Sueda et al. show that in quiescent neural stem cells, Hes1 levels are oscillatory, although the peaks and troughs are higher than those in active neural stem cells, causing Ascl1 expression to be continuously suppressed.
Keywords: active neural stem cell, Ascl1, Hes1, notch signaling, oscillatory expression, quiescent neural stem cell
Abstract
Somatic stem/progenitor cells are active in embryonic tissues but quiescent in many adult tissues. The detailed mechanisms that regulate active versus quiescent stem cell states are largely unknown. In active neural stem cells, Hes1 expression oscillates and drives cyclic expression of the proneural gene Ascl1, which activates cell proliferation. Here, we found that in quiescent neural stem cells in the adult mouse brain, Hes1 levels are oscillatory, although the peaks and troughs are higher than those in active neural stem cells, causing Ascl1 expression to be continuously suppressed. Inactivation of Hes1 and its related genes up-regulates Ascl1 expression and increases neurogenesis. This causes rapid depletion of neural stem cells and premature termination of neurogenesis. Conversely, sustained Hes1 expression represses Ascl1, inhibits neurogenesis, and maintains quiescent neural stem cells. In contrast, induction of Ascl1 oscillations activates neural stem cells and increases neurogenesis in the adult mouse brain. Thus, Ascl1 oscillations, which normally depend on Hes1 oscillations, regulate the active state, while high Hes1 expression and resultant Ascl1 suppression promote quiescence in neural stem cells.
Somatic stem/progenitor cells actively divide and give rise to many mature cells in embryonic tissues, whereas they are usually dormant/quiescent in many adult tissues. For example, muscle stem/progenitor cells derived from the dermomyotome of the somite actively proliferate and generate skeletal muscles in embryos, whereas satellite cells in the adult skeletal muscle, known as muscle stem cells, are quiescent (Shi and Garry 2006). When muscles are injured, satellite cells are activated and contribute to muscle regeneration. Similarly, in the developing nervous system, neural stem/progenitor cells (neuroepithelial cells and radial glial cells) actively divide and sequentially produce many neurons and glial cells (Alvarez-Buylla et al. 2001; Götz and Huttner 2005). Neural stem cells are also present in two regions of the adult mouse brain: the subgranular zone (SGZ) of the hippocampal dentate gyrus and the subventricular zone (SVZ) of the lateral ventricles (Doetsch 2003; Kriegstein and Alvarez-Buylla 2009). These adult neural stem cells are mostly quiescent/dormant and only occasionally divide to produce some new neurons, which integrate into the pre-existing neural circuits (Seri et al. 2001; Lagace et al. 2007; Imayoshi et al. 2008a; Bonaguidi et al. 2011; Encinas et al. 2011; Pilz et al. 2018). The molecular nature of such differences between the embryonic active state and adult quiescent state of stem cells remains to be analyzed.
The process of activating quiescent neural stem cells in the adult brain has been intensively analyzed. A small fraction of neural stem cells (type 1 cells in the SGZ and type B cells in the SVZ) is activated to produce transit-amplifying cells (type 2 cells in the SGZ and type C cells in the SVZ), which proliferate and soon generate neuroblasts (type 3 cells in the SGZ and type A cells in the SVZ). These neuroblasts then migrate into the granular layer of the dentate gyrus (from the SGZ) and the olfactory bulb (from the SVZ). The proneural gene Ascl1 plays a critical role in the activation of quiescent neural stem cells and subsequent formation of neuroblasts in the mouse brain. Ascl1 is expressed at low levels by some activated neural stem cells (type 1 and type B cells) and at high levels by transit-amplifying cells (type 2 and type C cells) (Pastrana et al. 2009; Kim et al. 2011; Andersen et al. 2014). Furthermore, live-imaging analysis showed that Ascl1-expressing neural stem cells exclusively generate neurons in the adult mouse hippocampus (Pilz et al. 2018). In contrast, in the absence of Ascl1, all neural stem cells remain quiescent, and type 2 cells are never formed in the hippocampus, indicating that Ascl1 is absolutely required for activation of quiescent neural stem cells (Andersen et al. 2014). However, it was shown that overexpression of Ascl1 in adult hippocampal neural stem cells leads to exclusive generation of oligodendrocytes at the expense of neurons (Jessberger et al. 2008). Thus, the detailed mechanisms of how Ascl1 expression is controlled and how Ascl1 activates quiescent neural stem cells to induce neurogenesis are unknown.
Accumulating evidence indicates that Notch signaling plays an essential role in maintaining quiescent neural stem cells in the adult brain. Inactivation of the Notch pathway up-regulates Ascl1 expression, activates neural stem cells, and transiently enhances neurogenesis, but neural stem cells are soon depleted, ending neurogenesis prematurely (Ables et al. 2010; Ehm et al. 2010; Imayoshi et al. 2010; Andersen et al. 2014). Activation of Notch signaling induces the transcriptional repressor Hes1, and Hes1 suppresses Ascl1 expression, which may contribute to the quiescence of adult neural stem cells. However, Notch signaling is also required for maintaining active neural stem cells in the embryonic brain (Mason et al. 2005; Mizutani et al. 2007; Imayoshi et al. 2010). How Notch signaling regulates both the active and quiescent states of neural stem cells is unknown. One possible mechanism may be involved in the expression dynamics of Hes1. In multipotent embryonic neural stem cells, Hes1 expression autonomously oscillates, and these oscillations periodically repress Ascl1 expression, thereby driving Ascl1 oscillations (Shimojo et al. 2008; Imayoshi et al. 2013). Optogenetic gene expression analysis showed that sustained Ascl1 expression induces cell cycle exit and neuronal differentiation, whereas oscillatory Ascl1 expression activates the proliferation of neural stem cells (Imayoshi et al. 2013), suggesting that Hes1 oscillation-induced Ascl1 oscillation may be involved in activating neural stem cells. These observations raised the possibility that the expression patterns of the Notch effector Hes1 might be different in active and quiescent neural stem cells.
To understand the mechanism controlling active versus quiescent states of neural stem cells, we examined the expression and functions of Hes1 and Ascl1 in the adult brain and found that Hes1-induced sustained suppression of Ascl1 expression contributes to the quiescent state of adult neural stem cells. We also found that inducing Ascl1 oscillation can activate neural stem cells and generate new neurons in the adult brain.
Results
Hes1 and Ascl1 expression patterns in the adult mouse brain
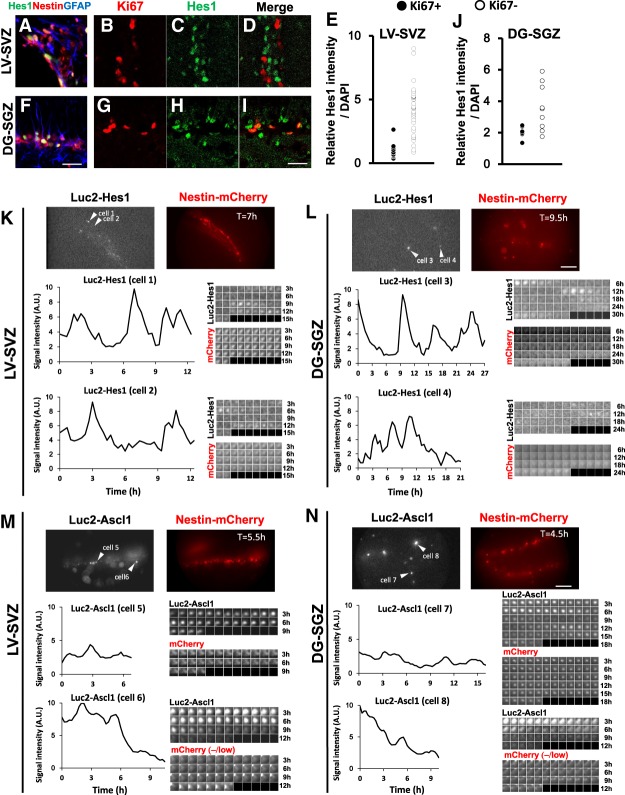
We first investigated Hes1 expression in the brains of adult Nestin-mCherry mice, in which neural stem cells were labeled with mCherry. Hes1 was specifically expressed at variable levels by Nestin-mCherry+;GFAP+ neural stem cells in both the SVZ and SGZ (Fig. 1A,F). To quantify the Hes1 expression levels, we next used Venus-Hes1 fusion knock-in mice, in which Venus (GFP variant) cDNA was knocked-in in-frame into the 5′ region of the Hes1 gene so that the Venus-Hes1 fusion protein was expressed (Imayoshi et al. 2013). In these mice, Venus expression correlated very well with the endogenous Hes1 expression (Imayoshi et al. 2013), and we used a GFP antibody to detect Hes1 expression. Levels of Hes1 were higher and more variable in quiescent neural stem cells (Ki67−) than in active neural stem cells (Ki67+) (Fig. 1B–E,G–J). To examine the Hes1 expression dynamics, we next used the Hes1 reporter mice, in which firefly luciferase (Luc2) cDNA was inserted in-frame into the 5′ region of the Hes1 gene in a bacterial artificial chromosome (BAC) clone so that the Luc2-Hes1 fusion protein was expressed (Imayoshi et al. 2013). The expression of the reporter in these mice was very similar to endogenous expression (Imayoshi et al. 2013). Neural stem cell lines (NS cells) established from the ventral telencephalon of these mice were cultured, and their luciferase activity was monitored. These cells were actively dividing in the presence of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) but became quiescent in the presence of bone morphogenetic protein (BMP) and bFGF, as observed in other neural stem cell cultures (Mira et al. 2010; Martynoga et al. 2013). Time-lapse imaging analysis indicated that Hes1 protein expression oscillated at lower levels in these NS cells when they were in an active state (Supplemental Fig. S1A). When these cells were switched to a quiescent condition, Hes1 expression was still oscillatory but highly up-regulated (Supplemental Fig. S1B,C). Importantly, under this condition, Hes1 expression was not completely repressed even at trough phases but rather was as high as peak levels of Hes1 oscillations in active NS cells. When these cells were returned to an active condition, Hes1 expression was down-regulated and oscillated at lower levels (Supplemental Fig. S1D). We next performed time-lapse imaging of adult brain slices of the Hes1 reporter mice crossed with Nestin-mCherry mice. In both the SGZ and SVZ regions, Hes1 expression oscillated at high levels in most of the mCherry+ neural stem cells (Fig. 1K,L; Supplemental Fig. S2A,B). Together, these results indicated that Hes1 expression is oscillatory but high in quiescent neural stem cells.
Figure 1.
Variable levels of Hes1 and Ascl1 in neural stem cells in the adult mouse brain. (A,F) Immunostaining of Hes1 in the SVZ of the lateral ventricle (LV-SVZ; A) and the SGZ of the dentate gyrus (DG-SGZ; F) of Nestin-mCherry mice (2 mo of age). (B–E,G–J) Hes1 levels in active (Ki67+) and quiescent (Ki67−) neural stem cells in the LV-SVZ (B–E) and the DG-SGZ (G–J) of the Venus-Hes1 fusion knock-in mice (2 mo of age) were examined. (E,J) Hes1 levels in active (Ki67+) and quiescent (Ki67−) neural stem cells were quantified. (K,L) Bioluminescence imaging and quantification of Luc2-Hes1 levels in Nestin-mCherry+ cells in slice cultures of the LV-SVZ (K) and the DG-SGZ (L). (M,N) Bioluminescence imaging and quantification of Luc2-Ascl1 levels in slice cultures of the LV-SVZ (M) and DG-SGZ (N). Luc2-Ascl1 levels were quantified in both Nestin-mCherry+ neural stem cells and Nestin-mCherry−/low transit-amplifying cells. Note that Luc2-Ascl1 levels are higher in Nestin-mCherry−/low transit-amplifying cells than in Nestin-mCherry+ neural stem cells. Next to cell 6, there is a Nestin-mCherry+ neural stem cell, which is negative for Luc2-Ascl1 expression. Scale bars: 25 µm (A–D,F–I); 100 µm (K–N).
We next examined Ascl1 expression using the Ascl1 reporter mice, in which Luc2 cDNA was inserted in-frame into the 5′ region of the Ascl1 gene in a BAC clone so that the Luc2-Ascl1 fusion protein was expressed (Imayoshi et al. 2013). NS cells established from the ventral telencephalons of these mice were cultured. Immunostaining showed that Ascl1 expression exhibited a “salt and pepper” pattern in the active state (Supplemental Fig. S1E) but was negative in the quiescent state (Supplemental Fig. S1F). Time-lapse imaging of luciferase activity indicated that Ascl1 protein expression was mostly negative in these NS cells when they were in a quiescent state, whereas it was up-regulated and oscillated when these cells were switched to an active condition (Supplemental Fig. S1G). We next performed time-lapse imaging of adult brain slices of the Ascl1 reporter mice crossed with Nestin-mCherry mice. In both the SGZ and SVZ regions, Ascl1 expression oscillated in subsets of mCherry+ neural stem cells at lower levels (Fig. 1M [cell 5], N [cell 7]; Supplemental Fig. S2C [cells 7 and 8], D [cells 10 and 11]). The expression occurred at higher levels in mCherry-negative/low transit-amplifying cells (Fig. 1M [cell 6], N [cell 8]; Supplemental Fig. S2C [cell 9], D [cell 12]). It was shown previously that Ascl1 is expressed by only a third of the active neural stem cell population as well as by transit-amplifying cells (Andersen et al. 2014). Thus, it is likely that because Ascl1 expression is oscillatory, it occurs in the majority (if not all) of activated neural stem cells even though a snapshot shows that only one-third of them express Ascl1.
Activation of adult neurogenesis by inactivation of Hes genes
The above expression analyses suggested that Hes1 expression was high in quiescent neural stem cells. We next examined whether Hes1 is required to maintain these cells in the adult brain. Hes1 was conditionally knocked out by crossing Hes1-floxed mice with Nes-CreERT2 mice, in which tamoxifen-inducible Cre recombinase was specifically expressed by neural stem cells (Imayoshi et al. 2006). Tamoxifen was administered to Nes-CreERT2;Hes1-floxed mice at 2 mo of age to induce Hes1 ablation in neural stem cells, but we did not see any significant defects in the maintenance of quiescent neural stem cells (data not shown). This is probably because the Hes1 deficiency was compensated for by other Hes and Hes-related genes, such as Hes5 and Hey1, as reported previously (Hatakeyama et al. 2004; Imayoshi et al. 2008b).
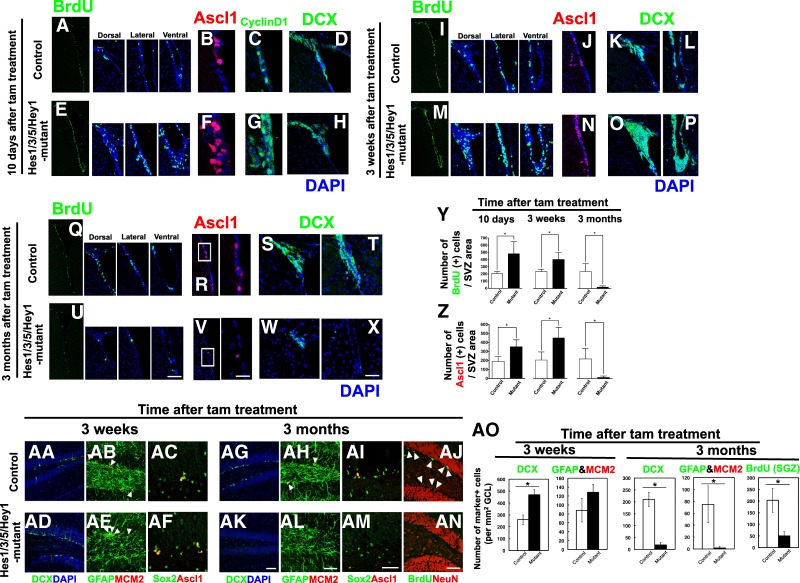
We next generated Hes1floxed/floxed;Hes3−/−;Hes5−/−;Hey1−/− mice and Nes-CreERT2;Hes1floxed/floxed;Hes3−/−;Hes5−/−;Hey1−/− mice. In the former mice, the maintenance of quiescent neural stem cells was not significantly affected, probably because Hes1 compensated for the Hes3, Hes5, and Hey1 deficiencies, and therefore these mice were used as controls (Fig. 2; Supplemental Fig. S3). To inactivate Hes1, tamoxifen was administered to Nes-CreERT2;Hes1floxed/floxed;Hes3−/−;Hes5−/−;Hey1−/− mice at 2 mo of age (referred to here as Hes1/3/5/Hey1 mutant mice), and brains were examined 10 d later. In the Hes1/3/5/Hey1 mutant SVZ, the numbers of Ascl1-positive cells and BrdU-incorporating and CyclinD1+-dividing cells significantly increased compared with the brains of control mice (Fig. 2A–C,E–G, Y [left panel], Z [left panel]). At this stage, neuroblasts (DCX+) in the mutants were not much different than in the controls (Fig. 2D,H). Three weeks after tamoxifen treatment, the numbers of both Ascl1-positive and BrdU-incorporating dividing cells and DCX+ neuroblasts significantly increased compared with in the controls (Fig. 2I–P,Y [middle panel], Z [middle panel]). However, 3 mo after tamoxifen treatment, Ascl1 expression mostly disappeared, and the numbers of BrdU-incorporating dividing cells and DCX+ neuroblasts significantly decreased in Hes1/3/5/Hey1 mutants compared with in the controls (Fig. 2Q–X,Y [right panel], Z [right panel]). Similarly, in the mutant hippocampal dentate gyrus, the number of DCX+ neuroblasts increased compared with in the controls 3 wk after tamoxifen treatment (Fig. 2AA,AD,AO). Furthermore, the number of active neural stem cells (GFAP+;MCM2+;Ascl1+) tended to be higher in the mutant dentate gyrus than in the controls (Fig. 2AB,AC,AE,AF,AO). However, 3 mo after tamoxifen treatment, the numbers of both DCX+ neuroblasts and GFAP+;MCM2+ neural stem cells were lower in Hes1/3/5/Hey1 mutants than in the controls (Fig. 2AG,AH,AK,AL,AO). Sox2+;Ascl1+ neural stem cells also tended to decrease in the mutant SGZ compared with in the controls (Fig. 2AI,AM). Furthermore, BrdU was administered for seven consecutive days to label both transit-amplifying cells and slowly dividing neural stem cells, but the number of BrdU+ cells significantly decreased in the mutant SGZ compared with in the controls (Fig. 2AJ,AN,AO). Thus, in adult Hes1/3/5/Hey1 mutant mice, Ascl1-dependent neurogenesis increases only transiently but then prematurely ceases due to depletion of neural stem cells. These results suggested that Hes and Hes-related genes cooperatively inhibit Ascl1-induced neurogenesis and regulate the maintenance of neural stem cells in the adult brain.
Figure 2.
Up-regulation of Ascl1 and premature loss of neural stem cells in the adult brains of Hes1/3/5/Hey1 mutant mice. Coronal sections of the SVZ of the lateral ventricles (A–Z) and the SGZ of the hippocampal dentate gyrus (AA–AO) of Hes1floxed/floxed;Hes3−/−;Hes5−/−;Hey1−/− mice (control) and Hes1/3/5/Hey1 mutant mice were examined by immunohistochemistry. BrdU was administered for 2 h before sacrifice. (A–H) Control (A–D) and Hes1/3/5/Hey1 mutant mice that were treated with tamoxifen 10 d before (E–H). (I–P) Control (I–L) and Hes1/3/5/Hey1 mutant mice that were treated with tamoxifen 3 wk before (M–P). (Q–X) Control (Q–T) and Hes1/3/5/Hey1 mutant mice that were treated with tamoxifen 3 mo before (U–X). Boxed regions in R and V are enlarged at the right. (Y,Z) Quantification of BrdU+ (Y) and Ascl1+ (Z) cells in control and Hes1/3/5/Hey1 mutant mice that were treated with tamoxifen 10 d (left), 3 wk (middle), and 3 mo (right) before BrdU administration. (AA–AN) Control (AA–AC,AG–AJ) and Hes1/3/5/Hey1 mutant mice that were treated with tamoxifen 3 wk (AD–AF) and 3 mo (AK–AN) before. (AJ,AN) BrdU was given for seven consecutive days before sacrifice. (AO) Quantification of DCX+, GFAP+;MCM2+, and BrdU+ cells in control and Hes1/3/5/Hey1 mutant mice that were treated with tamoxifen 3 wk (left) and 3 mo (right) before. (*) P < 0.05, Student's t-test. Scale bars, 50 µm.
We also treated these mice with tamoxifen at embryonic day 9.5 and found enhanced expression of proneural genes, including Ascl1; accelerated neurogenesis; and premature depletion of virtually all BrdU-incorporating active neural stem cells in the Hes1/3/5/Hey1 mutant mice (Supplemental Fig. S3D–F,H,M–P), whereas the control mice were mostly normal (Supplemental Fig. S3A–C,G,I–L). Thus, Hes and Hes-related genes are required to maintain both embryonic and adult neural stem cells.
Essential roles of Hes genes in maintaining adult neural stem cells
The above results suggest that following inactivation of Hes and Hes-related genes in the adult brain, all neural stem cells become transit-amplifying cells and neurons, leading to a transient increase in neurogenesis, but then neural stem cells are depleted, which eventually leads to an arrest of neurogenesis. To further confirm this notion, we quantified the label-retaining cells—those representing slowly dividing (or quiescent) neural stem cells—in Hes1/3/5/Hey1 mutant mice that had been injected with tamoxifen 10 d before. BrdU was given to both Hes1/3/5/Hey1 mutant mice and control mice for 14 consecutive days to label not only transit-amplifying cells but also slowly dividing neural stem cells (Fig. 3A). After 14 d of BrdU administration (t = 0), all dividing cells, including slowly dividing neural stem cells, were labeled by prolonged exposure to BrdU, whereas, 12 d after BrdU administration (t = 12), slowly dividing neural stem cells selectively retained BrdU because the BrdU was diluted out in fast-cycling transit-amplifying cells. In agreement with the above results (Fig. 2), there were significantly more dividing cells in Hes1/3/5/Hey1 mutant mice than in the controls at t = 0 (Fig. 3B,C,F). At t = 12, there were numerous label-retaining cells in the control mice (Fig. 3D,G), whereas the number of BrdU+ cells was severely reduced in Hes1/3/5/Hey1 mutant mice (Fig. 3E,G). These control mice had a number of BrdU-incorporating cells similar to that of wild-type control mice (Fig. 3F,G). These results indicate that slowly dividing neural stem cells are severely reduced in number as early as 10 d after the Hes/Hey genes are inactivated.
Figure 3.
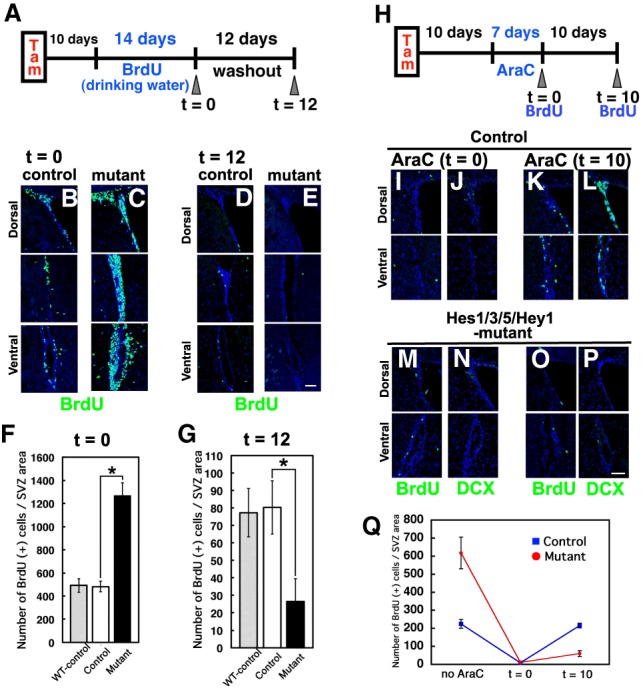
Depletion of slowly dividing neural stem cells and impairment of regeneration after cytosine-β-D-arabinofuranoside (AraC) treatment in Hes1/3/5/Hey1 mutant adult mouse brains. (A) Experimental design. (B–E) Dividing progenitors (t = 0; B,C) and slowly dividing cells, ones that retained labeling even after 12 d (t = 12; D,E), in the SVZ were labeled with BrdU in control (B,D) and Hes1/3/5/Hey1 mutant (C,E) mice. (F,G) Quantification of the number of BrdU-incorporating cells in the SVZ at t = 0 and t = 12 after BrdU administration for 14 consecutive days. WT-control (Hes1+/+;Hes3+/+;Hes5+/+;Hey1+/+) mice are shown as a reference. (H) Experimental design. (I–P) Coronal sections of the SVZ showing BrdU-incorporating cells soon after the removal of the osmotic pump (t = 0; I,J,M,N) and 10 d after the pump removal (t = 10; K,L,O,P) in AraC-treated control (I–L) and AraC-treated Hes1/3/5/Hey1 mutant (M–P) mice. (Q) Quantification of the number of BrdU-incorporating cells in the SVZ. BrdU was administered for 4 h before sacrifice. (*) P < 0.05, Student's t-test. Scale bars, 50 µm.
It has been shown that treatment with the antimitotic drug cytosine-β-D-arabinofuranoside (AraC) kills transit-amplifying cells but does not affect slowly dividing or quiescent neural stem cells (Morshead et al. 1994; Doetsch et al. 1999). Thus, while neurogenesis is transiently impaired by AraC treatment, the damaged brains are later reconstituted by slowly dividing or quiescent neural stem cells (Doetsch et al. 1999). We next examined this regeneration process in control and Hes1/3/5/Hey1 mutant mice that had been injected with tamoxifen 10 d before (Fig. 3H). After 7 d of AraC treatment (t = 0), cells that incorporated BrdU in BrdU pulse-labeling experiments (transit-amplifying cells) and DCX+ neuroblasts were mostly missing in both control and Hes1/3/5/Hey1 mutant mice (Fig. 3I,J,M,N,Q). At day 10 (t = 10), however, the BrdU+ transit-amplifying cell population in the control brains returned to normal numbers, indicating that neurogenesis had resumed (Fig. 3K,L,Q). In contrast, in the Hes1/3/5/Hey1 mutant brains, BrdU+ transit-amplifying cells did not reappear, and thus neurogenesis had not resumed by t = 10 (Fig. 3O–Q). This failed resumption was likely due to the lack of slowly dividing or quiescent neural stem cells at this stage in Hes1/3/5/Hey1 mutant mice. These results indicated that Hes/Hey genes are required for neuronal regeneration in AraC-treated brains by maintaining slowly dividing or quiescent neural stem cells.
Sustained Hes1 expression represses Ascl1 expression and inhibits neurogenesis
We next examined whether sustained Hes1 expression is sufficient for suppression of both Ascl1 expression and neurogenesis in the adult brain. We generated Rosa26 knock-in mice, in which continuous Hes1 and GFP expression was induced from the Rosa26 locus by Cre expression (Fig. 4A, bottom panel). These mice were crossed with Nes-CreERT2 mice to induce continuous Hes1 and GFP expression specifically in neural stem cells by tamoxifen treatment (R26-Hes1-iresGFP mice). As a control, we used Rosa26 knock-in mice, in which only CFP expression was induced from the Rosa26 locus by Cre expression (R26-CFP) (Fig. 4A, top panel). Tamoxifen was applied at 2 mo of age, and brains were examined 1 wk later. In the SVZs of control mice, many CFP-labeled cells incorporated BrdU (Fig. 4B,J). Furthermore, while some of the CFP-labeled cells expressed GFAP, a stem cell marker (Fig. 4D), many of them expressed Ascl1 or DCX (Fig. 4C,E,K), suggesting that CFP-labeled neural stem cells became active and produced transit-amplifying cells and neuroblasts within 1 wk in control mice. In contrast, in the SVZs of R26-Hes1-iresGFP mice, many GFP-labeled cells appeared, but virtually all of them expressed GFAP (Fig. 4H). Furthermore, very few of the GFP-labeled cells incorporated BrdU (Fig. 4F,J) or coexpressed Ascl1 or DCX (Fig. 4G,I,K). In the hippocampal dentate gyrus of R26-Hes1-iresGFP mice, sustained Hes1 expression did not efficiently occur. Therefore, we used lentivirus to induce sustained Hes1 expression (Fig. 4L). Many cells infected with the control virus (EF-Venus) expressed MCM2, Ascl1, GFAP, or DCX (Fig. 4M–P,U), whereas most cells infected with the Hes1 virus (EF-Hes1-Venus) expressed GFAP but not MCM2, Ascl1, or DCX (Fig. 4Q–U). These results indicated that sustained Hes1 expression is sufficient to not only repress Ascl1 expression but also inhibit neurogenesis, thereby maintaining quiescent neural stem cells in the adult brain.
Figure 4.
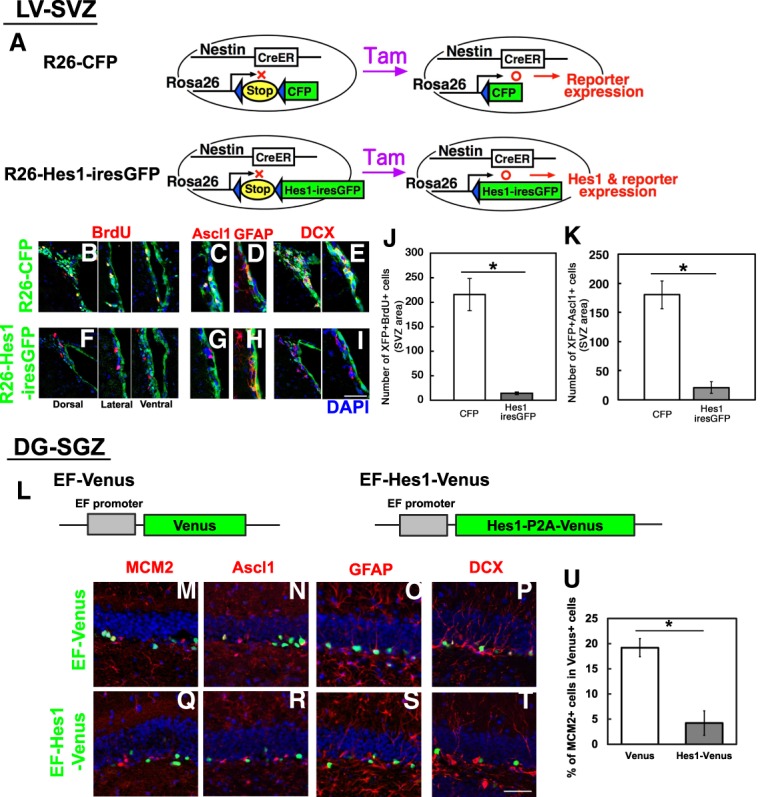
Suppression of Ascl1 expression and neurogenesis in the adult mouse brain by sustained expression of Hes1. (A) Experimental design. (B–I) Immunohistological analysis of the SVZ of R26-CFP (B–E) and R26-Hes1-iresGFP (F–I) mice that had been treated with tamoxifen 1 wk before. BrdU was administered for 2 h before sacrifice. (J,K) Quantification of BrdU-incorporating (J) and Ascl1-expressing (K) cells among CFP- or GFP-labeled cells. (L) Structures of lentiviruses. (M–T) Immunohistological analysis of the SGZ 1 wk after infection with EF-Venus virus (M–P) or EF-Hes1-Venus virus (Q–T). (U) Quantification of MCM2+ cells among virus-infected cells (Venus+). (*) P < 0.05, Student's t-test. Scale bars: 50 µm (B–I); 30 µm (M–T).
Optogenetic induction of Ascl1 oscillations activates neural stem cells in the adult brain
The above results suggest that high Hes1 expression and resultant Ascl1 suppression contribute to the maintenance of quiescent neural stem cells in the adult brain, which raised the possibility that inducing Ascl1 oscillations may activate quiescent neural stem cells and initiate neurogenesis. We tested this possibility by using the previously developed optogenetic Ascl1-inducing system, in which blue light-activated hGAVPO (Wang et al. 2012) induced Ascl1 expression (Supplemental Fig. S4A; Imayoshi et al. 2013). This system was introduced with lentiviruses (hGAVPO-mCherry virus and UAS-Ascl1 virus) into cultured NS cells that were then illuminated with blue light. Although this system efficiently induced Ascl1 expression in active NS cells, it only induced low levels of Ascl1 expression in quiescent NS cells (Supplemental Fig. S4C). In contrast, blue light-activated hGAVPO efficiently induced luciferase expression in quiescent NS cells (data not shown), suggesting that low levels of Ascl1 protein in a quiescent state was due to its fast degradation (Urbán et al. 2016). Indeed, it was shown previously that BMP signaling decreases Ascl1 protein stability (Viñals et al. 2004).
Because the Ascl1-E47 fusion protein is more active and stable than Ascl1 (Geoffroy et al. 2009), we next optogenetically induced the Ascl1-E47 fusion protein with hGAVPO-mCherry virus and UAS-Ascl1-E47-Venus virus (Supplemental Fig. S4B) and found that the Ascl1-E47 fusion protein was more efficiently induced in quiescent NS cells than Ascl1, reaching a level similar to that in active NS cells (Supplemental Fig. S4D). Using this system, we optogenetically induced the expression of Ascl1-E47 fusion protein in cultured quiescent NS cells. It was shown previously that 1 min of blue light exposure at 3-h intervals induced oscillatory expression, while 1 min of blue light exposure at 30-min intervals induced sustained expression (Imayoshi et al. 2013). Induction of Ascl1 oscillations efficiently activated quiescent NS cells to generate Ki67+DCX− cells at day 4 and day 7 (Supplemental Fig. S5A,B,D,F). In contrast, induction of sustained Ascl1 expression more efficiently generated Ki67+DCX+ and Ki67−DCX+ cells at day 4 and day 7 and depleted Ki67+DCX− cells by day 7 (Supplemental Fig. S5A,C,E–G). These results suggest that Ascl1 oscillations are able to activate cultured quiescent NS cells and maintain active NS cells more efficiently than sustained expression of Ascl1.
To induce Ascl1 oscillations in adult neural stem cells, we next introduced the same lentiviral system into the SGZ of the hippocampal dentate gyrus in adult mouse brains and implanted optic fibers to shine a blue light (Fig. 5A). We used 6-mo-old and 13- to 14-mo-old mice because very few neural stem cells express Ascl1 at these stages. Light pulses were applied to induce Ascl1 oscillations with 2.5-h periodicity for 1 or 2 wk (Fig. 5B; Supplemental Fig. S6A–C). After 1 wk of light stimulation, 39.1% ± 8.3% and 32.8% ± 3.5% of the cells infected with both hGAVPO-mCherry and UAS-Ascl1-E47-Venus viruses expressed Ki67 and Ascl1, respectively, in 6-mo-old mice (Fig. 5D–K,D′-K′,M,N). Furthermore, 15.6% ± 2.6% of the double-infected cells were positive for DCX (Fig. 5C–E,C′–E′,L), suggesting that neurogenesis was activated. In contrast, virtually none of the cells infected with hGAVPO-mCherry virus alone expressed Ascl1 or Ki67 (Fig. 5M,N), and most of these single-infected cells were negative for DCX (Fig. 5L). Similarly, neurogenesis was activated in cells infected with both hGAVPO-mCherry and UAS-Ascl1-E47-Venus viruses, but not in single-infected cells, in 13- to 14-mo-old mice after 1 wk of light stimulation (Supplemental Fig. S6D–L,D′–L′). These findings suggest that optogenetic induction of Ascl1 oscillations efficiently activated quiescent neural stem cells to initiate neurogenesis in adult mice.
Figure 5.
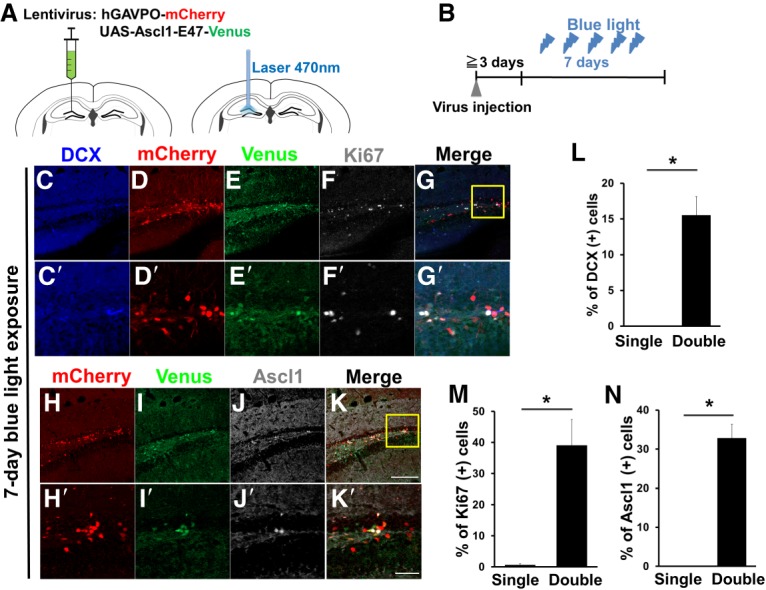
Optogenetic induction of Ascl1 oscillations in neural stem cells in the adult mouse brain (6 mo old; n = 3). (A,B) Experimental design. (A) After virus injection (left), optic fiber connected to Optoflash was implanted to illuminate with blue light (right). (B) Ascl1 oscillations with 2.5-h periodicity were induced, and brain sections were examined after 7 d of illumination. (C–K) Immunohistological analysis of cells in the adult mouse hippocampal dentate gyrus infected with hGAVPO-mCherry virus and UAS-Ascl1-E47-Venus virus. Boxed regions in G and K are enlarged in C′–G′ and H′–K′, respectively. (L–N) Quantification of marker expression in cells infected with both hGAVPO-mCherry virus and UAS-Ascl1-E47-Venus virus (double) or with hGAVPO-mCherry virus alone (single). (*) P < 0.05, Student's t-test. Scale bars: 100 µm (C–K); 30 µm (C′–K′).
To examine the long-term effects of Ascl1 oscillations, we continued light stimulation for a longer period. However, after 2 wk of light stimulation, significantly fewer double-infected cells expressed Ascl1 (Supplemental Fig. S6M–Q), indicating that the double-infected cells initially expressed Ascl1 by responding to light illumination, but most of them lost this ability within 2 wk. We also found that when sustained Ascl1 expression was optogenetically induced, there were very few double-infected cells that expressed Ascl1 even after 1 wk of light stimulation (data not shown). Furthermore, we noticed that cultured NS cells gradually lost their light responsiveness for Ascl1 expression (data not shown). Thus, it is likely that the hGAVPO-UAS system loses light responsiveness over time for unknown reasons, and therefore we were not able to examine longer effects of Ascl1 oscillations on neural stem cells.
Hes5 promoter-driven Ascl1 expression activates quiescent neural stem cells in the adult brain
To overcome the above difficulty, we tried an alternative approach to induce oscillatory Ascl1-E47 expression. It was reported that the Hes5 promoter can induce gene expression in neural stem cells (Lugert et al. 2010). We confirmed that injecting a lentivirus carrying Hes5 promoter-driven Venus cDNA successfully induced Venus expression in GFAP+;Sox2+ neural stem cells in the SGZ of the adult mouse hippocampus (Fig. 6A). Because the Hes5 promoter also induced oscillatory expression (Supplemental Fig. S7A), we used it to induce expression of the Ascl1-E47 fusion protein in cultured quiescent NS cells prepared from Delta-like1 (Dll1) reporter mice (Shimojo et al. 2016). Dll1 expression is directly controlled by Ascl1 (Castro et al. 2006). Therefore, Dll1 expression oscillates in active neural stem cells with oscillating Ascl1 expression, while it is sustained in differentiating neurons with sustained Ascl1 expression (Shimojo et al. 2008, 2016). There was no Dll1 reporter expression in NS cells when they were maintained in a quiescent state, but oscillatory Dll1 reporter expression occurred in NS cells when Hes5 promoter-driven Ascl1-E47 expression was induced (Supplemental Fig. S7B,C), suggesting that the Hes5 promoter successfully induced oscillatory Ascl1-E47 expression in quiescent neural stem cells.
Figure 6.
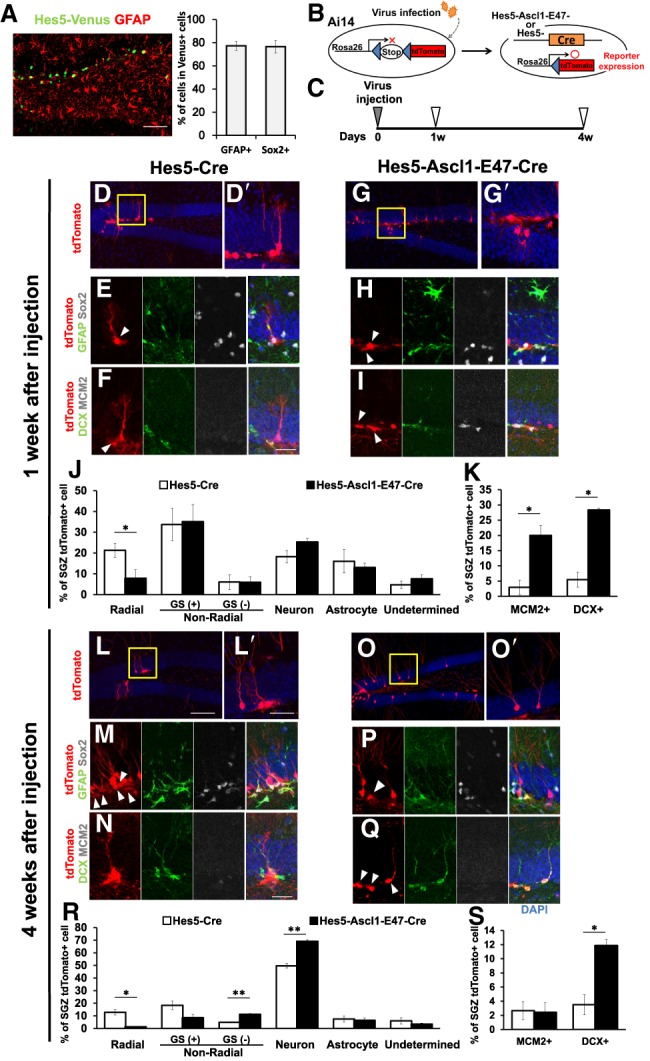
Induction of Ascl1-E47 expression by the Hes5 promoter in the adult mouse brain (5–8 mo old; n = 3). (A) Lentivirus carrying Hes5 promoter-driven Venus cDNA was injected into the hippocampal dentate gyrus of adult mice, and brains were examined 4 d later. (B,C) Experimental design. (D–I) Either Hes5-Cre virus (D–F) or Hes5-Ascl1-E47-Cre virus (G–I) was injected into the hippocampal dentate gyrus of Ai14 mice, and brains were examined 1 wk later. Boxed regions in D and G are enlarged in D′ and G′. (E) When the Hes5-Cre virus was infected, many tdTomato+ cells had a radial morphology and coexpressed GFAP and Sox2 (arrowhead). (F) Some coexpressed DCX, but MCM2 was mostly negative (arrowhead). (H,I) When the Hes5-Ascl1-E47-Cre virus was infected, many tdTomato+ cells had a nonradial morphology and coexpressed GFAP and Sox2 (H, arrowheads) or DCX and MCM2 (I, arrowheads). (J) Quantification of the indicated types of tdTomato+ cells in the SGZ. Radial cells are GFAP+;Sox2+ neural stem cells. Nonradial cells are either GFAP+/Sox2+-activated neural stem cells (GS+) or GFAP−;Sox2− transit-amplifying cells (GS−). Neurons and astrocytes were identified based on morphology and markers (DCX+ and/or NeuN+ for neurons and GFAP+ for astrocytes). There were very few NG2+ oligodendrocytes under this condition. (K) Quantification of the indicated marker expressions in tdTomato+ cells. (L–Q) Either Hes5-Cre virus (L–N) or Hes5-Ascl1-E47-Cre virus (O–Q) was injected into the hippocampal dentate gyrus of Ai14 mice, and brains were examined 4 wk later. Boxed regions in L and O are enlarged in L′ and O′. (M,P,Q) tdTomato+ cells coexpressing GFAP and Sox2 (M,P) or DCX (Q) are indicated by arrowheads. (R) Proportions of the indicated types of tdTomato+ cells in the SGZ and granule cell layer were quantified, as in J. (S) Quantification of the indicated marker expressions in tdTomato+ cells. (J,K,R,S) Open and closed bars represent cells infected with Hes5-Cre virus and Hes5-Ascl1-E47-Cre virus, respectively, in all graphs. (*) P < 0.05, (**) P < 0.01, Student's t-test. Scale bars: 50 µm (A,D,G,L,O); 20 µm (D′,E,F,G′,H,I,L′,M,N,O′,P,Q).
We also examined the effects of Hes5 promoter-driven Ascl1-E47 expression on in vitro NS cell cultures. Infection with a lentivirus that induced Ascl1-E47 expression under the control of the Hes5 promoter efficiently activated cultured NS cells (Ki67+DCX−) at day 4 and day 7 (Supplemental Fig. S7D,E), like the optogenetic induction of Ascl1-E47 oscillations. Infection with this virus also generated Ki67+DCX+ and Ki67−DCX+ cells at day 4 and day 7 (Supplemental Fig. S7D,F). These results indicated that Hes5 promoter-driven Ascl1-E47 expression is able to activate quiescent NS cells, which initiate proliferation and neuronal differentiation.
To analyze the in vivo effects of Hes5 promoter-driven Ascl1-E47 expression on adult neural stem cells, lentivirus-inducing Cre recombinase as well as Ascl1-E47 expression under the control of the Hes5 promoter (Hes5-Ascl1-E47-Cre virus) were prepared. As a control, we used lentivirus-inducing Cre recombinase only under the control of the Hes5 promoter (Hes5-Cre virus). These viruses were injected into the SGZs of the adult Ai14 mice, in which Cre recombinase labels not only infected cells but also their progeny with tdTomato (Fig. 6B; Madisen et al. 2010). We used Ai14 mice at 5–8 mo of age for the analysis because neurogenesis significantly decreases at this stage in the SGZ (Imayoshi et al. 2008a; Encinas et al. 2011). Brain sections were examined 1 and 4 wk later (Fig. 6C). One week after the Hes5-Cre control virus injection, many of the virus-infected cells (tdTomato+) had a radial morphology and coexpressed GFAP and Sox2 (Fig. 6D,D′,E,J, open bar), and very few of them expressed the cell cycle marker MCM2 (Fig. 6F,K, open bar), suggesting that these virus-infected cells were mostly quiescent neural stem cells. In contrast, 1 wk after the Hes5-Ascl1-E47-Cre virus injection, many virus-infected cells (tdTomato+) lost a radial morphology and down-regulated GFAP and Sox2 expression (Fig. 6G,G′,H,J, closed bar). Furthermore, 20.0% ± 3.3% of the virus-infected cells expressed the cell cycle marker MCM2 (Fig. 6I [arrowheads], K [closed bar]), suggesting that many neural stem cells were activated by the Hes5-Ascl1-E47-Cre virus within 1 wk, as observed for optogenetic induction of Ascl1-E47 oscillations. There were also more DCX+ neuroblasts at this stage (Fig. 6I,K, closed bar). Four weeks after the Hes5-Cre control virus injection, some tdTomato+ cells still had a radial morphology (Fig. 6L,L′,R, open bar) and were positive for GFAP and Sox2 but mostly negative for MCM2 (Fig. 6M,N,S, open bar), suggesting that these cells were quiescent neural stem cells. At this stage, there were only some DCX+ virus-infected neuroblasts (Fig. 6N,S, open bar), suggesting that the neurogenic activity of the control virus-infected neural stem cells remained low. In contrast, 4 wk after the Hes5-Ascl1-E47-Cre virus injection, the majority of the virus-infected cells (tdTomato+) differentiated into neurons (Fig. 6O,O′,R, closed bar). Furthermore, there were fewer GFAP+;Sox2+ virus-infected cells with a nonradial morphology (Fig. 6P, arrowhead), and 11.9% ± 0.9% of the virus-infected cells expressed DCX (Fig. 6Q [arrowheads], S [closed bar]), suggesting that the neurogenic activity of the virus-infected cells was maintained until this stage. Under this condition, only a minor population of the virus-infected cells differentiated into astrocytes or oligodendrocytes (Fig. 6R). These results suggest that Hes5 promoter-driven Ascl1-E47 expression activates neurogenesis and maintains neurogenic activity for a longer term in the adult brain.
Discussion
High levels of Hes1 expression regulate quiescent neural stem cells
The molecular nature of differences between the active and quiescent states of stem cells is not fully understood. Notch signaling is reportedly required to maintain both active and quiescent neural stem cells (Nyfeler et al. 2005; Ables et al. 2010; Ehm et al. 2010; Imayoshi et al. 2010; Veeraraghavalu et al. 2010), but how the same signaling pathway regulates such different states of neural stem cells remained to be determined. Here, we found that expression of the Notch signaling effector Hes1 oscillates in active neural stem cells but is high in quiescent neural stem cells (Fig. 7). Ascl1 expression also oscillates in active neural stem cells due to periodic repression by Hes1, while it is suppressed in quiescent neural stem cells by high Hes1 expression (Fig. 7). Ascl1 plays an important role in not only neuronal differentiation but also proliferation of neural stem cells, and these opposing functions are controlled by its sustained versus oscillatory expression: Oscillatory Ascl1 expression activates proliferation of neural stem cells, while sustained Ascl1 expression induces neuronal differentiation (Castro et al. 2011; Imayoshi et al. 2013). Furthermore, it was reported that Ascl1 is absolutely required for activation of quiescent neural stem cells (Andersen et al. 2014). Together, these findings suggest that high Hes1 expression and the resultant suppression of Ascl1 expression may contribute to the quiescent state of neural stem cells in the adult brain (Fig. 7). Indeed, inactivation of Hes1 and its related genes leads to Ascl1 expression and activation of quiescent neural stem cells, enhancing neurogenesis. In such activated neural stem cells, the function of Ascl1 could be oscillatory because the expression of Id1, which antagonizes Ascl1, is high (Nam and Benezra 2009) and could be oscillatory (William et al. 2007).
Figure 7.
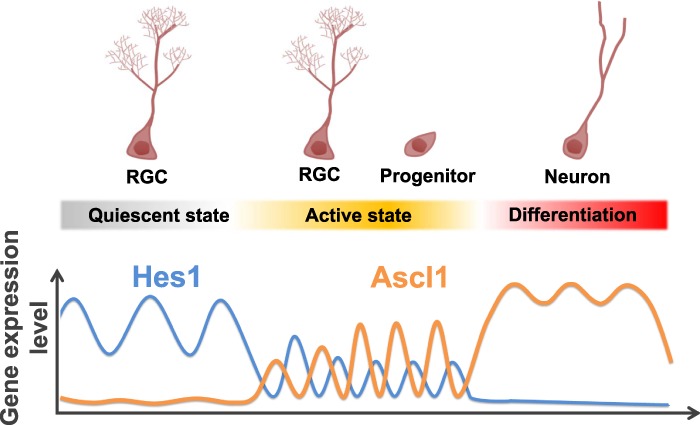
Expression dynamics of Hes1 and Ascl1 in quiescent and active neural stem cells and differentiating neurons. (RGC) Radial glia-like neural stem cell.
The detailed mechanism by which high levels of Hes1 are maintained in quiescent neural stem cells remains to be determined. Oscillatory expression of Hes1 is controlled by negative feedback: Hes1 represses its own expression by directly binding to its promoter (Hirata et al. 2002). It was reported that high levels of Id1 interact with Hes1 and inhibit its negative feedback, thereby up-regulating Hes1 expression (Bai et al. 2007; Boareto et al. 2017). Id1 expression is induced by BMP signaling (Miyazono and Miyazawa 2002), an essential pathway for the maintenance of quiescent neural stem cells (Mira et al. 2010). These results suggest that the BMP–Id–Hes axis may be the major pathway for suppressing Ascl1 and maintaining quiescent neural stem cells in the adult brain.
High Hes1 expression and quiescence
High levels of Hes1 are also observed in other types of quiescent cells. For example, high levels of Hes1 and the resultant suppression of Ascl1 and other proneural genes occur in the boundary regions of the embryonic brain, such as the isthmus, floor plate, and roof plate (Baek et al. 2006). In these regions, cells are mostly quiescent, suggesting that Hes1 and proneural factor oscillations may be important for cell cycle progression. Another example is the high levels of Hes1 found in quiescent hematopoietic stem/progenitor cells and quiescent muscle satellite cells (Yu et al. 2006; Mourikis et al. 2012). Furthermore, overexpression of Hes1 inhibits proliferation of hematopoietic stem/progenitor cells (Yu et al. 2006), suggesting that a high level of Hes1 is a common feature of cell quiescence. Similarly, it was reported that Hes1 is highly expressed by human fibroblasts when they enter quiescence due to serum deprivation or contact inhibition (Sang et al. 2008). Interestingly, these quiescent fibroblasts lost their ability to resume proliferation and entered senescence when Hes1 was knocked down, whereas high sustained Hes1 was sufficient to prevent these cells from entering senescence associated with prolonged cell cycle arrest (Sang et al. 2008). Hes1 also plays an important role in contact inhibition of proliferation in preadipocytes (Noda et al. 2011). Thus, high levels of Hes1 expression may be a general feature to prevent senescence and maintain quiescence of many cell types, including stem cells.
Ascl1 oscillations regulate active neural stem cells
Ascl1 is expressed in an oscillatory manner by active neural stem cells, and we found that inducing Ascl1 oscillations activates quiescent neural stem cells to generate new neurons in the adult brain. In both in vitro and in vivo experiments, optogenetic and Hes5 promoter-driven induction of Ascl1 oscillations activated neural stem cells to generate new neurons. When Hes5 promoter-driven Ascl1 oscillations were induced, the neurogenic activity was maintained for ∼4 wk, while it mostly declined in controls.
Ascl1 is required to generate both neurons and oligodendrocytes in the postnatal brain (Parras et al. 2004; Andersen et al. 2014). However, CAG promoter-induced overexpression of Ascl1 in adult hippocampal neural stem cells leads to exclusive generation of oligodendrocytes at the expense of neurons (Jessberger et al. 2008), although these neural stem cells normally generate neurons after Ascl1 expression (Pilz et al. 2018). In contrast, Hes5 promoter-driven induction of Ascl1 mostly generated neurons and virtually no oligodendrocytes in the adult hippocampus. The mechanism underlying these differences remains to be analyzed. One possibility concerns their different expression patterns: The Hes5 promoter is specific to neural stem cells and is therefore suppressed in differentiating cells, while the CAG promoter is active in both neural stem cells and differentiating cells. Ascl1 expression is normally down-regulated in differentiating neurons (Lo et al. 1991), and CAG promoter-induced continuous expression of Ascl1 might block neuronal differentiation and redirect neural stem cells toward the oligodendrocyte lineage at the expense of neurons. Another possibility is their different expression levels: The CAG promoter exhibits stronger activity than the Hes5 promoter, and high sustained Ascl1 expression might favor the oligodendrocyte lineage over the neuronal lineage. Alternatively, it is possible that Ascl1 functions together with Olig1 and Olig2, essential factors for oligodendrogenesis, whereas the Ascl1-E47 heterodimer does not. Further analyses are required to understand the mechanism by which Ascl1 differentially regulates specification of neurons and oligodendrocytes.
We demonstrated that the Hes5 promoter-driven Ascl1 expression system offers an efficient way to activate quiescent neural stem cells to induce neurogenesis in the adult brain. This method may be useful to manipulate endogenous stem cells for therapeutic purposes in patients with various brain disorders as an alternative to transplanting exogenous cells (Gaspard et al. 2008; Falkner et al. 2016).
Materials and methods
Transgenic mice
Hes1 flox mice, Hes3-null mice, Hes5-null mice, Nes-CreERT2 mice, Nestin-mCherry mice, Luc2-Hes1 mice, and Luc2-Ascl1 mice were generated before (Hatakeyama et al. 2004; Kokubo et al. 2005; Imayoshi et al. 2006, 2008b, 2013; Furutachi et al. 2015). Hey1-null mice and Ai14 mice were obtained from Yumiko Saga and Jackson Laboratory, respectively.
Tissue preparation and immunochemistry
Tissue preparation and immunochemical analyses were performed as described previously (Shimojo et al. 2016). The following primary antibodies (final dilution and source) were used: rabbit anti-Hes1 (1:500) (Kobayashi et al. 2009), mouse anti-βIII-tubulin (1:500; Babco), rat anti-BrdU (1:50; Oxford Biotech), goat antidoublecortin (DCX; 1:200; Santa Cruz Biotechnology), mouse anti-GFAP (1:200; Sigma), rabbit anti-GFAP (1:200; Sigma), mouse antimammalian achaete–schute homolog 1 (1:20; BD Pharmingen), mouse anti-Nestin (1:200; BD Pharmingen), rabbit anti-MCM2 (1:500; Abcam), mouse anti-cyclinD1 (1:200; Santa Cruz Biotechnology), goat anti-Sox2 (1:500; R&D Systems), rat anti-GFP (1:500; Nacalai Tesque), chicken anti-GFP (1:500; Abcam), and mouse anti-Ki67 (1:50; BD Biosciences).
Quantification of labeled cells and statistical analysis
The lateral SVZs and SGZs of more than three adult mice were analyzed in each experiment. A minimum of 10 coronal sections throughout the anterior–posterior extent was assessed for each animal. Stained cells were counted and expressed as the number of cells per 5 mm along the SVG or per square millimeter of the SGZ for each image. Student's t-test (one-tailed) was performed to calculate P-values.
Time-lapse imaging of NS cultures and brain slices
Time-lapse imaging of NS cultures and brain slices was performed as described previously (Imayoshi et al. 2013). For slice cultures, coronal brain slices (150-μm thickness) were transferred into cell culture inserts (Merck) on a glass-base dish with 135 mM NaCl2, 5 mM KCl, 10 mM HEPES, 1 mM CaCl2, and 1 mM MgCl2 bubbled with 100% O2 for 30 min at room temperature. Slices were immersed in type Ia collagen (Cellmatrix) diluted with slice culture medium (135 mM NaCl2, 5 mM KCl, 10 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 5% horse serum, 5% fetal bovine serum) and neutralizing buffer at room temperature. After 10 min of incubation at 37°C in 5% CO2 and 80% O2, slices were cultured at 37°C in slice culture medium containing 1 mM luciferin. After 6 h, the dish was placed on a stage of an inverted microscope and maintained at 37°C in 5% CO2 and 80% O2. Bioluminescence was acquired using a CCD camera, as described above.
Generation of R26-Hes1-iresGFP mice
Rosa26-loxP-stop-loxP-Hes1-iresEGFP knock-in embryonic stem cells (Kobayashi et al. 2009) were used to establish the mouse line, which was crossed with Nes-CreERT2 mice.
Tamoxifen treatment of mice
For activation of CreERT2, 10 mg of tamoxifen was administered orally to 2-mo-old mice once per day for four consecutive days. For controls, Rosa26-loxP-stop-loxP-CFP mice (Srinivas et al. 2001) were crossed with Nes-CreERT2 mice.
BrdU administration and AraC infusion
BrdU administration and AraC infusions were performed as described previously (Imayoshi et al. 2010).
Light stimulation in NS cell cultures by the hGAVPO system
Lentivirus was produced as described previously (Imayoshi et al. 2013). Blue light was delivered by LEDB-SBOXH (OptoCode) at 60 µmol/m2/sec for 1 min with 3-h intervals for oscillatory expression and for 1 min with 30-min intervals for sustained expression as described previously (Imayoshi et al. 2013; Isomura et al. 2017).
Lentiviral infection of adult mouse brain and light illumination with optic fibers
The coding sequence for codon-optimized Cre (iCre) or Ascl1-E47-P2A-iCre was subcloned into CSII-1.6-kb pHes5-MCS plasmid for lentivirus production. For optical controls of Ascl1 expression, hGAVPO-mCherry and UAS-Ascl1-E47-Venus viruses were applied. Viruses were delivered stereotactically into the dentate gyrus with the following coordinates: anteroposterior = −2 mm from bregma; lateral = ±1.5 mm; and ventral = 2.2 mm. Optic fiber connected with Optoflash (Bio Research Center Co., Ltd.) was implanted at the same injection sites immediately after viral injection with a dorsal–ventral depth of 1.6 mm from the skull. The light illumination condition was empirically determined because the light intensity of Optoflash is much weaker than OptoCode. For achieving oscillatory expression (2.5-h periodicity), blue light (470 nm) was delivered (one cycle consisted of 10 repeats of 2 min on at 5-min intervals followed by 80 min off). One week or 2 wk later, brain sections were immunohistologically examined.
Supplementary Material
Acknowledgments
We thank Yumiko Saga for Hey1-null mice, Hongkui Zeng for Ai14 mice, and Hitoshi Miyachi, Hiroyuki Okuno, and Kohei Jino for technical help. This work was supported by Core Research for Evolutional Science and Technology (CREST) (JPMJCR12W2 to R.K., and JPMJCR1752 to I.I.), Grant-in-Aid for Scientific Research on Innovative Areas (16H06480 to R.K., and 16H06529 to I.I.), and Grant-in-Aid for Scientific Research (B) (18H02449 to I.I.) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, and AMED-CREST (JP18gm1110002 to R.K.) and the Program for Technological Innovation of Regenerative Medicine (JP18bm0704020 to I.I.) from the Japan Agency for Medical Research and Development.
Author contributions: R.S., I.I., and Y.H. conducted the experiments. R.S., I.I., Y.H., and R.K. designed the experiments. R.S., I.I., and R.K. wrote the manuscript.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.323196.118.
Freely available online through the Genes & Development Open Access option.
References
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. 2010. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci 30: 10484–10492. 10.1523/JNEUROSCI.4721-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. 2001. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2: 287–293. 10.1038/35067582 [DOI] [PubMed] [Google Scholar]
- Andersen J, Urbán N, Achimastou A, Ito A, Simic M, Ullom K, Martynoga B, Lebel M, Göritz C, Frisén J, et al. 2014. A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron 83: 1085–1097. 10.1016/j.neuron.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek JH, Hatakeyama J, Sakamoto S, Ohtsuka T, Kageyama R. 2006. Persistent and high levels of Hes1 expression regulate boundary formation in the developing central nervous system. Development 133: 2467–2476. 10.1242/dev.02403 [DOI] [PubMed] [Google Scholar]
- Bai G, Sheng N, Bian W, Xie Z, Yokota Y, Benezra R, Kageyama R, Guillemot F, Jing N. 2007. Id sustains Hes1 expression to inhibit precocious neurogenesis by releasing negative autoregulation of Hes1. Dev Cell 13: 283–297. 10.1016/j.devcel.2007.05.014 [DOI] [PubMed] [Google Scholar]
- Boareto M, Iber D, Taylor V. 2017. Differential interactions between Notch and ID factors control neurogenesis by modulating Hes factor autoregulation. Development 144: 3465–3474. 10.1242/dev.152520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming G, Song H. 2011. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145: 1142–1155. 10.1016/j.cell.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DS, Skowronska-Krawczyk D, Armant O, Donaldson IJ, Parras C, Hunt C, Critchley JA, Nguyen L, Gossler A, Göttgens B, et al. 2006. Proneural bHLH and Brn proteins coregulate a neurogenic program through cooperative binding to a conserved DNA motif. Dev Cell 11: 831–844. 10.1016/j.devcel.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Castro DS, Martynoga B, Parras C, Ramesh V, Pacary E, Johnston C, Drechsel D, Lebel-Potter M, Garcia LG, Hunt C, et al. 2011. A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev 25: 930–945. 10.1101/gad.627811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. 2003. The glial identity of neural stem cells. Nat Neurosci 6: 1127–1134. 10.1038/nn1144 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716. 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- Ehm O, Göritz C, Covic M, Schäffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, et al. 2010. RBPJ(-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci 30: 13794–13807. 10.1523/JNEUROSCI.1567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park J-H, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. 2011. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8: 566–579. 10.1016/j.stem.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner S, Grade S, Dimou L, Conzelmann K-K, Bonhoeffer T, Götz M, Hübener M. 2016. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature 539: 248–253. 10.1038/nature20113 [DOI] [PubMed] [Google Scholar]
- Furutachi S, Miya H, Watanabe T, Kawai H, Yamasaki N, Harada Y, Imayoshi I, Nelson M, Nakayama KI, Hirabayashi Y, et al. 2015. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat Neurosci 18: 657–665. 10.1038/nn.3989 [DOI] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. 2008. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455: 351–357. 10.1038/nature07287 [DOI] [PubMed] [Google Scholar]
- Geoffroy CG, Critchley JA, Castro DS, Ramelli S, Barraclough C, Descombes P, Guillemot F, Raineteal O. 2009. Engineering of dominant active basic helix–loop–helix proteins that are resistant to negative regulation by postnatal central nervous system antineurogenic cues. Stem Cells 27: 847–856. 10.1002/stem.17 [DOI] [PubMed] [Google Scholar]
- Götz M, Huttner WB. 2005. The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6: 777–788. 10.1038/nrm1739 [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R. 2004. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development 131: 5539–5545. 10.1242/dev.01436 [DOI] [PubMed] [Google Scholar]
- Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R. 2002. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science 298: 840–843. 10.1126/science.1074560 [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Ohtsuka T, Metzger D, Chambon P, Kageyama R. 2006. Temporal regulation of Cre recombinase activity in neural stem cells. Genesis 44: 233–238. 10.1002/dvg.20212 [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. 2008a. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 11: 1153–1161. 10.1038/nn.2185 [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Shimogori T, Ohtsuka T, Kageyama R. 2008b. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development 135: 2531–2541. 10.1242/dev.021535 [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. 2010. Essential roles of Notch signaling in maintenance of neural stem cells in the developing and adult brains. J Neurosci 30: 3489–3498. 10.1523/JNEUROSCI.4987-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Isomura A, Harima Y, Kawaguchi K, Kori H, Miyachi H, Fujiwara TK, Ishidate F, Kageyama R. 2013. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science 342: 1203–1208. 10.1126/science.1242366 [DOI] [PubMed] [Google Scholar]
- Isomura A, Ogushi F, Kori H, Kageyama R. 2017. Optogenetic perturbation and bioluminescence imaging to analyze cell-to-cell transfer of oscillatory information. Genes Dev 31: 524–535. 10.1101/gad.294546.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Toni N, Clemenson GD Jr, Ray J, Gage FH. 2008. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci 11: 888–893. 10.1038/nn.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE. 2011. Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS ONE 6: e18472 10.1371/journal.pone.0018472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Mizuno H, Imayoshi I, Furusawa C, Shirahige K, Kageyama R. 2009. The cyclic gene Hes1 contributes to diverse differentiation responses of embryonic stem cells. Genes Dev 23: 1870–1875. 10.1101/gad.1823109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo H, Miyanaga-Tomita S, Nakazawa M, Saga Y, Johnson RL. 2005. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev Biol 278: 301–309. 10.1016/j.ydbio.2004.10.025 [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. 2009. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32: 149–184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, et al. 2007. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci 27: 12623–12629. 10.1523/JNEUROSCI.3812-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L-C, Johnson JE, Wuenschell CW, Saito T, Anderson DJ. 1991. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev 5: 1524–1537. 10.1101/gad.5.9.1524 [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, Haas CA, Kempermann G, Taylor V, Giachino C. 2010. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6: 445–456. 10.1016/j.stem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynoga B, Mateo JL, Zhou B, Andersen J, Achimastou A, Urbán N, van den Berg D, Georgopoulou D, Hadjur S, Wittbrodt J, et al. 2013. Epigenomic enhancer annotation reveals a key role for NFIX in neural stem cell quiescence. Genes Dev 27: 1769–1786. 10.1101/gad.216804.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HA, Rakowiecki SM, Raftopoulou M, Nery S, Huang Y, Gridley T, Fishell G. 2005. Notch signaling coordinates the patterning of striatal compartments. Development 132: 4247–4258. 10.1242/dev.02008 [DOI] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, Emeterio JS, Hortigüela R, Marqués-Torrejón MÁ, Nakashima K, et al. 2010. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7: 78–89. 10.1016/j.stem.2010.04.016 [DOI] [PubMed] [Google Scholar]
- Miyazono K, Miyazawa K. 2002. Id: a target of BMP signaling. Sci STKE 151: pe40 10.1126/stke.2002.151.pe40 [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. 2007. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449: 351–355. 10.1038/nature06090 [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. 1994. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 13: 1071–1082. 10.1016/0896-6273(94)90046-9 [DOI] [PubMed] [Google Scholar]
- Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. 2012. A critical requirement for Notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30: 243–252. 10.1002/stem.775 [DOI] [PubMed] [Google Scholar]
- Nam H, Benezra R. 2009. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell 5: 515–526. 10.1016/j.stem.2009.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda N, Honma S, Ohmiya Y. 2011. Hes1 is required for contact inhibition of cell proliferation in 3T3-L1 preadipocytes. Genes Cells 16: 704–713. 10.1111/j.1365-2443.2011.01518.x [DOI] [PubMed] [Google Scholar]
- Nyfeler Y, Kirch RD, Mantei N, Leone DP, Radtke F, Suter U, Taylor V. 2005. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J 24: 3504–3515. 10.1038/sj.emboj.7600816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. 2004. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J 23: 4495–4505. 10.1038/sj.emboj.7600447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, Cheng L-C, Doetsch F. 2009. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci 106: 6387–6392. 10.1073/pnas.0810407106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz G-A, Bottes S, Betizeau M, Jörg DJ, Carta S, Simons BD, Helmchen F, Jessberger S. 2018. Live imaging of neurogenesis in the adult mouse hippocampus. Science 359: 658–662. 10.1126/science.aao5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L, Coller HA, Roberts JM. 2008. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science 321: 1095–1100. 10.1126/science.1155998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. 2001. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21: 7153–7160. 10.1523/JNEUROSCI.21-18-07153.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Garry DJ. 2006. Muscle stem cells in development, regeneration, and disease. Genes Dev 20: 1692–1708. 10.1101/gad.1419406 [DOI] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. 2008. Oscillations in Notch signaling regulate maintenance of neural progenitors. Neuron 58: 52–64. 10.1016/j.neuron.2008.02.014 [DOI] [PubMed] [Google Scholar]
- Shimojo H, Isomura A, Ohtsuka T, Kori H, Miyachi H, Kageyama R. 2016. Oscillatory control of Delta-like1 in cell interactions regulates dynamic gene expression and tissue morphogenesis. Genes Dev 30: 102–116. 10.1101/gad.270785.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbán N, van den Berg DLC, Forget A, Andersen J, Demmers JAA, Hunt C, Ayrault O, Guillemot F. 2016. Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science 353: 292–295. 10.1126/science.aaf4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavalu K, Choi SH, Zhang X, Sisoda SS. 2010. Presenilin 1 mutants impair the self-renewal and differentiation of adult murine subventricular zone-neuronal progenitors via cell-autonomous mechanisms involving Notch signaling. J Neurosci 30: 6903–6915. 10.1523/JNEUROSCI.0527-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñals F, Reiriz J, Ambrosio S, Bartrons R, Rosa JL, Ventura F. 2004. BMP-2 decreases Mash1 stability by increasing Id1 expression. EMBO J 23: 3527–3537. 10.1038/sj.emboj.7600360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen X, Yang Y. 2012. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods 9: 266–269. 10.1038/nmeth.1892 [DOI] [PubMed] [Google Scholar]
- William DA, Saitta B, Gibson JD, Traas J, Markov V, Gonzalez DM, Sewell W, Anderson DM, Pratt SC, Rappaport EF, et al. 2007. Identification of oscillatory genes in somitogenesis from functional genomic analysis of a human mesenchymal stem cell model. Dev Biol 305: 172–186. 10.1016/j.ydbio.2007.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Alder JK, Chun JH, Friedman AD, Heimfeld S, Cheng L, Civin CI. 2006. HES1 inhibits cycling of hematopoietic progenitor cells via DNA binding. Stem Cells 24: 876–888. 10.1634/stemcells.2005-0598 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.