients with cirrhosis, particularly if treatment is contemplated for patients with low blood counts or decompensated disease.
Figure 3.
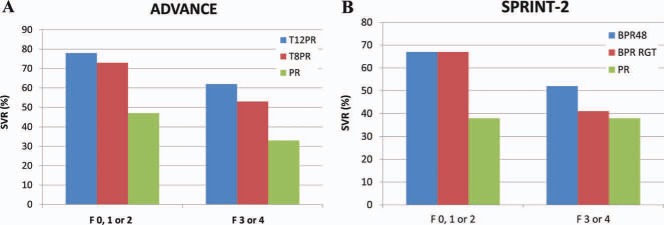
SVR rates for patients with no, mild, or portal fibrosis (Metavir F0, F1, or F2) or bridging fibrosis and cirrhosis (Metavir F3 or F4) in (A) the ADVANCE trial2 and (B) the SPRINT‐2 trial.3 Abbreviations: BPR, boceprevir and pegylated interferon/ribavirin; BPR48, boceprevir and pegylated interferon/ribavirin for 48 weeks; PR, pegylated interferon/ribavirin; RGT, response‐guided therapy; SPRINT‐2, Serine Protease Inhibitor Therapy 2; T8PR, telaprevir for 8 weeks and pegylated interferon/ribavirin; T12PR, telaprevir for 12 weeks and pegylated interferon/ribavirin.
The foregoing discussion applies to both IFN‐naive patients and IFN‐experienced patients. The addition of boceprevir or telaprevir to PEG‐IFN or RBV increases the SVR rate for IFN‐experienced patients, but the response range is wide, with very high SVR rates for previous relapsers and much lower rates for previous null responders7 (Fig. 4). Retreatment with triple therapy is clearly indicated for relapsers and is worthwhile for most partial responders. Previous null responders, who are expected to have a 30% chance of SVR, would be better off waiting for more effective therapies such as quadruple therapy with PEG‐IFN, RBV, and two DAAs. Patients with cirrhosis and a prior null response may not be able to wait for newer therapies, but the impulse for treatment now must be tempered by the sobering fact that the likelihood of SVR is only 14%, and the possibility of antiviral drug resistance is >50%.7
Figure 4.
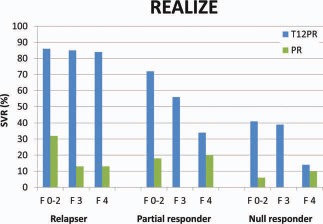
SVR rates for patients who experienced relapse or had a partial or null response to previous therapy in the REALIZE trial according to the pretreatment fibrosis stage.7 Abbreviations: PR, pegylated interferon/ribavirin; T12PR, telaprevir for 12 weeks and pegylated interferon/ribavirin.
The rapid development of DAAs for HCV and the improved SVR rate with the new standard of HCV therapy have energized patients and physicians, but the excitement must be balanced with a careful selection of patients to optimize benefits and to minimize harm.
Potential conflict of interest: Research grants: Abbott, Bristol‐Myers Squibb, Gilead, GlaxoSmithKline, Roche, Merck; Advisor: Bristol‐Myers Squibb, Gilead, GlaxoSmithKline
References
- 1. Sarazzin C, Kieffer TL, Bartels D, Hanzeika B, Muh U, Welker M, et al. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 2007; 132: 1767‐1777. [DOI] [PubMed] [Google Scholar]
- 2. Jacobson IM, McHutchinson JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364: 2405‐2416. [DOI] [PubMed] [Google Scholar]
- 3. Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364: 1195‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment‐induced viral clearance. Nature 2009; 461: 399‐401. [DOI] [PubMed] [Google Scholar]
- 5. Jacobson IM, Catlett I, Marcellin P, Bzowej NH, Muir AJ, Adda A, et al. Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial [abstract]. J Hepatol 2011; 54: S542. [Google Scholar]
- 6. Poordad F, Bronowicki JP, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski M, et al. IL28B polymorphism predicts virologic response in patients with hepatitis C genotype 1 treated with boceprevir (BOC) combination therapy [abstract]. J Hepatol 2011; 54: S6. [Google Scholar]
- 7. Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med 2011; 364: 2417‐2428. [DOI] [PubMed] [Google Scholar]


