Abstract
Objective
The objective of this study was to identify subpopulations vulnerable to skin cancer by occupations, among individuals with Fitzpatrick skin types III and IV.
Methods
Data were retrieved from the national mortality registry of Korean National Statistical Office (KNSO) from 1993 to 2012, including all medical certificates of death written and confirmed by physicians. Medical certificates of death from 1993 to 2012 were obtained from the national mortality registry of Korean National Statistical Office. These completed medical certificates are verified by the Korean Ministry of Government Administration and Home Affairs and formatted using 103 main and 236 specific causes of death as recommended by the World Health Organization. We calculated direct standardized mortality rate and standardized mortality ratio (SMR) using the indirect standardization method. The entire population as reflected in the 2005 national census was used as a reference population.
Results
Of 594 deaths from skin cancer, 227 (38.2%) were from non‐melanotic skin cancer (NMSC) and 367 (61.8%) from cutaneous melanoma (CM). Compared to office workers, agriculture/fishery/forestry workers had significantly higher SMRs for NMSC in men [SMR: 461, 95% confidential interval (CI): 329‐583] and women (SMR: 575, 95% CI: 317‐864). SMR was also increased in men who worked in exposed area (SMR of NMSC:553, 95% CI:222‐1018, SMR of CM:453, 95% CI: 133‐1009).
Conclusion
This is the first Asian study to suggest that agriculture/fishery/forestry workers have increased SMRs for NMSC and CM in exposed areas. Early diagnosis of skin cancer in this group is important.
Keywords: occupation, skin cancer, standardized mortality ratio, ultraviolet
Abbreviations
- BCC
basal cell carcinoma
- CI
confidence interval
- CM
cutaneous melanoma
- NMSC
non‐melanotic skin cancer
- SCC
squamous cell carcinoma
- SMR
standardized mortality ratio
- UVB
ultraviolet B radiation
1. INTRODUCTION
Skin cancer is less common in people of color than in Caucasians.1 This protective effect is associated with increased melanocyte activity and larger and well‐dispersed melanosomes.2, 3, 4 Nevertheless, the incidence of skin cancer is slowly increasing in Far East Asian countries such as Korea, Japan, and China. In Korea, skin cancer, including non‐melanotic skin cancer (NMSC) and cutaneous melanoma (CM), was the tenth most common cancer in 2014. According to the National Cancer Registry, the number of skin cancer patients increased from 3091 in 2006 to 5078 in 2014, and the fraction of skin cancer among all types of cancer from 1.4% to 2.0%. In Japan, the incidence of NMSC increased between the mid‐1950s and 2000.5 According to the National Cancer Registry of Singapore, the incidence of skin cancer increased between 1968 and 2006, especially among elderly Chinese.6
Occupational skin cancers have been due to industrial exposure to specific carcinogens such as polycyclic hydrocarbons (eg from coal tar products) or to arsenic.7, 8, 9 The carcinogens may lead cellular damage in the workplace with the skin of workers.10, 11 Industrial processes have improved in most developed countries to limit the chemical carcinogens.12, 13 However, workers with prolonged exposure to ultraviolet (UV) lights still have greater opportunity to UV irradiation without recognition to this industrial hazard.14, 15, 16
NMSC and CM are both related to UV exposure.14 Although skin carcinogenesis is still not fully understood, several papers demonstrated that genetic and molecular alterations are involved in this process.16, 17, 18 In terms of NMSC, ultraviolet B radiation (UVB)‐induced DNA damage in keratinocytes causes the molecular alteration, induces carcinogenesis, and triggers the production of cytokines such as interleukin‐10, which may contribute to tumor escape from the host immune response.18, 19, 20 Since these molecular mechanisms of UVB damage are also associated with the increased incidence of basal cell carcinoma (BCC) in Asians,21 UVB protection is likely as important for Asians as for Caucasians. In terms of CM, Viros et al22 show that mutant Trp53 accelerated BRAF‐driven melanomagenesis and that TP53 mutations are linked to evidence of UVR‐induced DNA damage in human melanoma.
Skin cancer is still not considered a public health concern in Asian countries. Moreover, reliable national‐level data on outdoor UVB exposure according to sex and occupation are lacking. Thus, we sought to determine the standardized mortality ratio of skin cancer, including NMSC and CM, and identify vulnerable subpopulations in needed of focused preventive measures.
2. METHODS
2.1. Data source and target population
Data were retrieved from the national mortality registry of Korean National Statistical Office (KNSO) from 1993 to 2012, including all medical certificates of death written and confirmed by physicians. Physicians are under obligation to record the cause of death and other staff to input other demographic characteristics such as age, sex, educational level, occupation, marital status, and residential area at the time of death. These completed medical certificates are verified by the Korean Ministry of Government Administration and Home Affairs and formatted using 103 main and 236 specific causes of death as recommended by the World Health Organization. The corrected data then become publicly available, with unrestricted online access.
This study analyzed the entire population aged from 25 to 55 years. This lower age limit was chosen because most Korean men serve in the army for 20 months, and the occupational status is very unstable in both men and women in their early 20s. To investigate mortality from skin cancer according to occupation, we also excluded individuals over 55 years old since economic activity decreases dramatically in this age group.
2.2. Skin cancer classification
The cause of death in the national mortality registry is formatted according to the International Classification of Diseases‐10 system (ICD‐10). Accordingly, we used the ICD‐10 codes “C43” for CM and “C44” for NMSC. Although the ICD‐10 system does not distinguish BCC from squamous cell carcinoma (SCC) under “C44,” location of the neoplasm can be determined using three‐digit ICD‐10 codes. Skin cancer of face and neck generally was regarded as sun‐exposed areas.23 Thus, we divided skin cancer into exposed and unexposed areas to evaluate the effect of UVB (Table 1).
Table 1.
The classification of skin in exposed and unexposed areas of body parts
| Skin cancers in exposed areas | Skin cancers in unexposed areas | ||
|---|---|---|---|
| C43.0/C44.0 | lip | C43.5/C44.5 | trunk |
| C43.1/C44.1 | eyelid, including canthus | C43.6/C44.6 | upper limb, including shoulder |
| C43.2/C44.2 | ear and external auricular canal | C43.7/C44.7 | lower limb, including hip |
| C43.3/C44.3 | other and unspecified parts of face | C43.8/C44.8 | overlapping of skin |
| C43.4/C44.4 | scalp and neck | ||
International classification of “C43.9/C44.9,” which means the unspecified cutaneous melanoma, is excluded in the subgroup analysis according to the body parts. However, we analyzed all cutaneous melanoma with C43 including this unspecified subgroup in following Table 2.
2.3. Occupation classification
We used the national classification system known as “the Korean Standard Classification of Occupations,” which comprises 10 major occupational groups used in death records. To increase the statistical power, we redefined the occupational categories as follows: “office workers,” “service/trade/manual workers,” and “agriculture/fishery/forestry workers” (Figure 1).
Figure 1.
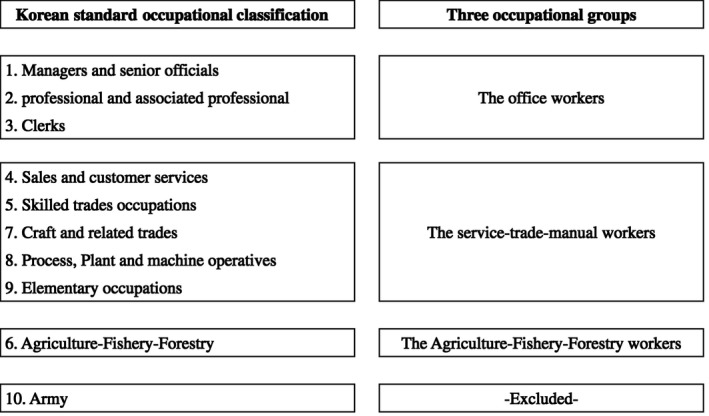
Schematic diagram for reorganization of “Korean Standard Classification of Occupations” as three occupational groups
2.4. Statistical analysis
We calculated direct standardized mortality rate and standardized mortality ratio (SMR) using the indirect standardization method. The entire population as reflected in the 2005 national census was used as a reference population. Direct standardized mortality rate was calculated by multiplying age‐specific mortality rates for each occupational category by the size of the corresponding reference subpopulation. In contrast, to calculate SMR, the age‐specific mortality rate determined for the reference subpopulation was multiplied by the subpopulation size in the occupational category according to each age group to give the expected death rate. Finally, the SMR was defined as the ratio of the total observed number of deaths to the total expected number of deaths in each occupational category. To obtain 95% confidence intervals (CIs) of SMR in age groups with <30 deaths, we applied the approximated mid‐p test after assuming the Poisson distribution in the target population.24 The resulting value was multiplied by 100, and the number of observed deaths was considered to be twice the expected value when SMR was 200.
3. RESULTS
3.1. All types of skin cancer
In total, 594 individuals died from skin cancer, including 227 (38.2%) from NMSC and 367 (61.8%) from CM (Table S1). Of them, 488 (82.2%) were men and 106 (17.8%) were women. The manual worker group had the lowest standardized mortality rate in both men (0.24 cases per 100 000) and women (0.07/100 000), while agriculture/fishery/forestry workers had the highest values in both men (0.60/100 000) and women (0.27/100 000). Men had higher standardized mortality rates than women in all the occupational categories.
Office workers were used as the reference category for SMR analysis. Compared to the reference group, agriculture/fishery/forestry workers had the highest SMRs in both men (SMR: 201, 95% CI: 153‐244) and women (SMR: 217, 95% CI: 139‐295), with statistical significance.
3.2. Non‐melanotic skin cancer (ICD‐code: C44)
Among the 227 deaths from NMSC, 195 (85.9%) were in men and 32 (14.1%) in women (Table 2). Manual/service/trade workers had the lowest standardized mortality rate in both men (0.10/100 000) and women (0.01/100 000), while agriculture/fishery/forestry workers had the highest rate in both men (0.44/100 000) and women (0.17/100 000). Men had higher direct standardized mortality rates than women in all the occupational categories.
Table 2.
Direct standardized mortality rate per 100 000 population and standardized mortality ratio by gender and occupation
| Areas | Gender | Occupation categories | Observed death | Direct standardized mortality rate (per 100 000 population) | Standardized mortality ratio (95% confidence interval) |
|---|---|---|---|---|---|
| (A) Non‐melanotic skin cancer | |||||
| Total areas | Men | Office workers (reference) | 52 | 0.08 (0.03‐0.14) | 100 (72‐125) |
| Manual‐Service‐Trade workers | 95 | 0.10 (0.04‐0.16) | 124 (98‐147) | ||
| Agriculture‐Fishery‐Forestry workers | 48 | 0.44 (0.30‐0.56) | 461 (329‐583) | ||
| Women | Office workers (reference) | 7 | 0.02 (0.00‐0.09) | 100 (40‐184) | |
| Manual‐Service‐Trade workers | 9 | 0.01 (0.00‐0.08) | 63 (28‐108) | ||
| Agriculture‐Fishery‐Forestry workers | 16 | 0.17 (0.08‐0.29) | 575 (317‐864) | ||
| Exposed areas | Men | Office workers (reference) | 8 | 0.01 (0.00‐0.05) | 100 (43‐177) |
| Manual‐Service‐Trade workers | 16 | 0.02 (0.00‐0.06) | 144 (79‐216) | ||
| Agriculture‐Fishery‐Forestry workers | 7 | 0.07 (0.03‐0.13) | 553 (222‐1018) | ||
| Women | Office workers (reference) | 2 | 0.01 (0.00‐0.06) | 100 (17‐303) | |
| Manual‐Service‐Trade workers | 2 | 0.00 (0.00‐0.06) | 53 (9‐159) | ||
| Agriculture‐Fishery‐Forestry workers | 1 | 0.01 (0.00‐0.07) | 167 (13‐751) | ||
| (B) Cutaneous melanoma (total area) | |||||
| Total areas | Men | Office workers (reference) | 52 | 0.08 (0.03‐0.14) | 100 (72‐125) |
| Manual‐Service‐Trade workers | 95 | 0.10 (0.04‐0.16) | 124 (98‐147) | ||
| Agriculture‐Fishery‐Forestry workers | 48 | 0.44 (0.30‐0.56) | 461 (329‐583) | ||
| Women | Office workers (reference) | 7 | 0.02 (0.00‐0.09) | 100 (40‐184) | |
| Manual‐Service‐Trade workers | 9 | 0.01 (0.00‐0.08) | 63 (28‐108) | ||
| Agriculture‐Fishery‐Forestry workers | 16 | 0.17 (0.08‐0.29) | 575 (317‐864) | ||
| Exposed areas | Men | Office workers (reference) | 5 | 0.01 (0.00‐0.04) | 100 (34‐205) |
| Manual‐Service‐Trade workers | 11 | 0.01 (0.00‐0.05) | 150 (73‐245) | ||
| Agriculture‐Fishery‐Forestry workers | 4 | 0.03 (0.01‐0.07) | 453 (133‐1009) | ||
| Women | Office workers (reference) | 4 | 0.01 (0.00‐0.08) | 100 (29‐223) | |
| Manual‐Service‐Trade workers | 1 | 0.00 (0.00‐0.05) | 10 (1‐46) | ||
| Agriculture‐Fishery‐Forestry workers | 1 | 0.00 (0.00‐0.06) | 50 (4‐227) | ||
Since we multiplied 100 to the calculated value of standardized mortality ratios, it is able to be interpreted that the number of observed deaths is twice more than that of the expected deaths when SMR is 200. In addition, if upper and lower confidential intervals are both above or below 100, there are meaningful statistical differences compared to the reference office workers group.
Compared to the reference group, agriculture/fishery/forestry workers had the highest SMRs in both men (SMR: 461, 95% CI: 329‐583) and women (SMR: 575, 95% CI: 317‐864), with statistical significance.
3.3. Cutaneous melanoma (ICD‐CODE: C43)
Among the 367 deaths from CM, 293 (79.8%) were in men and 74 (20.2%) in women (Table 2). Manual/service/trade workers had the lowest direct standardized mortality rate in both men (0.14/100 000) and women (0.06/100 000), while agriculture/fishery/forestry workers had the highest rate in both men (0.16/100 000) and women (0.10/100 000). Men had higher direct standardized mortality rates than women in all the occupational categories. Compared to the reference group, agriculture/fishery/forestry workers displayed no statistically significant differences in SMR in men (SMR: 92, 95% CI: 56‐130) and women (SMR: 118, 95% CI: 59‐189).
To evaluate the effect of UV exposure on skin cancer reliably, we analyzed NMSC and CM in exposed areas (Table 2). The corresponding SMR of exposed areas for male agriculture/fishery/forestry workers (SMR of NMSC:553, 95% CI:222‐1018, SMR of CM:453, 95% CI: 133‐1009) was significantly higher than that for office workers. In contrast, there were no statistically significant differences between the values for NMSC and CM in unexposed areas (data not shown).
4. DISCUSSION
Although skin cancer is less common in people with dark skin than in Caucasians, it is often associated with increased morbidity and mortality.1 In addition, the thinning of the ozone layer, aging of the society, and popularity of outdoor activities contribute to the incidence of skin cancer. Therefore, improved understanding of skin cancer in people of color is crucial. We investigated the relationship between skin cancer mortality rate and occupation and found statistically significant differences. Thus, agriculture/fishery/forestry workers had the highest SMRs for NMSC compared to office workers in both men and women. Furthermore, agriculture/fishery/forestry workers also showed the highest SMRs for CM in exposed areas in men, while there were no significant differences in SMRs for unexposed areas across the occupational categories.
Several epidemiological studies found that exposure to sunlight increased the risk of skin cancer.25, 26 Kricker et al investigated the association between occupation and NMSC occurrence using 10 published studies from Australia, USA, Canada, Ireland, and Italy.27 Although the association between NMSC and occupation‐related sunlight exposure was weaker, outdoor workers appeared to be at a higher risk, which increased with the level of exposure. Positive associations between skin cancer and total sun exposure were also reported in other studies, although most of them did not adjust for other factors. Gallagher et al conducted two separate studies on newly diagnosed cases of BCC and SCC between 1983 and 1984.28 Based on the Alberta Cancer registry for individuals between 25 and 70 years old, lifetime occupational and recreational childhood exposure were related to BCC. Moreover, lifetime occupational exposure was definitely positively associated with SCC, especially when the latest diagnostic methods were used. Outdoor workers show high risk of skin cancer, and that risk differs by outdoor activity.29 To summarize, there is a clear association between NMSC (both BCC and SCC) and UV exposure. This increased risk could be attributed both to occupational and recreational exposure.
Based on the key epidemiologic evidence described above, we suggest that biological, toxicological, and sociological aspects may be involved in the pathogenesis of skin cancer. First, prolonged UVB exposure during working hours might increase the SMR among agriculture/fishery/forestry workers. Although the relation between UVB exposure and skin cancer is weaker in Asians than in Caucasians, UVB still plays a significant role in NMSC and CM. For example, UVB‐induced interleukin‐10 expression is associated with inhibition of antitumor T‐cell immune response, leading to tumor growth in both melanoma and NMSC.30 Moreover, DNA damage by UV radiation triggers cutaneous carcinogenesis by altering the expression of oncogenes and tumor suppressor genes, including p53 and patched genes, which are responsible for transformation in keratinocytes.31, 32 Second, chemical and toxicological exposure may also explain the increased SMR in agriculture/fishery/forestry workers. For example, exposure to insecticides, herbicides and fungicides can increase odds ratios of SCC.33 Since all these chemicals are frequently used by agriculture/fishery/forestry workers, we cannot exclude their role in skin cancer. Finally, the availability of professional medical services can differ in urban and rural areas. Most agriculture/fishery/forestry workers reside in rural areas, resulting in restricted access to skin cancer screening. The National Health Interview Survey for cancer control reported that 4‐7% of agriculture/fishery/forestry workers undergo clinical skin examination during their lifetime, compared to 15% of general US workers.34 Furthermore, general practitioners not specializing in dermatology and plastic surgery are often unable to diagnose skin cancer early because of lack of symptoms. Even when some symptoms are present, the lack of clinical experience and skin cancer‐related education might delay targeted treatment, with symptomatic treatment used instead. In addition, since Korean dermatologists and plastic surgeons in private clinics mainly focus on cosmetic procedures rather than dermatologic diseases, most of such clinics are located in urban areas. All these factors may contribute to delayed diagnosis of skin cancer in agriculture/fishery/forestry workers, ultimately leading to increased morbidity and mortality.35
This study has the following strengths. First, this is the first Asian study reporting an association between occupation and SMR for NMSC and CM. In Korea, the cancer registry was launched later than in Japan and Singapore, and occupation‐dependent SMRs for skin cancer were not assessed in published studies. Second, the study analyzed the entire population of South Korea, suggesting high reliability and internal validity compared with single‐center studies or those using sample pooling. Third, the results may be generalized to Asians with Fitzpatrick skin types III and IV residing at the same latitude and altitude. Fourth, the subgroup analysis of CM in exposed/unexposed areas suggested that increased UV exposure might increase the risk of CM in Asians, while there was no significant difference in SMR for CM in unexposed areas for any occupation.
Our study also has several limitations. First, the exact population structure by age in each occupational group is unknown. Accordingly, we used a proxy population based on the 2005 national census data and assumed that changes in population structure by age were minimal during the study period. Therefore, the results may be somewhat inaccurate and not completely applicable to other years given the profound changes in the population structure. Second, we could not adjust for many demographic and socioeconomic factors, such as educational status, income level, marital status, smoking, and drinking. However, the purpose of this study was to identify occupational groups at increased risk of skin cancer, and the findings are meaningful at the large scale. Third, we could not distinguish between BCC and SCC within NMSC and between the pathologic subtypes of BCC, SCC, and CM. Although occupational solar exposure is mainly associated with SCC and nonoccupational or recreational exposure with BCC, these two types of skin cancer could not be distinguished because of the limitations of the ICD‐10 system. In addition, since the pathologic subtype of CM is critical for prognosis, the national death registry data should be combined with more specific individual medical records. Fourth, we could not measure a history of other exposures to carcinogens due to the data limitation. However, even though there are other carcinogenesis mechanisms except for UV‐induced DNA mutation, UV is one of the main occupational carcinogens. Therefore, we have tried to focus on that exposure. Finally, we could not measure the amount of UV exposure according to occupation directly. However, many previous studies also assumed that exposure was proportional to working hours spent outside. The nine occupational groups were categorized into three groups to ensure sun‐exposed occupational group. Although we have no validity methods to classify the sun‐exposed occupation, “Agriculture‐Fishery‐Forestry worker” usually more worked outside compare to other occupation. ISCO can be categorized into nonmanual versus manual workers, nonmanual worker included managers and senior officials, professional and associated professional and clerks. Other occupations are manual workers. Among manual workers, we though Agriculture‐Fishery‐Forestry worker is occupation group usually worked outside with sun exposure, hence we divided manual workers into two categories. However, our assumption has no validity, yet. More comprehensive occupational categories were needed to clarify the association between sun‐exposed and risk of skin cancer in Asian population. Skin cancers generally are treated by outpatient clinic, and the mortality of skin cancer is depend on its’ pathological stage.36 For example, 10‐year survival of stage 1 melanoma is 92%, that of stage 2 is 80% and that of stage 3 is 50%. Hence, our current results may underestimate mortality rate.
This is the first study showing that Asian agriculture/fishery/forestry workers have increased SMRs for NMSC and CM in exposed areas. In light of the increasing annual incidence of skin cancer and thinning of the ozone layer, skin cancer could become a public health concern in Korea in the near future. Thus, measures should be taken to improve early skin cancer detection in agriculture/fishery/forestry workers. Further study should evaluate the possible disparity in availability of skin cancer‐related health services.
DISCLOSURE
Approval of the research protocol: N/A. Informed Consent: Since we used national published secondary data in website, we did not get any individual consent form. Registry and Registration No. of the study: N/A. Animal Studies: N/A.
CONFLICT OF INTEREST
Authors declared that there was no conflict of interesting.
AUTHOR CONTRIBUTIONS
Jaeyong Shin and Kee Yang Chung led the writing; Eun‐Cheol Park and Jin‐Ha Yoon analyzed the data; Kyoung Ae Nam and Jin‐Ha Yoon MD, PhD conceived the ideas; Jin‐Ha Yoon collected the data.
Supporting information
ACKNOWLEDGEMENT
N/A.
Shin J, Chung KY, Park E‐C, Nam KA, Yoon J‐H. Occupational differences in standardized mortality ratios for non‐melanotic skin cancer and melanoma in exposed areas among individuals with Fitzpatrick skin types III and IV. J Occup Health. 2019;61:235–241. 10.1002/1348-9585.12040
REFERENCES
- 1. Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55(5):741‐760; 61‐4. [DOI] [PubMed] [Google Scholar]
- 2. Andersen KE, Maibach HI. Black and white human skin differences. J Am Acad Dermatol. 1979;1(3):276‐282. [DOI] [PubMed] [Google Scholar]
- 3. Montagna W, Carlisle K. The architecture of black and white facial skin. J Am Acad Dermatol. 1991;24(6 Pt 1):929‐937. [DOI] [PubMed] [Google Scholar]
- 4. Berardesca E, Maibach H. Racial differences in skin pathophysiology. J Am Acad Dermatol. 1996;34(4):667‐672. [DOI] [PubMed] [Google Scholar]
- 5. Ohtsuka H, Nagamatsu S. Changing trends in the number of deaths from nonmelanoma skin cancer in Japan, 1955–2000. Dermatology. 2005;210(3):206‐210. [DOI] [PubMed] [Google Scholar]
- 6. Sng J, Koh D, Siong WC, Choo TB. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61(3):426‐432. [DOI] [PubMed] [Google Scholar]
- 7. Diepgen T, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146:235‐6. [DOI] [PubMed] [Google Scholar]
- 8. Knobeloch LM, Zierold KM, Anderson HA. Association of arsenic‐contaminated drinking‐water with prevalence of skin cancer in Wisconsin's Fox River Valley. J Health Popul Nutr. 2006;206‐213. [PubMed] [Google Scholar]
- 9. Boström C‐E, Gerde P, Hanberg A, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sena JS, Girão R, Carvalho SMFd, et al. Occupational skin cancer: systematic review. Revista da Associação Médica Brasileira. 2016;62(3):280‐286. [DOI] [PubMed] [Google Scholar]
- 11. Yu RC, Hsu K‐H, Chen C‐J, Froines JR. Arsenic methylation capacity and skin cancer. Cancer Epidemiol Prev Biomarkers. 2000;9(11):1259‐1262. [PubMed] [Google Scholar]
- 12. Gawkrodger D. Occupational skin cancers. Occup Med. 2004;54(7):458‐463. [DOI] [PubMed] [Google Scholar]
- 13. Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8(3):444‐472. [DOI] [PubMed] [Google Scholar]
- 14. Diepgen T, Fartasch M, Drexler H, Schmitt J. Occupational skin cancer induced by ultraviolet radiation and its prevention. Br J Dermatol. 2012;167:76‐84. [DOI] [PubMed] [Google Scholar]
- 15. Chen AC, Halliday GM, Damian DL. Non‐melanoma skin cancer: carcinogenesis and chemoprevention. Pathology. 2013;45(3):331‐341. [DOI] [PubMed] [Google Scholar]
- 16. Bowden GT. Prevention of non‐melanoma skin cancer by targeting ultraviolet‐B‐light signalling. Nat Rev Cancer. 2004;4(1):23. [DOI] [PubMed] [Google Scholar]
- 17. Madan V, Lear JT, Szeimies R‐M. Non‐melanoma skin cancer. The Lancet. 2010;375(9715):673‐685. [DOI] [PubMed] [Google Scholar]
- 18. Pfeifer GP, Besaratinia A. UV wavelength‐dependent DNA damage and human non‐melanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11(1):90‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagano T, Kunisada M, Yu X, Masaki T, Nishigori C. Involvement of interleukin‐10 promoter polymorphisms in nonmelanoma skin cancers‐a case study in non‐Caucasian skin cancer patients. Photochem Photobiol. 2008;84(1):63‐66. [DOI] [PubMed] [Google Scholar]
- 20. Wehner MR, Shive ML, Chren M‐M, Han J, Qureshi AA, Linos E. Indoor tanning and non‐melanoma skin cancer: systematic review and meta‐analysis. BMJ. 2012;345:e5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748‐762. [DOI] [PubMed] [Google Scholar]
- 22. Viros A, Sanchez‐Laorden B, Pedersen M, et al. Ultraviolet radiation accelerates BRAF‐driven melanomagenesis by targeting TP53. Nature. 2014;511(7510):478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oh CM, Cho H, Won YJ, et al. Nationwide Trends in the Incidence of Melanoma and Non‐melanoma Skin Cancers from 1999 to 2014 in South Korea. Cancer Res Treat. 2018;50(3):729‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen GR, Yang SY. Mid‐P confidence intervals for the Poisson expectation. Stat Med. 1994;13(21):2189‐2203. [DOI] [PubMed] [Google Scholar]
- 25. Young C. Solar ultraviolet radiation and skin cancer. Occup Med. 2009;59(2):82‐88. [DOI] [PubMed] [Google Scholar]
- 26. Moan J, Grigalavicius M, Baturaite Z, Dahlback A, Juzeniene A. The relationship between UV exposure and incidence of skin cancer. Photodermatol Photoimmunol Photomed. 2015;31(1):26‐35. [DOI] [PubMed] [Google Scholar]
- 27. Kricker A, Armstrong BK, English DR. Sun exposure and non‐melanocytic skin cancer. Cancer Causes Control. 1994;5(4):367‐392. [DOI] [PubMed] [Google Scholar]
- 28. Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentation factors, and risk of nonmelanocytic skin cancer. II. Squamous cell carcinoma. Arch Dermatol. 1995;131(2):164‐169. [PubMed] [Google Scholar]
- 29. Zink A, Tizek L, Schielein M, Bohner A, Biedermann T, Wildner M. Different outdoor professions have different risks ‐ a cross‐sectional study comparing non‐melanoma skin cancer risk among farmers, gardeners and mountain guides. J Eur Acad Dermatol Venereol. 2018;32(10):1695‐1701. [DOI] [PubMed] [Google Scholar]
- 30. Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84(3):539‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodust PM, Stockfleth E, Ulrich C, Leverkus M, Eberle J. UV‐induced squamous cell carcinoma–a role for antiapoptotic signalling pathways. Br J Dermatol. 2009;161(Suppl 3):107‐115. [DOI] [PubMed] [Google Scholar]
- 32. Nishigori C. Cellular aspects of photocarcinogenesis. Photochem Photobiol Sci. 2006;5(2):208‐214. [DOI] [PubMed] [Google Scholar]
- 33. Dennis LK, Lynch CF, Sandler DP, Alavanja MC. Pesticide use and cutaneous melanoma in pesticide applicators in the agricultural heath study. Environ Health Perspect. 2010;118(6):812‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59(1):55‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moreno‐Ramirez D, Ojeda‐Vila T, Rios‐Martin JJ, et al. The role of accessibility policies and other determinants of health care provision in the initial prognosis of malignant melanoma: a cross‐sectional study. J Am Acad Dermatol. 2014;71(3):507‐515. [DOI] [PubMed] [Google Scholar]
- 36. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199‐6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


