Abstract
Background
Data regarding cardiac resynchronization therapy (CRT) in patients with multiple comorbidities are limited.
Objectives
This study evaluated the association of multiple comorbidities with the benefits of CRT over implantable cardioverter-defibrillator (ICD) alone.
Methods
We examined 1,214 MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) study patients with left bundle branch block (LBBB) and 0, 1, 2, or ≥3 comorbidities, including renal dysfunction, hypertension (HTN), diabetes, coronary artery disease, history of atrial arrhythmias, history of ventricular arrhythmias, current smoking, and cerebrovascular accident. In an adjusted analysis, we analyzed risk of heart failure (HF) events or death by comorbidity group in all patients and in patients with CRT with defibrillator (CRT-D) versus ICD. Then we examined percent change in left ventricular (LV) end-diastolic volume, LV end-systolic volume, LV ejection fraction, left atrial volume, and LV dyssynchrony at 1-year in CRT-D patients by comorbidity group.
Results
There was an inverse relationship between comorbidity burden and improvements in LV end-systolic volume, LV end-diastolic volume, left ventricular ejection fraction, left atrial volume, and LV dyssynchrony. In an adjusted model, there was an increasing risk of death or nonfatal HF events with increasing comorbidity burden regardless of treatment group (p < 0.001). During a mean follow-up of 4.65 years, there was no interaction with respect to comorbidity burden and the benefit of CRT-D versus ICD only for death or nonfatal HF events (interaction p = 0.943). In the groups with greatest comorbidity burden (2 and ≥3), the absolute risk reduction associated with CRT-D over ICD alone appeared greater than that seen for groups with less comorbidity burden (0 and 1).
Conclusions
During long-term follow-up of MADIT-CRT study patients with LBBB randomized to CRT-D, there were differences in HF or death risk and in the degree of reverse remodeling among comorbidity groups. However, the burden of comorbidity does not appear to compromise the clinical benefits of CRT-D compared with ICD alone.
Keywords: cardiac resynchronization therapy, heart failure, mortality
The benefits of cardiac resynchronization therapy (CRT) have been well established by clinical trials 1, 2, 3, 4, 5, 6. A plethora of subsequent analyses have addressed various aspects of CRT focusing on predictors of response to CRT, and the benefit of CRT in patients with an array of comorbidities 7, 8, 9, 10, 11, 12. The effect of CRT has been examined in the case of multiple comorbidities in only a few instances 13, 14, 15, 16. This is particularly important in the heart failure (HF) population because these patients often have multiple comorbid conditions. However, to date, studies of CRT patients with multiple comorbidities are limited by small sample size as well as single-center and retrospective design.
The MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) study was a large, randomized, multicenter trial that evaluated patients with low left ventricular ejection fraction (LVEF), wide QRS interval, and mild HF symptoms receiving either CRT with defibrillator (CRT-D) or implantable cardioverter-defibrillator (ICD) alone. Subjects included in the original analysis had various comorbid conditions typical of a HF population (5).
In this analysis of the data collected during the long-term follow-up of patients enrolled in MADIT-CRT, we seek to help physicians understand the expected clinical benefits of CRT-D in HF patients by addressing the following goals: 1) describe the burden of comorbidities in the MADIT-CRT study population; 2) determine whether the benefits of CRT-D seen in the MADIT-CRT study are associated with subjects’ comorbidity burden; and 3) describe any differences in cardiac structural and functional outcomes associated with comorbidity burden.
Methods
Study population
This was a retrospective post hoc analysis of the MADIT-CRT study database including long-term follow-up data for up to 7 years after randomization. The study design and primary results of the MADIT-CRT study have been published elsewhere 5, 17. In brief, the overall study population included 1,820 patients with HF, LVEF ≤30% and prolonged intraventricular conduction (QRS interval ≥130 ms). Patients with ischemic cardiomyopathy were eligible for inclusion if they had New York Heart Association (NYHA) functional class I or II symptoms; patients with nonischemic cardiomyopathy were eligible if they had NYHA functional class II symptoms. Patients were randomized in a 3:2 ratio to receive either CRT-D or ICD only, and they were stratified according to disease etiology (ischemic vs. nonischemic cardiomyopathy). Important exclusions included reversible causes of nonischemic cardiomyopathy (e.g., myocarditis), an existing indication for CRT, NYHA functional class III or IV in the 90 days preceding enrollment, pacemaker in situ, myocardial infarction, or coronary revascularization (coronary artery bypass graft surgery or percutaneous coronary intervention) in the 90 days preceding enrollment. We restricted our analysis to those patients with left bundle branch block (LBBB) at enrollment because previous data demonstrated benefit only in this group (18), and other post hoc analyses of the MADIT-CRT study data have restricted the study population in a similar way (19).
Definitions and endpoints
To determine the association of comorbidities with response to CRT, we selected 8 comorbidities as determined at the time of enrollment based on clinical relevance, prevalence in the population, and prior work on multiple comorbidities (20). These included renal disease (estimated glomerular filtration rate <60 ml/min/1.73 m2), diabetes mellitus, prior atrial arrhythmias, prior ventricular arrhythmias, hypertension (HTN), coronary artery disease (defined as prior myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting; or determination of ischemic cardiomyopathy by the enrolling physician) current smoking, and cerebrovascular disease 21, 22. Consideration was given to including obesity as comorbidity in our analysis, but prior work with the MADIT-CRT study population has not supported an association between obesity and outcomes 23, 24, 25. We divided the patient cohort into 4 categories based on comorbidity burden: 0, 1, 2, and ≥3 comorbidities. These categories were based on clinical relevance and a preliminary inspection of the data.
We evaluated the association of comorbidity burden with the primary endpoint from MADIT-CRT study: mortality and nonfatal HF events. In addition, we evaluated the association of comorbidity burden with CRT response as assessed by echocardiographic remodeling endpoints: left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), left atrial volume (LAV), left ventricular (LV) dyssynchrony, and LVEF as assed by 2-dimensional echocardiography by a blinded core laboratory.
Echocardiography methods
Methods for acquisition, evaluation, and reporting of echocardiograms in the MADIT-CRT study population have been previously reported (26). Briefly, echocardiograms were acquired according to a study-specific protocol at enrollment prior to device implantation and at 1 year. Recordings were evaluated offline by blinded investigators at an independent echocardiography core lab at the Brigham and Women’s Hospital.
LVEDV and LVESV were measured by using Simpson’s disk method in the apical 4- and 2-chamber views, and LVEF was calculated according to the established American Society of Echocardiography protocols (27). The coefficients of variation for these measures were 5.2%, 6.2%, and 5.5%, respectively (28). LV dyssynchrony was determined as the SD of regional time-to-peak transverse strain, measured during systole in the 12 anatomic wall segments of the apical 4- and 2-chamber views of the LV by using B-mode speckle tracking (TomTec Imaging Systems, Unterschleißheim, Germany) (29).
Statistical analysis
Baseline characteristics according to comorbidity group were compared using the chi-square test for categorical variables and the nonparametric Kruskal-Wallis test for continuous variables. The unadjusted primary endpoint of mortality or nonfatal HF events was summarized with Kaplan-Meier curves within each comorbidity group in the overall cohort of LBBB patients and within treatment groups (CRT-D and ICD only) by comorbidity burden.
A Cox proportional hazards multivariable regression model was used to compare mortality and nonfatal HF events among comorbidity groups. The model included main effect terms for comorbidity group and CRT-D treatment randomization and an interaction term for both. The model was further adjusted for clinically relevant covariates some of which were found using the best subsets variable reduction technique and with the stipulation that they also needed to be significant at <0.05. Additionally, female sex was forced into the model. Thus, the adjustment variables were age, sex, baseline LVESV, QRS interval, and PR interval. This model was used to estimate the association of the count of comorbidities with the hazard of death or nonfatal HF events by treatment assignment (CRT-D vs. ICD).
To assess the association between comorbidity burden and echocardiographic response to CRT, we restricted our population to only those patients who received CRT-D. The median change and interquartile range for the following parameters was assessed in each comorbidity group: LVEDV, LVESV, LVEF, LAV, and LV dyssynchrony. Differences among groups were assessed using the Kruskal-Wallis test, and an evaluation of trend from lowest to highest comorbidity burden was assessed using the nonparametric correlation measure, Spearman’s rho.
The association of 3 specific comorbidities of interest (renal dysfunction, HTN, and diabetes) and changes in echocardiographic measures was examined in additional detail because these comorbidities have been associated with specific changes in previous investigations.
Results
Patient characteristics
Characteristics of the 1,214 patients included in this analysis are reported according to comorbidity group in Table 1. Most patients had ≥1 comorbidity. Age tended to increase with comorbidity burden (mean age in group 0 was 57 years and mean age in group ≥3 was 67.7 years). Subjects with the highest burden of comorbidity were less likely to be women (24% in the ≥3 group vs. 38% in the 0 group). The groups were similar in regard to use of important cardiovascular medications except for aspirin and statins. Aspirin and statin use increased with increasing comorbidity burden because these agents would be more likely prescribed in a group with coronary artery disease and cerebrovascular disease, which were 2 of the pre-determined comorbidities. Baseline biometric, electrocardiographic, and echocardiographic measures were also similar between groups.
Table 1.
Patient Characteristics
| Comorbidity Group | ||||||
|---|---|---|---|---|---|---|
| 0 (n = 147) | 1 (n = 304) | 2 (n = 324) | ≥3 (n = 439) | Total (N = 1,214) | p Value | |
| Demographics | ||||||
| Female | 56 (38) | 119 (39) | 98 (30) | 105 (24) | 378 (31) | <0.001 |
| Age at enrollment, yrs | 57.0 ± 11.3 | 60.5 ± 11.8 | 65.7 ± 9.6 | 67.7 ± 9.0 | 64.1 ± 10.9 | <0.001 |
| White | 138 (94) | 275 (91) | 297 (93) | 398 (91) | 1,108 (91) | 0.662 |
| Comorbidities | ||||||
| Ischemic cardiomyopathy | 0 (0) | 49 (16) | 146 (45) | 335 (76) | 530 (44) | <0.001 |
| Prior CABG | 0 (0) | 18 (6) | 57 (18) | 190 (43) | 265 (22) | <0.001 |
| Prior PCI | 0 (0) | 21 (7) | 78 (24) | 161 (37) | 260 (21) | <0.001 |
| Prior MI | 0 (0) | 41 (14) | 89 (28) | 255 (60) | 385 (32) | <0.001 |
| NYHA functional class I | 0 (0) | 13 (4) | 45 (14) | 77 (18) | 135 (11) | <0.001 |
| NYHA functional class II | 0 (0) | 36 (12) | 101 (31) | 258 (59) | 395 (33) | <0.001 |
| Cerebrovascular accident | 0 (0) | 4 (1) | 12 (4) | 59 (13) | 75 (6) | <0.001 |
| Current smoking | 0 (0) | 28 (9) | 39 (12) | 65 (15) | 132 (11) | <0.001 |
| Past atrial arrhythmias | 0 (0) | 15 (5) | 25 (8) | 93 (21) | 133 (11) | <0.001 |
| Past ventricular arrhythmias | 0 (0) | 12 (4) | 9 (3) | 54 (12) | 75 (6) | <0.001 |
| GFR | 81.8 ± 18.7 | 75.2 ± 17.5 | 70.0 ± 19.3 | 60.8 ± 20.0 | 69.4 ± 20.4 | <0.001 |
| Diabetes | 0 (0) | 20 (7) | 79 (24) | 263 (60) | 362 (30) | <0.001 |
| Hypertension | 0 (0) | 135 (44) | 227 (70) | 393 (90) | 755 (62) | <0.001 |
| Medications at enrollment | ||||||
| ACE inhibitor or ARB | 141 (96) | 296 (97) | 314 (97) | 420 (96) | 1171 (96) | 0.603 |
| Aldosterone antagonist | 51 (35) | 118 (39) | 104 (32) | 138 (31) | 411 (34) | 0.173 |
| Amiodarone | 0 (0) | 4 (1) | 18 (6) | 50 (11) | 72 (6) | <0.001 |
| Aspirin | 74 (50) | 146 (48) | 214 (66) | 313 (71) | 747 (62) | <0.001 |
| Beta-blocker† | 137 (93) | 293 (96) | 305 (94) | 405 (92) | 1,140 (94) | 0.138 |
| Digitalis | 43 (29) | 92 (30) | 89 (27) | 112 (26) | 336 (28) | 0.526 |
| Diuretic | 76 (52) | 203 (67) | 218 (67) | 333 (76) | 830 (68) | <0.001 |
| Statins | 56 (38) | 149 (49) | 214 (66) | 350 (80) | 769 (63) | <0.001 |
| Biometric characteristics at enrollment | ||||||
| QRS interval, ms | 163.0 ± 18.7 | 165.3 ± 19.1 | 163.6 ± 19.6 | 160.8 ± 18.6 | 162.9 ± 19.1 | 0.017 |
| BMI, kg/m2 | 28.4 ± 5.2 | 28.5 ± 5.2 | 28.2 ± 5.1 | 28.9 ± 5.3 | 28.5 ± 5.2 | 0.737 |
| BNP level, ng/l* | 61.0 ± 70.0 | 97.3 ± 122.4 | 124.1 ± 155.8 | 140.6 ± 177.2 | 114.8 ± 150.0 | <0.001 |
| SBP, mm Hg | 119.9 ± 15.0 | 121.1 ± 16.7 | 123.3 ± 17.5 | 124.1 ± 17.6 | 122.6 ± 17.1 | 0.014 |
| 6-min walk distance, m | 402.6 ± 89.2 | 380.6 ± 109.4 | 357.8 ± 101.3 | 345.8 ± 104.7 | 364.5 ± 105.0 | <0 .001 |
| LVESV, ml | 175.7 ± 51.4 | 187.6 ± 61.2 | 180.9 ± 52.3 | 175.0 ± 45.8 | 179.8 ± 52.5 | 0.082 |
| LVEDV, ml | 246.9 ± 63.0 | 260.9 ± 75.6 | 252.6 ± 65.0 | 244.0 ± 56.2 | 250.9 ± 64.9 | 0.042 |
| LAV, ml | 88.2 ± 20.6 | 95.5 ± 22.8 | 93.0 ± 22.1 | 94.4 ± 22.3 | 93.5 ± 22.2 | 0.008 |
| Calculated LVEF, % | 29.4 ± 4.1 | 28.6 ± 3.5 | 28.8 ± 3.2 | 28.7 ± 3.4 | 28.8 ± 3.5 | 0.106 |
| PR interval, ms | 195 ± 28 | 192 ± 29 | 195 ± 3 0 | 202 ± 36 | 197 ± 32 | 0.010 |
Values are n (%) or mean ± SD.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; BNP = B-type natriuretic peptide; CABG = coronary artery bypass grafting; GFR = glomerular filtration rate; LAV = left atrial volume; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; MI = myocardial infarction; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; SBP = systolic blood pressure.
Only available for patients in the United States.
Excludes sotalol.
Also illustrated in Table 1 is the distribution of comorbidities at baseline. As expected, each comorbidity is generally more prevalent with increasing comorbidity burden. In Table 2, comorbidities are reported by treatment assignment (ICD only vs. CRT-D). With the exception of prior atrial arrhythmias (13% in ICD-only and 9% in CRT-D), comorbidities are well balanced between treatment groups.
Table 2.
Comorbidities by Treatment Assignment
| Comorbidity | Total (N = 1,214) | ICD Only (n = 490) | CRT-D (n = 724) |
|---|---|---|---|
| GFR <60 ml/min/1.73 m2 | 394 (32) | 164 (33) | 230 (32) |
| Diabetes | 362 (30) | 147 (30) | 215 (30) |
| Prior atrial arrhythmias | 133 (11) | 66 (13) | 67 (9) |
| Prior ventricular arrhythmias | 75 (6) | 33 (7) | 42 (6) |
| Hypertension | 755 (62) | 312 (64) | 443 (61) |
| Coronary artery disease | 566 (47) | 232 (47) | 334 (46) |
| Current smoking | 132 (11) | 58 (12) | 74 (10) |
| Cerebrovascular disease | 75 (6) | 36 (7) | 39 (5) |
Values are n (%).
CRT-D = cardiac resynchronization therapy with defibrillator; GFR = glomerular filtration rate; ICD = implantable cardioverter-defibrillator.
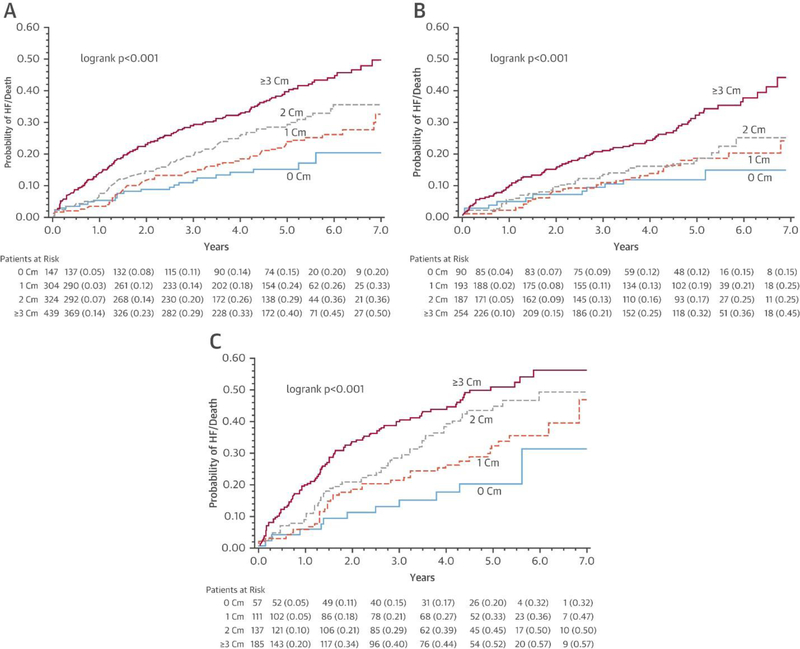
Comorbidity burden was significantly associated with the primary outcome from the MADIT-CRT study, death or nonfatal HF event, such that the risk of this endpoint was highest in the group with the most comorbidities and lowest in the group with no comorbidities regardless of treatment assignment (Figure 1A).
Figure 1.
Kaplan-Meier Curves for Mortality or Nonfatal HF Events by Treatment Group Stratified by Number of Comorbidities (0, 1, 2, ≥3)
(A) Overall, (B) cardiac resynchronization therapy with defibrillator (CRT-D), and (C) implantable cardioverter-defibrillator (ICD) only. In the overall MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) study population, the greatest comorbidity burden was associated with the greatest risk of death or heart failure (HF). This relationship was true when the population was divided by treatment assignment (CRT-D vs. ICD). In both cases, patients with ≥3 comorbidities had the highest risk, and those with 0 comorbidities had the lowest risk. In the ICD group, comorbidity burden had a clearer association with this risk whereas in the CRT-D group, there did not appear to be an important difference between the groups with 1 and 2 comorbidities. Cm = comorbidity.
Death or nonfatal HF event by comorbidity group and treatment group
When the population was stratified by treatment assignment (CRT-D vs. ICD only), the Kaplan-Meier estimates of death or nonfatal HF event differed by comorbidity group (Figures 1B and 1C). By visual inspection, this separation in outcomes was clearer in the ICD-only group, with increasing risk of death or HF associated with increasing comorbidity.
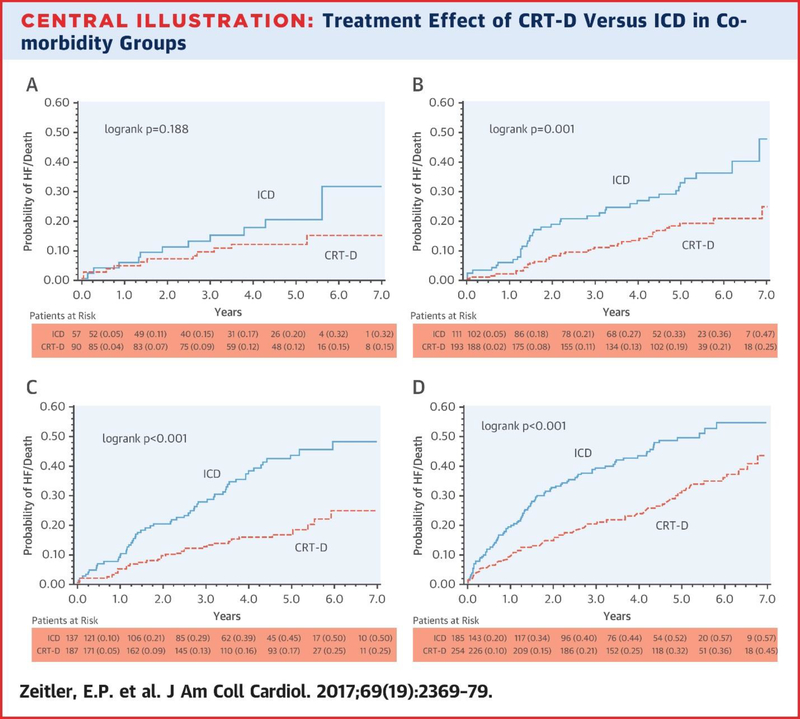
The mean length of follow-up for patients was 4.6 ± 1.75 years. There was a significantly lower risk of death or nonfatal HF event in patients with CRT-D compared with patients with ICD only in all comorbidity groups (Table 3, Central Illustration). This difference was statistically significant for the groups of patients with 1 comorbidity (hazard ratio [HR]: 0.50; 95% confidence interval [CI]: 0.31 to 0.81), 2 comorbidities (HR 0.44; 95% CI: 0.28 to 0.68), and ≥3 comorbidities (HR 0.52; 95% CI: 0.38 to 0.70), with p <0.05 for all. For the group with 0 comorbidities there was a 49% risk reduction of death or nonfatal HF event for patients with CRT-D compared with ICD-only patients, but the 95% CI crossed 1.00 (0.22 to 1.17; p = 0.113), and thus this was not a statistically significant difference. Although the absolute risk reduction appeared greatest in the groups with more comorbidity burden, there was no interaction with respect to comorbidity burden and the benefit of CRT-D compared with ICD only for the endpoint of death or nonfatal HF event (p for interaction = 0.943).
Table 3.
Risk of Death or Nonfatal Heart Failure Events During Long-Term Follow-Up Associated With Increasing Comorbidity Burden for Patients With CRT-D vs. ICD Only
| Comorbidity Group | Event Rate | Unadjusted HR* (95% CI) | Adjusted† HR* (95% CI) | p Value‡ |
|---|---|---|---|---|
| 0 | 15 (22/147) | 0.56 (0.24–1.30) | 0.51 (0.22–1.17) | 0.113 |
| 1 | 23 (71/304) | 0.48 (0.30–0.76) | 0.50 (0.31–0.81) | 0.005 |
| 2 | 28 (91/324) | 0.38 (0.25–0.58) | 0.44 (0.28–0.68) | <0.001 |
| ≥3 | 40 (177/439) | 0.53 (0.39–0.71) | 0.52 (0.38–0.70) | <0.001 |
| Interaction p-value | 0.943 | |||
Values are % (n/N) unless otherwise indicated.
Representing hazard of death or nonfatal heart failure events in the group with CRT-D compared with the group with ICD only.
Model adjusted for age, sex, baseline LVESV, QRS interval, and PR duration.
Interaction p = 0.943.
Central Illustration.
Treatment Effect of CRT-D Versus ICD in Comorbidity Groups
(A) Zero comorbidities, (B) 1 comorbidity, (C) 2 comorbidities, (D) ≥3 comorbidities. These 4 Kaplan-Meier curves divide the population by comorbidity group and compare probability of heart failure or death during 7 years of follow-up between treatment groups: implantable cardioverter-defibrillator (ICD) versus cardiac resynchronization therapy with defibrillator (CRT-D). In all cases, patients assigned to CRT-D had on average decreased probability of heart failure (HF) or death. This difference was statistically significant for the 1, 2, and ≥3 comorbidity groups. The greatest absolute risk reduction was observed in the groups with 2 or ≥3 comorbidities.
Because HTN was common in the population studied, we conducted a sensitivity analysis that excluded HTN as a comorbidity and obtained similar results (p for interaction = 0.654) (Online Table 1).
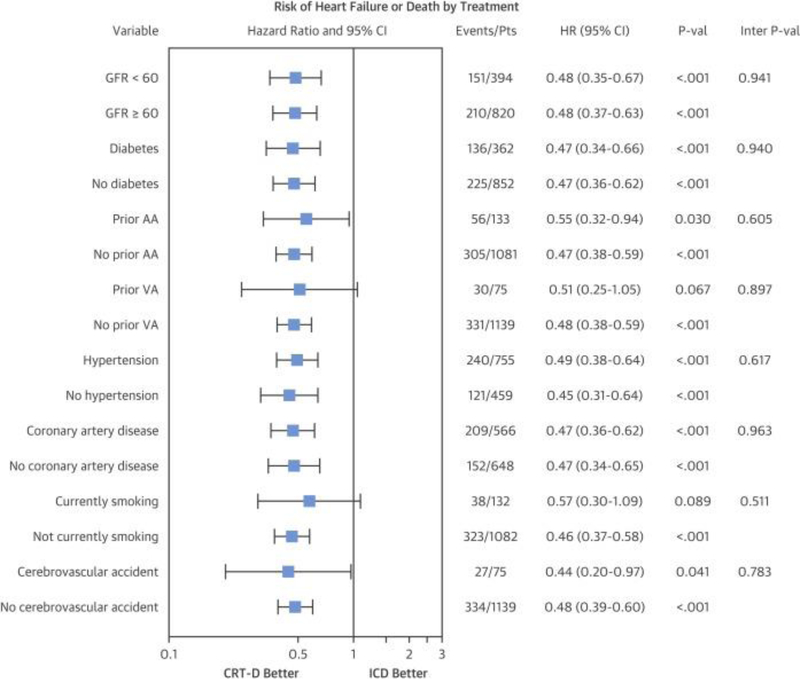
Moreover, in an unadjusted analysis, CRT-D was associated with an improvement in the primary endpoint for patients with any 1 of the following comorbidities: renal dysfunction, diabetes, history of prior atrial arrhythmias, HTN, coronary artery disease, or history of cerebrovascular accident (p < 0.05 for all) (Figure 2). Patients with history of prior ventricular arrhythmias or current smoking also experienced a reduced hazard of the primary endpoint, but not in a statistically significant way (p = 0.067 and 0.089, respectively).
Figure 2.
Forest Plot of Unadjusted Hazard of Mortality or Nonfatal HF Events by Treatment Group (CRT-D vs. ICD) According to Comorbidities
The hazard ratio (HR) for death or HF is plotted for each comorbidity independently. In nearly all cases, CRT-D is statistically favored. In those cases in which the 95% CI crosses unity, there is a clear trend favoring CRT-D. There is no interaction of any single comorbidity with the treatment assignment (ICD vs. CRT-D) with respect to the outcome of HF or death (interaction p > 0.50 for all). AA = atrial arrhythmia; CI = confidence interval; GFR = glomerular filtration rate; Pts = patients; VA = ventricular arrhythmia; other abbreviations as in Figure 1.
Changes in echocardiographic parameters by comorbidity
In the group that received CRT-D, changes in echocardiographic measures are reported in Table 4. For each measure, there were differences by comorbidity group (Kruskal-Wallis p < 0.05 for all). In general, there was an inverse relationship between burden of comorbidity and improvement in LVEDV, LVESV, LVEF, and LAV (p for trend <0.05 for all). The median change in LVEDV in the 0 comorbidity group was −26 ± 17.3%. This percent change decreased to −19 ± 11.8% in the ≥3 comorbidity group (p for trend <0.001). For 0, 1, 2, and ≥3 comorbidities, LVESV median percent change was −41 ± 21.9%, –36 ± 19%, −37 ± 19.4%, and −31 ± 17.5%, respectively (p for trend <0.001). Improvement in LVEF went from a median of 11% in the group with highest comorbidity burden to 13% in the group with 0 comorbidities (p for trend = 0.001), and a similar trend was seen in LAV with median change improving from −28% in the group with ≥3 comorbidities to −34% in the group with no comorbidities (p for trend = 0.001). The trend in change in LV dyssynchrony was less clear with respect to comorbidity burden (p for trend = 0.065).
Table 4.
Changes in Echocardiographic Measurements After CRT by Comorbidity Groups
| Comorbidity Group | n | LVEDV | LVESV | LVEF | LAV | LV Dyssynchrony |
|---|---|---|---|---|---|---|
| 0 | 108 | −28 (16.8), 29 | −42 (21.5), 29 | 14 (6.7), 29 | −34 (19.7), 29 | −22 (37.0), 47 |
| 1 | 197 | −22 (15.1), 54 | −36 (18.0), 54 | 12 (6.5), 54 | −31 (17.7), 55 | −37 (44), 108 |
| 2 | 190 | −23 (16.4), 53 | −37 (20.1), 53 | 12 (6.2), 53 | −30 (16.1), 53 | −34 (38.3), 102 |
| ≥3 | 241 | −19 (13.3), 76 | −32 (18.0), 76 | 11 (6.7), 76 | −28 (13.3), 76 | −19 (46.9), 142 |
| p value (KW) | 0.002 | 0.001 | 0.007 | 0.044 | 0.027 | |
| p value for trend | 0.001 | <0.001 | 0.002 | 0.004 | 0.146 | |
Values are median % change (interquartile range), n missing.
CRT = cardiac resynchronization therapy; KW = Kruskal-Wallis; other abbreviations as in Table 1.
Echocardiographic change with respect to renal dysfunction, HTN, and diabetes
A subset analysis of the change in echocardiographic measures in patients with renal dysfunction, HTN, diabetes, and combinations thereof was specifically investigated (Online Table 2). Of note, renal dysfunction and diabetes occurred infrequently in isolation. For example, diabetes was the only comorbidity in 20 (1.2%) patients but occurred in combination with other comorbidities in 724 (57%) patients. Changes in echocardiographic parameters for each of these 3 comorbidities in isolation was largely similar to changes seen in patients with any 1 comorbidity, and changes in patients with 2 or 3 of these comorbidities in combination mirrored changes in patients with any 2 or 3 comorbidities (Table 4, Online Table 2). When changes in these echocardiographic parameters were compared, there was no statistically significant difference between groups with any single comorbidity or combination thereof (p > 0.05 for all comparisons) (Online Table 2).
Discussion
We have presented several secondary analyses from the MADIT-CRT study that examine the association of multiple comorbidities with the benefits from CRT-D. There are 3 important findings. First, the MADIT-CRT study was composed of a relatively healthy population with more than one-third of patients having 0 or 1 comorbidity. Second, there was no interaction between burden of comorbidity and a reduction in death or nonfatal HF events associated with CRT-D compared with ICD. Third, although there was no association between level of comorbidity and change in the primary outcome of death or nonfatal HF events, there did appear to be a trend favoring improvements in echocardiographic measures associated with the lowest levels of comorbidity.
There were 1,214 subjects from the MADIT-CRT study cohort with LBBB included in our analyses. Of these, 12% had 0 comorbidities, 37% had 1 or 0 comorbidities, and 36% had 3 or more comorbidities suggesting that this population was relatively diverse. The most common comorbidity was HTN, affecting more than 60% of all patients and 90% of patients in the ≥3 group. Coronary artery disease was the second most common, including 44% of the total patients and more than three-quarters of patients in the ≥3 group. Diabetes was the third most common comorbidity including 30% of the total cohort. The other comorbidities each included 11% or less of the total population. There were more women in the lower comorbidity groups. This distribution of sex and comorbidities is important for 2 major reasons: 1) it puts our subsequent analyses discussed below in context of the MADIT-CRT population; and 2) allows for comparisons of our findings to more general clinical practice.
In each comorbidity group, the presence of CRT-D was associated with an improvement in the hazard of death or nonfatal HF events compared with ICD alone (Central Illustration). This difference was significant for the groups with ≥1 comorbidity, but not in the group with 0 comorbidities. However, it is important to note that the 0 comorbidity group was about one-half as large as the other groups, making it more difficult to detect a difference. Interestingly, in the groups with greatest comorbidity burden, the absolute risk reduction associated with CRT-D appeared larger. However, there was no interaction between comorbidity burden and the associated hazard of death or nonfatal HF events in the presence of CRT-D compared with ICD alone.
Furthermore, for 6 of the 8 comorbidities studied, CRT-D was associated with an improvement in the primary endpoint, and for the remaining 2 comorbidities, there was a clear trend favoring CRT-D (Figure 2). This clarifies the important role that CRT plays in saving and improving the lives of HF patients regardless of other common medical problems. Although the guidelines are clear that device-based therapy is not appropriate for patients with life expectancy <1 year (30), our findings should be reassuring that CRT-D can be an important intervention for relatively complex patients in the context of other evidence-based therapies.
When we examined the association between comorbidity burden and changes in cardiac size and function, some general trends emerged. For those measures we examined (LVEDV, LVESV, LVEF, LAV, and LV dyssynchrony), patients with the greatest comorbidity had the smallest associated improvements. In each case, differences in these measures were statistically significantly different between groups, and for LVEDV, LVESV, LVEF, and LAV there was a statistically significant trend of greater improvement with less comorbidity burden. This was paralleled by the increasing rate of the primary endpoint by comorbidity group (Figures 1B and 1C). However, reassuringly, there was no interaction between comorbidity burden and associated benefit of CRT-D over ICD alone, suggesting that despite increasing baseline risk of death or HF events with increasing comorbidity, the associated benefit of CRT-D is unchanged.
In subset analyses, we examined the relationship between changes in echocardiographic measures and 3 specific comorbidities in isolation and combination: renal dysfunction, HTN, and diabetes. These analyses were conducted based on previous literature and clinical suspicion suggesting that changes in cardiac structure and function with CRT may be different in these patients compared with the overall population. For example, some analyses have demonstrated poor cardiac reverse remodeling response to CRT in patients with renal dysfunction 31, 32, 33. The effect of diabetes on cardiac reverse remodeling with CRT reported in the literature is inconsistent 9, 34, 35. We did not detect differences in cardiac structure and function between groups with these 3 comorbidities, possibly suggesting a common pathway for injury and recovery for these 3 comorbidities such that marginal differences are obscured. Alternatively, because the groups were relatively small in this analysis, we may have had insufficient power to identify differences between groups.
Study limitations
First, these analyses were not pre-specified, so we can report associations only. Second, analyses were performed based on a clinical trial population, which may have limited generalizability due to both the pre-selected MADIT-CRT study population as well as evolution in the available technology and programming of CRT-D and ICD devices since the trial. Third, comorbidities were assessed only at the time of enrollment and were not assessed during the follow-up. The development (or resolution) as well as the trajectory of comorbidities is likely to have a significant impact on clinical outcomes in response to CRT. Nonetheless, the analysis presented remains highly clinically relevant because we approached the analysis from the perspective of a practicing physician who must make a decision regarding referral for CRT (or not) in the context of multiple comorbid conditions, often without complete information about severity, longevity, or trajectory. Fourth, all comorbidities included in these analyses were weighted equally despite differences in clinical risk; there is no consensus on how to “weight” different comorbidities. Fifth, there are missing data with regard to echocardiographic measurements; however, in previous analyses, patients with missing echocardiography data were similar to those with data available (36).
Conclusions
In this post hoc retrospective analysis of data from the long-term follow-up of the MADIT-CRT study, we investigated the association of multiple comorbidities with the outcome of mortality and nonfatal HF events. We found that the risk of death or HF events in patients treated with CRT-D or ICD only was highest in groups with the highest comorbidity burden. However, regardless of comorbidity burden, CRT-D therapy was associated with fewer death and HF events than ICD alone. Furthermore, changes in cardiac structure and function demonstrated that the greatest improvements were associated with the lowest burden of comorbidity. In summary, these findings support the ongoing application of CRT-D for eligible patients regardless of comorbidity burden.
Supplementary Material
Perspectives.
COMPETENCY IN PATIENT CARE
Patients with reduced LVEF, LBBB, and mild HF symptoms benefit from cardiac resynchronization device (CRT-D) therapy even when they have multiple medical comorbidities. Although the improvement in echocardiographic measures of ventricular function diminished as the burden of comorbidities increased, this was not matched by an attenuation of clinical benefit.
TRANSLATIONAL OUTLOOK
Further work is necessary to better understand which echocardiographic or other measures correlate most closely with clinical outcomes to guide selection of patients most likely to benefit from CRT-D therapy.
Abbreviations and Acronyms
- CRT-D
cardiac resynchronization therapy with defibrillator
- HF
heart failure
- HTN
hypertension
- ICD
implantable cardioverter-defibrillator
- LAV
left atrial volume
- LBBB
left bundle branch block
- LV
left ventricular
- LVEDV
left ventricular end-diastolic volume
- LVEF
left ventricular ejection fraction
- LVESV
left ventricular end-systolic volume
- NYHA
New York Heart Association
References
- 1.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med, 346 (2002), pp. 1845–1853. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med, 350 (2004), pp. 2140–2150. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med, 352 (2005), pp. 1539–1549 [DOI] [PubMed] [Google Scholar]
- 4.Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol, 52 (2008), pp. 1834–1843 [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med, 361 (2009), pp. 1329–1338. [DOI] [PubMed] [Google Scholar]
- 6.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med, 363 (2010), pp. 2385–2395. [DOI] [PubMed] [Google Scholar]
- 7.Delnoy PP, Ottervanger JP, Luttikhuis HO, et al. Comparison of usefulness of cardiac resynchronization therapy in patients with atrial fibrillation and heart failure versus patients with sinus rhythm and heart failure. Am J Cardiol, 99 (2007), pp. 1252–1257. [DOI] [PubMed] [Google Scholar]
- 8.Kies P, Bax JJ, Molhoek SG, et al. Comparison of effectiveness of cardiac resynchronization therapy in patients with versus without diabetes mellitus. Am J Cardiol, 96 (2005), pp. 108–111. [DOI] [PubMed] [Google Scholar]
- 9.Martin DT, McNitt S, Nesto RW, Rutter MK, Moss AJ Cardiac resynchronization therapy reduces the risk of cardiac events in patients with diabetes enrolled in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT). Circ Heart Fail, 4 (2011), pp. 332–338. [DOI] [PubMed] [Google Scholar]
- 10.Mathew J, Katz R, St John Sutton M, et al. Chronic kidney disease and cardiac remodelling in patients with mild heart failure: results from the REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction (REVERSE) study. Eur J Heart Fail, 14 (2012), pp. 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLeod CJ, Shen WK, Rea RF, et al. Differential outcome of cardiac resynchronization therapy in ischemic cardiomyopathy and idiopathic dilated cardiomyopathy. Heart Rhythm, 8 (2011), pp. 377–382. [DOI] [PubMed] [Google Scholar]
- 12.van Bommel RJ, Marsan NA, Delgado V, et al. Cardiac resynchronization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high operative risk. Circulation, 124 (2011), pp. 912–919. [DOI] [PubMed] [Google Scholar]
- 13.Bai R, Di Biase L, Elayi C, et al. Mortality of heart failure patients after cardiac resynchronization therapy: identification of predictors. J Cardiovasc Electrophysiol, 19 (2008), pp. 1259–1265. [DOI] [PubMed] [Google Scholar]
- 14.Kreuz J, Horlbeck F, Linhart M, et al. Independent predictors of mortality in patients with advanced heart failure treated by cardiac resynchronization therapy. Europace, 14 (2012), pp. 1596–1601. [DOI] [PubMed] [Google Scholar]
- 15.Theuns DA, Schaer BA, Soliman OI, et al. The prognosis of implantable defibrillator patients treated with cardiac resynchronization therapy: comorbidity burden as predictor of mortality. Europace, 13 (2011), pp. 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verbrugge FH, Dupont M, Rivero-Ayerza M, et al. Comorbidity significantly affects clinical outcome after cardiac resynchronization therapy regardless of ventricular remodeling. J Card Fail, 18 (2012), pp. 845–853. [DOI] [PubMed] [Google Scholar]
- 17.Moss AJ, Brown MW, Cannom DS, et al. Multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT): design and clinical protocol. Annals Noninvasive Electrocardiol, 10 (Suppl 4) (2005), pp. 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation, 123 (2011), pp. 1061–1072. [DOI] [PubMed] [Google Scholar]
- 19.Thomas S, Moss AJ, Zareba W, et al. Cardiac resynchronization in different age groups: a MADIT-CRT long-term follow-up substudy. J Card Fail, 22 (2016), pp. 143–149. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg BA, Al-Khatib SM, Edwards R, et al. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. J Am Coll Cardiol HF, 2 (2014), pp. 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barsheshet A, Goldenberg I, Moss AJ, et al. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. Eur Heart J, 32 (2011), pp. 1622–1630. [DOI] [PubMed] [Google Scholar]
- 22.Herscovici R, Moss AJ, Kutyifa V, et al. Risk factors and outcomes associated with the development of myocardial ischemic events in patients who receive cardiac resynchronization therapy. Am J Cardiol, 112 (2013), pp. 1896–1900. [DOI] [PubMed] [Google Scholar]
- 23.Daimee UA, Biton Y, Aktas MK, et al. Effect of significant weight change on inappropriate implantable cardioverter-defibrillator therapy. Pacing Clin Electrophysiol, 40 (2017), pp. 9–16. [DOI] [PubMed] [Google Scholar]
- 24.Aktas MK, Zareba W, Huang DT, et al. The effect of weight loss on clinical outcomes in patients implanted with a cardiac resynchronization therapy device-a MADIT-CRT substudy. J Card Fail, 20 (2014), pp. 183–189. [DOI] [PubMed] [Google Scholar]
- 25.Szepietowska B, Polonsky B, Sherazi S, et al. Effect of obesity on the effectiveness of cardiac resynchronization to reduce the risk of first and recurrent ventricular tachyarrhythmia events. Cardiovasc Diabetol, 15 (2016), p. 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kutyifa V, Kloppe A, Zareba W, et al. The influence of left ventricular ejection fraction on the effectiveness of cardiac resynchronization therapy: MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy). J Am Coll Cardiol, 61 (2013), pp. 936–944. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr, 18 (2005), pp. 1440–1463. [DOI] [PubMed] [Google Scholar]
- 28.Solomon SD, Foster E, Bourgoun M, et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation, 122 (2010), pp. 985–992. [DOI] [PubMed] [Google Scholar]
- 29.Pouleur AC, Knappe D, Shah AM, et al. Relationship between improvement in left ventricular dyssynchrony and contractile function and clinical outcome with cardiac resynchronization therapy: the MADIT-CRT trial. Eur Heart J, 32 (2011), pp. 1720–1729. [DOI] [PubMed] [Google Scholar]
- 30.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol, 61 (2013), pp. e6–e75. [DOI] [PubMed] [Google Scholar]
- 31.Mathew J, Katz R, St. Sutton M, et al. Chronic kidney disease and cardiac remodelling in patients with mild heart failure: results from the REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction (REVERSE) study. Eur J Heart Fail, 14 (2012), pp. 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adelstein EC, Shalaby A, Saba S Response to cardiac resynchronization therapy in patients with heart failure and renal insufficiency. Pacing Clin Electrophysiol, 33 (2010), pp. 850–859. [DOI] [PubMed] [Google Scholar]
- 33.Van Bommel RJ, Mollema SA, Borleffs CJ, et al. Impaired renal function is associated with echocardiographic nonresponse and poor prognosis after cardiac resynchronization therapy. J Am Coll Cardiol, 57 (2011), pp. 549–555. [DOI] [PubMed] [Google Scholar]
- 34.Szepietowska B, Kutyifa V, Ruwald MH, et al. Effect of cardiac resynchronization therapy in patients with insulin-treated diabetes mellitus. Am J Cardiol, 116 (2015), pp. 393–399. [DOI] [PubMed] [Google Scholar]
- 35.Hoke U, Thijssen J, van Bommel RJ, et al. Influence of diabetes on left ventricular systolic and diastolic function and on long-term outcome after cardiac resynchronization therapy. Diabetes Care, 36 (2013), pp. 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldenberg I, Moss AJ, Hall WJ, et al. Predictors of response to cardiac resynchronization therapy in the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT). Circulation, 124 (2011), pp. 1527–1536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.