Abstract
Objectives
2‐Ethyl‐1‐hexanol (2EH), a fragrance ingredient and a raw material for the production of plasticizer di(2‐ethylhexyl) phthalate, is responsible for sick building syndrome (SBS). This review aims to clarify the 2EH characteristics as an indoor air pollutant such as indoor air concentration, emission mechanism, toxicity, and clinical effects.
Methods
Scientific publications in English that has been made available on PubMed as of June 2018 and ad hoc publications in regional languages were reviewed.
Results
Inhalation exposure to 2EH caused mucous membrane irritation in the eyes, nose, and throat in experimental animals. Studies in human volunteers revealed an increase in olfactory irritation and eye discomfort. There has been increasing evidence of 2EH being present in indoor air in buildings. The primary sources of 2EH emissions are not building materials themselves, but instead the hydrolysis of plasticizers and flooring adhesives. In particular, compounds like di(2‐ethylhexyl) phthalate present in polyvinyl chloride flooring materials are hydrolyzed upon contact with alkaline moisture‐containing concrete floors. That being said, it may be observed that indoor concentrations of 2EH increased every year during summer.
Conclusions
Unlike other volatile organic compounds that cause SBS, 2EH can be retained in indoor air for long durations, increasing the likelihood of causing undesirable health effects in building occupants exposed to it. As a precautionary measure, it is important to use flooring materials that do not emit 2EH by hydrolysis, or to dry concrete before covering with flooring materials.
Keywords: 2‐ethyl‐1‐hexanol, emission mechanism, indoor air pollution, indoor concentration, sick building syndrome, volatile organic compounds
1. INTRODUCTION
Indoor air pollution triggers symptoms of irritation, such as dryness in the skin and eyes, and pain in the nose and throat. It also causes psychoneurotic symptoms, such as dizziness, nausea, and headache. These symptoms, which are common among occupants of nonindustrial buildings such as offices and schools, have been defined as sick building syndrome (SBS). This syndrome is caused by chemical factors such as formaldehyde and other volatile organic compounds (VOCs),1, 2 biological factors such as mold and tick,3, 4 or physical factors such as temperature and humidity.5, 6 In recent years, the VOC 2‐ethyl‐1‐hexanol (2EH) (CAS No. 104‐76‐7) has drawn attention as one of the prominent causes of SBS.7
2‐Ethyl‐1‐hexanol is used mainly as a raw material for the production of di(2‐ethylhexyl) phthalate (DEHP), a plasticizer for polyvinyl chloride (PVC), and as a fragrance component in cosmetics,8 but is hardly detected in outdoor environments. 2EH is a known metabolite of the plasticizer DEHP, a hepatic carcinogen in rodents.9 Based on a long‐term study in rats in which the no‐observed‐effect level (NOEL) of 2EH was found to be 50 mg/kg/bw/day with a safety factor of 100, the acceptable daily intake (ADI) of 2EH in humans was established as 0‐0.5 mg/kg body weight by the Joint Food and Agriculture Organization‐World Health Organization Expert Committee on Food Additives.10
It was reported that 2EH is detected at high concentrations in buildings where occupants complained of SBS symptoms.7 Mucosal irritation in the eyes and nose were reported as the primary endpoints in studies examining the effects of 2EH exposure in humans and animals.11, 12 However, this compound may also affect human health at low concentrations. The recommended 8‐hour time‐weighted average occupational exposure limit of 2EH is 5.3 mg/m3 (1 ppm) in Europe13 and Japan.14 On the other hand, the preliminary reference concentration of 2EH concentration in general indoor environments is 0.1 mg/m3, same as the Guide Value I (precautionary value) in Germany.15 The reference concentration for general environment is also being considered in Japan.16
An important characteristic of 2EH as an indoor air pollutant is the seasonal fluctuation of its indoor air concentration; it is detected at higher concentrations in high‐temperature and humid seasons and at markedly lower concentrations during winter.17, 18 Therefore, unless appropriate countermeasures are taken to reduce 2EH emissions, its indoor air concentration will periodically be high, which could cause long‐term exposure to 2EH in people exposed to high indoor concentrations of 2EH.19
Risk assessment studies have reported several effects of oral20, 21, 22 and inhalation exposure23, 24 to 2EH in animals. However, these effects, along with other characteristics of 2EH as an indoor air pollutant, have not been summarized in any comprehensive review. Therefore, this review aims to summarize current findings of relevant publications on the indoor air concentration, emission mechanism, toxicity, and clinical effects of 2EH.
We reviewed literature in English that has been made available on PubMed and Google Scholar as of June 2018 and ad hoc publications in regional languages. We searched papers with the term “2‐ethyl‐1‐hexanol” and chose the literature were related to indoor air pollutant and toxicity.
2. PHYSICAL PROPERTIES AND USE OF 2EH
The physical and chemical properties of 2EH are summarized inTable 1. There are many household products manufactured using 2EH. The plasticizers DEHP and di(2‐ethylhexyl) adipate (DEHA), used in the processing of plastic and rubber, are produced with 2EH as a raw material. 2EH is also used as a raw material for the production of 2‐ethylhexyl acrylate, an adhesive component, and as a fragrance ingredient in decorative cosmetics, fine fragrances, toiletries (such as shampoos and soaps), and non‐cosmetic products, such as household cleaners and detergents.8 In the environment, 2EH is volatilized from soil or water surfaces into the atmosphere.25
Table 1.
The physical and chemical properties of 2EH
| Property | Value | Reference |
|---|---|---|
| Molecular formula | ‐ | |
| Structure formula |
|
‐ |
| CAS No. | 104‐76‐7 | ‐ |
| Molecular weight | 130.23 g/mol | ‐ |
| Physical form | Colorless, oily liquid with mild, sweat, and slightly floral‐rosy odor | 8 |
| Melting point | ‐75°C | 26 |
| Boiling point | 188.52°C | 8 |
| Density at 20°C | 0.834 g/cm3 | 8 |
| Vapor density | 4.49 g/cm3 | 26 |
| Vapor pressure | 0.06 mmHg at 20°C, 0.185 mmHg at 25°C | 8 |
| Solubility | Soluble in organic solvents | 27 |
| Solubility in water | 880 mg/L at 25°C, 1000 mg/L at 20°C | 27 |
| Odor threshold | 0.075 ppm (perception), 0.138 ppm (100% recognition) | 27 |
| Conversion factors for vapor (25°C 1013 hPa) | 1 ppm = 5.32 mg/m3 | 25 |
3. TOXICOKINETICS OF 2EH
2‐Ethyl‐1‐hexanol is absorbed by the gastrointestinal tract and skin. Alcohol dehydrogenase (ADH) rapidly oxidizes the hydroxyl group in 2EH, forming 2‐ethyl‐1‐hexanal. It is further oxidized by aldehyde dehydrogenase (ALDH), forming 2‐ethyl‐1‐hexanoic acid (2EHA), which is excreted mainly as a glucuronate conjugate in urine. ADH activity for 2EH was reported to be 8.6 nmol/mg/min and 4.2 nmol/mg/min in humans and mice, respectively. Furthermore, ALDH activity for 2EH was 3.6 nmol/mg/min and 5.6 nmol/mg/min in humans and mice, respectively.28
Within 24 hours of orally administrating 2EH at 8.3 mmol/kg, 86.9% of the compound was excreted in urine as the glucuronide conjugate metabolite.29, 30
Following oral administration at doses of up to 300 mg/kg, 2EH was efficiently absorbed in male CD rats. Within 28 hours, 2EH metabolite was excreted in exhaled breath (as CO2; 6%‐7%), feces (8%‐9%), and urine (80%‐82%). The major urinary metabolite of 2EH was 2EHA, generated by decarboxylation of partially β‐oxidized 2EH. The other identified metabolites were 2‐ethyl‐5‐hydroxyhexanoic acid, 2‐ethyl‐5‐ketohexanoic acid, and 2‐ethyl‐1,6‐hexanedioic acid. Almost all (96.1%) of the administered 2EH was excreted as a metabolite and only approximately 3% was excreted unchanged.31
In another study, dermal administration of 2EH at 1 g/kg resulted in only 5% of the compound being absorbed at a rate of 0.57 mg/cm2/h.32 In a comparative study, the percutaneous absorption rates of 2EH in male rats and humans were 0.22 mg/cm2/h and 0.038 mg/cm2/h, respectively, with a (rat/human) ratio of 5.78.33 2EH was detected in the exhaled breath at 4 μg/m3 (0.0008 ppm).34 2EH was also detected on the skin surface.35 The concentration of 2EH gas released from the hand skin was 39.9‐136.2 μg/m3 (7.7‐25.6 ppb).36 Moreover, 2EH was considerably more abundant in the stool of neonates than adults, suggesting that neonates may be more susceptible to risks from exposure than adults to plastic materials containing plasticizers.37
4. EXPOSURE SCENARIO
4.1. Sources of 2EH Emissions
The general population may be exposed to 2EH from inhalation of ambient air, ingestion of food and drinking water, or dermal absorption of this compound or other products containing 2EH.25 Studies have reported 2EH emission from various sources, such as carpets,38, 39 furnitures,40 computers,41 books,42, 43 and food wrappings.44 Building materials, such as insulation and gypsum board,45 wallpaper,46 paint,47 PVC flooring,48 and adhesives,49 are also sources of 2EH emissions.
Several reports point out that flooring is a prominent source of 2EH air pollution in buildings. The region of the highest 2EH concentrations in apartment houses was concrete slabs surface, which was directly in contact with a vinyl carpet.50 In a school conference room with a 2EH air concentration of 1902 µg/m3, the rates of 2EH emission from the carpet tile and concrete surface beneath the carpet were 2492 μg/h/m2 and 12,697 μg/h/m2, respectively, measured using the double‐cylinder chamber method.51 It was also reported that 2EH concentrations in the air increased with the amount of 2EH emitted from the floor.52 In a study investigating VOC emission using a field and laboratory emission cell (FLEC) method, 2EH was found to be 47%‐76% of the total VOCs emitted from the floor coverings.53 2EH was emitted from the surface of a concrete floor after its PVC floor covering was removed.54 One study revealed that 2EH emission by a PVC flooring material decreased over time during the 60‐day experiment,55 which contradicted findings from a different study which found that 2EH indoor concentration fluctuated over a long period of time—increasing in summer when the temperature rose and decreasing in winter when the temperature fell.17, 18 Therefore, in addition to primary 2EH emission by the 2EH‐containing products, other emission mechanisms should also be considered. Some of these mechanisms have been identified and are described in the next section.
4.2. Emission of 2EH from hydrolysis reaction
A study showed that 2EH is generated from hydrolysis of DEHP in an environment simulating a concrete slab with a relative humidity (RH) between 70% and 100% and pH between 11 and 13. In the study, DEHP hydrolysis and 2EH emission increased with an increase in pH.56 Since DEHP has a half‐life of 100 years at pH 8 and 30°C,57 it is hardly degraded under normal indoor environment. Additionally, DEHP on the surface of cement with higher moisture content emits higher amount of 2EH.58 Thus, there is very little doubt that the amount of 2EH emission by DEHP is related to the moisture content of the cement with which it has direct contact.51 Therefore, dampness seems to play a major role in determining the amount of 2EH emitted. A study examined the relationship between RH and 2EH indoor air concentration, and showed that in buildings with RH values of 58%‐75% and 21%‐22%, the 2EH indoor air concentrations are 9 μg/m3 and 3 μg/m3, respectively.59 Additionally, in a room with high amount of 2EH emission, the moisture content of its concrete floor was as high as 8.2%.51 Using the FLEC method, 2EH was detected after PVC flooring was directly attached to a concrete floor60 or after PVC flooring material was tightly attached to a self‐leveling (SL) material.51 The amount of 2EH emission increases as the moisture content of an SL material increases.61 Taken together, long‐term emission of 2EH can be attributed to the hydrolysis of DEHP contained in the flooring material,49 supported by the fact that 2EH concentration in the air decreases significantly after plastic coverings, adhesives, and leveling layers are removed from the floor, all while the rooms were warmed to 55°C and simultaneously ventilated by additional exhaust fans for a week.53
Several published studies have reported on various materials that emit 2EH.18, 62, 63 We postulated, based on the amount of 2EH emission from flooring that compounds containing a 2‐ethyl‐1‐hexyl moiety, such as DEHP contained in PVC, and 2‐ethylhexyl acrylate contained in adhesives, are hydrolyzed to emit 2EH when the backing of carpeting material was in contact with concrete floors.18, 51 At pH values 11 and 13, flooring materials composed of DEHP‐containing PVC and 2‐ethylhexyl acrylate‐containing adhesives emitted a large amount of 2EH. Additionally, adhesives that did not contain 2‐ethylhexyl acrylate also emitted 2EH when combined with PVC flooring, but did not when combined with linoleum flooring.64 These results confirmed our postulate that a contact between a compound having a 2‐ethylhexyl group and a concrete floor causes secondary emission of 2EH from a hydrolysis reaction (Figure 1), which seems to be dependent on the pH and moisture content of the concrete surface. A similar emission of n‐butanol and 2‐butanol from hydrolysis reaction can be observed.65, 66 It is theorized that moisture in concrete is retained when the concrete is covering with a flooring material. Consequently, the amount of 2EH emitted increases in summer when the temperature rises and decreases in winter when the temperature drops. As this cycle repeats itself over a long period of time, 2EH will continue to be emitted, making it theoretically impossible to altogether prevent gradual and prolonged 2EH emission by ventilation and bakeout. In order to fundamentally eliminate the problem associated with 2EH emission, it is necessary to thoroughly dry concrete before covering it with a flooring material.
Figure 1.
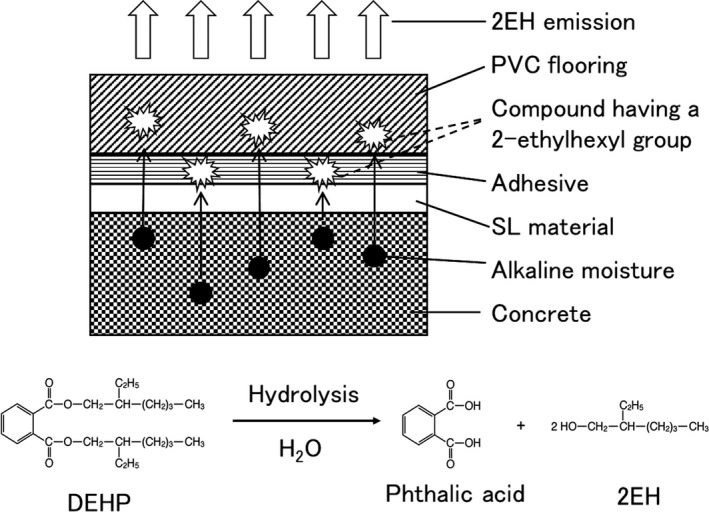
The emission mechanism of 2‐Ethyl‐1‐hexanol (2EH) by a hydrolysis reaction. Compounds having 2‐ethylhexyl group contained in floor materials and adhesives are hydrolyzed by the alkaline moisture content in concrete. For example, DEHP contained in PVC flooring is hydrolyzed, emitting 2EH into the room18
4.3. Emission of 2EH by microbiological reaction
It has been reported that some microorganisms in gypsum board or walls of a flood‐damaged house emit 2EH.67, 68 Aspergillus versicolor , which can grow on rich malt extract medium and several synthetic media, also emits 2EH.69 When cultivated in indoor dust for 7 days at an RH of 84%‐86%, and 2 days at an RH of 96%‐98%, A. versicolor generated several microbial VOCs, including 2EH, by metabolizing various hydrocarbons and fatty acids contained in the dust.70 Furthermore, Rhodococcus rhodochrous was reported to decompose the plasticizers DEHA71 and DEHP,72 thereby generating 2EH. Mycobacterium sp. also decomposes DEHP to produce 2EH.73 A pathway of 2EH production through the biological degradation of DEHP and DEHA has been proposed.74 Taken together, microorganisms have also been related to 2EH emission. In fact, several studies claim that 2EH in indoor air is a product of the decomposition of plasticizers by microorganisms.75 That said, all evidence in those studies were limited to in vitro findings. Therefore, the contribution of microbiological decomposition of plasticizers to 2EH indoor air concentration is still unverified. Detailed investigations are required.
5. INDOOR CONCENTRATIONS OF 2EH
2‐Ethyl‐1‐hexanol has been reported in the indoor air of buildings, mainly in Japan, Northern Europe, and North America (Table 2). High level of 2EH concentration has become a particular problem in a newly constructed university building in Japan, where 2EH concentrations were 1086 μg/m3 and 1183 μg/m3.7, 18 A study in Sweden reported that concentrations of 2EH up to 1000 μg/m3 were detected in offices.76 Two reports that measured the concentration of 2EH in North America showed 0.3‐48 μg/m3 and <7.95 μg/m3.77, 78
Table 2.
Summary of indoor air concentration of 2‐Ethyl‐1‐hexanol
| Country | Location | Study period | Concentration (μg/m3) | Reference |
|---|---|---|---|---|
| Japan | University | March 2001 | 164‐1086 | 7 |
| Japan | University | Summer 2002 | 25‐1183 | 18 |
| Sweden | Office building | Not reported | 1000 (max) | 74 |
| Japan | Nondomestic buildings | June 2002‐October 2004 | 16.5 (geometric mean) | 19 |
| USA | Office building | Summer 1995 | 0.3‐48 | 77 |
| Winter 1997‐1998 | ||||
| USA | Houses | 1997‐1998 | <7.95 | 78 |
| Japan | Large‐scale buildings | 2003‐2007 | 13.5 (geometric mean) | 79 |
| Japan | University | August‐September 2003 | 132 (max) | 80 |
| Japan | School | August 2007 | 12‐302 | 81 |
| Japan | Shopping center | June 2006 | 69.2 (max), 6.2 (geometric mean) | 82 |
| Japan | Museums | September and October 2005 | 1.30 (max) | 83 |
| Japan | Dwellings | October 2013 and January 2014 | 5.1 (max) | 84 |
| Sweden | Residential buildings | Not reported | 1‐86 | 50 |
| West Germany | Dwellings | Not reported | 2 (average) | 25 |
| Japan | Large‐scale buildings | July 2004‐September 2007 | Summer 55.4, winter 13.7 (geometric mean) | 17 |
| Switzerland | University | January and June 2000 | 4‐17 | 85 |
| Europe | Buildings | Summer 2012‐winter 2013 | Summer 4.7, winter 3.9 (average) | 86 |
| Finland | Office building | December 2000‐March 2001 | ca. 100 | 53 |
| Sweden | Buildings | Not reported | 17 (max), 9.8 (average) | 87 |
| Sweden | Rehabilitation center | Not reported | 0.3‐0.6 | 88 |
| Japan | Temporally houses | June 2011 | 69 ± 12.8 | 89 |
| Sweden | Hospitals | November 1996‐January 1997 | 4.8‐19.8 | 90 |
| Sweden | Hospitals | January‐February 1997 | 5‐20 | 91 |
| Sweden | Hospitals | January‐February 1997 | 2‐32 | 92 |
2‐Ethyl‐1‐hexanol was detected in 92 out of 99 rooms in the 42 buildings studied, with an average indoor concentration of 16.5 μg/m3 compared to an outdoor 2EH concentration of 1.9 μg/m3.19 In a survey of 175 rooms such as offices, sales floor, and classrooms in 57 buildings that were no older than 1 year, 2EH was detected in 99% of the rooms, with an average indoor concentration of 13.5 μg/m3.79
2‐Ethyl‐1‐hexanol has also been detected in other university building, school, shopping centers, and museums.80, 81, 82, 83 Furthermore, 2EH was detected in the living rooms at a maximum indoor concentration of 5.1 μg/m3.84 In another study, the 2EH indoor concentrations in 150 rooms of an apartment building were 1‐86 μg/m3.50 In Germany, the average 2EH concentration in 230 houses was 2 µg/m3 (<0.1‐10 µg/m3).25
The geometric mean of 2EH concentration was significantly higher in samples collected in summer (55.4 μg/m3) than in those collected during winter (13.7 μg/m3) (P < 0.01).56 Several similar trends have been reported.85, 86 With regard to building conditions that could facilitate 2EH emission, a damp office building had a higher 2EH concentration than a dry office building.53, 87 A rehabilitation center with wet linoleum flooring materials had 2EH concentrations of 0.3‐0.6 μg/m3.88 A survey conducted in five temporary houses that used PVC flooring materials showed a 2EH indoor concentration of 69 ± 12.8 μg/m3.89 Furthermore, three surveys were conducted in the same four geriatric hospital buildings, which were of various ages. 2EH was detected in two buildings aged 3 and 11 years with PVC floorings and dampness.90, 91, 92 On the other hand, the two other buildings, aged 1 and 71 years, showed no sign of dampness and had 2EH concentrations below the detection limit (<1 μg/m3).
Thus, it was concluded from these studies that 2EH concentration is not related to the age of buildings. Rather, the use of PVC, the dampness and higher temperature are known to contribute to 2EH emission. The reason it was detected frequently and at such high concentrations in Japan has not been clarified.
6. EFFECTS ON HUMAN HEALTH
6.1. Environmental exposure
A summary of 2EH effects on human health is presented inTable 3. Increased occurrence of ocular and nasal symptoms was observed in subjects working in buildings where 2EH was detected at levels between 5 and 20 μg/m3.91 Asthma symptoms may occur due to the humidity in concrete floor constructions that affect 2EH emission.92 In a humid building where people developed nasal mucosal inflammation, it was observed that fungi and bacteria were also abundant, wherein average 2EH concentration was 9.8 μg/m3.87
Table 3.
Summary of effects on human health
| Country | Location | Symptoms | Concentration (μg/m3) | Reference |
|---|---|---|---|---|
| Sweden | Hospital | Nasal and ocular symptoms | 5‐20 | 91 |
| Sweden | Hospital | Asthma symptoms | 2‐32 | 92 |
| Sweden | Building | Nasal mucosal inflammation | 9.8 (average), 17 (max) | 87 |
| Japan | University | Coughing, throat irritation, and sore eyes | 164‐1086 | 7 |
| Japan | University | Problems with the nasal passages, throat, and lower airways | 25‐1183 | 62 |
| Finland | Office building | Respiratory, conjunctival, and dermal symptoms; adult‐onset asthma was approximately nine times higher | Mean 2 (range 1‐3) | 53 |
| Finland | School | Irritation symptoms in the respiratory tract and eyes | 1‐4 | 54 |
| Sweden | Rehabilitation center | Ocular, nasal, and respiratory symptoms | 0.3‐0.6 | 88 |
| Japan | University | Ocular pain and headache | 37.1‐62.1 | 93 |
| Switzerland | University | Sickness and headache | 4‐17 | 85 |
At a university in Japan, where 2EH concentration was 1086 μg/m3 in maximum, a case of a female professor who complained of coughing, throat irritation, and sore eyes was reported. 2EH was detected at a prominently high concentration of 408‐1866 μg/m3. Other staff members also complained of area‐associated SBS symptoms in rooms where 2EH concentrations were higher than 160 μg/m3.7 In comparison with the SBS symptom prevalence, there was no significant difference between classrooms where 2EH concentration reached 65.5 μg/m3 and 4.8 μg/m3. However, symptoms of the nose, throat, and lower respiratory tract were observed only in rooms with high 2EH concentrations.18 Faculty members who used a conference room with 2EH concentration of over 336 μg/m3 showed a high prevalence of such complaints.7 Therefore, it was estimated that the threshold at which symptoms appeared excessively in a population should be in the range of 65.5‐336 μg/m3.18
In Finland, several respiratory and dermal symptoms and irritation in the eyes were reported in environments with 2EH concentration of 1‐4 μg/m3.53, 54
In a rehabilitation center in Sweden, where airborne concentrations of 2EH were very low (0.3‐0.6 μg/m3), the staff who had been previously exposed to VOCs as well as 2EH developed SBS symptoms after 2 days of re‐exposure regardless of a 4‐month period without VOC exposure.88
In a newly built university building in Japan, as the indoor concentration of 2EH decreased by ventilation, the number of occupants who complained about headache and eye irritations decreased.93
At a technical university in Switzerland, employees and students had complained about deteriorated indoor air quality after the building was renovated. Some employees even suffered from sickness and headache. Indoor concentration of 2EH was 4‐17 µg/m3.85
As described above, there are reports which claim that 2EH is present indoors, even in a general living environment, possibly causing irritation and inflammation in the mucous membranes of the respiratory tract and nasal cavity. However, the dose‐response relationship and the discrepancy in the lowest‐observed‐adverse‐effect‐level (LOAEL) among the countries remain to be further clarified.
6.2. Experimental inhalation or topical exposure settings
To assess the acute effects of 2EH, volunteers were exposed to 2EH vapor (1 mg/m3) for 2 hours. During exposure, the volunteers reported a significant increase in nasal and eye discomfort. No differences in response were observed between the sexes, or between the atopic and nonatopic treatments.94
Twenty‐four young men were assessed before, during, and after the 4‐hour exposure. As 2EH concentration increased in three levels, 8.14, 56.6, and 116 mg/m3, nasal flow reduction and substance P concentration were increased.95
To evaluate the effect of 2EH on sensory irritation, 2EH at mean concentrations of 1.5, 10, and 20 ppm (7.98, 53.2, and 106 mg/m3, respectively) were used for either constant or variable for the 4‐hour exposure. The study revealed a strong dose‐response relationship between the concentration of the airborne solvent and blinking rate. The study suggested a critical dose for 1‐hour constant exposure lied between 10 and 20 ppm, and the LOAEL for eye irritation due to 4‐hour exposure was 10 ppm under variable concentration conditions at a peak concentration of 20 ppm.12
Experiments with human volunteers at three time‐weighted average 2EH concentrations (1.5, 10, and 20 ppm) were performed for 4 hours under conditions of either constant or variable concentrations. At 10 ppm, nasal irritation increased with time, and 20 ppm resulted in remarkable irritation. Additionally, attention reduction was considered to occur around 20 ppm. Therefore, the LOAEL for irritability and nasal irritation was 10 ppm. Olfactory‐ and trigeminal‐mediated symptoms and intensities of odor, eye, and nasal irritations showed a dose‐dependent response. Over the course of the 4‐hour exposure, only olfactory symptoms decreased, while nasal irritations remained nearly unchanged and eye irritations slightly increased.96, 97
With regard to skin sensitization to 2EH, a maximization test was carried out on 29 volunteers. Tested at 4% in petrolatum, 2EH produced no irritation or sensitization after 48 hours in a closed‐patch test on human subjects.98
7. IN VIVO EFFECTS ON ANIMALS
7.1. Inhalation exposure
The effects of 2EH inhalation on animals are summarized in Table 4. Inhalable 2EH at 1210 mg/m3 (227 ppm) was administered by a single 6‐hour inhalation exposure to groups of Swiss mice, Wistar rats, and English Short Hair guinea pigs. 2EH‐induced local irritation was occurred in the mucous membranes of the eyes, nose, throat, and respiratory tract. However, these responses were temporary, and all animals had recovered within an hour of terminating exposure.11 In another study, mice exposed to 2EH at 234 mg/m3 (44 ppm) by inhalation exhibited a decrease in respiratory rate (RD50) by 50%.99
Table 4.
Summary of inhalation and oral exposure
| Concentration/dose | Period | Exposure | Species | Effects | Reference |
|---|---|---|---|---|---|
| 227 ppm | 6 h | Inhalation | Mouse, rat, and guinea pig | Mucous irritation in the eyes, nose, throat, and respiratory passages | 11 |
| 0, 15, 40, 120 ppm | 90 d | 6 h/d Inhalation | Rat | NOAEL of 120 ppm | 23 |
| 0, 20, 60, 150 ppm | 3 mo | 8 h/d Inhalation | Mouse | Inflammation and degeneration of the olfactory epithelium at ≥20 ppm | 24 |
| 100 mg/kg | Single | Intragastric administration | Rat | No direct effect on protein kinase C activity | 107 |
| 3.8 mmol/kg/d | 3 d | Gastric intubation | Rat | Increased cytochrome P450 4A1 levels | 108 |
| 2% (20 mg/kg/d) | 3 wk | In food | Rat | Significant decreases in triglyceride and cholesterol serum levels | 109 |
| 88 g/d/hen | Single | In food | Hen | Lowered plasma level of free cholesterols, reduced liver fats | 110 |
| 833 mg/kg/d | 3 wk | Gastric intubation | Rat | Increased liver weight | 111 |
| 4 mmol/kg/d (520.8 mg/kg/d) | 7 d | Gavage | Rat | Increases in both wet liver weight and antipyrine clearance | 112 |
| 1000 mg/kg/d | 3 wk | Gavage | Rat | Thirty percent increase in liver‐to‐body weight ratio; increase in peroxisome cell fraction and in peroxisome density | 22 |
| 130 mg/kg/d | 14 d | Gavage | Rat | Not induce hepatomegaly, peroxisome proliferation, and hyperlipidemia | 113 |
| 0‐1.75 g/kg/d | 14 d | Gavage | Mouse, rat | Increases in relative liver weights and peroxisomal β‐oxidation | 114 |
| 0, 25, 125, 250, 500 mg/kg/d (5 consecutive days/wk) | 13 wk | Gavage | Mouse, rat | Reduced body weight gain and increased relative liver, kidney, stomach, and testes weights at 500 mg/kg/d | 20 |
| NOEL of 125 mg/kg/d | |||||
| 0, 50, 200, 750 mg/kg/d (5 consecutive days/wk) | 18 mo | Gavage | Mouse, rat | Reduced body weight gain and increased relative liver and stomach weights at 500 mg/kg/d | 21 |
| 0, 50, 150, 500 mg/kg/d (5 consecutive days/wk) | 24 mo | Gavage | Rat | Decrease in body weight gain and dose‐dependent increases in relative liver, stomach, brain, kidney, and testis weights at 150 mg/kg/d and 500 mg/kg/d | 21 |
A 90‐day subchronic inhalation toxicity study of 2EH was performed in Wistar rats. In total, 10 males and 10 females per group were exposed to 2EH vapors at concentrations of 15, 40, and 120 ppm for 6 hours/day over a 90‐day period. No 2EH‐related adverse effects were observed. The highest concentration tested under these conditions (120 ppm) was described as the no‐observed‐adverse‐effect‐level (NOAEL) of 2EH in both male and female rats.23
Male ICR mice were exposed to 0, 20, 60, or 150 ppm 2EH for 8 hours/day each week, 5 days every week over 3‐month period. After a week of exposure to 2EH, the mice showed inflammation and degeneration in the olfactory epithelium, and mice exposed to 2EH at ≥20 ppm showed a significant concentration‐dependent reduction in the number of olfactory receptor neurons and globose basal cells. The olfactory bulb showed a reduction in the diameter of glomeruli and in the number of olfactory nerves at 3 months. These histopathology data suggested that 2EH has persistent effects on the olfactory system.24
7.2. Oral exposure
The effects of 2EH oral exposure on animals are summarized in Table 4. The acute oral lethal dose 50% (LD50) of 2EH in rats were reported to be 3.3 g/kg,100 2.05 (range 1.52‐2.77) g/kg,101 2.46 g/kg,102 7.1 (range 5.5‐9.1) g/kg,103 3.2 g/kg,104 3.29 (range 2.87‐3.79) g/kg,105 and 3.73 g/kg,11 whereas in mice it was reported to be 2.500 g/kg.106
A tumorigenic effect of 2EH was examined by determining its effect on protein kinase C activity. It was revealed that 2EH exerted no direct effect on protein kinase C activity in vivo.107
In another study, Wistar rats were treated with 2EH at 494 mg/kg by gastric intubation once a day for 3 days. At 24 hours after the last dose, the level of cytochrome P450 4A1, activity of lauric acid ω‐hydroxylase and palmitoyl‐CoA oxidase in the rats were increased. However, 2EH did not alter the activity of lauric acid (ω‐1)‐hydroxylase.108
Male Fischer 344 rats were fed diets containing 2EH at 20 mg/kg/day for 3 weeks, and significant decreases in the levels of serum triglyceride and cholesterol were observed.109
In laying hens, the diet which contained 2% (88 g/day/hen) 2EH lowered the plasma level of free cholesterols, liver fats but not significantly alter liver weight.110
Other studies using rats revealed that 2EH increase liver weight,111 antipyrine clearance,112 and peroxisome cell fraction.22
Male rats were administered with 2EH (1 mmol/kg/day) for 14 days. This treatment did not induce hepatomegaly, peroxisome proliferation, and hyperlipidemia in the rats.113
Male and female rats (Wistar‐ and Fischer 344‐derived) were orally administered with 2EH for 14 consecutive days. At doses above 1.05 g/kg/day, 2EH was toxic, and resulted in their death. Relative liver weights (liver‐to‐body weight ratios) administered at above 0.70 g/kg/day were increased in a dose‐dependent manner.114
2‐Ethyl‐1‐hexanol was administered by oral gavage to male and female Fischer 344 rats and B6C3F1 mice (0, 25, 125, 250, and 500 mg/kg/day) for 13 weeks. In the rats, 500 mg/kg/day reduced body weight gain, increased relative liver, kidney, stomach, and testes weights, and moderate changes at gross and microscopic levels in the liver and forestomach were observed. In the mice, 2EH at 500 mg/kg/day increased relative stomach weights in males and produced few gross and microscopic changes in the forestomach and liver (female). A NOEL of 125 mg/kg/day was established for 2EH in rats and mice.20
2‐Ethyl‐1‐hexanol at 0, 50, 200, and 750 mg/kg were administered to mice five times a week for 18 months. At 750 mg/kg, a slight increase in non‐neoplastic focal hyperplasia in the forestomach vs vehicle controls was shown. Besides, relative liver and stomach weights and incidence of hepatocellular carcinomas were increased. No metastases were observed.21
The authors also reported the chronic effects in rats treated with 0, 50, 150, and 500 mg/kg 2EH by gavage five times a week for 24 months. Reduced body weight gain with 2EH at above 150 mg/kg and an increased incidence of lethargy and unkemptness were observed at 50 mg/kg. There were dose‐related increases in relative liver, stomach, brain, kidney, and testis weights. Apart from marked aspiration‐induced bronchopneumonia in rats at 500 mg/kg, the hematologic, gross, and microscopic changes indicative of tumors were comparable among all rat groups.21
7.3. Topical exposure
The acute dermal LD50 value was 2.38 (1.51‐2.76) g/kg102 in rats, over 2.6 g/kg11 in rabbits. Signs of percutaneous toxicity were not observed, and skin irritation was moderate when 2EH (at 0.10, 0.316, 1.00, and 3.16 ml/kg) was dermally administered to the closely clipped, intact abdominal skin of albino rabbits.11 Additionally, 2EH was administered to rabbit eyes and the subsequent corneal injury was graded as 5 on a scale of 10,102, 115 indicating severe acute eye irritation.11
2‐Ethyl‐1‐hexanol diluted by polyethylene glycol (1%, 3%, 10%, 30%, and 100%) was administered to rabbit eyes. The potent ocular irritant 2EH produced moderate eye irritation from concentrations between 3% and 30%, and severe eye irritation at 100%.116
7.4. Intraperitoneal exposure
Intraperitoneal treatment of rats with 0.32 g/kg 2EH decreased plasma ketone bodies (from 0.8 to 1.6 mmol/L), increased hepatic triglycerides, and increased lipids predominantly in periportal regions of the liver lobule.117
After intraperitoneal injection, 2EH did not induce a significant production of hydrogen peroxide generated by peroxisome proliferators in the rat hepatocytes.118
8. EFFECTS ON REPRODUCTION AND TESTIS
Since there is no report in humans regarding reproductive toxicity effects, Japan Society for Occupational Health classified 2EH as group 3: Substances suspected to cause reproductive toxicity, based on the animal experimental data showing the effects on fetal growth and skeleton formation.14
Sprague‐Dawley rats were exposed to 2EH vapor for 7 hours/day on gestational days (GD) 1‐19 at 850 mg/m3 (160 ppm). 2EH reduced maternal food intake, but there were no significant decreases in weight gain, water intake, number of fetuses, and fetal weight.119
Teratological studies were conducted using Wistar rats orally treated with 2EH at up to 1660 mg/kg on GD 12. Teratogenic fetal malformation was increased,120 but there was no clear description in the article whether an appropriate comparison with the control group was made or not.
Developmental effects of 2EH in Wistar rats at 0, 130, 650 and 1300 mg/kg (10 animals per group) by gavage, from GD 6 to 15, were investigated. 2EH showed significant maternal toxicity with autopsy effects at 1300 mg/kg and six animals were found dead on GD 9, 10 and 13. In this group, there was also an increased number of early resorptions and high post‐implantation loss. The mean fetal body weight markedly decreased and an increased frequency of fetuses with malformations was observed. Furthermore, the number of fetuses bearing skeletal variations, retardations and dilated renal pelvis increased. A 650 mg/kg dose of 2EH showed slight clinical signs/symptoms in the mother without maternal body weight changes. Fetal body weights were slightly reduced, and the number of fetuses with skeletal variations and retardations increased. Six fetuses among the three litters in this group showed asymmetric dumbbell‐shaped thoracic vertebrae. The NOAEL for the maternal and fetuses was 130 mg/kg.121
2‐Ethyl‐1‐hexanol was orally administered to female mice at 1525 mg/kg/day from GD 6 to 15. Of 49 maternal mice, 17 died, and maternal body weight decreased. In addition, the number of births, the survival rate, and the weight of the infant significantly decreased.122
2‐Ethyl‐1‐hexanol was administered via occluded dermal application for 6 hours/day on GD 6 through 15 to pregnant Fischer 344 rats at 0‐2520 mg/kg/day. The NOAEL for the maternal toxicity of 2EH was 252 mg/kg/day based on skin irritation, and 840 mg/kg/day based on systemic toxicity. The NOAEL for developmental toxicity was at least 2520 mg/kg/day, with no teratogenicity.123
The rate of Sertoli cell proliferation was assessed in male CD Sprague‐Dawley pups. At 24 hours after treatment with 2EH at 166.4 mg/kg, the number of Sertoli cells in the testicular sections was not diminished. 2EH does not alter the morphology of Sertoli cells and gonocytes.124
It was investigated whether 2EH is responsible for testicular damage. No testicular damage was observed in young rats orally administered with 2EH at 351 mg/kg/day for 5 days.125 Additionally, administration of 2EH at 130 mg/kg/day for 14 days resulted in no testicular atrophy.113
In another study, 2EH were orally administered at 0, 50, 200, and 750 mg/kg to B6C3F1 mice 5 times a week for 18 months. The relative testicular weight was slightly increased in the groups treated with over 50 mg/kg/day 2EH. Similarly, 2EH was orally administered at 0, 50, 150, and 500 mg/kg five times a week to Fisher 344 rats for 24 months. 2EH induces a dose‐dependent increase in testis weight.21
Mixed cultures of Sertoli and germ cells were prepared from the testes of 27‐ to 30‐day‐old Sprague‐Dawley rats and the testicular toxicity was examined. The addition of 2EH at 2 × 10−4 M to the culture medium did not cause an increase in the rate of germ‐cell detachment, compared with non‐treated condition.126
Sertoli cells, which produce lactate and pyruvate are thought to be the initial target of testicular atrophy.127 The effect of 2EH on lactate and pyruvate production was studied, but their production was unaffected by 2EH at 200 μmol/L.128
The antiandrogenic potential of 2EH in vitro with a mouse Leydig tumor cell line, MA‐10 cells, was evaluated. 2EH did not have significant effects on cell viability and steroidogenesis.129
9. MUTAGENICITY, CARCINOGENICITY, AND GENOTOXICITY
Doses of 16.7, 58.3, and 175 mg/kg/day to male Fischer 344 rats were administered by gavage for 5 consecutive days. 2EH did not induce detectable chromosomal aberrations.130 Oral gavage doses of 2EH were administered 5 times a week to B6C3F1 mice at up to 750 mg/kg for 18 months and Fischer 344 rats at up to 500 mg/kg for 24 months. 2EH was not oncogenic in rats, but there were weak trends of adverse hepatocellular carcinoma incidence in mice at higher doses.21
There are several in vitro bacterial studies. Some group reported that 2EH was found to be mutagenic131 and cause DNA damage.132 However, other groups reported that 2EH was found to be non‐mutagenic in the Ames test and Rec assay.133, 134, 135, 136, 137, 138
Using a modified Ames Salmonella/microsome assay to determine mutagenicity, urine was pooled from male Sprague‐Dawley rats dosed daily for 15 days with 1000 mg/kg of 2EH. No mutagenic substances were excreted in the urine.139 2EH also exhibited no chromosome damage140 or mutagenic activity.136
In a carcinogenesis bioassay of DEHP and related compounds, it was reported that 2EH was not bound to hepatic DNA of Fischer 344 rats 24 hours following oral gavage administration.141 In vitro promoting activity of DEHP and its hydrolysis product, 2EH, were studied using promotable mouse epidermis‐derived JB6 cells, which revealed that 2EH did not promote the anchorage of JB6 cells.142
10. STUDY ON MODE OF ACTION OF 2EH
The in vitro effects of 2EH are summarized in Table 5. The administration of 2EH at a concentration of 1% to mitochondrial fractions from the liver of male Wistar rats exhibited insignificant inhibitory effect on State 3 respiration.143
Table 5.
Summary of the in vitro studies
| Experimental conditions | Effects | Reference |
|---|---|---|
| The mitochondrial fraction of rat liver was treated with 1% 2EH | Low inhibitory effect on the state 3 respiration | 143 |
| Adult rat hepatocytes were cultured for 48 h in the presence of 0.2 and 1 mmol/L 2EH | Increased numbers of peroxisomes | 144, 145 |
| Increased activities of carnitine acetyltransferase and 7‐ethoxycoumarin O‐deethylase (at 1 mmol/L) | ||
| Primary rat hepatocytes were cultured with 0‐0.5 mmol/L 2EH for 72 h | No effect on CN‐‐insensitive palmitoyl‐CoA oxidation | 146 |
| Cells of mice, rats, guinea pigs, and marmosets were cultured with 0.5 mmol/L 2EH for 72 h | Increased cyanide‐insensitive fatty acyl CoA oxidase activity in mice and rats | 147 |
| Rat Kupffer cells were cultured with 1.25‐3 mmol/L 2EH for 3 d | Increased intracellular calcium level at 3 mmol/L | 148 |
| Rat Kupffer cells were treated with 0.9 mmol/L 2EH | No effect on superoxide production | 149 |
| The cytosol of mouse and rat liver was treated with 15 mmol/L 2EH | Cytosolic GST was three times more potent in the mice than in the rats | 150 |
| Mouse liver was incubated with 0.25‐1.00 mmol/L 2EH | Significant inhibition of ADH activity but no appreciable effect on ALDH activity | 151 |
| Rat liver was incubated with 2.5‐15.0 mmol/L 2EH | Significant inhibition of the activities of aminopyrine N‐demethylase and aniline hydroxylase | 152 |
| Mice spleen cells were incubated with 10−8‐10−3 mol/L 2EH for 24 h | IL‐2 was induced in CD4 cells, but not in CD8 cells | 153 |
| Mice trigeminal ganglia neurons cells were incubated with 1‐10 mmol/L 2EH | Activation expressed TRPA1 in a concentration‐dependent manner | 154,155 |
| Perfused rat liver was incubated with 0.1‐3 mmol/L 2EH | Extensive cell damage due to lactose dehydrogenase leakage | 156 |
| Perfused rat liver was incubated with 200 µmol/L 2EH | The rate of ketone body production was decreased to about 60% | 117 |
| Mitochondria isolated from perfused rat livers were treated with 70 µmol/L and 3 mmol/L 2EH | Inhibition of the oxygen uptake in the periportal regions, but not in the centrilobular regions | 157, 158, 159 |
Adult rat hepatocytes cultured for 48 hours in the presence of 0.2 mmol/L 2EH contained more number of peroxisomes than controls. The activity of carnitine acetyltransferase (a mixed peroxisomal/mitochondrial marker) and 7‐ethoxycoumarin O‐deethylase (microsomal marker) increased ninefold and twofold, respectively, by the presence of 1 mmol/L 2EH.144, 145
The effect on peroxisomal enzyme activity in primary rat hepatocyte was determined after incubation with 2EH at 0‐0.50 mmol/L for 72 hours. 2EH at these concentrations had no effect on the oxidation of the peroxisomal marker, cyanide‐insensitive palmitoyl‐CoA. Therefore, it was inferred that 2EH had no effect on peroxisomal β‐oxidation.146
One study examined the possibility of species differences in response to 2EH. Hepatocytes were isolated from male mice, rats, guinea pigs, and marmosets, and incubated with 2EH. Although 2EH increased the activity of cyanide‐insensitive fatty acyl CoA oxidase in mice and rats, did not increase in guinea pig and marmoset.147
Kupffer cells were isolated and incubated with 2EH, but no effect of 2EH on intracellular calcium and superoxide production.148, 149
The inhibitory effect of 2EH on mouse and rat liver cytosolic GST activities was monitored in vitro. The study revealed that inhibition of GST by 2EH in mice was three times more potent than in rats.150
The activities of ADH and ALDH in mouse liver after 0.25, 0.50, and 1.00 mmol/L 2EH treatments were examined. The in vitro study revealed a significant inhibition of ADH activity by 2EH at concentrations of 0.50 and 1.00 mmol/L, but no appreciable effect on the activity of ALDH.151
2‐Ethyl‐1‐hexanol at concentrations between 2.5 and 15.0 mmol/L significantly inhibited the activity of aminopyrine N‐demethylase and aniline hydroxylase of rat liver.152
To investigate the effects of 2EH on immune responses, spleen cells from female BALB/c mice were incubated with 2EH. The activities of interleukin (IL)‐6 and immunoglobulin were not induced by 2EH. IL‐2 was induced by 2EH in CD4 cells, but not in CD8 cells. 2EH induced activation of CD4 cells, which was accompanied by the activation of transcription factors, suggesting that 2EH functions as a modulator of immune response.153
The effects of 2EH on heterologously expressed transient receptor potential (TRP) ion channels that cause sensory irritations in primary cell cultures of mice trigeminal ganglia neurons were investigated. 2EH activates heterologously expressed TRPA1 in a concentration‐dependent manner (1‐10 mmol/L). In Ca2+ imaging, 2EH acted as an agonist of multiple channels (TRPA1, TRPV1, GPCRs) which activate the trigeminal neurons.154, 155
Although 2EH causes toxicity exclusively to periportal regions of the perfused liver, the toxicity is dependent on oxygen tension in isolated sublobular regions of the liver lobule. It is therefore unlikely for the selective injury to periportal regions in studies with perfused liver to be caused by drug delivery.156 It was reported that 2EH inhibits β‐oxidation of fatty acids in mitochondria, but not in peroxisomes.117
A second group also assessed 2EH toxicity in the liver. Livers from starved female Sprague‐Dawley rats were perfused with 2EH (at 3 mmol/L) dissolved in Krebs‐Henseleit buffer (pH 7.4, 37°C) saturated with 95% O2, 5% CO2. Following infusion of 2EH, O2 uptake and ketone body formation were diminished by 50% and 80%, respectively. Furthermore, cell damage, as assessed by the appearance of LDH in the effluent perfusate, was apparent. Only O2‐rich upstream regions of the liver lobule were damaged. This toxicity is dependent on oxygen tension in isolated sublobular regions of the liver lobule. Peroxisome proliferators accumulate in the liver due to their lipophilicity. They inhibit actively respiratory mitochondria in the periportal region of the hepatic lobule and cause partially toxicity.157, 158, 159
11. CONCLUDING REMARKS
In this review, we focused on the toxicity of 2EH from the viewpoint of an indoor air pollutant.
2EH is metabolized to 2‐ethyl‐1‐hexanal, and then to 2EHA, after which it is rapidly excreted from the body. However, drug‐metabolizing enzyme activity reportedly varies greatly among individuals.28 Thus, long‐term exposure to 2EH, especially in populations with low metabolic activities, may cause health effects even below the minimum concentration that causes toxic effects.
In both Japan and Northern Europe, 2EH was detected in buildings where patients complained of SBS symptoms.7, 54 2EH has been reported to induce mucosal irritation and effects on the central nervous system. Thus, 2EH is considered among the causative agents of SBS symptoms.
Reports on the effects in animals of inhalation exposure to 2EH are limited. In particular, there is no report on the liver effects of its inhalation exposure. Orally ingested 2EH increases the number of peroxisomes. Peroxisome proliferators activate peroxisome proliferator‐activated receptor α (PPARα) and affect lipid metabolism, inflammation, glucose homeostasis, cell proliferation, and apoptosis.160 Because 2EHA, a metabolite of 2EH, acts as a PPARα agonist,161 it may be responsible for the effects observed upon 2EH oral administration on the liver.
In most buildings where the 2EH indoor air concentrations are high, plasticizer‐containing flooring materials have a direct contact with concrete. There are multiple sources of 2EH in rooms, that is, primary emission from PVC products and/or building materials, and secondary emission resulting from chemically induced hydrolysis and/or microbial decomposition of plasticizers and/or adhesives.
It was reported that 2‐butanol is generated through the hydrolysis of several acrylic adhesives.65 n‐Butanol is emitted from the floor,66 produced from di‐n‐butyl phthalate,162 and 2‐butanol from isobutyl phthalate.162 As a measure against VOCs emissions like that of 2EH, it is very important to use a flooring or other building material that does not emit VOCs even from the hydrolysis reaction, or to confirm that the moisture content in the concrete is sufficiently lowered before flooring the room.
DISCLOSURE
Approval of the research protocol: N/A. Informed consent: N/A. Registry and registration no. of the study/trial: N/A. Animal studies: N/A.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This work was partly supported by JSPS KAKENHI Grant number 16K15375, Japan.
Wakayama T, Ito Y, Sakai K, et al. Comprehensive review of 2‐ethyl‐1‐hexanol as an indoor air pollutant. J Occup Health. 2019;61:19–35. 10.1002/1348-9585.12017
Contributor Information
Yuki Ito, Email: yukey@med.nagoya-cu.ac.jp.
Michihiro Kamijima, Email: kamijima@med.nagoya-cu.ac.jp.
REFERENCES
- 1. Norbäck D, Torgen M, Edling C. Volatile organic compounds, respirable dust, and personal factors related to prevalence and incidence of sick building syndrome in primary schools. Brit J Ind Med. 1990;47(11):733‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sundell J, Anderson B, Anderson K, Lindvall T. Volatile organic compounds in ventilating air in buildings at different sampling points in the buildings and their relationship with the prevalence of occupant symptoms. Indoor Air. 1993;3(2):82‐93. [Google Scholar]
- 3. Verhoeff AP, Burge HA. Health risk assessment of fungi in home environments. Ann Allergy Asthma Immunol. 1997;78(6):544‐556. [DOI] [PubMed] [Google Scholar]
- 4. Smedje G, Norbåck D, Edling C. Subjective indoor air quality in schools in relation to exposure. Indoor Air. 1997;7(2):143‐150. [Google Scholar]
- 5. Reinikainen LM, Jaakkola JJ, Seppänen O. The effect of air humidification on symptoms and perception of indoor air quality in office workers: a six‐period cross‐over trial. Arch Environ Health. 1992;47(1):8‐15. [DOI] [PubMed] [Google Scholar]
- 6. Nordström K, Norbäck D, Akselsson R. Effect of air humidification on the sick building syndrome and perceived indoor air quality in hospitals: a four month longitudinal study. Occup Environ Med. 1994;51(10):683‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamijima M, Sakai K, Shibata E, et al. 2‐Ethyl‐1‐hexanol in indoor air as a possible cause of sick building symptoms. J Occup Health. 2002;44(3):186‐191. [Google Scholar]
- 8. McGinty D, Scognamiglio J, Letizia CS, Api AM. Fragrance material review on 2‐ethyl‐1‐hexanol. Food Chem Toxicol. 2010;48(Suppl 4):S115‐129. [DOI] [PubMed] [Google Scholar]
- 9. US Department of Health and Human Services . Carcinogenesis Bioassay of di(2‐ethylhexyl)phthalate (CAS No. 117‐81‐7) in F344 Rats and B6C3F1 Mice (Feed Studies). National Toxicology Program Technical Report; 1982. [PubMed]
- 10. Ekelman K. 2‐ehtyl‐1‐hexanol. [Online]. 1998. http://www.inchem.org/documents/jecfa/jecmono/v32je04.htm. Accessed June 7, 2018
- 11. Scala RA, Burtis EG. Acute toxicity of a homologous series of branched‐chain primary alcohols. Am Ind Hyg Assoc J. 1973;34(11):493‐499. [DOI] [PubMed] [Google Scholar]
- 12. Kiesswetter E, van Thriel C, Schäper M, Blaszkewicz M, Seeber A. Eye blinks as indicator for sensory irritation during constant and peak exposures to 2‐ethylhexanol. Environ Toxicol Pharmacol. 2005;19(3):531‐541. [DOI] [PubMed] [Google Scholar]
- 13. European Commission, Employment, Social Affairs and Inclusion . Recommendation from the Scientific Committee on occupational exposure limits for 2‐ethylhexanol European Commission Report. 2011.
- 14. The Committee for Recommendation of Occupational Exposure Limits, Japan Society for Occupational Health. Recommendation of occupational exposure limits . Recommendation of occupational exposure limits. J Occup Health. 2018;60(4):333‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anonymous . Guide values for 2‐ethylhexanol in indoor air. Bundesgesundheitsblatt. 2013;56:590‐599. (in German). [DOI] [PubMed] [Google Scholar]
- 16. Ministry of Health, Labour and Welfare, Japan . Draft guidelines on indoor air pollution. [Online]. 2018. https://www.mhlw.go.jp/content/11121000/000348512.pdf. Accessed September 26, 2018.
- 17. Sakai K, Kamijima M, Shibata E, Ohno H, Nakajima T. Annual transition and seasonal variation of indoor air pollution levels of 2‐ethyl‐1‐hexanol in large‐scale buildings in Nagoya, Japan. J Environ Monitor. 2009;11(11):2068‐2076. [DOI] [PubMed] [Google Scholar]
- 18. Kamijima M, Shibata E, Sakai K, et al. Indoor air pollution due to 2‐ethyl‐1‐hexanol. Nihon Koshu Eisei Zassi (Jpn J Public Health). 2005;52(12):1021‐1031. (in Japanese). [PubMed] [Google Scholar]
- 19. Sakai K, Kamijima M, Shibata E, Ohno H, Nakajima T. Indoor air pollution by 2‐ethyl‐1‐hexanol in non‐domestic buildings in Nagoya, Japan. J Environ Monitor. 2006;8(11):1122‐1128. [DOI] [PubMed] [Google Scholar]
- 20. Astill BD, Deckardt K, Gembardt C, et al. Prechronic toxicity studies on 2‐ethylhexanol in F334 rats and B6C3F1 mice. Fund Appl Toxicol. 1996;29(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 21. Astill BD, Gingell R, Guest D, et al. Oncogenicity testing of 2‐ethylhexanol in Fischer 344 rats and B6C3F1 mice. Fund Appl Toxicol. 1996;31(1):29‐41. [DOI] [PubMed] [Google Scholar]
- 22. Barber ED, Topping DC. Subchronic 90‐day oral toxicology of di(2‐ethylhexyl) terephthalate in the rat. Food Chem Toxicol. 1995;33(11):971‐978. [DOI] [PubMed] [Google Scholar]
- 23. Klimisch HJ, Deckardt K, Gembardt C, Hildebrand B. Subchronic inhalation toxicity study of 2‐ethylhexanol vapour in rats. Food Chem Toxicol. 1998;36(3):165‐168. [DOI] [PubMed] [Google Scholar]
- 24. Miyake M, Ito Y, Sawada M, et al. Subchronic inhalation exposure to 2‐ethyl‐1‐hexanol impairs the mouse olfactory bulb via injury and subsequent repair of the nasal olfactory epithelium. Arch Toxicol. 2016;90(8):1949‐1958. [DOI] [PubMed] [Google Scholar]
- 25. National Institutes of Health, Hazardous Substance Data Bank . 2‐ethyl‐1‐hexanol. [Online]. 2014. https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm. Accessed June 7, 2018.
- 26. Cheng L. Assessment report on 2‐ethylhexanol for developing ambient air quality objectives Toxico‐Logic Consulting Inc. Report, 2004. [Google Scholar]
- 27. Bevan C. Monohydric alcohols‐C7 to C18, aromatic, and other alcohols In: Bingham E, Cohrssen B, Powell CH, eds. Patty’s Toxicology. 5th ed New York: John Wiley & Sons; 2001:470‐476. [Google Scholar]
- 28. Ito Y, Kamijima M, Hasegawa C, et al. Species and inter‐individual differences in metabolic capacity of di(2‐ethylhexyl)phthalate (DEHP) between human and mouse livers. Environ Health Prev Med. 2014;19(2):117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamil IA, Smith JN, Williams RT. Studies in detoxication. 46. The metabolism of aliphatic alcohols; the glucuronic acid conjugation of acyclic aliphatic alcohols. Biochem J. 1953;53(1):129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamil IA, Smith JN, Williams RT. Studies in detoxication. 47. The formation of ester glucuronides of aliphatic acids during the metabolism of 2‐ethylbutanol and 2‐ethylhexanol. Biochem J. 1953;53(1):137‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albro PW. The metabolism of 2‐ethylhexanol in rats. Xenobiotica. 1975;5(10):625‐636. [DOI] [PubMed] [Google Scholar]
- 32. Deisinger PJ, Boatman RJ, Guest D. Metabolism of 2‐ethylhexanol administered orally and dermally to the female Fischer 344 rat. Xenobiotica. 1994;24(5):429‐440. [DOI] [PubMed] [Google Scholar]
- 33. Barber ED, Teetsel NM, Kolberg KF, Guest D. A comparative study of the rates of in vitro percutaneous absorption of eight chemicals using rat and human skin. Fund Appl Toxicol. 1992;19(4):493‐497. [DOI] [PubMed] [Google Scholar]
- 34. Krotoszynski BK, Bruneau GM, O'neill HJ. Measurement of chemical inhalation exposure in urban population in the presence of endogenous effluents. J Anal Toxicol. 1979;3(6):225‐234. [Google Scholar]
- 35. Ruzsanyi V, Mochalski P, Schmid A, et al. Ion mobility spectrometry for detection of skin volatiles. J Chromatogr B. 2012;911(1):84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hisanaga M, Takao T, Tetsuo O, Ito H. Determination of volatile organic compounds in human skin gas by GC/MS. Bunseki‐kagaku. 2012;61(1):57‐61. (in Japanese). [Google Scholar]
- 37. Costello B, Ewen R, Ewer A, et al. An analysis of volatiles in the headspace of the faeces of neonates. J Breath Res. 2008;2(3):037023. [DOI] [PubMed] [Google Scholar]
- 38. Hodgson AT, Wooley JD, Daisey JM. Emissions of volatile organic compounds from new carpets measured in a large‐scale environmental chamber. J Air Waste Manage. 1993;43(3):316‐324. [DOI] [PubMed] [Google Scholar]
- 39. Fang L, Clausen G, Fanger PO. Impact of temperature and humidity on chemical and sensory emissions from building materials. Indoor Air. 1999;9(3):193‐201. [DOI] [PubMed] [Google Scholar]
- 40. European Commission, Joint Research Centre . Evaluation of VOC emissions from building products. Office for Official Publications of the European Communities, 1997. [Google Scholar]
- 41. Bako‐Biro Z, Wargocki P, Weschler CJ, Fanger PO. Effects of pollution from personal computers on perceived air quality, SBS symptoms and productivity in offices. Indoor Air. 2004;14(3):178‐187. [DOI] [PubMed] [Google Scholar]
- 42. Lattuati‐Derieux A, Bonnassies‐Termes S, Lavédrine B. Identification of volatile organic compounds emitted by a naturally aged book using solid‐phase microextraction/gas chromatography/mass spectrometry. J Chromatogr A. 2004;1026(1–2):9‐18. [DOI] [PubMed] [Google Scholar]
- 43. Gibson LT, Ewlad‐Ahmed A, Knight B, Horie V, Mitchell G, Robertson CJ. Measurement of volatile organic compounds emitted in libraries and archives: an inferential indicator of paper decay? Chem Cent J. 2012;6(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Panseri S, Chiesa L, Zecconi A, Soncini G, De Noni I. Determination of volatile organic compounds (VOCs) from wrapping films and wrapped PDO Italian cheeses by using HS‐SPME and GC/MS. Molecules. 2014;19(7):8707‐8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Claeson AS, Sandstrom M, Sunesson AL. Volatile organic compounds (VOCs) emitted from materials collected from buildings affected by microorganisms. J Environ Monitor. 2007;9(3):240‐245. [DOI] [PubMed] [Google Scholar]
- 46. Katsumata H, Murakami S, Kato S, et al. Measurement of semi‐volatile organic compounds emitted from various types of indoor materials by thermal desorption test chamber method. Build Environ. 2008;43(3):378‐383. [Google Scholar]
- 47. Wal J, Hoogeveen A, Wouda P. The Influence of temperature on the emission of volatile organic compounds from PVC flooring, carpet, and paint. Indoor Air. 1997;7(3):215‐221. [Google Scholar]
- 48. Järnström H, Saarela K, Kalliokoski P, Pasanen A‐l. Comparison of VOC and ammonia emissions from individual PVC materials, adhesives and from complete structures. Environ Int. 2008;34(3):420‐427. [DOI] [PubMed] [Google Scholar]
- 49. Chino S, Kato S, Seo J, Ataka Y. Study on emission of decomposed chemicals of esters contained in PVC flooring and adhesive. Build Environ. 2009;44(7):1337‐1342. [Google Scholar]
- 50. Follin T. Measuring during airing out pollutions from concrete slabs In: Proceedings of the 7th International Conference on Indoor Air Quality and Climate. Vol. 3. Tokyo: Organizing Committee of 7th International Conference on indoor Air Quality and Climate; 1996: 65–70. [Google Scholar]
- 51. Kamijima M, Shibata E, Sakai K, et al. Study on the emission source of 2‐ethyl‐1‐hexanol in buildings. Indoor Environ. 2003;6(2):160‐163. (in Japanese). [Google Scholar]
- 52. Ishidao T, Ishimatsu S, Hori H. Measurement of volatile organic compounds concentrations in indoor air of school buildings in a university (Part 2). J Work Environ. 2005;26(5):52‐57. (in Japanese). [Google Scholar]
- 53. Tuomainen A, Seuri M, Sieppi A. Indoor air quality and health problems associated with damp floor coverings. Int Arch Occup Environ Health. 2004;77(3):222‐226. [DOI] [PubMed] [Google Scholar]
- 54. Putus T, Tuomainen A, Rautiala S. Chemical and microbial exposures in a school building: adverse health effects in children. Arch Environ Health. 2004;59(4):194‐201. [DOI] [PubMed] [Google Scholar]
- 55. Wolkoff P. Impact of air velocity, temperature, humidity, and air on long‐term voc emissions from building products. Atmos Environ. 1998;32(14–15):2659‐2668. [Google Scholar]
- 56. Björk F, Eriksson C‐A, Karlsson S, Khabbaz F. Degradation of components in flooring systems in humid and alkaline environments. Constr Building Mater. 2003;17(3):213‐221. [Google Scholar]
- 57. Wolfe N, Steen W, Burns L. Phthalate ester hydrolysis: Linear free energy relationships. Chemosphere. 1980;9(7–8):403‐408. [Google Scholar]
- 58. Tomoto T, Moriyoshi A, Sakai K, Shibata E, Kamijima M. Identification of the sources of organic compounds that decalcify cement concrete and generate alcohols and ammonia gases. Build Environ. 2009;44(9):2000‐2005. [Google Scholar]
- 59. Markowicz P, Larsson L. Influence of relative humidity on VOC concentrations in indoor air. Environ Sci Pollut R. 2015;22(8):5772‐5779. [DOI] [PubMed] [Google Scholar]
- 60. Wilke O, Jann O, Brodner D. VOC‐ and SVOC‐emissions from adhesives, floor coverings and complete floor structures. Indoor Air. 2004;14(Suppl 8):98‐107. [DOI] [PubMed] [Google Scholar]
- 61. Yokota T, Chino S, Kato S, Murakami S, Atake Y, Seo J‐H. Study on emission of decomposed chemicals of plasticizer contained in PVC flooring. J Environ Eng. 2007;72(617):47‐52. (in Japanese). [Google Scholar]
- 62. Chino S, Kato S, Seo J, Ataka Y. Measurement of reaction product emitted from flooring material. J Environ Eng. 2008;73(624):215‐220. (in Japanese). [Google Scholar]
- 63. Chino S, Kato S, Seo J, Ataka Y. Measurement of chemical compounds emitted from the floor constructed various kinds of PVC floorings. J Environ Eng. 2009;74(636):185‐191. (in Japanese). [Google Scholar]
- 64. Sjoberg A, Ramnas O. An experimental parametric study of VOC from flooring systems exposed to alkaline solutions. Indoor Air. 2007;17(6):450‐457. [DOI] [PubMed] [Google Scholar]
- 65. Yonemoto S, Nishimoto S, Kojima T, et al. Study on generation mechanism of 2‐ethylhexanol from vinyl chloride floor sheet construct and its suppression method. Jpn Soc Finish Tech. 2006;22:19‐22. (in Japanese). [Google Scholar]
- 66. Kamijima M, Sakai K, Yokoyama K, et al. 1‐Butanol emission from building floors. Jpn J Hyg. 2006;61:250. (in Japanese). [Google Scholar]
- 67. Sunesson AL, Nilsson CA, Andersson B, et al. Volatile metabolites produced by two fungal species cultivated on building materials. Ann Occup Hyg. 1996;40(4):397‐410. [DOI] [PubMed] [Google Scholar]
- 68. Van Lancker F, Adams A, Delmulle B, et al. Use of headspace SPME‐GC‐MS for the analysis of the volatiles produced by indoor molds grown on different substrates. J Environ Monitor. 2008;10(10):1127‐1133. [DOI] [PubMed] [Google Scholar]
- 69. Bjurman J, Kristensson J. Volatile production by Aspergillus versicolor as a possible cause of odor in houses affected by fungi. Mycopathologia. 1992;118(3):173‐178. [Google Scholar]
- 70. Pasanen P, Korpi A, Kalliokoski P, Pasanen A‐L. Growth and volatile metabolite production of Aspergillus versicolor in house dust. Environ Int. 1997;23(4):49‐57. [Google Scholar]
- 71. Nalli S, Cooper DG, Nicell JA. Biodegradation of plasticizers by Rhodococcus rhodochrous . Biodegradation. 2002;13(5):343‐352. [DOI] [PubMed] [Google Scholar]
- 72. Pasanen P, Korpi A, Kalliokoski P, Pasanen A‐L. Interaction of metabolites with R. rhodochrous during the biodegradation of di‐ester plasticizers. Chemosphere. 2006;65(9):1510‐1517. [DOI] [PubMed] [Google Scholar]
- 73. Nakamiya K, Hashimoto S, Ito H, Edmonds JS, Yasuhara A, Morita M. Microbial treatment of bis (2‐ethylhexyl) phthalate in polyvinyl chloride with isolated bacteria. J Biosci Bioeng. 2005;99(2):115‐119. [DOI] [PubMed] [Google Scholar]
- 74. Horn O, Nalli S, Cooper D, Nicell J. Plasticizer metabolites in the environment. Water Res. 2004;38(17):3693‐3698. [DOI] [PubMed] [Google Scholar]
- 75. Nalli S, Horn OJ, Grochowalski AR, Cooper DG, Nicell JA. Origin of 2‐ethylhexanol as a VOC. Environ Pollut. 2006;140(1):181‐185. [DOI] [PubMed] [Google Scholar]
- 76. Andersson B, Andersson K, Nilsson C‐A. Mass spectrometric identification of 2‐ethylhexanol in indoor air: recovery studies by charcoal sampling and gas chromatographic analysis at the micrograms per cubic metre level. J Chromatogr A. 1984;291:257‐263. [Google Scholar]
- 77. Girman JR, Hadwen GE, Burton LE, et al. Individual volatile organic compound prevalence and concentrations in 56 buildings of the building assessment survey and evaluation (BASE) study. Proc Indoor Air. 1999;II:460‐465. [Google Scholar]
- 78. Hodgson AT, Rudd AF, Beal D, Chandra S. Volatile organic compound concentrations and emission rates in new manufactured and site‐built houses. Indoor Air. 2000;10(3):178‐192. [DOI] [PubMed] [Google Scholar]
- 79. Sakai K, Kamijima M, Shibata E, et al. Indoor air pollution by volatile organic compounds in large buildings. Nihon Koshu Eisei Zassi (Jpn J Public Health). 2010;57(9):825‐834. (in Japanese). [PubMed] [Google Scholar]
- 80. Ishidao T, Ishimatsu S, Hori H. Measurement of volatile organic compounds concentrations in indoor air of school buildings in a university. J Work Environ. 2004;25(5):66‐71. (in Japanese). [Google Scholar]
- 81. Ichiba T, Takahashi T, Yamashita Z, et al. Approach to sick building problem in school. Jpn J Hyg. 2009;64(1):26‐31. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 82. Manabe R, Kunugita N, Katoh T, et al. Investigation of air pollution in a shopping center and employees’ personal exposure level. Jpn J Hyg. 2008;63(1):20‐28. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 83. Akiyama Y, Kunugita N, Katoh T, et al. Indoor air quality of an art museum and a museum. J Jpn Soc Atmos Environ. 2008;43(6):323‐331. (in Japanese). [Google Scholar]
- 84. Takeuchi S, Tanaka‐Kagawa T, Saito I, et al. Differential determination of plasticizers and organophosphorus flame retardants in residential indoor air in Japan. Environ Sci Pollut R. 2018;25(8):7113‐7120. [DOI] [PubMed] [Google Scholar]
- 85. Reiser R, Meile A, Hofer C, Knutti R. Indoor air pollution by volatile organic compounds (VOC) emitted from flooring material in a technical university in Switzerland In: Proceedings of the 9th International Conference on Indoor Air Quality and Climate. California: Organizing Committee of 9th International Conference on indoor Air Quality and Climate; 2002:1004‐1009 [Google Scholar]
- 86. Mandin C, Trantallidi M, Cattaneo A, et al. Assessment of indoor air quality in office buildings across Europe ‐ The OFFICAIR study. Sci Total Environ. 2017;579:169‐178. [DOI] [PubMed] [Google Scholar]
- 87. Wålinder R, Wieslander G, Norbäck D, Wessen B, Venge P. Nasal lavage biomarkers: effects of water damage and microbial growth in an office building. Arch Environ Health. 2001;56(1):30‐36. [DOI] [PubMed] [Google Scholar]
- 88. Wieslander G, Kumlin A, Norback D. Dampness and 2‐ethyl‐1‐hexanol in floor construction of rehabilitation center: Health effects in staff. Arch Environ Occup Health. 2010;65(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 89. Oikawa D, Takao Y, Murata S, Takeuchi W, Shimoyama K, Sekine Y. Measurement of carbonyl and volatile organic compounds in indoor air of temporary houses constructed in Miyagi prefecture. Indoor Environ. 2011;14(2):113‐121. (in Japanese). [Google Scholar]
- 90. Nordström K, Norbäck D, Wieslander G. Subjective indoor air quality in geriatric hospitals. Indoor Built Environ. 1999;8(1):49‐57. [Google Scholar]
- 91. Wieslander G, Norbäck D, Nordström K, Wålinder R, Venge P. Nasal and ocular symptoms, tear film stability and biomarkers in nasal lavage, in relation to building‐dampness and building design in hospitals. Int Arch Occup Environ Health. 1999;72(7):451‐461. [DOI] [PubMed] [Google Scholar]
- 92. Norback D, Wieslander G, Nordstrom K, Wålinder R. Asthma symptoms in relation to measured building dampness in upper concrete floor construction, and 2‐ethyl‐1‐hexanol in indoor air. Int J Tuberc Lung Dis. 2000;4(11):1016‐1025. [PubMed] [Google Scholar]
- 93. Mori M, Hara K, Miyakita T, Ishitake T. Association of indoor air quality with physical health of users in a newly built school building in a university. Jpn J Hyg. 2011;66(1):122‐128. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 94. Ernstgard L, Norback D, Nordquist T, et al. Acute effects of exposure to 1 mg/m3 of vaporized 2‐ethyl‐1‐hexanol in humans. Indoor Air. 2010;20(2):168‐175. [DOI] [PubMed] [Google Scholar]
- 95. van Thriel C, Seeber A, et al. Physiological and psychological approaches to chemosensory effects of solvents. Toxicol Lett. 2003;140–141:261‐271. [DOI] [PubMed] [Google Scholar]
- 96. van Thriel C, Kiesswetter E, Schaper M, et al. An integrative approach considering acute symptoms and intensity ratings of chemosensory sensations during experimental exposures. Environ Toxicol Pharmacol. 2005;19(3):589‐598. [DOI] [PubMed] [Google Scholar]
- 97. van Thriel C, Kiesswetter E, Schaper M, et al. From neurotoxic to chemosensory effects: new insights on acute solvent neurotoxicity exemplified by acute effects of 2‐ethylhexanol. Neurotoxicology. 2007;28(2):347‐355. [DOI] [PubMed] [Google Scholar]
- 98. Opdyke D. Fragrance raw material monographs. 2‐Ethylhexanol. Food Cosmetic Toxicol. 1979;17:775‐777. [Google Scholar]
- 99. Schaper M. Development of a database for sensory irritants and its use in establishing occupational exposure limits. Am Ind Hyg Assoc J. 1993;54(9):488‐544. [DOI] [PubMed] [Google Scholar]
- 100. Hodgson JR. Results of peroxisome induction studies on tri(2‐ethylhexyl)trimellitate and 2‐ethylhexanol. Toxicol Ind Health. 1987;3(2):49‐61. [DOI] [PubMed] [Google Scholar]
- 101. Nishimura H, Saito S, Kishida F, Matsuo M. Analysis of acute toxicity (LD50‐value) of organic chemical to mammals by solubility parameter (1) acute oral toxicity to rats. Jpn J Ind Health. 1994;36(5):314‐323. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 102. Smyth HF, Carpenter CP, Weil CS, Pozzani UC, Striegel JA, Nycum JS. Range‐finding toxicity data: list VII. Am Ind Hyg Assoc J. 1969;30(5):470‐476. [DOI] [PubMed] [Google Scholar]
- 103. Shaffer C, Carpenter CP, Smyth H Jr. Acute and subacute toxicity of di (2‐Ethylhexyl) phthalate with note upon its metabolism. J Ind Hyg Toxicol. 1945;27(5):130‐135. [Google Scholar]
- 104. Dave G, Lidman U. Biological and toxicological effects of solvent extraction chemicals: Range finding acute toxicity in the rainbow trout (Salmo gairdnerii Rich.) and in the rat (Rattus norwegicus L.). Hydrometallurgy. 1978;3(3):201‐216. [Google Scholar]
- 105. Schmidt P, Gohlke R, Rothe R. Toxicity of various C8‐aldehydes and alcohols. Z Ges Hyg. 1973;19(7):485‐490. (in German). [PubMed] [Google Scholar]
- 106. Nishimura H, Saito S, Kishida F, Matsuo M. Analysis of acute toxicity (LD50‐value) of organic chemical to mammals by solubility parameter (2) acute oral toxicity to mice. Jpn J Ind Health. 1994;36(6):421‐427. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 107. Bojes HK, Thurman RG. Peroxisomal proliferators inhibit acyl CoA synthetase and stimulate protein kinase C in vivo. Toxicol Appl Pharmacol. 1994;126(2):233‐239. [DOI] [PubMed] [Google Scholar]
- 108. Dirven HA, van den Broek PH, Peters JG, Noordhoek J, Jongeneelen FJ. Microsomal lauric acid hydroxylase activities after treatment of rats with three classical cytochrome P450 inducers and peroxisome proliferating compounds. Biochem Pharmacol. 1992;43(12):2621‐2629. [DOI] [PubMed] [Google Scholar]
- 109. Moody DE, Reddy JK. Serum triglyceride and cholesterol contents in male rats receiving diets containing plasticizers and analogues of the ester 2‐ethylhexanol. Toxicol Lett. 1982;10(4):379‐383. [DOI] [PubMed] [Google Scholar]
- 110. Wood DL, Bitman J. The effect of feeding di‐(2‐ethylhexyl) phthalate and related compounds on lipids in the laying hen. Poult Sci. 1984;63(3):469‐477. [DOI] [PubMed] [Google Scholar]
- 111. Yamada A. Toxicity of phthalic acid esters and hepatotoxicity of di‐(2‐ethyl‐ hexyl) phthalate. J Food Hyg Soc Jpn. 1974;15(3):147‐152. (in Japanese). [Google Scholar]
- 112. Pollack GM, Shen DD, Dorr MB. Contribution of metabolites to the route‐ and time‐dependent hepatic effects of di‐(2‐ethylhexyl)phthalate in the rat. J Pharmacol Exp Ther. 1989;248(1):176‐181. [PubMed] [Google Scholar]
- 113. Rhodes C, Soames T, Stonard MD, Simpson MG, Vernall AJ, Elcombe CR. The absence of testicular atrophy and in vivo and in vitro effects on hepatocyte morphology and peroxisomal enzyme activities in male rats following the administration of several alkanols. Toxicol Lett. 1984;21(1):103‐109. [DOI] [PubMed] [Google Scholar]
- 114. Keith Y, Cornu MC, Canning PM, Foster J, Lhuguenot JC, Elcombe CR. Peroxisome proliferation due to di‐(2‐ethylhexyl) adipate, 2‐ethylhexanol and 2‐ethylhexanoic acid. Arch Toxicol. 1992;66(5):321‐326. [DOI] [PubMed] [Google Scholar]
- 115. Carpenter CP, Smyth HF Jr. Chemical burns of the rabbit cornea. Am J Ophthalmol. 1946;29(11):1363‐1372. [DOI] [PubMed] [Google Scholar]
- 116. Kennah HE, Hignet S, Laux PE, et al. An objective procedure for quantitating eye irritation based upon changes of corneal thickness. Fundam Appl Toxicol. 1989;12(2):258‐268. [DOI] [PubMed] [Google Scholar]
- 117. Badr MZ, Handler JA, Whittaker M, Kauffman FC, Thurman RG. Interactions between plasticizers and fatty acid metabolism in the perfused rat liver and in vivo. Inhibition of ketogenesis by 2‐ethylhexanol. Biochem Pharmacol. 1990;39(4):715‐721. [DOI] [PubMed] [Google Scholar]
- 118. Kambia K, Dine T, Gressier B, Dupin‐Spriet T, Luyckx M, Brunet C. Evaluation of the direct toxicity of trioctyltrimellitate (TOTM), di(2‐ethylhexyl) phthalate (DEHP) and their hydrolysis products on isolated rat hepatocytes. Int J Artif Organs. 2004;27(11):971‐978. [DOI] [PubMed] [Google Scholar]
- 119. Nelson BK, Brightwell WS, Krieg EF Jr. Developmental toxicology of industrial alcohols: a summary of 13 alcohols administered by inhalation to rats. Toxicol Ind Health. 1990;6(3–4):373‐387. [DOI] [PubMed] [Google Scholar]
- 120. Ritter EJ, Scott WJ Jr, Randall JL, Ritter JM. Teratogenicity of di(2‐ethylhexyl) phthalate, 2‐ethylhexanol, 2‐ethylhexanoic acid, and valproic acid, and potentiation by caffeine. Teratology. 1987;35(1):41‐46. [DOI] [PubMed] [Google Scholar]
- 121. Hellwig J, Jackh R. Differential prenatal toxicity of one straight‐chain and five branched‐chain primary alcohols in rats. Food Chem Toxicol. 1997;35(5):489‐500. [DOI] [PubMed] [Google Scholar]
- 122. Hardin BD, Schuler RL, Burg JR, et al. Evaluation of 60 chemicals in a preliminary developmental toxicity test. Teratogen Carcin Mut. 1987;7(1):29‐48. [DOI] [PubMed] [Google Scholar]
- 123. Tyl RW, Fisher LC, Kubena MF, et al. The developmental toxicity of 2‐ethylhexanol applied dermally to pregnant Fischer 344 rats. Fundam Appl Toxicol. 1992;19(2):176‐185. [DOI] [PubMed] [Google Scholar]
- 124. Li LH, Jester WF Jr, Laslett AL, Orth JM. A single dose of Di‐(2‐ethylhexyl) phthalate in neonatal rats alters gonocytes, reduces sertoli cell proliferation, and decreases cyclin D2 expression. Toxicol Appl Pharmacol. 2000;166(3):222‐229. [DOI] [PubMed] [Google Scholar]
- 125. Sjoberg P, Bondesson U, Gray TJ, Plöen L. Effects of di‐(2‐ethylhexyl) phthalate and five of its metabolites on rat testis in vivo and in in vitro. Acta Pharmacol Toxicol. 1986;58(3):225‐233. [DOI] [PubMed] [Google Scholar]
- 126. Gray TJ, Beamand JA. Effect of some phthalate esters and other testicular toxins on primary cultures of testicular cells. Food Chem Toxicol. 1984;22(2):123‐131. [DOI] [PubMed] [Google Scholar]
- 127. Dostal LA, Chapin RE, Stefanski SA, Harris MW, Schwetz BA. Testicular toxicity and reduced Sertoli cell numbers in neonatal rats by di (2‐ethylhexyl) phthalate and the recovery of fertility as adults. Toxicol Appl pharmacol. 1988;95(1):104‐121. [DOI] [PubMed] [Google Scholar]
- 128. Moss EJ, Cook MW, Thomas LV, Gray T. The effect of mono‐(2‐ethylhexyl) phthalate and other phthalate esters on lactate production by Sertoli cells in vitro. Toxicol Lett. 1988;40(1):77‐84. [DOI] [PubMed] [Google Scholar]
- 129. Piché CD, Sauvageau D, Vanlian M, Erythropel HC, Robaire B, Leask RL. Effects of di‐(2‐ethylhexyl) phthalate and four of its metabolites on steroidogenesis in MA‐10 cells. Ecotox Environ Safe. 2012;79:108‐115. [DOI] [PubMed] [Google Scholar]
- 130. Putman DL, Moore WA, Schechtman LM, Hodgson JR. Cytogenetic evaluation of di‐(2‐ethylhexyl)phthalate and its major metabolites in Fischer 344 rats. Environ Mutagen. 1983;5(2):227‐231. [DOI] [PubMed] [Google Scholar]
- 131. Seed JL. Mutagenic activity of phthalate esters in bacterial liquid suspension assays. Environ Health Perspect. 1982;45:111‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Saido K, Taguchi H, Yada S, et al. Thermal decomposition products of phthalates with poly (vinyl chloride) and their mutagenicity. Macromol Res. 2003;11(3):178‐182. [Google Scholar]
- 133. Warren JR, Lalwani ND, Reddy JK. Phthalate esters as peroxisome proliferator carcinogens. Environ Health Perspect. 1982;45:35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tomita I, Nakamura Y, Aoki N, Inui N. Mutagenic/carcinogenic potential of DEHP and MEHP. Environ Health Perspecy. 1982;45:119‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zeiger E, Haworth S, Speck W, Mortelmans K. Phthalate ester testing in the National Toxicology Program's environmental mutagenesis test development program. Environ Health Perspect. 1982;45:99‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kirby PE, Pizzarello RF, Lawlor TE, Haworth SR, Hodgson JR. Evaluation of di‐(2‐ethylhexyl)phthalate and its major metabolites in the Ames test and L5178Y mouse lymphoma mutagenicity assay. Environ Mutagen. 1983;5(5):657‐663. [DOI] [PubMed] [Google Scholar]
- 137. Shimizu H, Suzuki Y, Takemura N, Goto S, Matsushita H. The results of microbial mutation test for forty‐three industrial chemicals. Jpn J Ind Health. 1985;27(6):400‐419. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 138. Agarwal DK, Lawrence WH, Nunez LJ, Autian J Mutagenicity evaluation of phthalic acid esters and metabolites in Salmonella typhimurium cultures. J Toxicol Environ Health. 1985;16(1):61‐69. [DOI] [PubMed] [Google Scholar]
- 139. DiVincenzo GD, Hamilton ML, Mueller KR, Donish WH, Barber ED. Bacterial mutagenicity testing of urine from rats dosed with 2‐ethylhexanol derived plasticizers. Toxicology. 1985;34(3):247‐259. [DOI] [PubMed] [Google Scholar]
- 140. Phillips BJ, James TE, Gangolli SD. Genotoxicity studies of di(2‐ethylhexyl)phthalate and its metabolites in CHO cells. Mutat Res. 1982;102(3):297‐304. [DOI] [PubMed] [Google Scholar]
- 141. Albro PW, Corbett JT, Schroeder JL, Jordan S, Matthews HB. Pharmacokinetics, interactions with macromolecules and species differences in metabolism of DEHP. Environ Health Perspect. 1982;45:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ward JM, Diwan BA, Ohshima M, et al. Tumor‐initiating and promoting activities of di(2‐ethylhexyl) phthalate in vivo and in vitro. Environ Health Perspect. 1986;65:279‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Takahashi T. Biochemical studies on phthalic esters–II. Effects of phthalic esters on mitochondrial respiration of rat liver. Biochem Pharmacol. 1977;26(1):19‐24. [DOI] [PubMed] [Google Scholar]
- 144. Gray TJ, Beamand JA, Lake BG, Foster JR, Gangolli SD. Peroxisome proliferation in cultured rat hepatocytes produced by clofibrate and phthalate ester metabolites. Toxicol Lett. 1982;10(2–3):273‐279. [DOI] [PubMed] [Google Scholar]
- 145. Gray T, Lake BG, Beamand JA, Foster JR, Gangolli SD. Peroxisomal effects of phthalate esters in primary cultures of rat hepatocytes. Toxicology. 1983;28(1–2):167‐179. [DOI] [PubMed] [Google Scholar]
- 146. Mitchell AM, Lhuguenot JC, Bridges JW, Elcombe CR. Identification of the proximate peroxisome proliferator(s) derived from di(2‐ethylhexyl) phthalate. Toxicol Appl Pharmacol. 1985;80(1):23‐32. [DOI] [PubMed] [Google Scholar]
- 147. Cornu MC, Lhuguenot JC, Brady AM, Moore R, Elcombe CR. Identification of the proximate peroxisome proliferator(s) derived from di (2‐ethylhexyl) adipate and species differences in response. Biochem Pharmacol. 1992;43(10):2129‐2134. [DOI] [PubMed] [Google Scholar]
- 148. Hijioka T, Keller BJ, Thurman RG. Wy‐14,643 but not 2‐ethylhexanol increases intracellular free calcium in cultured Kupffer cells. Toxicol Lett. 1991;59(1–3):239‐244. [DOI] [PubMed] [Google Scholar]
- 149. Rose ML, Rivera CA, Bradford BU, et al. Kupffer cell oxidant production is central to the mechanism of peroxisome proliferators. Carcinogenesis. 1999;20(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 150. Law MY, Moody DE. In vitro inhibition of mouse and rat glutathione S‐transferases by di(2‐ethylhexyl) phthalate, mono(2‐ethylhexyl) phthalate, 2‐ethylhexanol, 2‐ethylhexanoic acid and clofibric acid. Toxicol In Vitro. 1991;5(3):207‐210. [DOI] [PubMed] [Google Scholar]
- 151. Agarwal DK, Agarwal S, Seth PK. Interaction of di‐(2‐ethylhexyl) phthalate with the pharmacological response and metabolic aspects of ethanol in mice. Biochem Pharmacol. 1982;31(21):3419‐3423. [DOI] [PubMed] [Google Scholar]
- 152. Seth PK. Hepatic effects of phthalate esters. Environ Health Perspect. 1982;45:27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yoshida Y, Liu J, Sugiura T, et al. The indoor air pollutant 2‐ethyl‐hexanol activates CD4 cells. Chem Biol Interact. 2009;177(2):137‐141. [DOI] [PubMed] [Google Scholar]
- 154. Lehmann R, Hatt H, van Thriel C. Alternative in vitro assays to assess the potency of sensory irritants‐Is one TRP channel enough? Neurotoxicology. 2017;60:178‐186. [DOI] [PubMed] [Google Scholar]
- 155. Lehmann R, Schobel N, Hatt H, van Thriel C. The involvement of TRP channels in sensory irritation: a mechanistic approach toward a better understanding of the biological effects of local irritants. Arch Toxicol. 2016;90(6):1399‐1413. [DOI] [PubMed] [Google Scholar]
- 156. Liang DC, Keller BJ, Misra UK, Thurman RG. Oxygen tension is a major determinant of hepatotoxicity due to 2‐ethylhexanol in isolated tissue cylinders from periportal and pericentral regions of the liver lobule from phenobarbital‐treated rats. Toxicol Appl Pharmacol. 1991;107(2):344‐349. [DOI] [PubMed] [Google Scholar]
- 157. Keller BJ, Yamanaka H, Liang DC, et al. O2‐dependent hepatotoxicity due to ethylhexanol in the perfused rat liver: mitochondria as a site of action. J Pharmacol Exp Ther. 1990;252(3):1355‐1360. [PubMed] [Google Scholar]
- 158. Keller BJ, Liang D, Thurman RG. 2‐Ethylhexanol uncouples oxidative phosphorylation in rat liver mitochondria. Toxicol Lett. 1991;57(1):113‐120. [DOI] [PubMed] [Google Scholar]
- 159. Keller BJ, Yamanaka H, Thurman RG. Inhibition of mitochondrial respiration and oxygen‐dependent hepatotoxicity by six structurally dissimilar peroxisomal proliferating agents. Toxicology. 1992;71(1–2):49‐61. [DOI] [PubMed] [Google Scholar]
- 160. Burri L, Thoresen GH, Berge RK. The role of PPAR activation in liver and muscle. PPAR Res. 2010;2010:542359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Maloney EK, Waxman DJ. trans‐Activation of PPARα and PPARγ by structurally diverse environmental chemicals. Toxicol Appl Pharmacol. 1999;161(2):209‐218. [DOI] [PubMed] [Google Scholar]
- 162. Uhde E, Salthammer T. Impact of reaction products from building materials and furnishings on indoor air quality—a review of recent advances in indoor chemistry. Atmos Environ. 2007;41(15):3111‐3128. [Google Scholar]


