Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- DN
dysplastic nodule
- FLC
fibrolamellar hepatocellular carcinoma
- GPC‐3
glypican‐3
- GS
glutamine synthetase
- H&E
hematoxylin and eosin
- HCC
hepatocellular carcinoma
- HSP70
heat shock protein 70
- LEL
lympho‐epithelial‐like carcinoma
- pCEA
polyclonal carcinoembryonic antigen
Paradoxically, with the recognized increase in hepatocellular carcinoma, liver biopsy is used less frequently for diagnosis unless there are “atypical” imaging characteristics.1, 2 Thus, unless pathologists practice in a center with high liver surgery volume, experience with this tumor may diminish. However, whether for tissue acquisition for management decisions of the atypical lesions, the incidental lesion encountered in a “blind” percutaneous biopsy, or for final diagnostic reporting of resected or transplanted hepatectomies, familiarity with the histopathologic lesions of hepatocellular carcinoma remains important for practicing pathologists. As noted in recent reviews, clinical management choices and decisions, and prognosis of primary liver carcinoma are fields of rapid growth. Arguments are now being made that for optimum clinical outcomes, management also necessitates the most reliable classification of tumors, including histopathologic evaluation.3, 4, 5 This review discusses current criteria, useful immunohistochemistry stains, and differential diagnostic considerations for hepatocellular carcinoma.
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC), a malignant tumor of hepatocytes, may exhibit any or all of the cytologic and/or architectural characteristics of hepatocellular differentiation, along with features of malignancy. Identification of intercellular bile and/or canaliculi are diagnostic of the cellular origin of the tumor. Figures 1, 2, 3, 4, 5, 6 show hematoxylin and eosin (H&E)–stained images of “routine” HCC.
Figure 1.
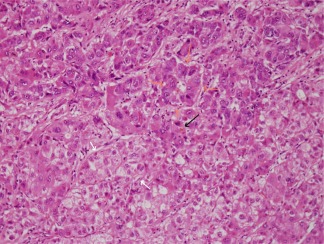
H&E‐stained HCC. There are examples of broad trabeculae (white arrows), pseudoglands or pseudoacini (orange arrows), and a bile plug (black arrow). Nuclear contours are irregular and large nucleoli are notable. H&E, magnification ×20.
Figure 2.
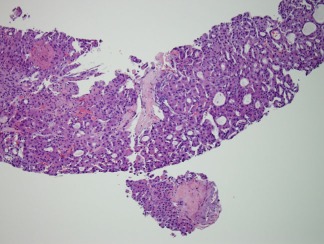
HCC biopsy shows both thin trabeculae and pseudoacini. The lack of normal parenchymal portal tracts or terminal hepatic venules is the initial clue as to the neoplastic nature of this hepatocellular tumor. The pleomorphism and pseudoacini would not be acceptable for adenoma were this in a noncirrhotic liver. H&E, magnification ×20.
Figure 3.
HCC with large pseudoglands/pseudoacini, deeply eosinophilic cells, and thick fibrous bands coursing through the tumor. This could be confused for fibrolamellar HCC if care is not taken to know the context (cirrhosis, not shown) and features of the cells and stroma. H&E, magnification ×20.
Figure 4.
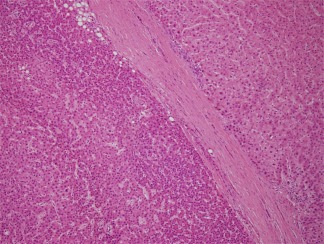
Well‐differentiated HCC (left) is separated from the nontumorous liver (right) by a dense capsule. Without appreciating the dense capsule that lacks ductular reaction, this well‐differentiated HCC could be confused for a high‐grade dysplastic nodule. There is marked nuclear crowding and a high N/C ratio; all of this can best be appreciated in comparison to the adjacent nontumorous liver. H&E, magnification ×10.
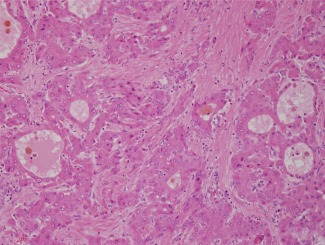
Figure 5.
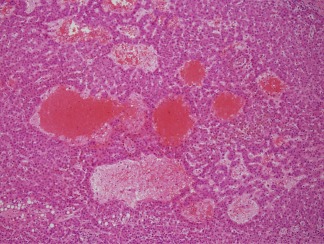
Peliotic foci are found within the central portion of the well‐differentiated HCC shown in Fig. 4. Peliotic formations are not diagnostic of HCC, as they may also occur in hepatocellular adenomas. H&E, magnification ×20.
Figure 6.
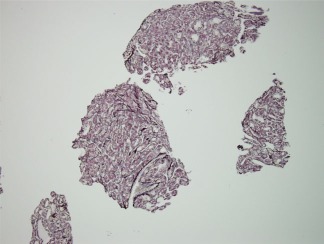
Reticulin stain in a biopsy of HCC highlights loss of reticulin as well as demonstration of pseudoglands. Gomori's reticulin, magnification ×20.
Architectural Considerations
The trabecular and pseudoacinar patterns of HCC are the most easily recognized. The former recapitulates hepatic cords, but the width is ≥3 nuclei; in some cases, “floating trabeculae” may be appreciated in which there are apparent cross‐sections of cords that are not attached or are floating freely. If considered three‐dimensionally, this could represent a cross‐section from a complex growth of tumor with multiple projections. In nontumorous liver, this appearance is seen only in large vein outflow obstruction with sinusoidal dilatation, but the cords are ≤2 nuclei wide. Pseudoacinar formations may resemble “cholestatic rosettes” when small; pseudoacini may also be dilated and large, infrequent to numerous, empty or filled with eosinophilic material. More challenging to recognize as HCC is the compact (solid) pattern in which nearly confluent sheets of tumor are present. A characteristic feature of so‐called progressed HCC is the presence of unpaired arteries within the tumor nodule. Finally, intravascular or intraductal tumor and stromal invasion are uncommon but diagnostic of malignancy. Stromal invasion is best recognized on large tissue sections of encapsulated HCC in a background of cirrhosis; keratin 76 and keratin 197 show progressive loss of the perinodular ductular reaction from cirrhotic nodules to dysplastic nodules to HCC. In some cases, apparent overrun of residual portal tracts may also be appreciated in stromal invasion in HCC.
Cytologic Considerations
Malignant hepatocytes manifest almost all the features of benign hepatocytes; the exception is accumulation of iron granules. HCC in the setting of iron overload is iron‐free. The cytoplasm may be deeply eosinophilic, basophilic, or clear. Cytoplasmic aggregates may include steatosis, fibrinogen (so‐called pale bodies), glycogen (clear cells) or glycoprotein inclusions (+/− periodic acid Schiff with diastase‐positive), and Mallory‐Denk bodies; intranuclear inclusions include glycogen, cytoplasmic pseudo‐inclusions, or large nucleoli. Nuclear contours are often irregular, and the nuclear/cytoplasmic ratio is increased. Giant, multinucleated cells may occur in HCC. An important concept in HCC is that once tumors are no longer well‐differentiated, pleomorphism of cytologic phenotype may occur throughout the tumor.
Useful Stains
Table 1 lists commonly used stains for routine HCC. It is worth noting that no single stain is entirely sensitive or specific,8 and the premier stain for diagnosis remains hematoxylin and eosin. Reticulin and immunohistochemistry stains can be used, with care, to confirm (or rarely, exclude) the diagnosis, as discussed below. Immunohistochemistry also plays a large role in the evaluation of phenotypic differentiation of liver carcinoma, as discussed below. Figures 7, 8, 9, 10 show stains used commonly for HCC.
Table 1.
Stains for HCC
| Stain/IHC | Primary Usefulness in HCC | Other Useful Findings | Comments |
|---|---|---|---|
| Reticulin | Highlights loss of normal cord architecture; referred to as “loss of reticulin” | Best used in comparison with nontumorous liver; easily misinterpreted and does not necessarily distinguish adenoma and HCC | |
| K8 and K18 | Keratins of hepatocytes; likely not positive in most adenocarcinomas | ||
| K7 | May label cells in up to 50% of cases of HCC | Very strong and diffuse labeling is highly suggestive of biphenotypic differentiation | |
| K20 | Has been found in scattered cells in up to 20% of cases of HCC | Not useful in the K7/K20 algorithm for evaluating carcinomas | |
| CD34 | Labels the endothelial cells of the sinusoids of HCC but not nontumorous liver | Is a marker of “capillarization,” loss of endothelial cell fenestration | Caution: may be positive focally and/or diffusely in hepatocellular adenoma |
| pCEA | Labels canaliculi between benign and malignant hepatocytes; will not be as numerous as nontumorous liver | Cholangiocarcinoma is positive in cytoplasm | Nontumorous liver is strong positive control |
| CD10 | Canalicular marker; specific but not as sensitive as pCEA | For clear cell carcinoma, CD10 can be useful to evaluate for cytoplasmic reactivity expected in renal cell carcinoma | Nontumorous liver is strong positive control |
| HEPAR1 | Labels mitochondrial enzyme (granular) in mature hepatocytes; thus, may be negative in HCC | Very limited application in evaluation; may be positive in other cell types | |
| GPC‐3 | Useful when positive, not useful when negative; reported to be negative in hepatocellular adenoma | Caution: can be “patchy”; has been reported in chronic hepatitis C and in DNs | |
| GS | Useful when positive, not useful when negative; may be positive in adenoma | Zone 3 hepatocytes are normally strongly positive for GS | Characteristic “map‐like” pattern in FNH |
| HSP70 | Useful in combination with GPC‐3 and GSa | Caution: may be positive in DNs | |
| Annexin‐2 | Positive in hepatocytes and/or sinusoids | Caution: may also be positive in similar patterns in DNs and hepatocellular adenoma |
Abbreviations: IHC, immunohistochemistry; K, keratin.
See text.
Figure 7.
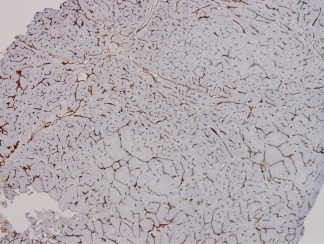
CD34 immunohistochemistry demonstrates the neovascularization of this HCC. Nontumorous hepatic sinusoids do not react with CD34 antibodies, as the endothelial cells are fenestrated and do not rest on a basement membrane. This pattern of sinusoidal reactivity, however, is not in itself diagnostic of HCC, as it may also occur diffusely or in areas of hepatocellular adenoma. Anti‐CD34 immunohistochemistry, magnification ×10.
Figure 8.

The darkly stained structures in this photomicrograph are keratin 19 (K19)‐positive. On the right side, there is a cirrhotic nodule virtually surrounded by K19‐positive ductular cells, whereas the tumor on the left of the photomicrograph has no K19 ductular reaction around the edges. For further description, see Ref.7. Anti‐K19 immunohistochemistry, magnification ×10.
Figure 9.
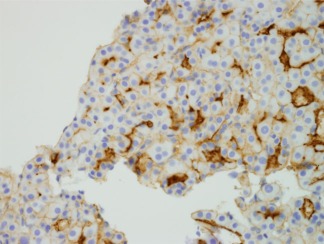
Polyclonal carcinoembryonic antigen (pCEA) immunostaining. pCEA cross‐reacts with biliary glycoprotein and is reactive in the canaliculi of benign and malignant hepatocytes. Thus, it is a very strong marker of hepatocellular differentiation, as no other cell type has canaliculi. When nontumorous liver is present in a biopsy or resection, it serves as a useful control. It is clear from this example that in HCC the canalicular structures are not uniform. Anti‐pCEA, magnification ×40.
Figure 10.

GPC‐3, an oncofetal protein that is not found in hepatocellular adenoma but may be present in HCC and DNs and has been reported in chronic hepatitis C. The reactivity is as shown: irregular within the tumor and within the cells. The reactivity may be pericanalicular, granular in the cytoplasm, or membranous. Anti‐GPC‐3, magnification ×20.
Histologic Subtypes of Hepatocellular Carcinoma
It is increasingly recognized that morphologic subtypes of liver carcinoma exist beyond the obvious broad distinctions of hepatocellular and cholangiocarcinoma. Commonly accepted subtypes are fibrolamellar HCC (FLC), scirrhous HCC, sarcomatoid HCC, and the so‐called inflammatory HCC or lympho‐epithelial‐like carcinoma (LEL).9 The first, FLC, is characterized by large polygonal tumor cells with prominent nuclei and dark nucleoli along with bands of lamellar fibrosis coursing through it. Tumor cells may contain pale body inclusions. FLC and LEL both are found predominantly in noncirrhotic livers. Scirrhous HCC, on the other hand, may be noted in livers with foci of otherwise typical HCC; the scirrhous component may be subcapsular. Neither the cytology of the tumor cells nor the character of the fibrous component are to be confused with FLC; in addition, it has been noted that treated HCC may become scirrhous in some areas.9 These tumors are considered separately from the sclerosing HCC that is associated with hypercalcemia.9 Sarcomatoid HCC is diagnosed as such when the majority of tumor cells are spindled; this phenotype may also be a result of treatment. With enough sections, identifiable typical HCC is commonly found.8, 9 LEL may or may not be related to Epstein‐Barr viral infection as other lympho‐epithelial carcinomas are.9 Varying quantities of mononuclear cells are present within these tumors. Many of the tumors have cholangiolar differentiation. Immunogenic stimuli are not understood.
New Topics in Pathology of Hepatocellular Carcinoma
Thanks to the careful work of our Japanese colleagues, there is now recognition of two types of HCC that are both <2 cm but have separate long‐term outcomes.10 The first, distinctly nodular, advanced,10 or progressed HCC,5 is the classical form of encapsulated HCC with unpaired arteries, usually moderately differentiated but with tumoral cytologic heterogeneity, and the long‐term prognosis similar to its larger counterparts. CD34 is positive in sinusoids of the tumor. There is a small but documented risk of intrahepatic tumor metastases, and invasion into portal veins. The second form of small HCC is known as early HCC.10, 11 The lesion is vaguely nodular, does not have a capsule, and even though unpaired arteries are present, portal tracts are as well. These tumors receive both arterial and portal blood supply, and CD34 is not uniformly present. Compared with surrounding parenchyma, portal tract numbers are fewer. Microscopically, the tumors are well‐differentiated with thin trabeculae, with or without pseudoacini, and blend imperceptibly into adjacent nontumorous parenchyma. Steatosis is common in early HCC; the N/C ratio is increased, lending an overall crowded appearance to the involved area. This lesion may be a challenge to distinguish from dysplastic nodules, and there may be East‐West discordance in individual cases.10, 11 The definitive demonstration of carcinoma is stromal invasion into the intratumoral portal tracts, but sufficient tumor sampling may not be present in a biopsy sample. Early HCC, as defined, carries a prognosis of slower tumor progression.9 A schematic of the progression of dysplastic nodules to early and progressed HCC was published and discussed recently.12 Figure 11 is a reprint of the schematic.
Figure 11.
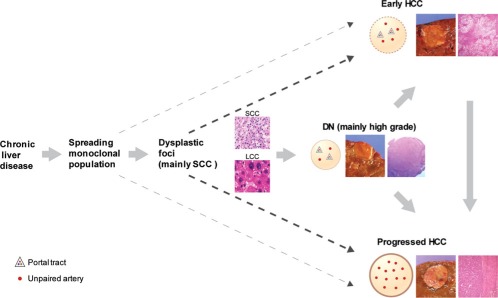
Schematic of the currently accepted concepts of progression of clonal populations in chronic liver disease to DNs, early HCC, and progressed HCC. Reprinted with permission from Archives of Pathology & Laboratory Medicine.12 Copyright College of American Pathologists.
With increased application of immunohistochemical panels and molecular studies, several investigators have shown the existence of liver carcinomas that “share” genetic and phenotypic expression of both hepatocellular and biliary differentiation, regardless of histologic findings. The tumors may be quite homogenous within themselves, or may be heterogenous with areas of clear‐cut classical HCC or, less likely, intrahepatic cholangiocarcinoma. Many of the tumors seemingly have nearly blended histologic features in differing fields, while others show an abrupt transition between the two. In many cases, tumor nests, cords, and gland‐like structures are present within sclerotic stroma. Terminology continues to be worked out by investigators in this field. The 2010 World Health Organization Classification of Tumors of the Digestive System9 refers to the entire group as combined hepatocellular‐cholangiocarcinoma with subgroups of classical type, and subtypes with stem cell features. Within the latter, there are three further subdivisions: typical, intermediate cell, and cholangiocellular types. Roncalli et al.5 have proposed three types: (1) HCC with stem/progenitor cell immunophenotype; (2) mixed hepatobiliary carcinoma, classical type; and (3) mixed hepatobiliary carcinoma with stem/progenitor cell phenotype and immunophenotype. These tumors are under intensive investigation, as it is apparent they are neither uncommon nor restricted to cirrhotic (or noncirrhotic) livers.
Differential Diagnostic Considerations of HCC
The differential diagnostic considerations of nodules in the liver largely depend on the status of the background nontumorous liver. The possibilities for cirrhotic liver differ from those of noncirrhotic liver, as shown in Fig. 12. The potentially most challenging lesions for pathologists to distinguish from HCC in cirrhotic livers are high‐grade dysplastic nodules (DN),10, 12 and in noncirrhotic livers, hepatocellular adenomas.13 Proposals for the first type have been made, including detailed analysis of various routine histologic features such as cord thickness, vascular and capsular invasion and nuclear features,14 or utilization of immunomarkers with heat shock protein 70 (HSP70), glutamine synthetase (GS), and glypican‐3 (GPC‐3) in various combinations.15 One group has suggested the use of annexin‐2.16 Di Tommaso et al.17 showed that in all DNs in cirrhotic livers studied, positivity for all three (HSP70, GS, and GPC‐3) was never seen, but the combination correctly identified 43.7% of HCCs; 72.7% of DN were negative for all three markers. The value of two or three positive markers in liver biopsies has also been published.11, 17 Annexin‐2 was most useful in its diffuse sinusoidal pattern (82% of HCCs versus 0 DNs) compared with the hepatocellular or zonal sinusoidal patterns.16 References are provided for an in‐depth review of adenoma and HCC.13
Figure 12.
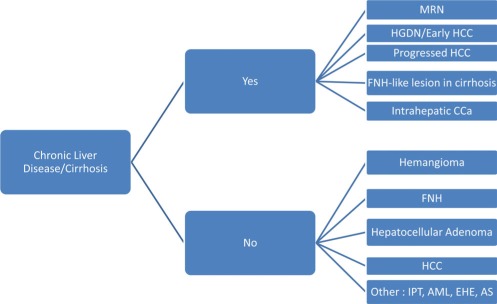
Differential diagnosis of nonmetastatic nodules in adult liver. Abbreviations: AML, angiomyolipoma; AS, angiosarcoma; CCa, cholangiocarcinoma; EHE, epithelioid hemangioendothelioma; FNH, focal nodular hyperplasia; HCC, hepatocellular carcinoma; HGDN, high‐grade dysplastic nodule; IPT, inflammatory pseudotumor; MRN, macroregenerative nodule.
Potential conflict of interest: Nothing to report.
References
- 1. Bruix J. Liver cancer: Still a long way to go. Hepatology 2011;54:1‐2. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parisi G. Should a radiological diagnosis of hepatocellular carcinoma be routinely confirmed by a biopsy? Yes. Eur J Intern Med 2012;23:34‐6. [DOI] [PubMed] [Google Scholar]
- 4. Schirmacher P, Bedossa P, Roskams T, Tiniakos DG, Brunt EM, Zucman‐Rossi J, et al. Fighting the bushfire in HCC trials. J Hepatol 2011;55:276‐277. [DOI] [PubMed] [Google Scholar]
- 5. Roncalli M, Park YN, Di Tommaso L. Histopathological classification of hepatocellular carcinoma. Dig Liver Dis 2010;42( suppl 3):S228‐S234. [DOI] [PubMed] [Google Scholar]
- 6. Park YN, Kojiro M, Di Tommaso L, Dhillon AP, Kondo F, Nakano M, et al. Ductular reaction is helpful in defining early stromal invasion, small hepatocellular carcinomas, and dysplastic nodules. Cancer 2007;109:915‐923. [DOI] [PubMed] [Google Scholar]
- 7. Lennerz JK, Chapman WC, Brunt EM. Keratin 19 epithelial patterns in cirrhotic stroma parallel hepatocarcinogenesis. Am J Pathol 2011;179:1015‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bedossa P, Paradis V. Hepatocellular carcinoma In: Saxena R, ed.Practical Hepatic Pathology: A Diagnostic Approach.St. Louis, MO:W.B. Saunders;2011:489‐501. [Google Scholar]
- 9. Theise ND, Curado MP, Franceschi S, Hytiroglou P, Kudo M, Park YN, et al. Hepatocellular carcinoma In: Bosman FT, Carneiro F, Hruban RH, Theise ND, eds.WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France:International Agency for Research on Cancer;2010:205‐216. [Google Scholar]
- 10. Kojiro M, Roskams T. Early hepatocellular carcinoma and dysplastic nodules. Semin Liver Dis 2005;25:133‐142. [DOI] [PubMed] [Google Scholar]
- 11. Roskams T, Kojiro M. Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis. Semin Liver Dis 2010;30:17‐25. [DOI] [PubMed] [Google Scholar]
- 12. Park YN. Update on precursor and early lesions of hepatocellular carcinomas. Arch Pathol Lab Med 2011;135:704‐715. [DOI] [PubMed] [Google Scholar]
- 13. Bioulac‐Sage P, Cubel G, Balabaud C, Zucman‐Rossi J. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin Liver Dis 2011;31:91‐103. [DOI] [PubMed] [Google Scholar]
- 14. Quaglia A, Jutand MA, Dhillon A, Godfrey A, Togni R, Bioulac‐Sage P, et al. Classification tool for the systematic histological assessment of hepatocellular carcinoma, macroregenerative nodules, and dysplastic nodules in cirrhotic liver. World J Gastroenterol 2005;11:6262‐6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Tommaso L, Franchi G, Park YN, Fiamengo B, Destro A, Morenghi E, et al. Diagnostic value of HSP70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology 2007;45:725‐734. [DOI] [PubMed] [Google Scholar]
- 16. Longerich T, Haller MT, Mogler C, Aulmann S, Lohmann V, Schirmacher P, et al. Annexin A2 as a differential diagnostic marker of hepatocellular tumors. Pathol Res Pract 2011;207:8‐14. [DOI] [PubMed] [Google Scholar]
- 17. Di Tommaso L, Destro A, Seok JY, Balladore E, Terracciano L, Sangiovanni A, et al. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol 2009;50:746‐754. [DOI] [PubMed] [Google Scholar]


