Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- CI
chronic inflammation
- N/A
not applicable
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Liver biopsy interpretation continues to be central to patient care and investigative work in nonalcoholic fatty liver disease (NAFLD). Imperfect as it is, liver biopsy remains the best method for assessing the diagnostic categories of disease as well as the degrees of ongoing activity and established fibrosis and remodeling. From the initial studies that solidified the clinicopathological characteristics of nonalcoholic steatohepatitis (NASH) into the lexicon of hepatology to the current time, refinements of the histological features in adults and children have been proposed and studied. This review highlights our current knowledge.
Overview of NAFLD Pathology
As with any liver disease, the pathologist's diagnostic interpretation in NAFLD is ultimately an amalgamation of information: lesions of significance and patterns of injury and fibrosis. The process is not, therefore, simply additive, and the determination of a diagnosis is unique and is separate from the derivation of semiquantitative injury scores. Although they are related, the two should not be considered interchangeable.1 Table 1 summarizes the lesions and locations for NAFLD and NASH.
Table 1.
Lesions and patterns of NAFLD and NASH in adults and children
| Lesion | NAFLD | NASH | Patterns |
|---|---|---|---|
| Macrovesicular steatosis | Required | Required | Adults: zone 3, panacinar, azonal |
| Children: zone 1, panacinar | |||
| Lobular inflammation | +/− | Required | Adults: may or may not be accentuated in zone 3 |
| Hepatocellular ballooning | − | Required | Adults: zone 3 in prefibrotic stage(s) |
| Children: zone 3 or zone 1 | |||
| Apoptosis | +/− | Commonly present | Adults: correlates with severity of activity |
| Portal CI | +/− | Commonly present | Adults: correlates with severity of activity and fibrosis |
| Children: correlates with severity of fibrosis | |||
| Microvesicular steatosis, nonzonal patches | N/A | Uncommon | Adults: correlates with severity of activity and fibrosis |
| Children: unknown | |||
| Megamitochondria | N/A | +/− | No specific patterns reported. |
| Glycogenated nuclei | +/− | Common | Not specifically studied |
| Zone 3 perisinusoidal fibrosis | N/A | Earliest pattern in adults | Children: may or may not occur |
| Portal/periportal fibrosis | N/A | Common in children | Adults: described in bariatric surgery patients |
| Children: common as earliest pattern | |||
| Bridging fibrosis/cirrhosis | N/A | +/— | May or may not retain lesions of active steatohepatitis |
Abbreviation: N/A, not applicable.
Adult NAFLD
Before the onset of advanced fibrosis and remodeling, many of the components of NAFLD/NASH in adults are concentrated in zone 3, the perivenular region of the acinus: steatosis, hepatocyte ballooning, and perisinusoidal fibrosis in particular (Fig. 1). Steatotic droplets may be single or multiple, and they may be noted to coalesce. It is relatively simple to delineate individual droplets, and the cell's nucleus may be eccentric. This is called macrovesicular steatosis. A degree of steatosis ≥ 5% is considered required for NAFLD/NASH. True microvesicular steatosis may also occur, but when it is present, it is in nonzonal patches; the individual droplets are not discernable, and the nucleus remains in its central location. Megamitochondria may be found in hepatocytes with microvesicular fat. Lobular inflammation, which is common in NAFLD but is required in NASH, consists of a variety of features, including Kupffer cell aggregates without (microgranulomas) or with fat globules (lipogranulomas), small foci of mononuclear cell clusters, and occasional eosinophils or neutrophils. Large inflammatory foci are frequent in areas of fibrosis in zone 3, and they initially may be confused with portal tracts because of the presence of an arterial branch.2 Hepatocyte ballooning remains poorly understood and yet is considered the third required lesion for a diagnosis of steatohepatitis. Ballooned hepatocytes are typically larger than neighboring hepatocytes, have reticulated, flocculent cytoplasm, and are devoid of the normal keratins (8 and 18) by immunohistochemistry evaluation.3 These cells also may contain Mallory‐Denk bodies, the keratin 8/18–positive, ubiquitinated filaments that appear as a dense, eosinophilic, and ropey band. Another common lesion in steatohepatitis is apoptosis; this is identified as a rounded cytoplasmic fragment free within sinusoids with or without the hyperchromatic nuclear material.
Figure 1.
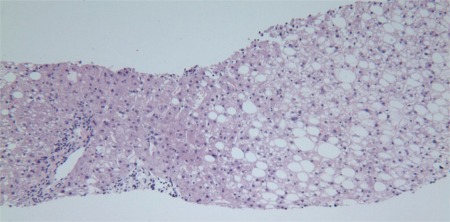
Adult NAFLD. This photomicrograph illustrates the involvement of zone 3 hepatocytes with mixed large‐ and small‐droplet steatosis and the sparing of zone 1 (periportal) hepatocytes (hematoxylin and eosin, ×20).
Portal chronic inflammation (CI) may occur in all aspects of NAFLD and NASH. Portal inflammation may be absent, mild, or more than mild in NASH. Portal CI may increase in comparison with lobular changes after effective interventional treatments.
Iron granules may occur in hepatocytes or within the reticuloendothelial cells of the liver in NAFLD. When either is marked, disease processes of aberrant iron metabolism should be considered.
Nuclear glycogenation is common in all forms of NAFLD.
Patterns of Fibrosis: Adult NASH
The initial deposition of excess matrix in NASH occurs in acinar zone 3; the pattern has been likened to chicken‐wire fencing and is called perisinusoidal or pericellular. The collagen may be too delicate to be perceived with routine hematoxylin and eosin stains and may require a Masson's trichrome stain (Fig. 2). This is the recommended stain because much of the work in the field has been done with this stain. However, the deposition may be dense and readily perceived with hematoxylin and eosin. The next step in progression is portal/periportal fibrosis; this is readily recognized by entrapped hepatocytes at the edges of involved portal tracts in addition to a subtle or apparent ductular reaction (increased ductular profiles admixed with mild inflammation and fibrosis).4 Bridging fibrosis may occur between any of the vascular structures: central‐central perisinusoidal fibrosis or a central‐portal or portal‐portal septal formation. The latter two forms also have ductular reactions. The final stage, cirrhosis, includes the complete formation of septa surrounding nodules; residual perisinusoidal fibrosis may or may not remain. Lesions of active steatohepatitis may or may not persist at this stage5; however, zonality is no longer present.
Figure 2.
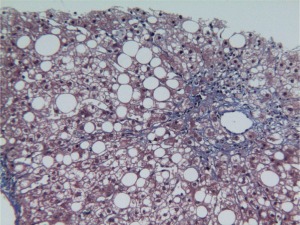
Adult NASH. Delicate strands of collagen can be seen in this example of zone 3 perisinusoidal fibrosis (Masson's trichrome, ×20).
Pediatric NAFLD
NAFLD and NASH in children may be identical to NAFLD and NASH in adults; more often, the features differ in the following ways: steatosis is more severe, ballooning is less common (or absent), steatosis accumulates near portal tracts (zone 1) or in a panacinar distribution, and portal and periportal fibrosis and portal CI may occur without perivenular perisinusoidal fibrosis (Figs. 3 and 4). Thus, the diagnosis of steatohepatitis (as described for adults) often cannot be made. Descriptive diagnostic categories have been proposed by the National Institute of Diabetes and Digestive and Kidney Diseases–sponsored NASH Clinical Research Network: a borderline zone 1 pattern, a borderline zone 3 pattern, and definite steatohepatitis.6
Figure 3.
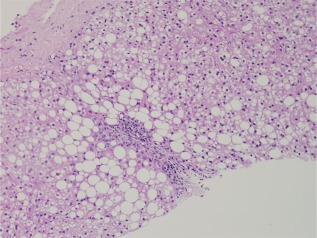
Pediatric NAFLD. In contrast to Fig. 1, this photomicrograph of pediatric NAFLD demonstrates the accentuated involvement of zone 1 (periportal) hepatocytes with steatosis (hematoxylin and eosin, ×20).
Figure 4.

Pediatric NAFLD: borderline type 1. This trichrome stain illustrates zone 1 steatosis, portal fibrosis, and a ductular reaction, which characterize pediatric NAFLD. This is an example of fibrosis stage 1c (portal, periportal only). Because there is no zone 3 ballooning, this cannot be diagnosed as definite NASH, but the features are more severe than those of steatosis without fibrosis (Masson's trichrome, ×20).
Challenges in Biopsy Interpretation
Glycogenosis, which is the accumulation of glycogen within hepatocytes, results in tinctorial and textural alterations that may be confused with ballooning because of altered cytoplasmic characteristics. Glycogen has a grayish tint and is opaque.
Ballooned hepatocytes have been determined by ultrastructural and immunohistochemical analyses to contain fat droplets as well as products of oxidative damage.8 By routine microscopy, occasional fat droplets may be seen in typical ballooned hepatocytes; however, cytoplasmic alteration (not size) is the criterion for determining ballooning.
Portal CI in NASH may appear to be disproportionate to the lobular lesions of necroinflammatory injury. In particular, lymphoid aggregates, marked interface activity, and a predominance of plasma cells all raise concerns of concurrent liver disease.9 Granulomas, bile duct infiltration, and prominent acute inflammation with pericholangitis are not features of NASH, and other disease processes should be considered.
If steatosis is absent but other features of fatty liver disease are present both clinically and histologically (e.g., perisinusoidal fibrosis and/or ballooned hepatocytes with Mallory‐Denk bodies), this author reports that the full criteria for the diagnosis are not present, but there is evidence that the lesion has occurred in the liver. Additional considerations include sampling and the possibility of alcoholic liver injury.
Scoring Systems
Semiquantitative methods of evaluation were developed for establishing reproducible methods of assessing responses to interventions. Extant methods separately score activity and fibrosis and follow the paradigm for chronic hepatitis (as reviewed by Brunt10). One was developed for NASH,11 whereas the more recent method addresses the features of the full spectrum of NAFLD.12 Neither was developed as a clinical tool.
References
- 1. Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander‐Tetri BA; for NASH Clinical Research Network . The NAS and the histopathologic diagnosis of NASH: distinct clinicopathologic meanings. Hepatology 2011;53:810‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gill RM, Belt P, Wilson L, Bass NM, Ferrell LD. Centrizonal arteries and microvessels in nonalcoholic steatohepatitis. Am J Surg Pathol 2011;35:1400‐1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lackner C, Gogg‐Kammerer M, Zatloukal K, Stumptner C, Brunt EM, Denk H. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol 2008;48:821‐828. [DOI] [PubMed] [Google Scholar]
- 4. Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander‐Tetri BA, Bhathal PS, et al. Progressive fibrosis in non‐alcoholic steatohepatitis—altered regeneration and the ductular reaction. Gastroenterology 2007;133:80‐90. [DOI] [PubMed] [Google Scholar]
- 5. Caldwell SH, Lee VD, Kleiner DE, Al‐Osaimi AM, Argo CK, Northup PG, et al. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol 2009;8:346‐352. [PMC free article] [PubMed] [Google Scholar]
- 6. Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology 2008;135:1961‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takahashi Y, Inui A, Fujisawa T, Takikawa H, Fukusato T. Histopathological characteristics of non‐alcoholic fatty liver disease in children: comparison with adult cases. Hepatol Res 2011;41:1066‐1074. [DOI] [PubMed] [Google Scholar]
- 8. Fujii H, Ikura Y, Arimoto J, Sugioka K, Iezzoni JC, Park SH, et al. Expression of perilipin and adipophilin in nonalcoholic fatty liver disease; relevance to oxidative injury and hepatocyte ballooning. J Atheroscler Thromb 2009;16:893‐901. [DOI] [PubMed] [Google Scholar]
- 9. Brunt EM, Ramrakhiani S, Cordes BG, Neuschwander‐Tetri BA, Janney CG, Bacon BR, et al. Concurrence of histologic features of steatohepatitis with other forms of chronic liver disease. Mod Pathol 2003;16:49‐56. [DOI] [PubMed] [Google Scholar]
- 10. Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology 2000;31:241‐246. [DOI] [PubMed] [Google Scholar]
- 11. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander‐Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467‐2474. [DOI] [PubMed] [Google Scholar]
- 12. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al.; for Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]


