Watch a video presentation of this article
Abbreviations
- ALT
alanine aminotransferase
- APRI
aspartate aminotransferase/platelet ratio index
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristic curve
- BAAT
body mass index, alanine aminotransferase, and triglycerides
- BARD
body mass index, alanine aminotransferase/aspartate aminotransferase ratio, and presence of diabetes
- BMI
body mass index
- CK‐18
cytokeratin 18
- DM
diabetes mellitus
- ELF
European liver fibrosis
- GGT
gamma‐glutamyl transpeptidase
- HA
hyaluronic acid
- HAIR
hypertension, alanine aminotransferase, and insulin resistance
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TG
triglyceride
- TIMP1
tissue inhibitor of metalloproteinase 1
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of abnormal liver test results in the United States.1 NAFLD is defined as the presence of steatosis in 5% or more of hepatocytes in the setting of no or little alcohol use. A thorough history is taken and an examination and laboratory testing are performed to exclude other liver diseases that also may present or coexist with hepatic steatosis. Although most patients with NAFLD have a benign course, a subset of these patients may have the progressive form of NAFLD called nonalcoholic steatohepatitis (NASH), which is characterized by the presence of ballooning degeneration and lobular inflammation with or without perisinusoidal fibrosis in addition to steatosis. Patients with NASH can progress to cirrhosis and are at increased risk of death resulting from liver disease.2 Liver biopsy is the gold standard to confirm NASH and fibrosis. This review briefly discusses the noninvasive biomarkers that may be useful and indications for performing liver biopsy in patients with NAFLD.
Noninvasive Markers
NAFLD typically is characterized by a hepatocellular pattern of liver‐related enzymes with mild elevations (1‐2 times the upper limit of normal) in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST).1 Despite having the disease, up to 50% of NAFLD patients can have normal ALT and AST levels.2 Therefore, several biomarkers have been proposed to aid in the diagnosis. These markers can be divided into those that are helpful in diagnosing NAFLD, NASH, or advanced fibrosis. Table 1 summarizes the different markers, panels, and their performance characteristics. Imaging biomarkers are beyond the scope of this review because of space constraints.
Table 1.
Biomarkers of NAFLD and NASH
| Test Name or Study | Markers | Purpose of Test | AUROC Training | AUROC Validation |
|---|---|---|---|---|
| ALT | ALT | Diagnosis of NAFLD | 0.68 | None |
| Steatosis test | ALT, α2‐macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin, GGT, cholesterol, TGs, glucose, age, sex, and BMI | Diagnosis of NAFLD | 0.79 | 0.72–0.86 |
| Liver fat score | AST, insulin, metabolic syndrome, DM type 2, and AST/ALT | Diagnosis of NAFLD | 0.87 | 0.86 |
| Hepatic steatosis index | ALT, AST, age, BMI, and sex | Diagnosis of NAFLD | 0.812 | 0.819 |
| Fatty liver index | BMI, waist circumference, TG level, and GGT | Diagnosis of NAFLD | 0.84 | None |
| CK‐18 | Distinguishing NASH from NAFLD | 0.93 | 0.83 | |
| NASH test | Age, sex, height, weight, and serum levels of TGs, cholesterol, α2‐macroglobulin, apolipoprotein A1, haptoglobin, GGT, ALT, AST, and total bilirubin | Distinguishing NASH from NAFLD | 0.79 | 0.79 |
| Younossi et al.7 | CK‐18, cleaved CK‐18, adiponectin, and resistin | Distinguishing NASH from NAFLD | 0.91 | 0.73 |
| Palekar et al.8 | Age, sex, AST, BMI, AST/ALT ratio, and serum HA | Distinguishing NASH from NAFLD | 0.763 | Not reported |
| HAIR | Hypertension, ALT, and insulin resistance | Distinguishing NASH from NAFLD in bariatric surgery patients | 0.9 with score of 3 associated | Not reported |
| NASH clinic scoring system | Hypertension, DM type 2, AST > 27 IU/L, ALT > 27 IU/L, sleep apnea, and nonblack race | Distinguishing NASH from NAFLD in bariatric surgery patients | 0.80 | 0.75 |
Markers for NAFLD
The presence of coexisting risk factors such as diabetes, metabolic syndrome, and obesity with elevated liver enzymes increases the odds of the presence of NAFLD as the underlying cause of elevated ALT and/or AST levels. Although ALT is a useful test, it is by no means reliable or accurate in predicting the presence of NAFLD. Therefore, several panels of biomarkers and clinical prediction rules have been developed to improve the diagnostic accuracy further for predicting NAFLD. Some of these are listed in Table 1.4
Markers for NASH
The diagnosis of NASH without a liver biopsy remains the most significant clinical challenge in the evaluation of a patient with hepatic steatosis. Several biomarkers have been examined to distinguish between simple steatosis and NASH. Some of the inflammatory markers include serum C‐reactive protein, interleukin‐6, ferritin, hyaluronic acid (HA), tumor necrosis factor α, leptin, adiponectin, and resistin.4 Apoptosis plays a key role in the pathogenesis of NASH. Among the markers of apoptosis, plasma cytokeratin 18 (CK‐18) is emerging as one of the promising biomarkers for the noninvasive detection of NASH.4 Since oxidative stress also plays an important role in the pathogenesis of NASH, several biomarkers of oxidative stress have been investigated. Among these, oxidized low‐density lipoprotein, thiobarbituric acid–reacting substances, superoxide dismutase, and glutathione peroxidase dismutase have been examined.4 The combination of biomarkers of apoptosis and oxidative stress has been studied and has led to modest improvement in the discrimination of NASH from steatosis alone.
The NASH test combined 13 variables [age, sex, height, weight, and serum levels of triglycerides (TGs), cholesterol, α2‐macroglobulin, apolipoprotein A1, haptoglobin, gamma‐glutamyl transpeptidase (GGT), ALT, AST, and total bilirubin] to achieve an area under the receiver operating characteristic curve (AUROC) of 0.79 with a specificity, sensitivity, positive predictive value, and negative predictive value of 94%, 33%, 66%, and 81%, respectively.4 Younossi et al. used a panel including 2 forms of CK‐18, adiponectin, and resistin.7 This panel achieved an AUROC of 0.91 in the training set; however, the AUROC dropped to 0.73 in the validation cohort.4, 7 Palekar et al. developed a panel that included 6 variables: age, sex, AST, body mass index (BMI), AST/ALT ratio, and serum HA.8 This model achieved an AUROC of 0.76.4, 8 Other panels also have been proposed to identify the presence of NASH in patients who are undergoing bariatric surgery; panels that may be useful in this select group of patients included the hypertension, alanine aminotransferase, and insulin resistance (HAIR) system and the NASH clinical scoring system (see Table 1).
Markers for Advanced Fibrosis and Cirrhosis
The use of biomarkers or panels for predicting advanced fibrosis in patients with NAFLD is much closer to routine clinical practice than the use of biomarkers for diagnosing NASH without a liver biopsy assessment.9 Single biomarkers that have been shown to predict fibrosis are HA and type IV collagen S, which have AUROCs of 0.82 and 0.80 and negative predictive values of 84% and 78%, respectively.4
Two of the most promising tests for diagnosing advanced fibrosis in NAFLD are the European liver fibrosis (ELF) score and the NAFLD fibrosis score.4, 6, 10 The ELF score includes HA, tissue inhibitor of metalloproteinase 1 (TIMP1), aminoterminal peptide of procollagen 3, and age. The simplified ELF score excluded the age and performed similarly in predicting fibrosis in NAFLD with an AUROC of 0.90. The addition of 5 parameters—BMI, albumin, platelets, presence of diabetes mellitus (DM), and AST/ALT ratio—improved the AUROC to 0.98. The NAFLD fibrosis score is helpful in the clinical setting because it uses routinely available variables in the clinical setting, including age, BMI, hyperglycemia, platelet count, serum albumin, and AST/ALT ratio. The NAFLD fibrosis score achieved AUROCs between 0.81 and 0.937. Its usefulness is not in diagnosing the stage of fibrosis but in predicting the presence of bridging fibrosis and cirrhosis in patients with NAFLD. Other notable biomarkers in predicting fibrosis in NAFLD are listed in Table 2, but they are not discussed in detail because of space constraints. Finally, simple tests such as the AST/platelet ratio and the AST/ALT ratio also have shown fair to good diagnostic test performance characteristics that may be applied clinically4 (Table 1).
Table 2.
Biomarkers of Fibrosis in Patients with Fatty Liver Disease
| Test Name or Study | Markers | Purpose of Test | AUROC Training | AUROC Validation |
|---|---|---|---|---|
| GGT | GGT | Distinguishing fibrosis | 0.74 | Not reported |
| ELF score | HA, TIMP1, and aminoterminal peptide of procollagen III (P3NP) | Distinguishing fibrosis (severe fibrosis) | Not reported | 0.9 |
| Modified ELF | ELF score + BMI, albumin, platelet, presence of DM, and AST/ALT ratio | Distinguishing fibrosis (severe) | Not reported | 0.98 |
| NAFLD fibrosis score | Age, BMI, hyperglycemia, platelet count, albumin, and AST/ALT ratio | Distinguishing fibrosis | 0.77–0.93, 0.85 in recent meta‐analysis | Not reported |
| Fibrometer | Glucose, AST, age, weight ferritin, ALT, and platelet count | Distinguishing fibrosis | 0.936 | 0.952 |
| F0 and F1 versus F2, F3, and F4 | 0.928 | 0.950 | ||
| F0, F1, and F2 versus F3 and F4 | 0.929 | 0.888 | ||
| F0, F1, and F3 versus F4 | ||||
| Fibro test | α2‐macroglobulin, haptoglobin, apolipoprotein A1, GGT, total bilirubin, and ALT | Distinguishing fibrosis | 086 | 0.75 |
| F0 and F1 versus F2, F3, and F4 | 0.932 | 0.81 | ||
| F0, F1, and F2 versus F3 and F4 | ||||
| BARD | BMI, AST/ALT ratio, and presence of DM | Distinguishing fibrosis | 0.81 | 0.78 |
| BAAT | BMI, ALT, and TG | Distinguishing fibrosis | 0.84 | Not reported |
| FIB‐4 | Age, AST, ALT, and platelet counts | Distinguishing fibrosis | 0.802 | 0.86 |
| APRI | AST/platelet × 100 | Distinguishing fibrosis | 0.86 for significant fibrosis, 0.861 for severe fibrosis, and 0.842 for cirrhosis | Not reported |
| AST/ALT ratio | Distinguishing fibrosis | 0.83 with cutoff of 0.8 | Not reported |
Abbreviations: AUROC: Area under Receiver Operating Characteristic, GGT: Gamma‐glutamyl transpeptidase ELF: European Liver Fibrosis Score, HA: Hyaluronic acid, BMI: Body Mass Index, DM: diabetes mellitus, AST: AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, BARD: BMI, AST/ALT ratio and presence of diabetes, BAAT: BMI, ALT and Triglyceride, TG: Triglyceride, FIB‐4: fibrosis index with 4 components: age (year) × AST (U/L)/ platelet count (109/L)× (ALT (U/L))(1/2); APRI: AST platelet ratio index.
In summary, the ELF score panel and the NAFLD fibrosis score seem to have good test performance characteristics for predicting advanced fibrosis, including bridging fibrosis, cirrhosis, or both.
Role of Liver Biopsy
Liver biopsy is still the gold standard for diagnosing NASH and assessing the stage of fibrosis in patients with NAFLD. However, it has many limitations, including cost, sampling error, morbidity, and, rarely, death. The position statement on NAFLD and NASH based on the 2009 European Association for the Study of Liver Diseases special conference recommended liver biopsy when noninvasive markers suggest advanced fibrosis and in indeterminate cases when the suspicion of fibrosis remains.11 Liver biopsy was also recommended when bariatric surgery or cholecystectomy is performed. The recently published practice guidelines on the management of nonalcoholic fatty liver disease by the American Association for the Study of Liver Diseases, the American College of Gastroenterology, and the American Gastroenterological Association recommended liver biopsy in patients who are at increased risk of steatohepatitis and fibrosis, such as those with metabolic syndrome.12 On the basis of several studies highlighted earlier, we recommend that liver biopsy be considered when the likelihood of the presence of NASH and advanced fibrosis is increased significantly among patients with NAFLD. We also propose that the presence of metabolic syndrome and diabetes increases the likelihood of the presence of NASH on liver biopsy.13 Therefore, these patients with NAFLD who have coexisting diabetes and/or metabolic syndrome and elevated ALT or AST levels may be considered for a liver biopsy examination (Table 3).9 This will help in the early identification of patients who are at the highest risk of progressive liver disease.14 Prompt recognition of NASH and the institution of emerging treatment strategies (such as vitamin E, exercise, and weight loss) in these patients will halt the progression of liver disease. Furthermore, biopsy‐proven NASH patients also are candidates for more aggressive lifestyle interventions, which will reduce the risk of future cardiovascular and liver disease progression. Other subpopulations of NAFLD patients who may be considered for liver biopsy include those with AST levels greater than ALT levels, older patients,16 those with low platelet counts or serum albumin levels when there is diagnostic uncertainty, and those with a competing diagnosis that requires exclusion, such as drug‐induced liver injury. Figure 1 displays the diagnostic algorithm that we recommend for assessing the need for performing liver biopsy in patients with NAFLD. We use routinely available models or markers that increase the pretest likelihood of finding more advanced liver disease on liver biopsy. These tests can aid in clinical decision making for patients with NAFLD. Some of these markers are a high AST/ALT ratio, a high AST/platelet ratio, low albumin levels, and low platelet levels. In addition, we take into account the preferences of our patients by helping them make an informed decision by reviewing their risk of progression and the clinical usefulness of the information that will be obtained with liver biopsy. This patient‐centered approach is especially important in the subgroup of patients for whom there are emerging data suggesting an increased risk of NASH or fibrosis, such as the elderly and patients who have a family history of diabetes or cirrhosis15 (Fig. 1).
Table 3.
Indications of Liver Biopsy in NAFLD
| Indication for Biopsy | Strength of Evidence |
|---|---|
| Clinical: | |
| Metabolic syndrome with abnormal liver enzymes | Strong evidence |
| DM type 2 with abnormal liver enzymes | Strong evidence |
| During bariatric surgery | Strong evidence |
| Cholecystectomy | Strong evidence |
| Laboratory test: | |
| AST > ALT | Strong evidence |
| Low platelet count | Strong evidence |
| Low albumin | Strong evidence |
| Special clinical considerations: | |
| Older age | Emerging evidence |
| Family history of diabetes | Proposed and needs to be validated |
Figure 1.
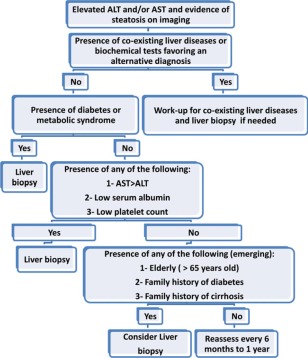
Evidence‐based recommendations regarding when to perform liver biopsy in patients with NAFLD.
Genetic testing for risk stratification of patients with NAFLD is not yet ready for routine clinical practice.
Conclusions
Biomarkers and clinical prediction rules for refining the management paradigm of NAFLD are emerging. Metabolic syndrome and diabetes are the key determinants of advanced histological examinations in NAFLD. Therefore, liver biopsy may be considered in this subset of patients with NAFLD. Until further refinement of biomarkers and clinical prediction rules, liver biopsy should be considered in patients with NAFLD who have an increased risk of advanced fibrosis and when the diagnosis is uncertain. Incremental advances in the noninvasive diagnosis of NASH will continue to shape the management paradigm of NAFLD and will change clinical practice in the coming years.
This study was supported in part by the American Gastroenterological Association Foundation–Sucampo–ASP Designated Research Award in Geriatric Gastroenterology (grant K23DK090303 to Rohit Loomba) and by a T. Franklin Williams Scholarship Award. Funding was provided by Atlantic Philanthropies, Inc., the John A. Hartford Foundation, and the Association of Specialty Professors.
References
- 1. Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002;123:1705–1725. [DOI] [PubMed] [Google Scholar]
- 2. Neuschwander‐Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 2003;37:1202–1219. [DOI] [PubMed] [Google Scholar]
- 3. Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011;54:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis. Aliment Pharmacol Ther 2011;33:525–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adams LA, Feldstein AE. Non‐invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis 2011;12:10–16. [DOI] [PubMed] [Google Scholar]
- 6. Musso G, Gambino R, Cassader M, Pagano G. Meta‐analysis: natural history of non‐alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non‐invasive tests for liver disease severity. Ann Med 2011;43:617–649. [DOI] [PubMed] [Google Scholar]
- 7. Younossi ZM, Jarrar M, Nugent C, et al. A novel diagnostic biomarker panel for obesity‐related nonalcoholic steatohepatitis (NASH). Obes Surg 2008;18:1430–1437. [DOI] [PubMed] [Google Scholar]
- 8. Palekar NA, Naus R, Larson SP, Ward J, Harrison SA. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver international: official journal of the International Association for the Study of the Liver 2006;26:151–156. [DOI] [PubMed] [Google Scholar]
- 9. Neuschwander‐Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 11. Ratziu V, Bellentani S, Cortez‐Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 2010;53:372–384. [DOI] [PubMed] [Google Scholar]
- 12. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–2023. [DOI] [PubMed] [Google Scholar]
- 13. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–131. [DOI] [PubMed] [Google Scholar]
- 14. Rafiq N, Bai C, Fang Y, et al. Long‐term follow‐up of patients with nonalcoholic fatty liver. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 2009;7:234–238. [DOI] [PubMed] [Google Scholar]
- 15. Loomba R, Tech MA, Unalp A, Wilson L, Lavine J, Doo E, et al.; for NASH Clinical Research Network. Association between diabetes, family history of diabetes and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012; April 13. doi:10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noureddin M, Vaughn IA, Neuschwander‐Tetri BA, Sanyal AJ, McCullough AJ, Merriman RB, et al. Clinical and histological determinants of nonalcoholic steatohepatitis (NASH) and advanced fibrosis in the elderly. Hepatology 2011;54(suppl):1118a. [DOI] [PMC free article] [PubMed] [Google Scholar]


