Abstract
IL-6 produced by human fibroblast-like synoviocytes (HFLS) promotes rheumatoid arthritis (RA), while lncRNA DILC regulates liver cancer stem cells by inhibiting IL-6. Therefore, lncRNA DILC may participate in RA. In the present study, we found that plasma lncRNA DILC was down-regulated, while IL-6 was up-regulated in RA patients than in healthy controls. Plasma levels of lncRNA DILC and IL-6 were significantly and inversely correlated only in RA patients. Overexpression of lncRNA DILC resulted in promoted apoptosis of HFLS isolated from RA patients, while lncRNA DILC siRNA silencing played an opposite role. In addition, overexpression of lncRNA DILC also resulted in inhibited IL-6 expression in HFLS isolated from RA patients. Therefore, lncRNA DILC may participate in RA by inducing apoptosis of HFLS and down-regulating IL-6.
Keywords: human fibroblast-like synoviocytes, IL-6, lncRNA DILC, rheumatoid arthritis
Introduction
The human genome transcribes both non-coding RNAs (ncRNAs) and protein-coding mRNAs [1]. Studies on the human genome have revealed that the number of genes-transcribed ncRNAs is much bigger than the number of protein-coding genes [2]. Different from the role of mRNAs as bridges linking DNA and protein, ncRNAs directly participate in biological processes, such as the regulation of cell growth and differentiation, in the form of RNA [3]. A growing body of literature has revealed the critical functions of ncRNAs, or lncRNAs (>200 nt) in human diseases [4,5]. However, the clinical application of lncRNAs in treatment of human diseases is limited by their obscure functions.
Rheumatoid arthritis (RA) affects about 1 out of 100 people during their life time [6]. RA causes a series of clinical disorders, such as comorbidities in vascular and progressive articular destruction [7]. The continuous development of RA also increases the risk of other more severe diseases, such as cardiovascular diseases [8]. It has been reported that the altered expression pattern of lncRNAs is closely correlated with disease activity [9], indicating the involvement of lncRNAs in these diseases. LncRNA DILC regulates liver cancer stem cells by inhibiting IL-6 [11], which contribute to the development of RA [12], indicating the potential involvement of lncRNA DILC in RA. Our preliminary deep sequencing data also revealed the down-regulated expression of DILC in RA patients (data not shown). Therefore, the present study was carried out to explore the functions of lncRNA DILC in RA.
Materials and methods
Research subjects
A total of 75 patients with RA (patient group) and 66 healthy volunteers (control group) were enrolled in Southwest Medical University. Those participants were admitted by Southwest Medical University from March 2016 to August 2018. Patients’ inclusion criteria: (1) RA patients diagnosed in Southwest Medical University; (2) patients with complete medical history over the past 5 years; (3) patients understood the experimental principle and were willing to participate. Patients’ exclusion criteria: (1) RA patients who were treated within 3 months before admission; (2) multiple diseases were diagnosed. The 75 RA patients included 40 males and 35 females, and mean age was 49.4 ± 5.1 years. DAS28 score (disease activity) ranged from 3.99 to 5.57 (mean 4.77 ± 0.92). The 66 healthy volunteers included 36 males and 30 females, and the mean age was 49.8 ± 5.8 years. All participants signed informed consent. Ethics Committee of Southwest Medical University approved the present study.
Plasma and human fibroblast-like synoviocyte preparations
Blood (5 ml) was extracted from the elbow vein of each participant under fasting conditions before therapies. Blood was extracted from patients before any treatment. Plasma samples were prepared through conventional method.
According to the methods described by Lee et al. [13], human fibroblast-like synoviocytes (HFLSs) were isolated and cultivated. HFLSs at passages 5–7 were collected for subsequent experiments.
Real-time quantitative PCR (RT-qPCR)
Plasma samples or HFLSs were directed mixed with RNAzol reagent (Sigma-Aldrich, St. Louis, MO, U.S.A.) to extract total RNAs. RevertAid RT Reverse Transcription Kit (Thermo Fisher Scientific) was used to perform reverse transcription to synthesize cDNAs. Real-time quantitative PCR (RT-qPCR) was performed to detect the expression of lncRNA DILC and IL-6 mRNA with all PCR reaction systems prepared using Luna® Universal One-Step RT-qPCR Kit (NEB, Ipswich, MA, U.S.A.). Primers of lncRNA DILC, IL-6, and endogenous control GAPDH were designed and synthesized by GenePharma (Shanghai, China). LncRNA DILC and IL-6 mRNA expression was normalized to GAPDH based on 2−ΔΔCT method.
Enzyme-linked immunosorbent assay (ELISA)
Human IL-6 Quantikine ELISA Kit (D6050, R&D Systems) was used to measure plasma levels of IL-6. IL-17 levels were normalized to pg/ml before subsequent analysis.
Cell transfection
Vectors expressing lncRNA DILC was constructed by inserting lncRNA DILC genome DNA into pCI mammalian expression vector, which was done by Sangon (Shanghai, China). LncRNA DILC siRNA and negative control siRNA were also designed and synthesized by Sangon (Shanghai, China). Lipofectamine 2000 reagent (Invitrogen, Thermo Fisher Scientific, Inc.) was used to perform all cell transfections with 10 nM vectors. Cells with no transfections were control cells. Negative controls cells were cells transfected with empty vectors or negative control siRNAs. Cells were collected 24 h after transfection before subsequent experiments.
Cell apoptosis assay
Cell apoptosis assays were performed using cell collected at 24 h after transfection. Briefly, single cell suspensions (3 × 104 cells/ml) were prepared using serum-free cell culture medium. Cells were transferred to a 6-well plate with 2 ml cell suspensions per well. Cells were cultivated for 48 h to allow cell apoptosis. After digestion with 0.25% trypsin, cells were stained with V-FITC (Dojindo, Japan) and propidium iodide (PI) (Dojindo, Japan). Finally, flow cytometry was performed to detect apoptotic cells.
Total protein extraction and Western blotting
Total Protein Extraction Kit (NBP2-37853, Novus Biologicals) was used to extract total proteins from HFLSs at 24 h after the transfection of lncRNA DILC expression vectors. Protein concentrations were measured by BCA method, followed by electrophoresis performed using 10% SDS–PAGE gel. After gel transfer to PVDF membranes, membranes were incubated in 5% non-fat milk for 2 h at 25°C for blocking. After that, membranes were first incubated with primary antibodies of rabbit anti-human IL-6 (ab6672, 1:1000, Abcam) and GAPDH (ab9485, 1: 1000, Abcam), and secondary antibody of goat anti-rabbit IgG-HRP (1:1000, MBS435036, MyBioSource). Signals were developed using ECL (Sigma-Aldrich, U.S.A.) and normalized using Image J v1.47 software.
Statistical analysis
Mean ± standard deviation was used to represent the data from three biological replicates. GraphPad Prism 6 software was used to perform all statistical analyses. Comparisons between plasma levels of lncRNA DILC and IL-6 between RA patients and healthy controls were performed by unpaired t test. Comparisons of cell apoptosis and IL-6 expression among different transfection groups were performed by one-way ANOVA and Tukey test. Pearson’s correlation coefficient was used to analyze the correlations between plasma levels of lncRNA DILC and IL-6. Differences were statistically significant at P<0.05.
Results
Plasma lncRNA DILC and IL-6 showed opposite expression pattern in RA patients
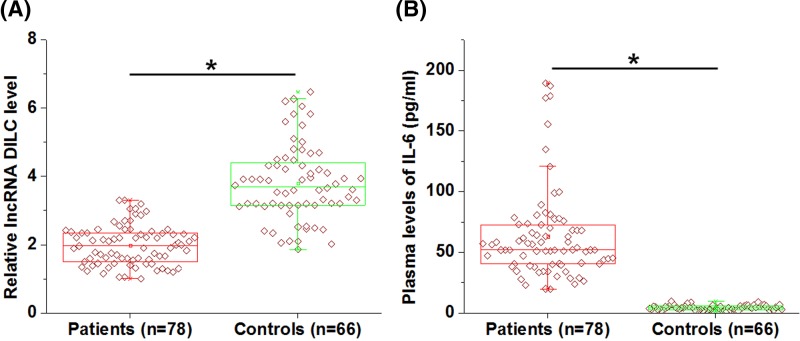
Plasma levels of lncRNA DILC and IL-6 in 78 RA patients and 66 healthy volunteers were measured by RT-qPCR and ELISA, respectively. It was observed that plasma lncRNA DILC was significantly down-regulated (Figure 1A), while IL-6 was up-regulated (Figure 1B) in RA patients than in healthy controls (P<0.05).
Figure 1. Plasma lncRNA DILC and IL-6 showed opposite expression pattern in RA patients.
Data of RT-qPCR and ELISA showed that plasma lncRNA DILC was significantly down-regulated (A), while IL-6 was up-regulated (B) in RA patients than in healthy controls (P<0.05).
Plasma levels of lncRNA DILC and IL-6 were significantly and inversely correlated only in RA patients
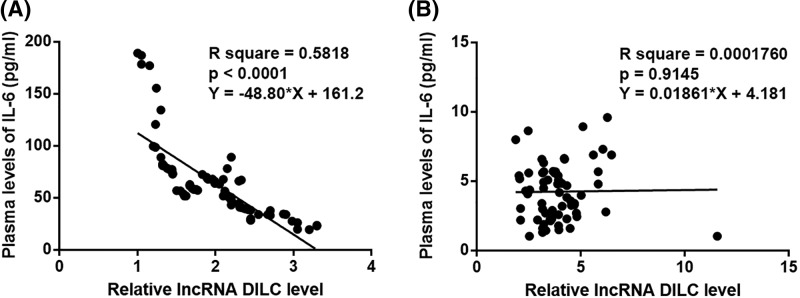
Pearson’s correlation coefficient was used to analyze the correlations between plasma levels of lncRNA DILC and IL-6. A significant and inverse correlation between plasma levels of lncRNA DILC and IL-6 was observed in RA patients (Figure 2A). However, the correlation between plasma levels of lncRNA DILC and IL-6 was not significant in healthy controls (Figure 2B).
Figure 2. Plasma levels of lncRNA DILC and IL-6 were significantly and inversely correlated only in RA patients.
Pearson’s correlation coefficient analysis showed that plasma levels of lncRNA DILC and IL-6 were significantly and inversely correlated in RA patients (A), but not in healthy controls (B).
LncRNA DILC positively regulated apoptosis of HFLS isolated from RA patients
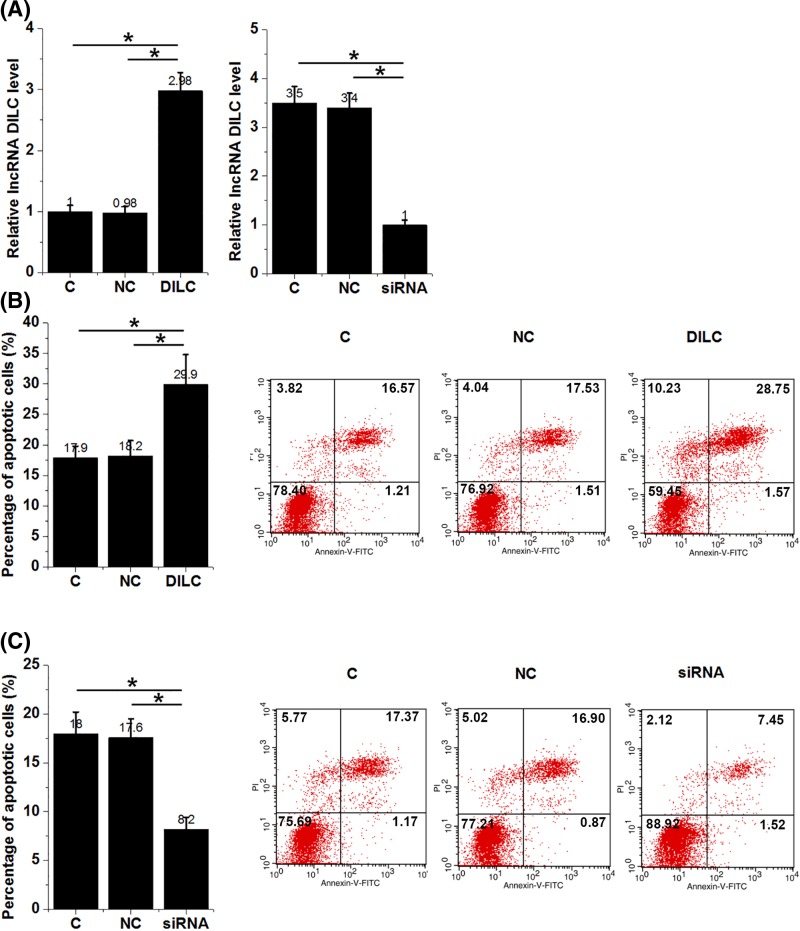
Cell apoptosis assay was performed to detect cell apoptosis after the transfection of lncRNA DILC overexpression vectors or siRNAs. As shown in Figure 3A, overexpression and knockdown of lncRNA DILC were reached at 24 h after transfection (Figure 3A, P<0.05). Compared with control (C) and negative control (NC) groups, overexpression of lncRNA DILC led to increased apoptosis (Figure 3B, P<0.05), while lncRNA DILC knockdown led to decreased apoptosis (Figure 3C, P<0.05) of HFLS isolated from RA patients.
Figure 3. LncRNA DILC regulated apoptosis of HFLS isolated from RA patients.
Overexpression and knockdown of lncRNA DILC were reached at 24 h after transfection (A). Overexpression of lncRNA DILC led to increased (B), while lncRNA DILC knockdown led to decreased (C) apoptosis of HFLS isolated from RA patients (*P<0.05).
Overexpression of lncRNA DILC led to decreased IL-6 protein level in HFLS isolated from RA patients
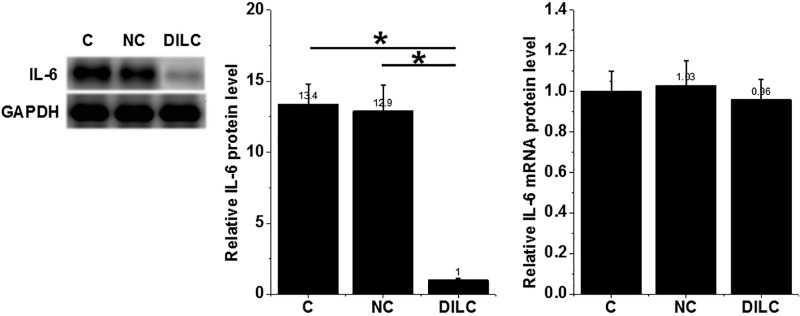
Compared with control (C) and negative control (NC) groups, overexpression of lncRNA DILC led to decreased IL-6 protein level in HFLS isolated from RA patients (P<0.05) (Figure 4). However, overexpression of lncRNA DILC failed to significantly affect IL-6 expression at mRNA level.
Figure 4. Overexpression of lncRNA DILC led to decreased IL-6 protein level in HFLS isolated from RA patients.
Overexpression of lncRNA DILC led to decreased IL-6 protein level in HFLS isolated from RA patients (*P<0.05), but failed to significantly affect IL-6 expression at mRNA level.
Discussion
The treatment of RA is challenged by the unclear pathogenesis. The key finding of the present study is that lncRNA DILC is likely an inhibitor of RA, and the actions of lncRNA DILC in RA is likely mediated by the down-regulation of IL-6 and promoted apoptosis of HFLS.
RA is a chronic inflammatory disease that is characterized by the accelerated production of pro-inflammatory factors [13]. The increased production of IL-6 by HFLS in RA patients mediates inflammatory responses and aggregates disease conditions [11]. Therefore, inhibition of pro-inflammatory factors including IL-6 production is considered as a promising therapeutic target for RA [14–16]. Our study also observed the significantly up-regulated plasma levels of IL-6 in RA patients than in healthy controls. In some extreme cases, plasma levels of IL-6 were more than 30 times higher than the median value in healthy controls, indicating the existence of severe inflammatory responses in RA patients.
HFLS is a major contributor in the development of RA [17,18]. The activation of HFLS in RA patients secretes various pro-inflammatory factors, such as IL-6 and IL-8, to promote the development of RA [11]. Therefore, inhibition of HFLS is critical for the recovery of RA patients [19]. In the present study we first reported the down-regulation of lncRNA DILC in RA patients. Interestingly, our study also proved that lncRNA DILC promoted the apoptosis of HFLS, suggesting that the overexpression of lncRNA DILC may serve as a therapeutic target for RA.
It is known that the expression of IL-6 in human diseases can be regulated by certain lncRNAs [20]. A recent study reported that lncRNA DILC can bind to the promoter region of IL-6 to inhibit its expression [10]. Interesting, our study did not observed significantly reduced IL-6 mRNA in HFLS after lncRNA DILC overexpression. In contrast, lncRNA DILC overexpression led to significantly reduced IL-6 protein levels in HFLS. To observe the potential changes in expression levels of IL-6 mRNA at early time points after DILC overexpression, we further detected the expression of IL-6 mRNA at 2, 4, and 8 h after transfection, but still no significant changes were observed (data not shown). It is known that lncRNAs may regulate gene expression at post-transcriptional, translational, and epigenetic levels [4,5]. Therefore, DILC may regulate IL-6 at translational level or DILC may regulate the levels of IL-6 in HFLS by affecting protein degradation or accumulation. In addition, DILC and IL-6 were significantly and inversely correlated only in RA patients, but not in healthy controls, indicating the possible existence of RA-related pathological factors mediating the interaction between DILC and IL-6.
It is worth nothing that we did not observed significant effects of IL-16 on cell apoptosis. In addition, no significant changes in expression levels of several classic apoptotic factors, such as caspase-9, caspase-3, p53, Bax, and Bcl2 [21–23] were observed after DIHC overexpression (data not shown). Therefore, the mechanism of the regulated apoptosis of HFLS by DILC is still unknown. Our future studies will try to consider other apoptosis-related factors. In conclusion, lncRNA DILC was down-regulated in RA and overexpression of lncRNA DILC may improve RA by down-regulating IL-6 and inhibiting the apoptosis of HFLS.
Abbreviations
- HFLS
human fibroblast-like synoviocyte
- ncRNA
non-coding RNA
- RA
rheumatoid arthritis
- RT-qPCR
real-time quantitative PCR
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work is supported by Southwest Medical University Foundation [grant number 2017-ZRQN-112].
Author Contribution
G.W., L.T., and Y.L. designed the study. G.W., L.T., and X.Z. performed the experiments. G.W. performed the statistical analysis and wrote the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Southwest Medical University. All patients and healthy volunteers provided written informed consent prior to their inclusion within the study. The research has been carried out in accordance with the World Medical Association Declaration of Helsinki.
References
- 1.Mattick J.S. and Makunin I.V. (2006) Non-coding RNA. Hum. Mol. Genet. 15, R17–R29 10.1093/hmg/ddl046 [DOI] [PubMed] [Google Scholar]
- 2.Eddy S.R. (2001) Non–coding RNA genes and the modern RNA world. Nat. Rev. Genet. 2, 919–929 10.1038/35103511 [DOI] [PubMed] [Google Scholar]
- 3.Mattick J.S. (2001) Non‐coding RNAs: the architects of eukaryotic complexity. EMBO Rep. 2, 986–991 10.1093/embo-reports/kve230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteller M. (2011) Non-coding RNAs in human disease. Nat. Rev. Genet. 12, 861–874 10.1038/nrg3074 [DOI] [PubMed] [Google Scholar]
- 5.Chen X., Yan C.C., Zhang X. and You Z.H. (2016) Long non-coding RNAs and complex diseases: from experimental results to computational models. Brief. Bioinform. 18, 558–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y., Costenbader K.H., Gao X., Hu F.B., Karison E.W. and Lu B. (2015) Mediterranean diet and incidence of rheumatoid arthritis in women. Arthritis Care Res. 67, 597–606 10.1002/acr.22481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McInnes I.B. and Schett G. (2017) Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389, 2328–2337 10.1016/S0140-6736(17)31472-1 [DOI] [PubMed] [Google Scholar]
- 8.Radner H., Lesperance T., Accortt N.A. and Solomon D.H. (2017) Incidence and prevalence of cardiovascular risk factors among patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis. Arthritis Care Res. 69, 1510–1518 10.1002/acr.23171 [DOI] [PubMed] [Google Scholar]
- 9.Yuan M., Wang S., Yu L., Qu B., Xu L., Liu L.. et al. (2017) Long noncoding RNA profiling revealed differentially expressed lncRNAs associated with disease activity in PBMCs from patients with rheumatoid arthritis. PLoS ONE 12, e0186795 10.1371/journal.pone.0186795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Sun W., Shen W., Xia M., Chen C., Xiang D.. et al. (2016) Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J. Hepatol. 64, 1283–1294 10.1016/j.jhep.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 11.Nanki T., Nagasaka K., Hayashida K., Saita Y. and Miyasaka N. (2001) Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Immunol. 167, 5381–5385 10.4049/jimmunol.167.9.5381 [DOI] [PubMed] [Google Scholar]
- 12.Lee S Y., Kwok S.K., Son H.J., Ryu J.G., Kim E.K., Oh H.J.. et al. (2013) IL-17-mediated Bcl-2 expression regulates survival of fibroblast-like synoviocytes in rheumatoid arthritis through STAT3 activation. Arthritis Res. Ther. 15, R31 10.1186/ar4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker K.F. and Isaacs J.D. (2018) Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum. Dis. 77, 175–187 10.1136/annrheumdis-2017-211555 [DOI] [PubMed] [Google Scholar]
- 14.Kim G.W., Lee N.R., Pi R.H., Lim Y.S., Lee Y.M., Lee J.M.. et al. (2015) IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch. Pharm. Res. 38, 575–584 10.1007/s12272-015-0569-8 [DOI] [PubMed] [Google Scholar]
- 15.Zhai K-F, Duan H., Luo L., Cao W.G., Han F.K., Shan L.L.. et al. (2017) Protective effects of paeonol on inflammatory response in IL-1β-induced human fibroblast-like synoviocytes and rheumatoid arthritis progression via modulating NF-κB pathway. Inflammopharmacology 25, 523–532 10.1007/s10787-017-0385-5 [DOI] [PubMed] [Google Scholar]
- 16.Zhai K., Duan H., Chen Y., Khan G.J., Cao W.G., Gao G.Z.. et al. (2018) Apoptosis effects of imperatorin on synoviocytes in rheumatoid arthritis through mitochondrial/caspase-mediated pathways. Food Funct. 9, 2070–2079 10.1039/C7FO01748K [DOI] [PubMed] [Google Scholar]
- 17.Bartok B. and Firestein G.S. (2010) Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255 10.1111/j.0105-2896.2009.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko K., Miyabe Y., Takayasu A., Fukuda S., Miyabe C., Ebisawa M.. et al. (2011) Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res. Ther. 13, R158 10.1186/ar3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S., Jin Z. and Lu X. (2017) MicroRNA-192 suppresses cell proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes by downregulating caveolin 1. Mol. Cell. Biochem. 432, 123–130 10.1007/s11010-017-3003-3 [DOI] [PubMed] [Google Scholar]
- 20.Xu Z., Yang F., Wei D., Liu B., Chen C., Bao Y.. et al. (2017) Long noncoding RNA-SRLR elicits intrinsic sorafenib resistance via evoking IL-6/STAT3 axis in renal cell carcinoma. Oncogene 36, 1965–1977 10.1038/onc.2016.356 [DOI] [PubMed] [Google Scholar]
- 21.Chen J.H., Cao J.L., Chu Y.L., Wang Z.L., Yang Z.T. and Wang H.L. (2008) T-2 toxin-induced apoptosis involving Fas, p53, Bcl-xL, Bcl-2, Bax and caspase-3 signaling pathways in human chondrocytes. J. Zhejiang Univ. Sci. B 9, 455–463 10.1631/jzus.B0820013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prokop A., Wieder T., Sturm I., Essmann F., Seeger K., Wuchter C.. et al. (2000) Relapse in childhood acute lymphoblastic leukemia is associated with a decrease of the Bax/Bcl-2 ratio and loss of spontaneous caspase-3 processing in vivo. Leukemia 14, 1606 10.1038/sj.leu.2401866 [DOI] [PubMed] [Google Scholar]
- 23.Cregan S.P., Fortin A., MacLaurin J.G., Callaghan S.M., Cecconi F., Yu S.W.. et al. (2002) Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J. Cell Biol. 158, 507–517 10.1083/jcb.200202130 [DOI] [PMC free article] [PubMed] [Google Scholar]