Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- MET
metabolic equivalent
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Nonalcoholic fatty liver disease (NAFLD) is one of the most common diseases in industrialized countries and represents an imbalance between energy intake and energy output. NAFLD statistically parallels other chronic medical conditions that are predicated on energy balance, such as obesity, diabetes, hyperlipidemia, and hypertension, which are collectively known as metabolic syndrome. Treatment for NAFLD is most critical for those patients with nonalcoholic steatohepatitis (NASH) because they are at greater risk for progression to cirrhosis as well as hepatocellular carcinoma. Optimal treatment for NAFLD begins with weight loss and exercise and the positive corollaries that usually follow: improved insulin sensitivity, increased adiponectin expression, and improved lipid profiles. Improvement in liver enzymes historically has been monitored as a measure of therapeutic success. However, therapeutic response ideally is measured by improvements in hepatic histological results because serum aminotransferases do not always correlate with the disease state. There is currently no Food and Drug Administration–approved pharmacotherapy for the treatment of NASH, and similarly, there is no one specific dietary approach, although lifestyle modification via diet and exercise has shown potential benefit.
Weight loss by dietary changes with or without exercise or with bariatric surgery has persistently been shown to lead to biochemical and histological improvements in NAFLD. In a pilot study of 23 patients with biopsy‐proven NASH who were given 1 year of intense diet counseling, 9 of the 15 patients who completed the study showed histological improvement, with 7% weight loss in the histological responder group versus 2% weight gain in the nonresponders.1 This labor‐intensive approach to the treatment of NAFLD emphasizes the importance of weight loss but even more so the difficulty with patient motivation because less than half of the participants lost enough weight to alter the course of the disease.
Pharmaceutical agents used as an adjunct to lifestyle modification have reinforced the concept that effective weight loss leads to improvement in NASH histological results, but this is difficult both to achieve and to sustain. Orlistat, an inhibitor of pancreatic lipase, was studied in a randomized placebo controlled trial of 50 biopsy‐proven NASH patients, and the study demonstrated that 36 weeks of a regimen of placebo plus vitamin E and dietary counseling (1400‐kCal diet) produced results similar to those of a regimen of orlistat plus vitamin E and dietary counseling. Histological improvement was demonstrated in patients from both groups who lost 9% or more of their body weight.2 From this and other studies, a tangible target for providers and patients with NAFLD is a weight loss of 5% to 10% of total body weight over a 6‐ to 12‐month period.
There is no ideal diet to obtain the target goal of 5% to 10% body weight loss, and further investigation is needed to determine the clinical and histological efficacy of the various popular diets in NASH populations, including Weight Watchers, carbohydrate restriction without fat restriction (Atkins diet), macronutrient and glycemic load modification (Zone diet), and fat restriction (Ornish diet). In obese patients with a body mass index of more than 35 kg/m2, no significant difference in weight loss was seen with either dietary approach.3 In the absence of studies in NAFLD patients, the specific dietary program is less important than tailoring a diet to a patient's lifestyle to achieve and maintain long‐term weight loss.
Macronutrient composition also seems to be important in both promoting and inhibiting the development of NASH. Observational studies have shown NAFLD patients to have higher consumption of omega‐6 fatty acids and lower consumption of omega‐3 polyunsaturated fatty acids.4 Animal studies have shown that omega‐3 polyunsaturated fatty acids promote insulin sensitivity, reduce intrahepatic triglyceride content, and ameliorate steatohepatitis.5
The positive association between high‐fructose corn syrup via soft drink consumption and NAFLD also is evident.6 In a large‐scale study of 427 NAFLD patients, fructose‐containing beverages were associated significantly with a higher fibrosis stage,6 and this is thus an important modifiable risk factor. Most recently, caffeinated coffee intake has been associated independently with decreased rates of fibrosis in NASH, although prospective studies demonstrating improvement in NASH with coffee intake are lacking.8
Physical activity as a lifestyle modification also has been studied in the treatment of NAFLD. The intensity and type of exercise seem to play a role in NAFLD treatment in a manner independent of weight loss. In a cohort study of 813 adults with biopsy‐proven NAFLD enrolled in the NASH Clinical Research Network, vigorous exercise was associated with decreased adjusted odds of having NASH (odds ratio = 0.65, 95% confidence interval = 0.43–0.98).9 Vigorous exercises were activities with a metabolic equivalent value of 6 or more, such as using a treadmill or step machine. Doubling the time spent in vigorous physical activity was associated with decreased fibrosis, although neither moderate‐intensity exercise nor total exercise per week was associated with NASH or the stage of fibrosis; this suggests that intensity may be more important than the duration or total volume of exercise.
Resistance training also may be an important component to an exercise regimen in NAFLD populations. In a study of 19 NAFLD patients, 3‐times‐weekly resistance training for 8 weeks resulted in a 13% reduction in steatosis measured with magnetic resonance spectroscopy and in 12% increased insulin sensitivity in the absence of weight reduction.10 Compared with aerobic training, resistance training may increase lean body mass while decreasing total body fat, which increases insulin sensitivity. The initiation of increased physical activity is the first step to the treatment of NAFLD, with the goal program including both vigorous exercise and resistance training.
The treatment of NAFLD is a patient‐centered process that requires a patient to accept responsibility for lifestyle modifications. Evidence is emerging that allows physicians to guide patients better with diet and exercise counseling (See Fig. 1). Pharmaceutical therapies for the treatment of NAFLD actively are being sought, but weight loss remains the only intervention with proven outcomes.
Figure 1.
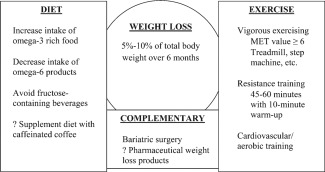
Integration of diet, exercise, and complementary medicine for the treatment of nonalcoholic fatty liver disease. Abbreviation: MET, metabolic equivalent.
Potential conflict of interest: Nothing to report.
The views expressed herein are those of the authors and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, Department of Defense, or the US Government.
References
- 1. Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One‐year intense nutritional counseling results in histological improvement in patients with non‐alcoholic steatohepatitis: a pilot study. Am J Gastroenterol 2005;100:1072‐1081. [DOI] [PubMed] [Google Scholar]
- 2. Harrison SA, Fecht W, Brunt EM, Neuschwander‐Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology 2009;49:80‐86. [DOI] [PubMed] [Google Scholar]
- 3. Dansinger M, Gleason J, Griggith J, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction. JAMA 2005;93:43‐53. [DOI] [PubMed] [Google Scholar]
- 4. Zelber‐Sagi S, Nitzan‐Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, et al. Long term nutritional intake and the risk for non‐alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol 2007;47:711‐717. [DOI] [PubMed] [Google Scholar]
- 5. Levy JR, Clore JN, Stevens W. Dietary n‐3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 rats. Hepatology 2004;39:608‐616. [DOI] [PubMed] [Google Scholar]
- 6. Abdelmalek MF, Suzuki A, Guy C, Unalp‐Arida A, Colvin R, Johnson RJ, et al.; for Nonalcoholic Steatohepatitis Clinical Research Network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010;51:1961‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population using ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124‐131. [DOI] [PubMed] [Google Scholar]
- 8. Molloy JW, Calcagno CJ, Williams CD, Jones FJ, Torres DM, Harrison SA. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 2012;55:429‐436. [DOI] [PubMed] [Google Scholar]
- 9. Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB; for NASH CRN Research Group. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol 2011;106:460‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, et al. Resistance exercise reduces liver fat and its mediators in non‐alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]


