Abstract
Epidemiologic evidence suggests that individuals during their prenatal development may be especially vulnerable to the effects of environmental factors such as stress that predisposes them to psychiatric disorders including alcohol use disorder (AUD) later in life. Currently, the epigenetic mechanisms of anxiety comorbid with AUD induced by prenatal stress (PRS) remain to be elucidated. Here, we examined anxiety-like and alcohol drinking behaviors in adult offspring of prenatally stressed dam (PRS-mice) using elevated plus maze, light/dark box and two-bottle free-choice paradigm. It was found that PRS-mice exhibit heightened anxiety-like behaviors and increased alcohol intake in adulthood and these behavioral deficits were associated with a significant decrease in dendritic spine density (DSD) in medial prefrontal cortex (mPFC) relative to their controls (NS mice). To determine the mechanisms by which PRS reduces DSD, we examined the expressions of key genes associated with synaptic plasticity, including activity regulated cytoskeleton associated protein (Arc), spinophilin (Spn), postsynaptic density 95(Psd95), tropomyosin receptor kinase B (TrkB), protein kinase B (Akt), mammalian target of rapamycin (mTOR) and Period 2 (Per2) in mPFC of PRS- and NS-mice. The mRNA levels of these genes were significantly decreased in PRS-mice. Methylated DNA and chromatin immunoprecipitation studies revealed that DNA methylation or reduced histone H3K14 acetylation on promoters of above genes suggesting that epigenetic dysregulation may be responsible for the deficits in their expression. Findings from this study suggest that prenatal stress induced abnormal epigenetic mechanisms and synaptic plasticity-related events may be associated with anxiety-like and alcohol drinking behaviors in adulthood.
Keywords: prenatal stress, epigenetics, DNA methylation, dendritic spine, synaptic plasticity, anxiety, alcohol intake
1. Introduction
The alcohol use disorder (AUD) is highly prevalent among people with mood disorders, including anxiety and depression and contribute a gross public health burden globally (Bijl and Ravelli, 2000; Burns and Teesson, 2002; Hasin et al., 2007). Recent studies show that people with anxiety/depressive disorders have an increased risk to develop alcohol use disorders (de Graaf et al., 2003; Robinson et al., 2009; Schuckit and Hesselbrock, 1994; McDolnald and Meyer, 2011). Several studies have also demonstrated strong association with anxiety-like and alcohol drinking behaviors in preclinical models (Pandey et al, 2004, Pandey et al., 2017). Also, adolescent intermittent alcohol exposure leads to development of anxiety-like and alcohol drinking behaviors in rats during adulthood (Kyzar et al., 2016; 2017). In addition, accumulated evidence suggests that molecular mechanisms of AUDs and anxiety behaviors may be associated with epigenetic dysregulation of candidate genes in specific neurocircuitry via chromatin remodeling characterized by aberrant DNA methylation and histone modifications that leads to altered gene expressions (Tsankova et al., 2007; Mehler, 2008; Day et al., 2015; Pandey et al.,2017; Starkman et al., 2012; Qiang et al., 2014; Warnault et al., 2013; Ponomarev, 2013; Manzardo et al., 2013; Barbier et al., 2015).
Epidemiologic evidence accumulated over decades suggests that individuals during their prenatal development may be especially vulnerable to the effects of environmental factors that predispose them to psychiatric disorders including alcoholism later in life (Becker et al., 2011; Gordon, 2002; Sinha, 2007, 2008; Uhart and Wand, 2009; Charil et al., 2010; Fine et al., 2014; Markham and Koenig, 2011; Mulder et al., 2002; Fumagalli et al., 2007; Winstock, 2008). Currently, precise molecular mechanisms in the specific brain regions due to prenatal stress-induced epigenetic changes in the development of the comorbidity of anxiety and alcoholism remain unclear. We recently found that adult offspring (mice) born from prenatally stressed dam (referred as PRS-mice) exhibited anxiety-like behaviors characterized by reduced social interaction and it has been established that these behavioral changes may be attributed to altered DNA methylation profiles and disrupted chromatin structures in genes associated with mental disorders such as Bdnf, Gad1 and Relin in the medial frontal cortex (mPFC) (Dong et al., 2014, 2016; Zheng et al., 2016, Matrisciano et al., 2013). However, it is not clear whether long-lasting epigenetic reprogramming induced by prenatal stress leads to alteration of dendritic spine density and genes associated with synaptic plasticity since abnormal synaptic plasticity plays an important role in the pathogenesis of neuropsychiatric disorders including anxiety, depression and alcoholism (Segal, 2005; Fagiolini et al., 2009). In addition, since anxiety often predisposes one to increase of alcohol consumption, it is necessary to explore whether prenatal stress induced anxiety-like behaviors is comorbid with altered alcohol drinking behaviors. Therefore, in this study, we first examined the effects of prenatal stress on behavioral phenotypes of anxiety and alcohol consumption in adult offspring using PRS mouse model. We also investigated dendritic spine densities and epigenetic changes in the genes associated with spine formation and plasticity in the mPFC of PRS and control adult mice. We focused our study in mPFC, as this region has been shown to play a major role in vulnerability to stress and also is associated with neuropsychiatric disorders including AUD (Duman et al., 2016; Bludau et al., 2016; Heilig et al.,2017). Our novel results suggest prenatal stress can lead to long-lasting epigenetic modifications of synaptic plasticity-associated genes, thereby causing synaptic remodeling in mPFC and producing behavioral phenotypes of anxiety and alcoholism in adulthood offspring.
2. Methods
2.1. Animals and PRS procedure
All procedures were performed according to NIH guidelines for animal research (Guide for the Care & Use of Laboratory Animals, NRC, 1996) and were approved by the Animal Care Committee of the University of Illinois at Chicago. Pregnant mice (Swiss albino ND4, Harlan, Indianapolis, IN, USA) were individually housed with a 12-h light–dark cycle, and food and water ad libitum. Control dams were left undisturbed throughout gestation, whereas stressed dams were subjected to repeated episodes of restraint stress, as described previously (Dong et al., 2014, 2016; Zheng et al., 2016, Matrisciano et al., 2013). The stress procedure consisted of restraining the pregnant dam in a transparent tube (12 × 3 cm) under a bright light for 45 min three times per day from the seventh day of pregnancy until delivery. After weaning (PND 21), male mice were selected for the study and housed five per cage separately by condition. A maximum of one or two male pups was taken from each litter for each measure to remove any litter effects (Becker and Kowall, 1977; Chapman and Stern, 1979). All behavioral tests, including drinking experiments were performed in one set of mice whereas biochemical measurements in the brain were conducted in separate sets of mice that were not subjected to behavioral tests.
2.2. Behavioral Experiments
2.2.1. Elevated Plus-Maze Test (EPM):
To examine the anxiety behavior of gestational-stress offspring, the elevated plus-maze was performed in similar way as described by us (Pandey et al., 2015). Briefly, it consisted of two open and two closed arms and was made of Plexiglas. The closed arms had transparent Plexiglas walls at the sides and end. The floor was made of black Plexiglas and elevated to a height of 50 cm above the floor. At the start of each test, mice were placed individually on the central platform and their behavior monitored by computer for 10 minutes. The number of entries for each arm and the time spent in each arm were recorded and analyzed. The percentage of open arm entries (open arm entries x100/total arm entries) and percentage of time spent in open arm (time spent in open arm x100/time spent in open and closed arms) were used as indices of anxiety. The number of closed arm entries is represented as general activity of mice.
2.2.2. Light/Dark Box Exploration test (LDB):
The LDB consists of a dark compartment without illumination and a light compartment with illumination (0.25 Amp; light-emitting diode light). Both compartments are connected through an opening. On the day of testing, each mouse was allowed a 5-min pretest habituation period in the room before testing. Then, the mouse was gently placed in the dark compartment with its head facing away from the opening. The mouse was observed for a 5-min test period, and the time spent in each compartment was monitored and recorded by computer. The percentage of time spent in either the dark compartment or light compartment was calculated for each animal. Total ambulation in the light and dark compartments was represented as the general activity of the mouse (Pandey et al., 2015, Sakharkar et al., 2014).
2.2.3. Alcohol Preference:
Alcohol preference was measured by the two-bottle free-choice paradigm (Pandey et al., 2004). Mice were placed in individual cages and have ad libitum access to food and water, in two bottles, and were habituated to drink water from either bottle. Bottle positions were changed daily so that the mice would not develop a position habit. Once they started drinking water equally from either bottle, mice were provided with 3% (v/v) alcohol solution in one bottle and water in the other bottle daily for 3 days, and then concentrations of ethanol were increased to 7% for 3 days, 9% for 3 days and to 12% for another 3 days. Consumption of ethanol and water (ml) was measured daily at 6:00 PM, and fresh water and ethanol (3, 7, 9, or 12%) solution in water were provided every day at the start of dark cycle. The mean percentage of alcohol intake and the percentage of water intake were calculated from their total fluid intake for 3 days for 3, 7, 9, and 12% alcohol. We measured body weight of mice before and after each dose of alcohol. The alcohol intake was presented as g/kg/day.
2.3. Histological study
Spine Density Measurement:
The Golgi-Cox staining procedure was performed to measure the dendritic spine density in the pyramidal neurons of mPFC using the FD Rapid Golgi Stain Kit (Pandey et al., 2008). Brains were rapidly immersed in impregnation solution for at least 2 week. Then 200 μm brain sections were cut, mounted and stained according to the protocol provided by Kit manufacturer. After staining, sections were dehydrated and cleared in xylene solution and then cover slipped using mounting medium. Sections were observed under a light microscope at high magnification (100x). Spines from neurons where dendrites are connected to soma and showing complete impregnation were marked and then counted using IMAGE J Program (Orlowski and Bjarkam, 2012). Spines from dendrites (a total of 9 dendrites) from three adjacent brain sections were counted and then averaged for each mouse. Total of 5 mice from each group was analyzed. The dendritic spine density was represented as mean ± SEM of the number of dendritic spines/10μm of dendritic length. All reconstructions were conducted with a ZEISS Axioskop2 microscope.
2.4. Biochemical measurements
2.4.1. Quantitative real-time PCR:
The quantitative PCR measurements were carried out using the Applied Biosystems Real-Time PCR System with a SYBR green master mix (Fermentas, Glen Burnie, MD, USA). After behavioral tests, total RNA from the mPFC of PRS and NS mice, was isolated using TRIZOL reagent (Life Technologies, Grand Island, NY, USA), and was further purified using the RNeasy kit (QIAGEN, Valencia, CA, USA). The expressions of Arc, PSD95, Spn, TrkB mTor, Akt, were measured using RT-qPCR. ActB gene was chosen for normalizing mRNA expression. To confirm amplification specificity, the PCR products were subject to a melting curve analysis, in which only one peak was observed. Each sample was run in duplicate and repeated twice. Primers were designed to span at least one intron–exon boundary. PCR efficiency was carried out to confirm the specificity of the primers. The relative gene expressions were calculated using ΔΔCt method. The primer sequences used to amplify the genes analyzed are summarized in supplementary Table 1.
2.4.2. Immunoblotting:
Total protein from mPFC, extracted using RIPA lysis buffer and quantified by Enhanced BCA Protein Assay Kit (Beyotime P0010S), was separated by SDS-PAGE and transferred to PVDF membrane. After being blocked in TBS buffer containing 0.05% Tween-20 and 5% skim milk, the membranes were incubated overnight at 4°C with the following primary antibodies: anti-Arc (Abcam; ab118929; 1:1000), anti-PSD95 (Abcam, ab18258, 1:1000), anti-spinophilin (Upstate 06–852, 1:1000), anti-TrkB (Sigma-Aldrich, 07–225, 1:1000), anti-mTOR (Abcam, ab2972, 1:1000), anti-Akt (Cell Signaling, #9272, 1:1000) and anti-Per2 (Novus biologicals NB100–125; 1:1000), After incubation with the corresponding secondary antibody, the immunoreactive signals were visualized by LI-COR ODYSSEY Fc Western Blotting Detection System ( and quantitated using Image Studio Ver5.2. The levels of these proteins in the stress offspring versus non-stress offspring were normalized by β-actin protein levels. In order to estimate background caused by non-specific binding of secondary antibody, a secondary control without the primary antibody was performed.
2.4.3. DNA Methylation Procedure:
DNA methylation (enrichment of 5-methylcytosine, 5-mC) on the promoters of genes listed above were measured using MeDIP (Methylated DNA immunoprecipitation Diagenode, Denville, NJ, USA) as previously described by us (Dong et al., 2014, 2016). Genomic DNA isolated was sonicated to 200bp using Bioruptor (Diagenode) and subjected to immunoprecipitation using specific 5-mC antibody followed by qPCR. Primers (Table 1) were designed to be specific to the CpG enriched regions near the promoters of the genes. Input genomic DNA that was not subjected to the methylation enrichment procedure was used as a control. The percentage of methylated vs. unmodified promoter was calculated using the following equation: % [(meDNA−IP/total input)] = 2[(Ct(10% input)−3.32)−Ct(meDNA−IP)] × 100%.
Table 1.
| Primer sequences for mRNA expression | ||
| Genes | Forward primer (5’−3’) | Reward Primer (5’−3’) |
| Akt | CCTCTGCTTTGTCATGGAGTAT | CACAATCTCCGCACCATAGAA |
| Arc | CCTGAGCCACCTGGAAGAGTA | GGCCCATTCATGTGGTTCTG |
| Mtor | GGTGTGGCATGTGGTTCTGT | CCATCCAATCTGATGCTGGA |
| Per2 | TTGGTGTGTGGGTTGTTGTG | CTACCTGGTCAAGGTGCAAGAG |
| PSD95 | TGACGACCCATCCATCTTTATC | CCCGGACATCCACTTCATT |
| Spn | GATCCAAGTATTCAGCACCTACTC | CACTCGCTTCTCTAGCTCATATTC |
| TrkB | ACTAAGATCCACGTCACCAATC | CAGGGTGTAGTCTCCGTTATTC |
| b-Actin | TAAGGCCAACCGTGAAAAGATGAC | ACCGCTCGTTGCCAATAGTGATG |
| Primer sequences for MeDIP and ChIP assays | ||
| Genes | Forward primer (5’−3’) | Reward Primer (5’−3’) |
| Akt | CTACTGGAGGGAGGTCTTCTAT | GTGATCTACCCATCTCTGGTTC |
| Arc | AATAACCTGCCTTAGCCTCATC | CCGAGTGACTAATGTGCTCTG |
| Mtor | CTCACGACTGATTGGCTCTT | GGGAGGAACATCCACCAATAA |
| Per2 | GCTGGTCAGTTTAGGAAGTAGG | GGATGTCTCACACCGTCAATAA |
| PSD95 | CATTGCCCTGAAGAACGC | ATGGATCTTGGCCTCGAA |
| Spn | ATGAATCCAAGAAGGAGGACTTC | GATGAAGAAGACGAAGAGGACG |
| TrkB | CAAAGACCCGTTACCGACTT | GTGATCTACCCATCTCTGGTTC |
2.4.4. Chromatin Immunoprecipitation Assay:
We performed ChIP assays based on protocols previously described (Dong et al., 2014, 2016). Briefly, about 10 mg of tissue was used for this procedure. Tissue slices were fixed with 1% formaldehyde at 37°C for 15 min to crosslink acetylated histones with the target genomic DNAs. After being washed six times with cold PBS containing protease inhibitors, slices were homogenized in 200–400 μl of SDS lysis buffer. To obtain consistent chromatin fragmentation, the lysates were sonicated by a Sonic Dismembrator, Model 500 (Fisher Scientific) at 70% of output power for 10 s on ice and repeated 4 times. The sizes of the majority of sonicated genomic DNA fragments included 250 to 500 bp. The ChIP procedure was carried out by using the ChIP assay kit and protocol (Upstate Cell Signaling Solutions). The concentration of ChIP grade anti-acetyl-histone H3 (lysine 14) (AcH3K14) antibody (Millipore, Billerica) was suggested by the manufacturer. An aliquot (1–2%) of the sonicated lysate without antibody served as an Input. At the end of the ChIP procedure, the protein/DNA cross-linked nucleosomal chromatin complex immunoprecipitated by antiboy was reverse cross-linked. Protein-free DNA then was extracted for detection and quantification of genes above. The percentages of immunoprecipitated DNA were calculated as described above for the MeDIP.
2.5. Statistical analysis
Significant differences between two groups (PRS vs NS) were assessed by Student t-test for LDB, EPM, DSD, mRNA, Western blot, MeDIP and ChIP assays (two tailed), or 2 -way repeated ANOVA followed by Bonferroni post hoc comparisons for alcohol drinking behavior (using group and ethanol as two factors) using IBM SPSS Statistic 24 (SPSS, Chicago, IL, USA). Values are represented as mean ± S.E.M. The criterion for statistical significance was p<0.05. The statistical significance (PRS vs NS) was represented as: *p < 0.05, ** p < 0.01 and ***p < 0.001.
3. Results
3.1. Prenatal stress induces anxiety like behaviors in adulthood
We previously reported that (Dong et al., 2014, 2016; Zheng et al., 2016, Matrisciano et al., 2013) prenatal stress induces behavioral deficits in adult offspring, including hyperlocomotion, stereotype behavior, social interaction and fear conditioning deficits. Here, we used another test for anxiety measures. In EPM test as shown in Fig 1A, PRS mice exhibited a significant decrease in both duration of stay in and number of entries into open arms (Fig. 1A) when compared with their NS counterparts p < 0.05 for open arm entries, **p <0.01 for duration in open arms, Student t-test, N = 8 per group. There were no significant differences of closed arm entries between two groups, suggesting no changes in the general activity of the mice in EPM test. Similar results were found in LDB test where PRS mice spent significantly more time in dark than light compartments (Fig. 1B) in comparison with NS group *p < 0.05, Student t-test, N = 8 per group (Fig. 1B). The total ambulation in LDB of PRS mice did not significantly differ from NS mice (Fig. 1C) showing no changes in the general activity of the mice. These findings suggest that prenatal stress can lead to anxiety-like phenotypes in adult offspring.
Figure 1:
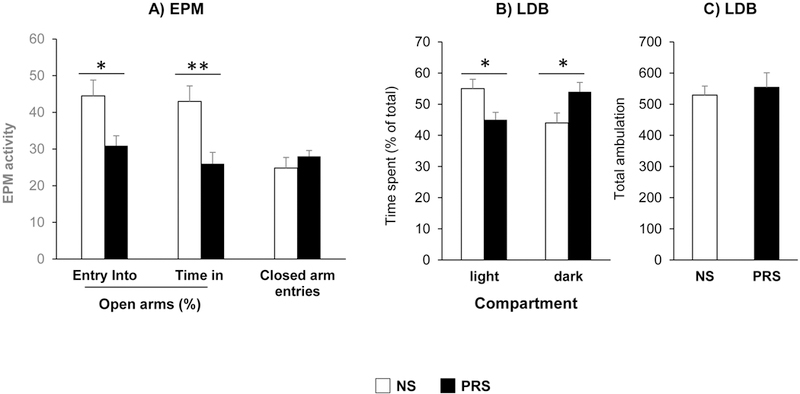
Prenatal stress induces anxiety-like behavior in adult offspring at age of postnatal day 75 (PND75). Anxiety-like behaviors were measured using the elevated plus maze (EPM) and Light/Dark Box (LDB) tests. Compared with NS mice, PRS mice exhibited low percentage of entering into, and time spent in open arms in EPM test (A), high preference to dark compartment in LDB test (B) and similar ambulation of LDB exploration (C) Data are presented as mean ± SEM. For EPM, *p < 0.05 (open arm entries), **p < 0.01 (duration in open arms) vs. NS mice, respectively, N= 8 for each group. For LDB, *p < 0.05, NS vs PRS mice, N= 8 for each group.
3.2. PRS Promotes Alcohol Drinking Behaviors in Adult Offspring
Accumulated evidence suggests that anxiety may result in a risk of alcoholism (Becker et al., 2011; Uhart and Wand, 2009; Fumagalli et al., 2007; Weinstock, 2008). We therefore next tested the drinking behaviors in PRS mice using two bottle free choice paradigm. It was found that PRS mice showed higher alcohol consumption when pharmacologically relevant concentrations of 3–12% ethanol were offered (Fig. 2A). At the concentration of 7–12%, PRS-mice consumed about 3-fold more alcohol than their control counterparts [F(1, 14) = 26.547; ***p < 0.001, 2-way repeated ANOVA, N = 8 per group]. The drinking behavior of PRS mice is characterized by increasing intake of alcohol solution [F(1, 14) = 20.533; ***p < 0.001, 2-way repeated ANOVA, N = 8 per group] and decreasing water intake [F(1, 14) = 20.578; ***p < 0.001, 2-way repeated ANOVA, N = 8 per group] (Fig. 2B and C) but there were no significant differences between two groups (NS vs PRS) in total fluid intake (Fig. 2D). These data suggest that prenatal stress induces higher alcohol preference in adult offspring as compared with control non-stress adult offspring.
Figure 2:
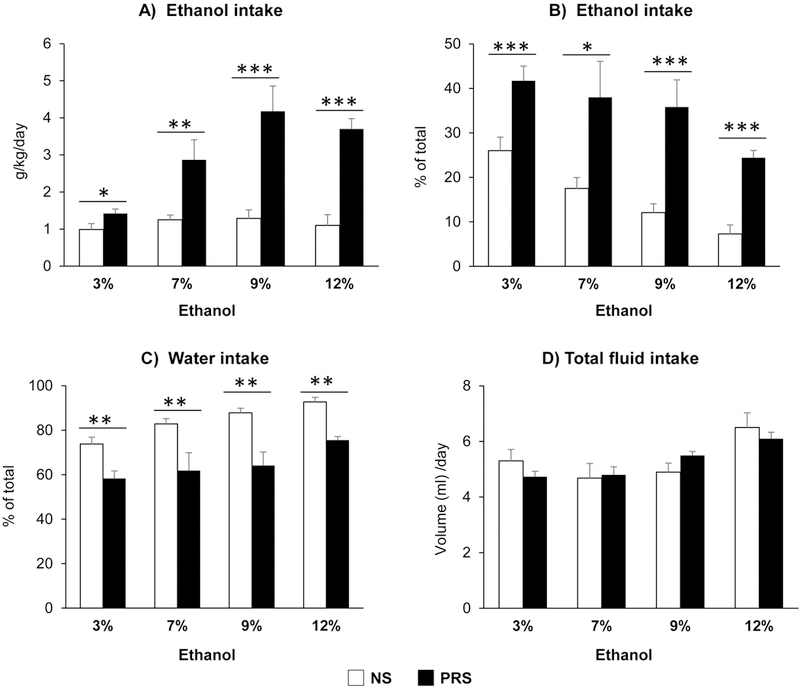
Prenatal stress induces alcohol intake in adult offspring (PND75). Compared with NS mice, PRS mice consumed more alcohol (g/kg/d) (A) [NS vs PRS mice, *p < 0.05 (3%); **p < 0.01(7%); ***p < 0.001(9%) and ***p < 0.001 (12%), N = 8 for each group] and exhibited high preferences to alcohol (B) [NS vs PRS mice, ***p < 0.001 (3%); *p < 0.05(7%); *p < 0.001(9%) and **p < 0.001(12%), N=8 for each group] and less to water intake (C) [NS vs PRS mice, **p < 0.01 (3%); **p < 0.01(7%); **p < 0.01 (9%) and **p < 0.01 (12%), N=8 for each group] in the two-bottle free-choice test. There was no significant difference in total fluid intake between two groups (D). Values are the mean ± SEM.
Prenatal stress disrupts synaptic plasticity related events
Studies reveal that impaired synaptic function characterized by substantial dendritic abnormalities in the pyramidal neurons of mPFC is found to be associated with psychiatric behavioral phenotypes in PRS mice (Duman et al., 2016; Christoffel et al., 2011; Korb and Finkbeiner, 2011; Segal, 2005). To determine whether prenatal stress results in impaired dendritic spine formation, we assessed dendritic spine density using Golgi-Cox staining method. The impregnated pyramidal neurons were identified by their triangular somal shape, the presence of an apical dendrite and numerous dendritic spines. Nine pyramidal neurons from each animal were selected for count spines. All reconstructions were conducted with a ZEISS Axioskop2 microscope. Figure 3A and B show the typical images of dendritic spines on apical dendrite of pyramidal neurons in mPFC of NS and PRS mice from which significant differences in morphology and density of spines between two groups were viewed. The result of quantitative analyses showed that there is about 30% less (***p < 0.001, Student t-test, N = 5 per group) dendritic spine density in PRS neurons than in NS controls (Fig. 3E).
Figure 3:
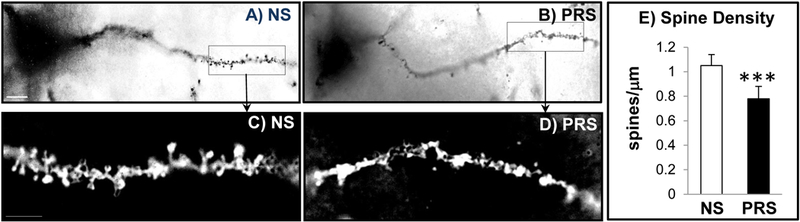
Prenatal stress (PRS) induces reduction of dendritic spines on mPFC neurons. A and B are representative photomicrographs of dendritic spines of pyramidal neurons from mPFC of NS and PRS mice (PND75). C and D are the magnified views of boxed areas in A and B. E is the bar diagram showing quantification of dendritic spine density in mPFC of adult NS and PRS mice. Values are the mean ± SEM. ***p < 0.001, N=5 mice for each group. Scale bars in A and B, 10μm; in C and D, 5μm).
To explore the possible molecular mechanisms by which prenatal stress leads to reduction of dendritic spines, we focused on the expressions of key genes related to synaptic formation, stability and function, including activity regulated cytoskeleton associated protein (Arc), spinophilin (Spn), postsynaptic density 95(Psd95), tropomyosin receptor kinase B (TrkB). As shown in Fig. 4A, the mRNAs transcribed by above genes were decreased by about 30–50% in mPFC of PRS mice as compared with NS counterparts (Arc: **p < 0.05; Spn: **p < 0.01; Psd95: *p < 0.05; TrkB: **p < 0.01; Student t-test, N =8 per group). To confirm RT-PCR observation, we conducted immunoblotting with specific antibodies. The results show that all proteins encoded by above synaptic genes were significantly decreased in PRS mice (ARC: ***p < 0.001; SPN: **p < 0.01; PSD95: *p < 0.05; TRKB: *p < 0.05; Student t-test, N = 5 per group) (Fig. 4B). These findings provide molecular evidence supporting the notion that reduced dendritic spine density may be related to reductions in synaptic plasticity associated gene expression in the cortical structures of prenatal stress mice.
Figure 4:
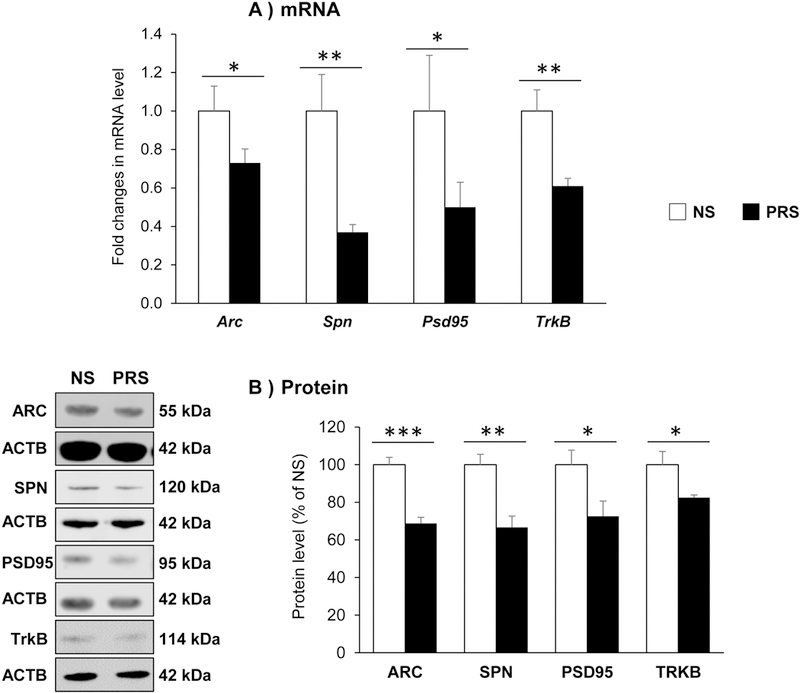
Prenatal stress induces a decrease in the expressions of specific synaptic genes (A) and their encoded proteins (B) in the mPFC offspring of PND75. The data are expressed as mean ± SEM. For genes: Arc (*p < 0.05), Spn (**p < 0.01), Psd95 (*p < 0.05) and TrkB (**p < 0.01); for proteins: ARC (***p < 0.001), SPN (**p < 0.01), PSD95 (*p < 0.05) and TRKB (*p < 0.05) NS vs PRS mice, N=8 in each group for gene expression and N = 5 in each group for Western immunoblotting.
3.4. Prenatal stress induces epigenetic dysregulation on genes associated with altered synaptic function.
Our previous findings indicated that in PRS brain, DNMT1 and DNMT3a are significantly overexpressed, which suggests that the reduction of candidate genes associated with prenatal stress may occur via promoter hypermethylation (Dong et al., 2014, 2016; Zheng et al., 2016, Matrisciano et al., 2013). To test the hypothesis, we examined the promoter methylation status of Arc, Spn, Psd95 and TrkB using MeDIP by measuring the enrichment of 5mC with specific antibody. As shown in Fig. 5A, there was significant enrichment of 5-mC found on Arc and Spn but not Psd95 and TrkB promoters (Arc: *p < 0.05; Spn: *p < 0.05; Student t-test, N = 8 per group) in PRS-mice compared to their NS counterparts, indicating that decreased expression of Arc and Spn may largely due to hypermethylation on their promoters.
Figure 5:
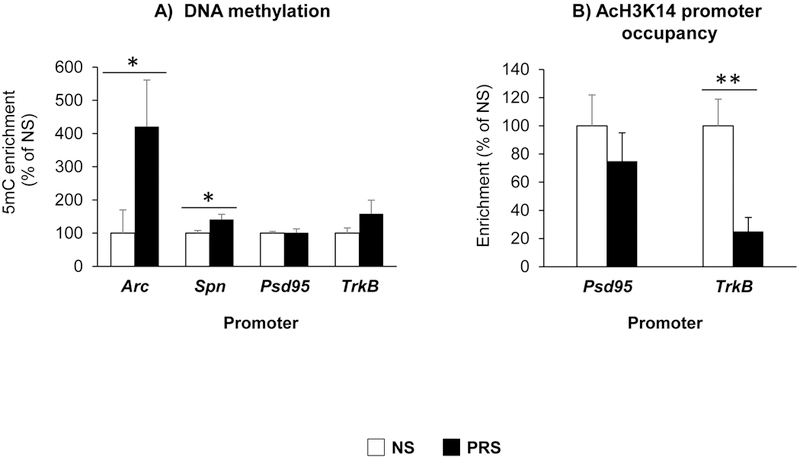
In mPFC of PND 75 old offspring, prenatal stress induces, (A) altered DNA methylation (defined as levels of 5mC) on Arc (*p < 0.05), Spn (*p < 0.05) promoters, NS vs PRS mice, N = 8 per group; (B) decreased histone H3K14 acetylation (AcH3K14) on TrkB (**p < 0.01) promotors. The data are expressed as mean ± SEM. NS vs PRS mice, N = 8 for each group.
To further explore the possible mechanisms of Psd95 and TrkB downregulation, we analyzed histone acetylation levels around their promoters using ChIP assay. By using ChIP grade specific antibody that recognize acetylation at the lysine-14 (K14) residue of histone H3 (AcH3K14) (Zheng et al., 2016), we found that there was significant reduction in the acH3K14 (TrkB: **p < 0.01; Student t-test, N = 8 per group) occupancy associated with TrkB but not Psd95 promoters (Fig. 5B). The results provide evidence that decreased acH3K14 on TrkB promoter may lead to its reduction in PRS mice.
3.5. Prenatal stress induces downregulation of Akt - mTOR signaling cascade and circadian gene Per 2.
Akt (protein kinase B) and mTOR (mammalian target of rapamycin) are important components playing multiple roles in regulating local protein synthesis (Li et al., 2010; Akama and McEwen, 2003), including Psd95. To investigate whether decreased PSD95 in PRS mice is linked to downregulation of Akt and mTOR, we measured their mRNA and protein expressions. As shown in Fig. 6A and B, there were significant reductions of Akt and mTOR mRNA/protein in PRS mice as compared with NS group (Akt: *p < 0.01; mTOR: **p < 0.05; Student t-test, N = 8 per group) (AKT: *p < 0.05; MTOR: *p < 0.05; Student t-test, N = 5 per group). MeDIP data (Fig. 6C) revealed high levels of 5mC on their promoters, suggesting that hyper DNA methylation may be responsible for their downregulation (Akt: ***p < 0.05; mTor: *p < 0.001; Student t-test, N = 8 per group). These finding suggest that Akt and mTOR signaling cascade was disrupted by prenatal stress, which may contribute to the reduction of Psd95.
Figure 6:
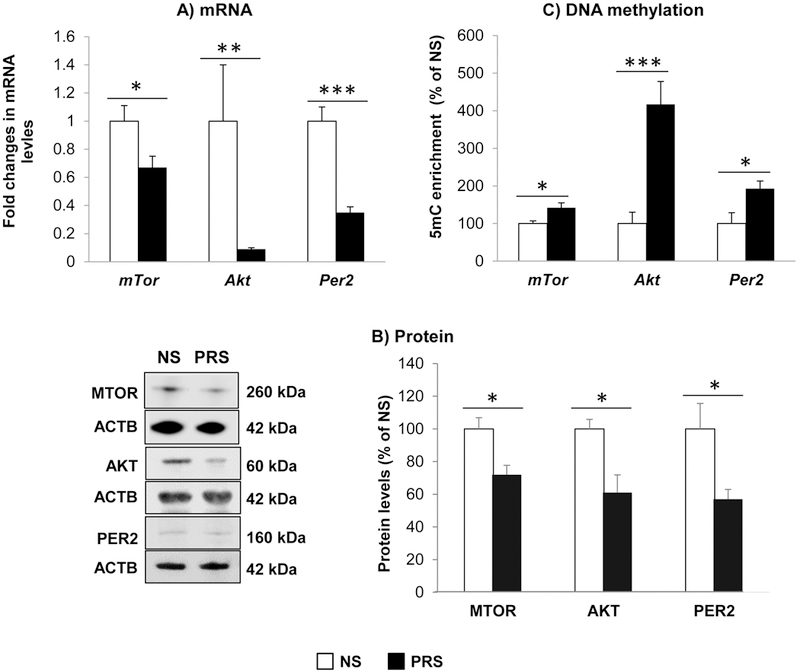
Prenatal stress induces: (A) decrease in the expressions of mTor (*p < 0.05), Akt (**p < 0.01), and Per 2 (***p < 0.001); (B) their encoded proteins [MTOR (*p < 0.05), AKT (*p < 0.05), and PER 2 (*p < 0.05); and (C) DNA hyermethylation (defined as levels of 5mC) on their promoters: mTor (*p < 0.05), Akt (***p < 0.001), and Per 2 (*p < 0.05) in mPFC of PND 75 old offspring. The data are expressed as mean ± SEM. NS vs PRS mice, N = 8 in each group for RT-PCR and MeDIP, N = 5 in each group for Western immunoblotting.
Recent studies revealed that circadian genes, including Period (Per) can influence alcohol consumption behavior (Spanagel et al., 2005; Blomeyer et al., 2013; Gamsby et al., 2013). To determine whether prenatal stress also produce changes in the expression of these genes, we measured mRNA and protein levels of Per2. The results showed that Per2 levels significantly decreased in PRS mice as compared with NS group (Fig. 6A-C) (Per2: *p < 0.001, N = 8; PER2:* p < 0.05; N = 5; Student t-test). MeDIP data show high levels of 5mC on its promoter, suggesting that DNA methylation may lead to downregulation of Per2 expression (Per2: *p < 0.05; Student t-test, N = 8 per group). These findings provide evidence that prenatal stress can influence circadian gene that may be associated with the phenotype of excessive alcohol intake observed in PRS mice.
4. Discussion
In this study, using prenatal stress mouse model of neurodevelopmental disorders (Dong et al., 2014, 2016; Zheng et al., 2016, Matrisciano et al., 2013) we demonstrated that PRS mice exhibit not only anxiety-like but also alcohol drinking behaviors. These findings support the notion that early life stress is a risk factor in the development of AUD comorbid with anxiety disorders (Becker et al., 2011; Gordon, 2002; Markham and Koenig, 2011; Mulder et al., 2002; Fumagalli et al., 2007; Winstock, 2008). Another finding of this study is that PRS-induced deficits in histone acetylation and increased DNA methylation possibly leading to decreased expression of synaptic plasticity-associated genes in mPFC. To establish association between deficits in synaptic genes and impairs synaptic function in PRS adult offspring, we examined dendritic spine density using Golgi-Cox staining in adult offspring (PND75). Interestingly, a significant reduction of dendritic spine density of pyramidal neurons is found in the mPFC of PRS-mice compared to NS counterparts. We believe that this morphological change induced by prenatal stress may be involved in the behavioral deficits observed in PRS mice, including excessive alcohol intake. The decreased spine density in PRS mice is supported by the findings from qPCR and immunoblotting that the genes/proteins associated with synaptic formation, stability and function such as Arc, spinophilin, PSD95 and TrkB are remarkably decreased in the same brain region. Among many proteins implicated in synaptic function, Arc is a master regulator of neuronal function (Korb and Finkbeiner, 2011) and as a critical effector molecule downstream of many signaling pathways. Arc, plays an important role in facilitating LTP consolidation, modulating dendritic spine density and regulating cognitive functions (Shepherd and Bear, 2011). Dysregulation of Arc expression can lead to synaptic dysfunction which is observed in a number of neurological disorders (Li et al., 2015). Downregulation of Arc in the central nucleus of amygdala (CeA) by its antisense oligonucleotides lead to reduced DSD and provoked anxiety-like behaviors and increased alcohol intake in rats (Pandey et al., 2008), suggesting a crucial role of Arc in the pathophysiology of AUD. Furthermore, lower Arc levels and DSD were also found in the amygdaloid structures of alcohol preferring rats as compared with non-preferring rats (Moonat et al., 2011). Here, we observed that PRS-induced deficits in Arc and other genes due to aberrant chromatin architecture might be involved in anxiety and higher alcohol consumption phenotypes in adult offspring. Spinophilin, as one of typical synaptic markers, facilitates spine growth. The deficit of this protein indicates an impairment of synaptic formation (Feng et al., 2000). PSD95 is notable in its integral role within the postsynaptic machinery mediating glutamate receptor anchoring and synaptic stability (Chen et al., 2011). TrkB, an important receptor for BDNF, modulates spine density and morphology (Kellner et al., 2014, Cao et al., 2013, Bramhan and Messaoudi 2005). To further explore the mechanisms by which above described synaptic genes are reduced by prenatal stress, we examined the status of DNA methylation on their promoter regions as we previously reported PRS-mice exhibits altered DNA methylation profiles at genes typically expressed in glutamatergic neurons and in GABAergic neurons (Dong et al., 2014, 2016). We have established that in the cortico-limbic structures of PRS-mice these promoters are hypermethylated and their transcription is downregulated (Dong et al., 2014, 2016). Thus, we hypothesized that decreased synaptic genes measured in this study may be due to promoter hyper DNA methylation. The MeDIP data demonstrate that although high levels of 5mC were found on the promoters of genes measured, the significant group effect was observed in Spn and Arc but not Psd95 and TrkB. It has been reported that Arc expression is regulated by BDNF-TrkB signaling pathway (Cao et al., 2013; Bramham et al., 2008). Thus, we can conclude that downregulation of Arc induced by prenatal stress may be result of promoter methylation and disruption of BDNF-TrkB signaling cascades. Since histone modifications, especially acetylation at lysine 14 on histone3, plays an important role for transcriptional activity, we then performed ChIP to explore the status of AcH3K14 on the promoters of Psd95 and TrkB. Data revealed a reduced binding of AcH3K14 on TrkB promoter, suggesting that low level of acetylated histone on its promoter may limit its expression.
The above results also indicate that the decreased PSD95 expression may be through different other than epigenetic mechanisms. To account for this, we examined the expressions of Akt and mTOR because these two genes are the key components of pathways which are essential for regulation of synaptic structure, maturation and function (Akama and McEwen, 2003). Activation of mTOR signaling pathway via Akt leads to de novo synthesis of synaptic proteins, including PSD95 (Akama and McEwen, 2003; Li et al., 2010). Our qPCR and immunoblotting data indicate that Akt and mTOR are notably decreased in PRS mice. Findings from MeDIP provide evidence that aberrant promoter methylation may be responsible for their downregulation. Thus, disruption of synaptic Akt-mTOR signaling pathway in PRS mice may be responsible for the decreased expression of PSD95.
Recent advance in AUD studies suggest that disruptions in circadian functions are associated with a wide variety of disorders, including anxiety and excessive alcohol intake. For example, downregulation of circadian clock components such as Period 2 (Per2) play a direct role in regulating ethanol consumption (Spanagel et al., 2005; Blomeyer et al., 2013; Gamsby et al., 2013). This prompted us to examine whether expression of Per2 is also altered in the mPFC of PRS mice. We found that Per2 was significantly reduced in PRS mice through an aberrant promoter methylation. Thus, disruption of circadian rhythms may be at least in part involved in excessive ethanol intake.
Taken together, we provide evidence that prenatal stress induces anxiety-like behaviors and excessive ethanol intake in adult offspring through epigenetic dysregulation of genes and signaling pathway associated with synaptic formation, and stability as well as function (Fig. 7). However, more studies are required to explore their causality. In this study, we specially focused on mPFC, as this brain region play a critical role in stress response and AUDs (Duman et al., 2016; Bludau et al., 2016; Heilig et al., 2017). However, the findings from this region are not enough to draw a conclusion that the causality of behavioral deficits induced by prenatal stress is only attributed by the mPFC. The role of other brain regions, such as hippocampus and amygdala cannot be ruled out since these two regions are also associated with psychiatric disorders and AUDs. Therefore, further studies are needed on epigenetic changes in these brain regions and compared them with mPFC in this model to address the region specificity of the findings.
Figure 7: Schematic diagram of putative mechanisms by which prenatal stress induces excessive alcohol intake comorbid with anxiety behaviors in adult offspring.
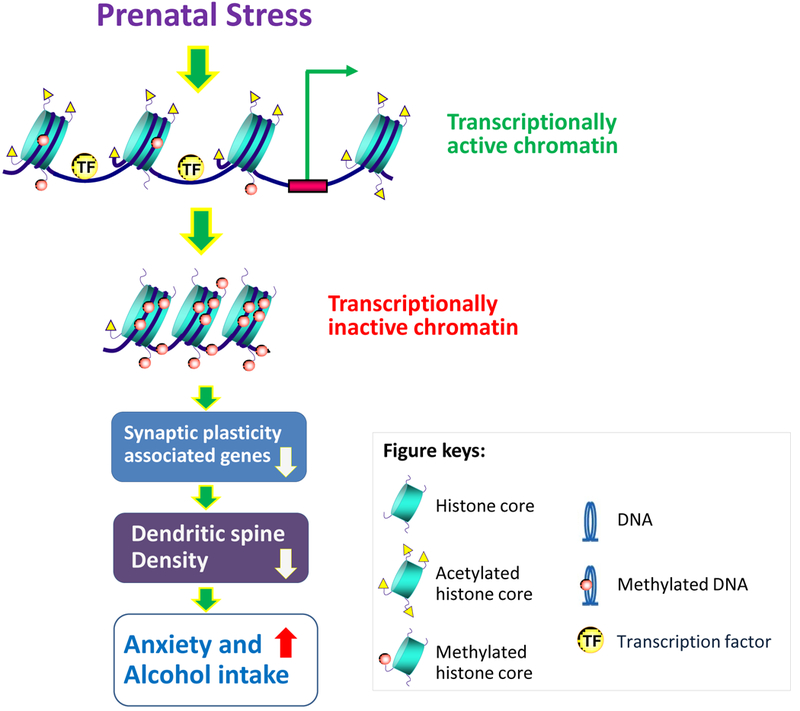
Epigenetic regulation of gene expression is controlled by remodeling of chromatin structure. Specifically, chromatin condensation and relaxation block or allow the accessibility of gene promoters to the transcriptional machinery. Prenatal stress induces increased DNMTs and HDACs (Dong et al., 2014, 2016, Zheng et al., 2016), leading to closed chromatin and to transcriptional silencing of genes associated with synaptic formation and plasticity, which may be responsible for the dendritic spine density, possibly operative in regulating anxiety-like and excessive alcohol intake phenotype in adult offspring.
5. Conclusions
The findings from the present study suggest that prenatal stress can induce alterations of multiple genes and networks in the mPFC of adult offspring, leading to anxiety-like behaviors and excessive alcohol intake. The PRS mice may serve as a useful model for exploring the development of AUD comorbid with anxiety and may be useful in preclinically screening for the potential efficacy of drugs acting on altered epigenetic mechanisms.
Supplementary Material
Highlights.
Prenatal stress induces excessive alcohol intake and anxiety-like behaviors in adult offspring.
Prenatal stress downregulates genes associated with spine formation and plasticity via epigenetic mechanisms.
Prenatal stress leads to decreased dendritic spine density in the medial frontal cortex of adult offspring.
Acknowledgments
This work was supported by the NIAAA P50AA022538 grant to SCP, AG and ED and NIAAA UO1AA-019971 grant and VA senior Research Career Scientist award to SCP.
Footnotes
Conflicts of Interest
The authors declare no potential conflicts of interest in this research.
References
- Akama KT, McEwen BS Estrogen stimulates posynaptic density-95 rapid protein synthesis via the Akt/proteinkinase B pathway. J Neurosci 23 (2003), pp. 2333–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Tapocik JD, Juergens N, Pitcairn C, Borich A, Schank JR, Sun H, Schuebel K, Zhou Z, Yuan Q, Vendruscolo LF, Goldman D, Heilig M DNA methylation in the medial prefrontal cortex regulates alcohol-induced behavior and plasticity. J Neurosci, 35(2015), pp. 6153–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, and Doremus-Fitzwater TL Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology, 218(2011), pp. 131–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G, Kowall M Crucial role of the postnatal maternal environment in the expression of prenatal stress effects in the male rats. J Comp Physiol Psychol, 91(1977) pp. 1432–1446 [DOI] [PubMed] [Google Scholar]
- Bijl RV, Ravelli A Psychiatric morbidity, service use, and need for care in the general population: results of The Netherlands Mental Health Survey and Incidence Study. Am J Public Health, 90 (2000), pp. 602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Buchmann AF, Lascorz J, Zimmermann US, Esser G, Desrivieres S, Schmidt MH, Banaschewski T, Schumann G, Laucht M Association of PER2 genotype and stressful life events with alcohol drinking in young adults. PLoS One, 8(2013), pp. e59136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bludau S, Bzdok D, Gruber O, Kohn N, Riedl V, Sorg C, Palomero-Gallagher N, Müller VI, Hoffstaedter F, Amunts K, Eickhoff SB Medial prefrontal aberrations in major depressive disorder revealed by cytoarchitectonically informed voxel-based morphometry. Am J Psychiatry, 173(2016), pp. 291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis Prog. Neurobiol, 76 (2005), pp.99–125 [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, and Guzowski JF The immediate early gene Arc/Arg3.1 regulation, mechanisms, and function. J Neurosci, 28(2008), pp. 11760–11767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L , Teesson M Alcohol use disorders comorbid with anxiety, depression and drug use disorders. Findings from the Australian National Survey of Mental Health and Well Being. Drug Alcohol Depend, 68(2002), pp. 299–307 [DOI] [PubMed] [Google Scholar]
- Cao C, Rioult-Pedotti MS, Migani P, Yu CJ, Tiwari R, Parang K, Spaller MR, Goebel DJ, Marshall J Impairment of TrkB-PSD-95 Signaling in Angelman Syndrome. PLoS Biol, 11(2013), pp. e1001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C & King S Prenatal stress and brain development. Brain Res Rev, 65 (2010), pp. 56–79 [DOI] [PubMed] [Google Scholar]
- Chapman RH, Stern JM Failure of severe maternal stress or ACTH during pregnancy to affect emotionality of male rat offspring: implications of litter effects for prenatal studies. Dev Psychobiol, 12(1979) pp. 255–267 [DOI] [PubMed] [Google Scholar]
- Chen X, Nelson CD, Li X, Winters CA, Azzam R, Sousa AA, Leapman RD, Gainer H, Sheng M, Reese TS PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci, 31(2011), pp. 6329–6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Russo SJ Structural and synaptic plasticity in stress-related disorders. Rev Neurosci, 22(2011), pp. 535–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Kennedy AJ, Sweatt JD DNA methylation and its implications and accessibility for neuropsychiatric therapeutics. Annu Rev Pharmacol Toxicol, 55 (2015), pp. 591–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Dzitoyeva S, Matrisciano F, Tueting P, Grayson DR, Guidotti A BDNF epigenetic modifications associated with schizophrenia-like phenotype induced by prenatal stress in mice. Biol Psych, 77(2014), pp. 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Tueting P, Matrisciano F, Grayson DR, Guidotti A Behavioral and molecular neuroepigenetic alterations in prenatally stressed mice: relevance for the study of chromatin remodeling properties of antipsychotic drugs. Transl Psychiatry, 12 (2016), 6:e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-actingantidepressants. Nat Med, 22 (2016), pp. 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med, 22(2016), pp. 238–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol, 19 (2009), pp. 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P Spinophilin regulates the formation and function of dendritic spines Proc Natl Acad Sci U S A, 97(2000), pp.9287–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine R, Zhang J and Stevens HE. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Mol Psychiatry 19 (2014), pp.641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Racagni G, Riva MA Stress during development: impact on neuroplasticity and relevance to psychopathology Prog. Neurobiol, 81 (2007), pp.197–217 [DOI] [PubMed] [Google Scholar]
- Gamsby JJ, Templeton EL, Bonvini LA, Wang W, Loros JJ, Dunlap JC, Green AI, Gulick The D Circadian Per1 and Per2 genes influence alcohol Intake, reinforcement, and blood slcohol levels. Behav Brain Res, 249 (2013), pp. 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HW Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocrinology, 27(2002), pp.115–126 [DOI] [PubMed] [Google Scholar]
- de Graaf R , Bijl RV, Spijker J, Beekman AT, Vollebergh WA Temporal sequencing of lifetime mood disorders in relation to comorbid anxiety and substance use disorders--findings from the Netherlands Mental Health Survey and Incidence Study. Soc Psychiatry Psychiatr Epidemiol, 38(2003), pp.1–11 [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry, (2007), pp. 830–842 [DOI] [PubMed] [Google Scholar]
- Heilig M, Barbier E, Johnstone AL, Tapocik J, Meinhardt MW, Pfarr S, Wahlestedt C, Sommer WH Reprogramming of mPFC transcriptome and function in alcohol dependence. Genes Brain Behav, 16(2017), pp. 86–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner Y, Gödecke N, Dierkes T, Thieme N, Zagrebelsky M, Korte M The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front Synaptic Neurosci, 6 (2014), pp. 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb E, Finkbeiner S Arc in synaptic plasticity: from gene to behavior. Trends Neurosci, 34(2011), pp. 591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Floreani C, Teppen TL,and Pandey SC Adolescent alcohol exposure: burden of epigenetic reprogramming, synaptic remodeling, and adult psychopathology. Front Neurosci, 10 (2016), pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N , Lee B, Liu RJ, Banasr M , Dwyer JM, Iwata M, Li XY, Aghajanian G Duman RS mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 329 (2010), pp. 959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y , Pehrson AL, Waller JA , Dale E , Sanchez C, Gulinello M A critical evaluation of the activity-regulated cytoskeleton associated protein (Arc/Arg3.1)’s putative role in regulating dendritic plasticity, cognitive processes, and mood in animal models of depression. Front Neurosci, 9 (2015), pp.1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzardo AM, Henkhaus RS, Butler MG Global DNA promoter methylation in frontal cortex of alcoholics and controls. Gene, 498 (2013), pp. 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Koenig Prenatal stress JI: Role in psychotic and depressive diseases. Psychopharmacology, 214 (2011), pp. 89–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR. Davis JM, Nicoletti F, Guidotti A Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology, 68 (2013), pp.184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JL, Meyer TD Self-report reasons for alcohol use in bipolar disorders: why drink despite the potential risks? Clin Psychol Psychother, 18 (2011), pp. 418–425 [DOI] [PubMed] [Google Scholar]
- Mehler MF Epigenetic principles and mechanisms underlying nervous system functions in health and disease Prog. Neurobiol, 86 (2008), pp. 305–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ , Robles de Medina PG , Huizink AC , Van den Bergh BR , Buitelaar JK , Visser GH Prenatal maternal stress: effects on pregnancy and the (unborn) child Early Hum Dev, 70(2002), pp. 3–14 [DOI] [PubMed] [Google Scholar]
- Orlowski D, Bjarkam CR A simple reproducible and time saving method of semi-automatic dendrite spine density estimation compared to manual spine counting J Neurosci Methods, 208(2012), pp.128–133 [DOI] [PubMed] [Google Scholar]
- Pandey SC , Zhang H , Ugale R , Prakash A, Xu T , Misra K Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism J Neurosci, 28(2008), pp. 2589–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC , Roy A , Zhang H , Xu T Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinkingbehaviors J Neurosci, 24(2004), pp. 5022–5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood Neurobiol Dis, 82 (2015), pp.607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Kyzar EJ, Zhang H Epigenetic basis of the dark side of alcohol addiction Neuropharmacology, 122 (2017), pp. 74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I Epigenetic control of gene expression in the alcoholic brain Alcohol Res, 35(2013), pp. 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Li JG, Denny AD, Yao JM, Lieu M, Zhang K, et al. Epigenetic mechanisms are involved in the regulation of ethanol consumption in mice Int. J. Neuropsychopharmacol, 18 (2014), pii: pyu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Sareen J, Cox BJ, and Bolton J Self-medication of anxiety disorders with alcohol and drugs: Results from a nationally representative sample J Anxiety Disord, 23(2009), pp. 38–45 [DOI] [PubMed] [Google Scholar]
- Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson DR, Pandey SC Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats Int J Neuropsychopharmacol, 17(2014), pp. 2057–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, and Hesselbrock V Alcohol dependence and anxiety disorders: What is the relationship? AJP, 151(1994), pp.1723–1734 [DOI] [PubMed] [Google Scholar]
- Segal M Dendritic spines and long-term plasticity Nat Rev Neurosci, 6(2005), pp.277–284 [DOI] [PubMed] [Google Scholar]
- Shepherd JD , Bear MF New views of Arc, a master regulator of synaptic plasticity Nat Neurosci, 14(2011), pp.279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R The role of stress in addiction relapse Curr Psychiatry Rep, 9(2007), pp. 388–395. [DOI] [PubMed] [Google Scholar]
- Sinha R, Chronic stress drug use, and vulnerability to addiction Ann. N. Y. Acad. Sci, 1141 (2008), pp.105–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R , Pendyala G, Abarca C, Zghoul T , Sanchis-Segura C, Magnone MC , Lascorz J , Depner M , Holzberg D , Soyka M , Schreiber S , Matsuda F , Lathrop M , Schumann G , Albrecht U The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption Nat Med, 11(2005), pp.35–42 [DOI] [PubMed] [Google Scholar]
- Starkman BG, Sakharkar AJ, and Pandey SC Epigenetics—Beyond the genome in alcoholism Alcohol Res, 34(2012), pp. 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, and Nestler EJ Epigenetic regulation in psychiatric disorders. Nature Reviews Neuroscience, 8(2007), pp. 355–367 [DOI] [PubMed] [Google Scholar]
- Uhart M, and Wand Stress GS, alcohol and drug interaction: An update of human research Addict. Biol, 14(2009), pp.43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Levine A, Barak S, Ron D Chromatin remodeling—a novel strategy to control excessive ethanol drinking Transl. Psychiatry, 3 (2013), e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M The long-term behavioural consequences of prenatal stress Neurosci Biobehav Rev, 32(2008), pp.1073–1086 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Fan W, Zhang X, Dong E Gestational stress induces depression-like and anxiety-like phenotypes through epigenetic regulation of BDNF expression in offspring hippocampus Epigenetics, 11(2016), pp.150–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


