Abstract
Purpose: Sorafenib is the only approved drug in first-line treatment for hepatocellular carcinoma. Recently, the Phase III REFLECT trial proved lenvatinib not inferior to sorafenib, potentially establishing a new standard of care in this setting. The study showed that both have similar overall survivals, yet with longer time to progression for lenvatinib. Currently, the selection of one or other is not based on clinical or biological parameters for this reason we performed a network meta-analysis and we also analyzed the REFLECT trial and its implications in the current and future clinical practice.
Materials and methods: We performed the meta-analysis according to the Prisma statement recommendations. HR was the measure of association for time to progression and overall survival. The pooled analysis of HR was performed using a random effect model, fixing a 5% error as index of statistical significance.
Results: For HBV-positive patients, there was a clear trend in favor of lenvatinib over sorafenib (HR 0.82 95% credible interval [CrI] 0.60–1.15). For HCV-positive no differences between lenvatinib and sorafenib were observed (HR 0.91 95% CrI 0.41–2.01). The data showed that lenvatinib could be the best drug for HBV-positive patients in 59% of cases compared to only 1% of patients treated with sorafenib.
Conclusion: The identification of clinical or biological markers that could predict response or resistance to treatments is needed to guide treatment decision. This network meta-analysis demonstrates that the etiology is a good candidate and this result should be validated in a specific trial.
Keywords: sorafenib, hepatocellular carcinoma, randomized trial, biomarkers, erlotinib, linifanib, sunitinib, brivanib
Introduction
Sorafenib was approved thank to two positive trials, the SHARP study in 20071 and the Asia Pacific study in 2008.2 However, clinical trials investigating sunitinib,3 brivanib,4 and linifanib5 as first-line treatments, with sorafenib as a control arm, failed to meet their primary endpoint of improving overall survival (OS). Sorafenib is the only systemic drug approved in this setting of patients. Lenvatinib represents an alternative molecular-targeted therapy option for hepatocellular carcinoma (HCC) patients.6 It is an oral multireceptor tyrosine kinase inhibitor (TKI) of the activities of the vascular endothelial growth factor (VEGF) receptors (VEGFR1, VEGFR2, and VEGFR3), the fibroblast growth factor (FGF) receptors (FGFR1, FGFR2, FGFR3, and FGFR4) and the alpha and beta platelet-derived growth factor receptors.7–10
The VEGF-signaling pathway is the key regulator of tumor growth and metastasis;11 VEGFR2 induces major phenotypic changes of endothelial cells in angiogenesis, including proliferation, migration, survival, and tube formation.12
The most important difference between this drug and other TKIs, especially sorafenib, is the ability to potently inhibit FGFRs. The FGFR signaling pathway plays an important role in diverse cell functions, including proliferation, differentiation, apoptosis, and migration, and also in tumor proliferation, angiogenesis, migration, and survival.13-15FGFR pathway could be subject to various somatic aberrations, such as gene amplification, point mutations, translocations, and isoform switching, resulting in carcinogenesis.16,17In vivo, lenvatinib shows a more potent anti-tumor activity than in vitro.18,19
Preliminary evidence20 of tumor shrinkage in HCC patients resulted in the design of a single-arm, open-label, multicenter Phase II trial21 evaluating lenvatinib in patients with advanced HCC. In this trial, 46 patients were enrolled at 14 sites across Japan and Korea to receive lenvatinib. As for the primary endpoint, a median TTP of 7.4 months (95% CI 5.5–9.4) was obtained. The study showed a 37% ORR and a stable disease in 41% of patients with a 78% disease control rate (mRECIST criteria). Median OS was 18.7 months (95% CI 12.7–25.1).
Following the results of the Phase II trial, a randomized, open-label Phase III trial was designed6 with the aim to determine whether lenvatinib was not inferior to sorafenib in advanced HCC in term of OS. The non-inferiority margin was set at 1.08. A total of 954 patients were randomly assigned to receive either lenvatinib (n=478) or sorafenib (n=476). A median OS of 13.6 months (95% CI 12.1–14.9) and 12.3 months (95% CI 10.4–13.9) was reached in the lenvatinib arm and the sorafenib arm, respectively, with a HR)of 0.92 (95% CI 0.79–1.06), thus meeting the criteria for non-inferiority. The secondary endpoint of progression-free survival (PFS) of 7.4 months (95% CI 6.9–8.8) and 3.7 months (95% CI 3.6–4.6) was in favor of the lenvatinib arm (HR 0.66 P<0.0001). Similarly, the lenvatinib arm showed better ORR than the sorafenib arm (mRECIST criteria), with ORR of 24.1% (20.2–27.9) and 9.2% (6.6–11.8), respectively (OR 3.13 P<0.0001).
The lenvatinib arm and the sorafenib arm showed different toxicity profiles. The most common any-grade advserse events (AEs) for lenvatinib were hypertension (42%), diarrhea (39%), decreased appetite (34%), and decreased weight (13%), whereas palmar-plantar erythrodysesthesia (52%), diarrhea (46%), hypertension (30%), and decreased appetite (27%) for sorafenib. Treatment-related grade ≥3 AEs were observed in 57% and 49% of patients in the lenvatinib arm and in the sorafenib arm, respectively. Similarly, serious treatment-related AEs were more frequent in the lenvatinib arm with an incidence of 18% vs 10% in the sorafenib arm.
Actually the indication of Lenvatinib and Sorafenib are in first line in patients with advanced hepatocellular carcinoma. Currently, the selection of one or other is not based on clinical or biological parameters.
To date, there are no validated prognostic nor predictive markers of response to sorafenib in HCC, although hepatitis status seems to be a potential candidate.
In the subgroup analysis of the SHARP study1, sorafenib showed an advantage in term of OS on subgroup of patients positive for HCV (0.50; 95% CI 0.32-0.77), differently no different was observed in HBV-positive patients (0.76; 95% CI 0.38–1.50, P=not significant). The same results were obtained for TTP (HR =1.03 and 0.43 for HBV-positive and HCV-positive patients, respectively). These data were confirmed in the Asia-Pacific trial. In the pooled analysis of the SHARP and Asia- Pacific trials, Bruix et al confirmed that the absence of HCV was a potential prognostic factor for poorer OS (HR 0.7, P=0.02). The authors revealed that HBV-positive patients did not show a significant difference in treatment response compared to HBV-negative patients (HR =0.78; 95% CI 0.57–1.06) and OS (HR =1.128, P=0.4538).
A recent meta-analysis by Jackson et al22 highlighted that the benefit in OS depends on the patient’s status of hepatitis. OS improves in HBV-negative and HCV-positive patients when treated with sorafenib. However, as recently pointed out by Personeni et al,23 Jackson et al only considered negative data from randomized trials comparing sorafenib with other drugs (brivanib,4 linifanib5, and sunitinib3), disregarding both the SHARP1 and the Asia-Pacific2 trials that evaluated sorafenib against placebo. For this reason, we performed a meta-analysis of these two randomized studies and a network meta-analysis (NMA) between sorafenib and lenvatinib, assessing the different outcomes related to the different etiologies.
Materials and methods
Meta-analysis of SHARP trial and Asia-Pacific study
Study design and inclusion criteria
Clinical trials comparing sorafenib and placebo were searched in PubMed. Only randomized controlled trials (sorafenib vs placebo) that included patients with hepatocellular carcinoma were considered eligible and included in the quantitative analysis.
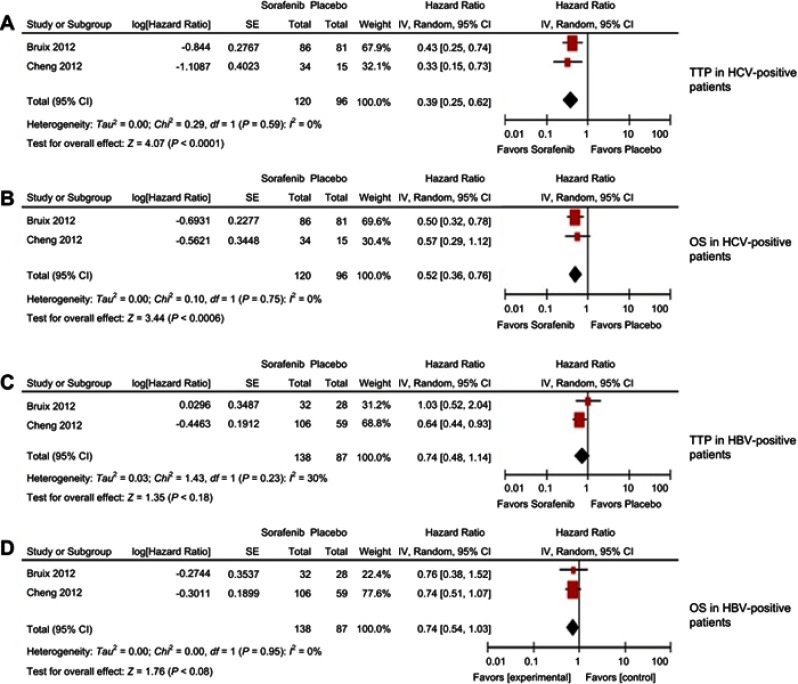
Figure 1 reports the search strategy followed in this meta-analysis. A bibliographic research was conducted of the PubMed, EMBASE, Cochrane Library, and Embase databases. Keyword used included ‘‘sorafenib and hepatocellular carcinoma and randomized trial and placebo.”
Figure 1.
Result of the meta-analysis; time to progression (A) and overall survival (B) for hepatitis C-positive patients; time to progression (C) and overall survival (D) for hepatitis B-positive patients.
Data extraction and management
Two review authors (ACG and ET) independently screened the titles and abstracts of all the selected studies. All the abstracts of potentially eligible trials were independently read by the same authors that decided if the study was selected. The full text of all selected papers was then analyzed by the same authors to select all the trials finally included in the pooled analysis. When discrepancies in trial search or selection occurred, they were discussed with a third researcher (GLF) to reach a final consensus. The quality of the studies included in this meta-analysis was assessed using the Cochrane risk of bias tool. The risk of bias in this meta-analysis was low.
Statistical analysis
We performed the meta-analysis according to the Prisma statement recommendations.10 Data were entered in a computer database for transfer and statistical analysis in Review Manager 5.2. Heterogeneity among the trials was assessed with descriptive aim using the I2 test. Any level <5% was considered as statistically significant. HR was the measure of association for time to progression (TTP) and OS. The pooled analysis of HR was performed using a random effect model, fixing a 5% error as index of statistical significance.
NMA of virus etiology
The model for the NMA was fit as previously suggested.24 Data were extracted from the publications or estimated as proposed by Parmar et al.25 Treatment effects were estimated by posterior means and 95% credible intervals (CrIs) using random effect, identity link function, and non-informative prior distributions (uniform and normal). We performed 25,000 iterations with burn-in number of 5,000 iterations and a thin interval of 20 to obtain the posterior distributions of model parameters. Convergence was assessed using the Brooks–Gelman–Rubin method. Posterior distributions were used to assess the probability of each treatment to be the best, second best, and so on. Inconsistency and heterogeneity were assessed using node-split models, I2, and Cochran Q tests. Significant heterogeneity was considered to be present for I2>50% or p-value >0.10. Der Simonian and Laird method and random effect were used. All the analyses were made with the R packages “Metaphor” and “Gemtc” (https://www.r-project.org/). The quality of the studies included in this meta-analysis was assessed using the Cochrane risk of bias tool. The risk of bias in this meta-analysis was low.
Results
Meta-analysis of SHARP trial and Asia-Pacific study
Two studies1,2 were analyzed for this present work. They included 120 and 138 HCC patients treated with sorafenib, and 96 and 87 with placebo for HCV and HBV analysis, respectively. Both studies are sub-analyses of a Phase III trial. The two studies were considered of high quality with low risk of bias.
The results of the meta-analysis showed a significant benefit of sorafenib for HCV-positive patients in terms of TTP (HR 0.39 CI 95% 0.25–0.62 P<0.0001) and OS (HR 0.52 CI 95% 0.36–0.76 P=0.0006) (Figure 1A and Figure 1B). HBV-positive patients showed a trend in favor of sorafenib rather than placebo for TTP (HR 0.74 CI 95% 0.48–1.14 p = 0.18) and OS (HR 0.74 CI 95% 0.544-1.03 p = 0.08) (Figure 1C and Figure 1D). No heterogeneity was detected for the outcomes.
NMA of virus etiology
The NMA was performed on a total of 1,788 patients on six study,1–26 of these 1160 patients were HCV-positive or HBV-positive. Of these, 251 (21.6%) HBV-positive patients and 91 (7.8%) HCV-positive patients received lenvatinib, whereas 390 (33.6%) HBV-positive patients and 229 (19.7%) HCV-positive patients received sorafenib. A total of 114 (9.8%) HBV-positive patients and 85 (7.3%) HCV-positive patients received placebo. All studies were considered of high quality with low risk of bias.
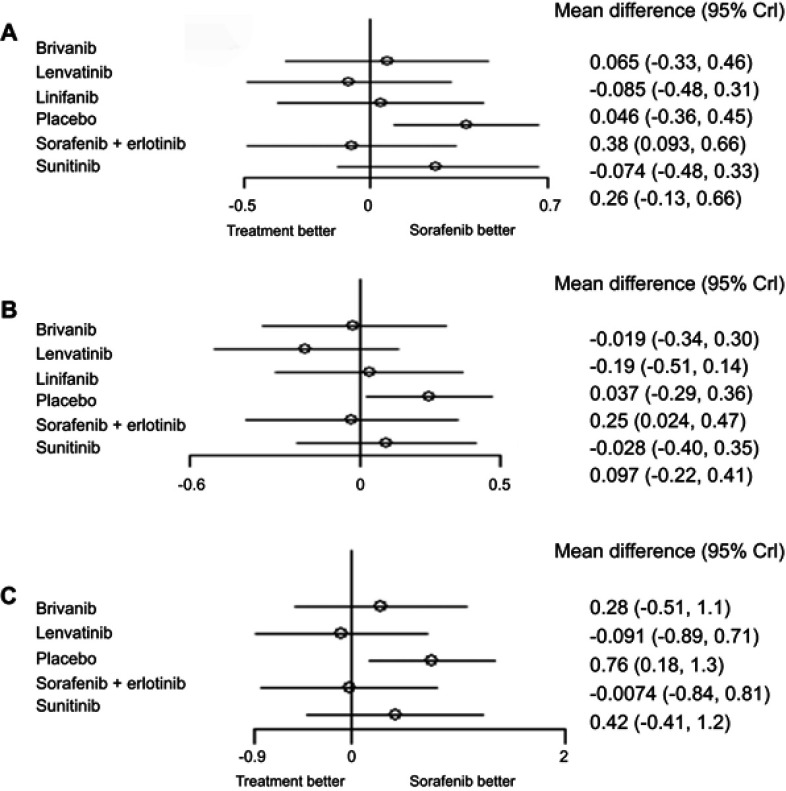
In the overall population no difference was observed between lenvatinib and sorafenib, despite if a slight trend toward a greater efficacy of lenvatinib (HR 0.92, 95% CrI 0.61–1.36) (Figure 2A). Both lenvatinib and sorafenib were significantly better than placebo.
Figure 2.
Results of Network Meta Analysis in all population (A); hepatitis B-positive patients (B) and hepatitis C-positive patients (C).
When we restricted the analysis to HBV-positive patients, a significant benefit in terms of OS was estimated for sorafenib (HR 0.78 95% CrI 0.62–0.97) with respect to placebo; for HBV-positive patients there was a clear trend in favor of lenvatinib over sorafenib (HR 0.82 95% CrI 0.60–1.15) (Figure 2B).
For HCV-positive no differences between lenvatinib and sorafenib were observed (HR 0.91 95% CrI 0.41–2.01) (Figure 2C). I2, Cochran's Q, and node-split models showed no evidence of heterogeneity nor inconsistency, strengthening the results of the NMA.
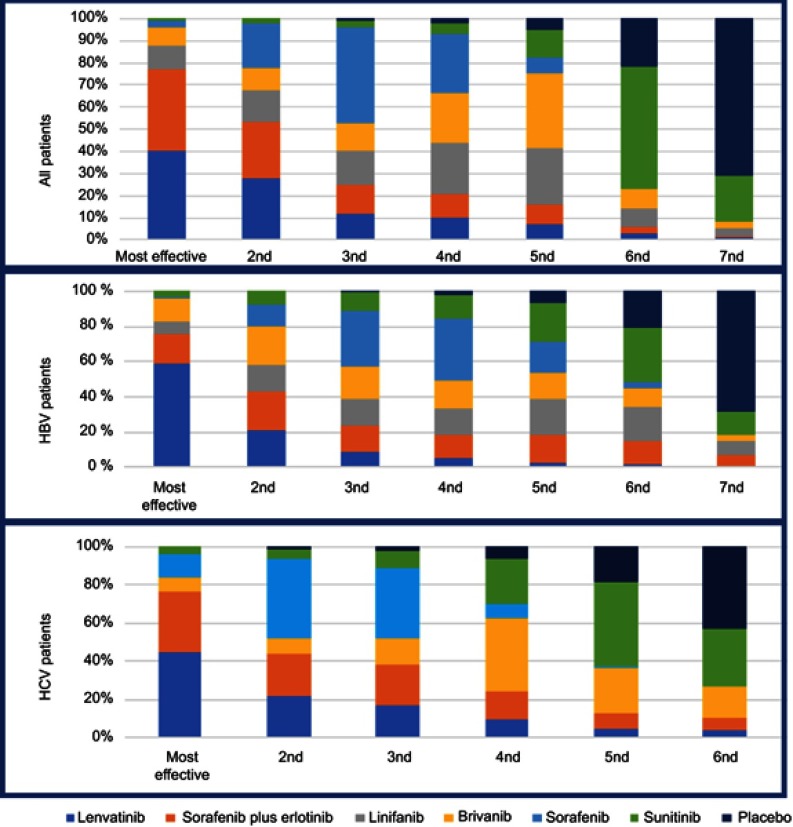
The rankogram in Figure 3 reports the probably best approach for these patients. The rankogram shows that Lenvatinib was probably the best approach for HBV-positive patients.
Figure 3.
Rankogram of theNetwork Meta analysis.
Discussion
Lenvatinib has a biological rationale for use in patients with advanced HCC. The REFLECT study was well designed, despite being open-label.6
What is key to our study is whether a non-inferiority study can change clinical practice. Table 1 lists the most important factors that may influence this choice of treatment. The first is the toxicity profile compared to the standard of care. Lenvatinib showed a non-negligible safety profile, proving no advantage against sorafenib: patients in the sorafenib arm had more dermatological AEs, but less hypertension than patients in the lenvatinib arm. Generally, dermatological AEs carry no risk of death, although they often compromise the patient’s quality of life (QoL), and can be resolved by dose decrease or treatment interruption. Patients receiving lenvatinib had a better QoL, as also demonstrated by a sub-analysis of the REFLECT study. However, hypertension can be rarely associated with serious complications regardless of treatment interruption. The second factor is the cost of the new drug compared to the standard of care: a lower price with similar efficacy and toxicity profile can well influence the doctor’s decision in clinical practice. Finally, patients who tolerate sorafenib may undergo regorafenib for disease progression as an effective second-line alternative, whereas no results are available about any effective option for disease progression beyond lenvatinib.
Table 1.
The main results of the REFLECT trial
| LENVATINIB | SORAFENIB | In favor of: | |
|---|---|---|---|
| Overall survival (months, 95% CI) | 13.6 (12.1–14.9) | 12.3 (10.4–13.9) | EQUAL |
| Time to progression (months, 95% CI) | 8.9 (7.4–9.2) | 3.7 (3.6–5.4) | LENVATINIB |
| Disease control rate (%, 95% CI) | 36.1 (75.5%, 71.7–79.4) | 28.8 (60.5%, 56.1–64.9) | EQUAL |
| Total treatment-emergent adverse events (%) | 99% | 99% | EQUAL |
| Treatment-related, treatment-emergent adverse events of grade ≥3 (%) | 57% | 49% | EQUAL |
| Serious treatment-emergent adverse events (%) | 43% | 30% | SORAFENIB |
| Palmar-plantar erythrodysesthesia grade ≥3 (%) |
3% | 11% | LENVATINIB |
| Hypertension grade ≥3 (%) |
23% | 14% | SORAFENIB |
| Option beyond didease progression | NO | YES | SORAFENIB |
| Cost | Unknown | Unknown | Unknown |
| Quality of life | Unknown | Unknown | LENVATINIB |
In the context, the identification of biomarkers or clinical parameters that could predict response or resistance to treatments is needed to guide treatment decision.
Our data from NMA highlighted that lenvatinib has a greater activity in HBV-positive patients. The data showed that lenvatinib could be the best drug for HBV-positive patients in 59% of cases compared to only 1% of patients treated with sorafenib.
This is a crucial point because actually we could have clinical parameters to select better the best treatment for the patients. Our findings seem to confirm previous suggestions on this topic although further confirmatory data may be necessary.
We believe that future prospective studies should aim to customize therapy based on the etiology. For example, a sub-analysis of the RESOURCE study27 highlights, as we have already pointed out,28 that the HR for OS was 0.58 (95% CI 0.41–0.82 P=0.0009) in HBV-positive patients against 0.79 (95% CI 0.49-1.26 P=0.1583) in HCV-positive patients. Similar data were observed for PFS (0.39 vs 0.59, respectively) and TTP (0.38 vs 0.57, respectively). Actually, we have more drug in advanced hepatocellular in the same line. Future study must be aimed to identify a best strategy in first and second line.
Conclusion
In conclusion, the identification of clinical or biological markers that could predict response or resistance to treatments is needed to guide treatment decision. This NMA demonstrates that the etiology is a good candidate and this result should be validated in a specific trial.
Acknowledgments
The authors would like to thank Veronica Zanoni for editorial assistance.
Author contributions
All authors contributed to data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspectes of the work.
Disclosure
ACG is on the advisory board for Eisai Co., Ltd. SC received personal fees from Bayer and grants from Eisai Co., Ltd, personal fees from Lilly and personal fees from Servier. MS received grants and personal fees from Merck, grants and personal fees from Bayer, personal fees from Eisai Co., Ltd., personal fees from Bristol-Myers Squibb, grants from Merck and personal fees from AMGEN. The authors report no other conflicts of ineterest in this work.
References
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the asia-pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372 [DOI] [PubMed] [Google Scholar]
- 4.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. doi: 10.1200/JCO.2012.48.4410 [DOI] [PubMed] [Google Scholar]
- 5.Cainap C, Qin S, Huang WT, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi: 10.1200/JCO.2013.54.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 7.Fala L. Lenvima (lenvatinib), a multireceptor tyrosine kinase inhibitor, approved by the FDA for the treatment of patients with differentiated thyroid cancer. Am Health Drug Benefits. 2015;8(Spec Feature):176–179. [PMC free article] [PubMed] [Google Scholar]
- 8.Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. doi: 10.1155/2014/638747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122(3):664–671. doi: 10.1002/ijc.23131 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18 eCollection 2014. doi: 10.1186/2045-824X-6-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. doi: 10.1038/nrc2403 [DOI] [PubMed] [Google Scholar]
- 12.Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is flk-1-dependent. J Biol Chem. 1999;274(43):31047–31054. [DOI] [PubMed] [Google Scholar]
- 13.Becker D, Lee PL, Rodeck U, Herlyn M. Inhibition of the fibroblast growth factor receptor 1 (FGFR-1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene. 1992;7(11):2303–2313. [PubMed] [Google Scholar]
- 14.Funato N, Moriyama K, Shimokawa H, Kuroda T. Basic fibroblast growth factor induces apoptosis in myofibroblastic cells isolated from rat palatal mucosa. Biochem Biophys Res Commun. 1997;240(1):21–26. doi: 10.1006/bbrc.1997.7588 [DOI] [PubMed] [Google Scholar]
- 15.Li M, Bernard O. FDC-P1 myeloid cells engineered to express fibroblast growth factor receptor 1 proliferate and differentiate in the presence of fibroblast growth factor and heparin. Proc Natl Acad Sci USA. 1992;89(8):3315–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dienstmann R, Rodon J, Prat A, et al. Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors. Ann Oncol. 2014;25(3):552–563. doi: 10.1093/annonc/mdt419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22(1):259–267. doi: 10.1158/1078-0432.CCR-14-3212 [DOI] [PubMed] [Google Scholar]
- 18.Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14(17):5459–5465. doi: 10.1158/1078-0432.CCR-07-5270 [DOI] [PubMed] [Google Scholar]
- 19.Finn RS, Kudo M, Cheng A, et al. Analysis of serum biomarkers (BM) in patients (pts) from a phase 3 study of lenvatinib (LEN) vs sorafenib (SOR) as first-line treatment for unresec hepatocellular carcinoma (uHCC). Ann Oncol. 2017;28(suppl_5):v605–v649. doi: 10.1093/annonc/mdx440.022 [DOI] [Google Scholar]
- 20.Ikeda M, Okusaka T, Mitsunaga S, et al. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2016;22(6):1385–1394. doi: 10.1158/1078-0432.CCR-15-1354 [DOI] [PubMed] [Google Scholar]
- 21.Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52(4):512–519. doi: 10.1007/s00535-016-1263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard J, EE Psarelli, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: A meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35(6):622–628. doi: 10.1200/JCO.2016.69.5197 [DOI] [PubMed] [Google Scholar]
- 23.Personeni N, Rimassa, L, Giordano, L Santoro, A. Sorafenib in hepatitis C virus-negative patients with hepatocellular carcinoma: don’t throw the baby out with the bathwater ! J Clin Oncol. 2017;35(19):2213–2214. doi: 10.1200/JCO.2017.72.7776 [DOI] [PubMed] [Google Scholar]
- 24.Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: A tutorial. BMC Med Res Methodol. 2010;10:54. doi: 10.1186/1471-2288-10-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. [DOI] [PubMed] [Google Scholar]
- 26.Zhu AX, Rosmorduc O, Evans TR. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinibin patients with avanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–566. doi: 10.1200/JCO.2013.53.7746 [DOI] [PubMed] [Google Scholar]
- 27.Bruix J, Qin S, Merle P. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 28.Casadei Gardini A, Frassineti GL, Foschi FG, Ercolani G, Ulivi P. Sorafenib and Regorafenib in HBV- or HCV-positive hepatocellular carcinoma patients: analysis of RESORCE and SHARP trials. Dig Liver Dis. 2017;49(8):943–944. doi: 10.1016/j.dld.2017.04.022 [DOI] [PubMed] [Google Scholar]