Abstract
Purpose: This study evaluated the risk of opioid dose escalation as it relates to sex differences among patients receiving opioids for long-term therapy.
Patients and methods: This retrospective cohort study was conducted in tertiary hospital settings in Malaysia using electronic prescription records. Opioid naïve patients, aged ≥18 years, who were undergoing long-term opioid therapy of ≥90 days, with at least one opioid prescription (buprenorphine, morphine, oxycodone, fentanyl, dihydrocodeine or tramadol) between 1st January 2011 and 31st December 2016, were included in the study. They were followed until (i) the end of the study period, (ii) death from any cause or (iii) discontinuation of therapy from their first opioid prescription without any intervals of ≥120 days between successive prescriptions. The risk of high opioid dose escalation to ≥100 mg/day and ≥200 mg/day relative to men and women was measured.
Results: A total of 4688 patients (58.8% women, 41.3% men) on long-term opioid therapy were identified. Among these patients, 248 (5.29%) were escalated to high opioid doses of ≥100 mg/day and 69 (1.47%) were escalated to ≥200 mg/day. The escalation to high-dose opioid therapy was more likely to occur in men than in women, even after adjustment for age (dose ≥100 mg/day [adjusted hazard ratio 2.32; 95% confidence interval (CI), 1.79 to 3.00; p<0.0001] and ≥200 mg/day [adjusted hazard ratio 6.10; 95% CI, 3.39 to 10.98; p<0.0001]).
Conclusion: The risk of opioid dose escalation differed between men and women, as men were at higher risk than women for high opioid dose escalation.
Keywords: opioids, dose escalation, opioid prescribing, male patient, female patient
Introduction
The benefits of long-term opioid use in chronic non-cancer pain (CNCP) are questionable, owing to the lack of evidence supporting its use in this condition.1,2 Despite the unproven benefits, opioid prescriptions for CNCP have increased tremendously over the past three decades,3,4 and this has also coincided with a sharp increase in the incidence of adverse events and opioid overdose-related deaths.5–8 In Malaysia, the overall increase in opioid use over the last six years has been relatively high, but clinically, it has affected fewer patients compared to other analgesics.9
High opioid dose is reportedly one of the main reasons contributing to the increase in opioid mortality.10–12 A previous study has shown that an opioid dose of ≥100 mg/day (milligram morphine equivalent [MME]) is associated with an 8.9-fold increase in overdose risk, as compared to 50–99 mg/day (a 3.7-fold increase) and 1–20 mg/day.13 In addition, 40% of opioid-related deaths are associated with an opioid dose of >100 mg/day morphine equivalents compared to 20% of deaths with an opioid dose of <100 mg/day.5
In an effort to minimise the undesirable effects of opioids, a few clinical practice guidelines have recommended that individual risk factors, including sex differences, be stratified.14 Studies have shown that women have different pain perception and drug-seeking behaviour from men.15,16 Compared with men, women are also more likely to be prescribed opioids,17,18 because they are more likely to report pain, and experience non-cancer pain more frequently.19 By identifying the differences in opioid use between men and women, opioid therapy can be optimised, and opioid-related adverse events could be minimised.
To date, most of the studies on opioid use and sex differences have been primarily conducted in North America, and limited research has been carried out in other parts of the globe, which also report considerable consumption of opioids. The present study aimed to examine the sex differences in opioid prescription in Malaysia. The risk of opioid dose escalation in male and female patients on long-term opioid therapy was also evaluated. With a knowledge of individual risk, clinicians would be able to identify the patients at higher risk, and implement appropriate prevention strategies.
Methods
This study was granted ethical approval by the Medical Research Ethical Committee, Ministry of Health, Malaysia. Informed consent was not required because patients were not directly involved in the study. Data were de-identified and results were reported in an aggregated manner.
Study design and setting
The data for this retrospective cohort study were obtained from the electronic pharmacy records of tertiary outpatient hospital settings in Malaysia. These hospitals provide services of various disciplines, including pain services, renal services, surgery and anaesthesiology. The facilities have 800‒1000 inpatient beds per hospital.
Electronic pharmacy records were accessed for data, such as drug names and strengths, quantities, frequencies, issuing departments, prescription dates, patient ages and sex. Patient age was calculated based on the date of the first prescription in the database.
Patients were identified based on their prescriptions for buprenorphine, morphine, oxycodone, fentanyl, dihydrocodeine and tramadol, which were issued between 1st January 2011 and 31st December 2016. Patients aged ≥18 years, who had not received any opioid prescription in the previous year were included. The first opioid prescription after the previous one-year interval was defined as the index prescription. From their first opioid prescription, these newly treated patients were followed until the discontinuation of opioid treatment (an interval between successive prescriptions of >120 days). The study period ended either on 31st December 2016 or death by any cause, whichever occurred first. The study included only the first episode of opioid use for patients who had many intervals of >120 days between successive prescriptions.
Cases of incomplete prescription information, or those in which opioids were prescribed as needed were excluded from the analysis. Prescriptions for methadone, which is used exclusively for opioid addiction, were also excluded.
Opioid days of supply
The days’ supply of opioid prescriptions was derived by dividing the quantity of opioids supplied by the number of daily doses (frequency). The annual number of days’ supply of opioid prescriptions was calculated by summing up all-day supply for each patient for a particular follow-up period. For patients with multiple opioid prescriptions issued on the same day, only the prescription with the largest number of days’ supply was included. If the prescriptions overlapped (the second prescription was issued before the duration of the first prescription came to an end), the overlapping period was subtracted.
Patients who were prescribed opioids ≥90 days/year, without any intervals of ≥120 days between successive prescriptions, were included as long-term opioid users.
Opioid daily dose
The opioid dose for each prescription was converted to oral MMEs by multiplying it by the conversion factor provided by the Centers for Disease Control and Prevention (CDC).20 The total opioid dose in MMEs for each patient was obtained by summing the opioid dose across all prescriptions for a particular patient per follow-up period. This total opioid dose was then divided by the total days of supply of the opioid prescription for a particular follow-up period, to derive the opioid dose per day for each patient in MMEs. If the patient received multiple opioid prescriptions on the same day, the doses were merged and a combined daily dose in MMEs was calculated. Patients with a total opioid dose of ≥100 mg/day were identified. The study also included patients who were escalated to an opioid dose of ≥200 mg/day, because in some guidelines, the cautionary dose for opioid-related mortality starts at this value.
Data management and statistical analysis
The primary outcome measure was the number of patients on long-term opioid therapy who were escalated to a high opioid dose of ≥100 or ≥200 mg/day. All analyses were stratified according to sex. A Cox proportional hazards model was used to estimate the risk of dose escalation for men relative to that for women over a maximum follow-up period of 6 years, and the analyses were controlled for the effect of age. The adjusted hazard ratios (HR) and 95% confidence intervals (CIs) were used to summarise the risk of dose escalation. A p-value <0.05 was considered statistically significant. All analyses were carried out using the Stata v15.1 software.21
Results
A total of 41 091 patients with 56 439 opioid prescriptions over the 6-year study period were analysed. Among these, 4688 (11.4%) were patients on long-term opioid therapy (14 962 prescriptions). Among the patients on long-term opioid therapy, the number of women (2754, 58.8%) was higher than the number of men (1934, 41.3%) (Table 1). A total of 248 (5.3%) patients were escalated to a high opioid dose of ≥100 mg/day, of which 61.2% (n=152/248) were men and 38.7% (n=96/248) were women. Among all long-term patients escalated to an opioid dose of ≥100 mg/day, 3.2% (n=152/4688) were men and 2.0% (n=96/4688) were women (Table 2). This finding indicates that one out of 15 men, and one out of 30 women underwent high opioid dose (≥100 mg/day) escalation. Older patients, aged between 60 and 80 years, were the predominant group in both male (48.2%) and female (42.5%) patients.
Table 1.
Patients demographics
| Sex | Women | Men | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Number of patients | 2754 | 58.75 | 1934 | 41.25 | 4188 | 100 |
| Age | ||||||
| Mean | 60.01 | 60.17 | 60.07 | |||
| Median | 60 | 62 | 61 | |||
| Mode | 62 | 71 | 70 | |||
| Range | 18–95 | 18–96 | 18–96 | |||
| SD | 14.35 | 14.78 | 14.53 | |||
| Age group | ||||||
| 18 to 40 years old | 263 | 9.55 | 227 | 11.74 | 490 | 10.45 |
| 41 to 50 years old | 367 | 13.33 | 238 | 12.31 | 605 | 12.91 |
| 51 to 60 years old | 772 | 28.03 | 412 | 21.3 | 1184 | 25.26 |
| 61 to 80 years old | 1169 | 42.45 | 933 | 48.24 | 2102 | 44.84 |
| >81 years old | 183 | 6.64 | 124 | 6.41 | 307 | 6.55 |
| Type of opioids | ||||||
| Buprenorphine | 76 | 0.89 | 4 | 0.06 | 80 | 0.53 |
| Dihydrocodeine | 114 | 1.33 | 38 | 0.59 | 152 | 1.02 |
| Fentanyl | 231 | 2.7 | 209 | 3.26 | 440 | 2.94 |
| Morphine | 1,069 | 12.5 | 1,158 | 18.06 | 2,227 | 14.88 |
| Oxycodone | 259 | 3.03 | 223 | 3.48 | 482 | 3.22 |
| Tramadol | 6,802 | 79.55 | 4,779 | 74.54 | 11,581 | 77.54 |
| Total prescriptions | 8,551 | 100 | 6,411 | 100 | 14,962 | 100 |
Table 2.
Cumulative hazard ratio between men and women with long term opioids
| Dose | Men | Women | Total | Unadjusted hazard ratio | Adjusted hazard ratio | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | (95%CI) | (95%CI) | |
| 100 mg/day | 152 | 61.2 | 96 | 38.7 | 248 | 2.32 (1.79 to 3.00) | 2.47 1.91 to 3.19) |
| 200 mg/day | 55 | 79.7 | 14 | 20.2 | 69 | 6.10 (3.39 to 10.98) | 6.46 (3.59 to 11.64) |
A total of 69 patients (1.47%) were escalated to an opioid dose of ≥200 mg/day, 79.7% (n=55/69) were men and 20.2% (n=14/69) were women. Among all long-term patients escalated to opioid dose of ≥200 mg/day, 1.17% (n=55/4688) were men and 0.29% (n=14/4688) were women. These figures indicate that one out of 35 men, and one out of 196 women underwent high opioid dose (≥200 mg/day) escalation. The median time to reach ≥100 or ≥200 mg/day was 126 days for men and 162 days for women.
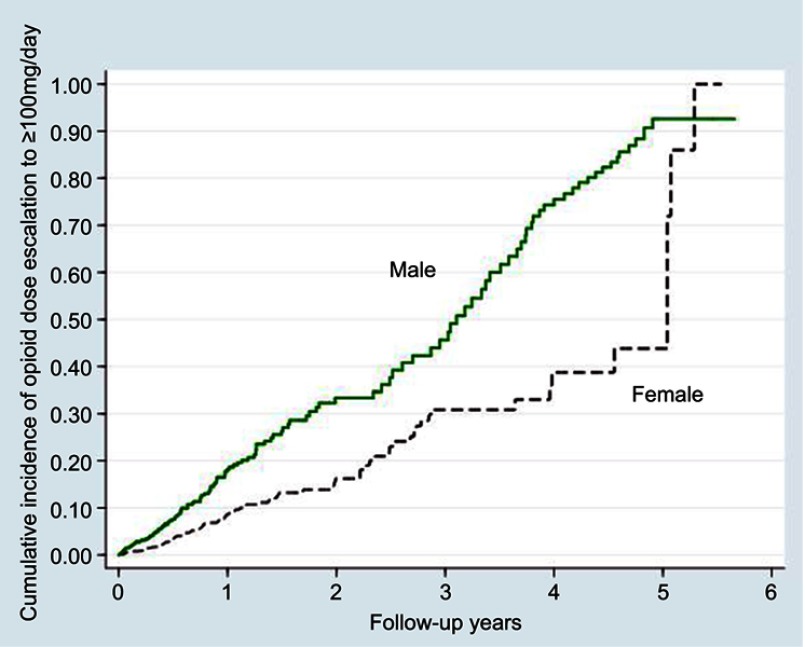
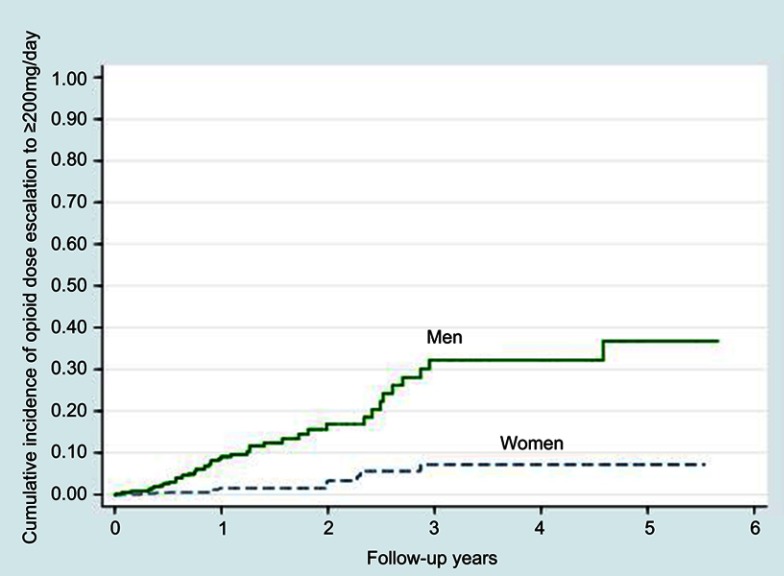
After adjustment for age, men were found to be twice more likely than women to be escalated to high-dose opioid therapy of 100 mg/day (adjusted HR 2.32; 95% CI, 1.79 to 3.00; p<0.0001) (Figure 1 and Table 2). Similar findings were noted for those escalated to 200 mg/day (adjusted HR 6.10; 95% CI, 3.39 to 10.98; p<0.0001) (Figure 2).
Figure 1.
Kaplan-Meier curves of high opioid dose escalation to an average daily dose exceeding 100 mg of morphine (or equivalent) among men and women.
Figure 2.
Kaplan-Meier curves of high opioid dose escalation to an average daily dose exceeding 200 mg of morphine (or equivalent) among men and women.
The most frequently prescribed opioid was tramadol (77.40%), followed by morphine (14.88%), oxycodone (3.22%) and fentanyl (2.94%). This sequence was consistent for both men and women (Table 1).
Discussion
The findings showed that female patients were prescribed long-term opioid therapy more frequently than men, but the risk of high opioid dose escalation was higher in men than in women. Among the patients on long-term opioid therapy, 5.3% were escalated to a high opioid dose of ≥100 mg/day, and 1.47% were escalated to ≥200 mg/day.
Women were more likely than men to receive long-term opioid therapy, which is consistent with findings reported in previous studies.17,19,22 One of the reasons for this observation is the fact that compared with men, women have lower pain sensitivity23,24 and are exposed to a greater number of chronic conditions that cause pain,25 and are associated with high use of pain medications. Female patients also tend to seek help from health professionals more frequently than male patients, thus making women more likely to be prescribed opioids.19
Although women were more likely to use opioids over a long term, the risk of high opioid dose escalation was higher (adjusted HR 2.32) in men than in women. As observed in the present study, 3.2% of the male patients and 2.0% of the female patients were escalated to a high opioid dose of ≥100 mg/day, which is consistent with previous studies that have reported a higher rate of opioid dose escalation in men compared to women.22,26 The current study also found that 1.47% of the patients on long-term opioid therapy were escalated to a dose of ≥200 mg/day, which is consistent with a previous study that reported that 1.8% of patients on chronic opioid therapy were escalated to a high dose of ≥200 mg/day.22 In the current study, the proportion of men escalated to a high dose of ≥200 mg/day was higher than that of women (1.17%, men vs 0.29%, women), which is similar to the findings of a previous study (2.3%, men vs 1.4%, women).
Dose escalation may be reflective of opioid tolerance. In a previous study, the rate of development of opioid tolerance in men was reportedly slightly higher than that in women,27 and this finding is associated with the role of gonadal hormones in the pharmacokinetic and pharmacodynamic effects of opioids. Moreover, a previous study showed that younger patients have higher rates of opioid dose escalation than older patients.26 Ageing can alter the pharmacokinetic profile by slowing down metabolism and reducing drug elimination, which might explain the lower dose requirement in the elderly.28 The analysis in the present study was controlled for age to ensure the rate of dose escalation was due to the effect of sex and not confounded by age.
The findings of this study support the need for extra vigilance when prescribing opioids in men, as they are at greater risk of high opioid dose escalation. This precaution is also applicable to all patients, regardless of sex, particularly if prescribing opioids over a long term, during which the efficacy is uncertain, and if a higher number of adverse events have been reported, especially for high opioid doses, relative to the benefits. The identification of risk allows the clinician to modify the opioid treatment for persons who are at increased risk, and thereby minimise undesirable effects.19
The use of patient-level data in stratifying the risk of dose escalation in men and women is the main strength of this study. However, some limitations should also be noted. The nature of prescription data, with the unavailability of information on diagnoses and comorbidities, may confound the associations outlined in this study. The findings from outpatient hospital settings might not be easily generalised to other settings, including private, primary care or inpatient settings. Information on opioid prescriptions from other sources was not available, which may have led to an underestimation of the number of patients on long-term opioid therapy who underwent dose escalation. The data on opioid prescribing used in this study did not allow us to determine whether the medications were actually dispensed and consumed.
Conclusion
Among the patients on long-term opioid therapy, men were at higher risk of high-dose escalation, compared to women. Opioid-related morbidity and mortality can be minimised by understanding the different risks associated with prescription opioid use based on sex differences. Further research is needed to identify other opioid-related risks and related outcomes in order to stratify the risks. Such an endeavour might effectively minimise the undesirable effects of opioid therapy.
Acknowledgments
CSZ was supported by a research grant from The Ministry of Education Malaysia (Fundamental Research Grant Scheme, FRGS 19-010-0618). NHT was supported by a research grant from The International Islamic University Malaysia (RIGS 17-076-0651). The authors would like to thank Professor Patrick Ball for reviewing and editing the manuscript.
Author contributions
All authors contributed to the data analysis, drafting or revising the article, gave final approval of the version to be published, agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chou R, Turner J, Devine E, et al. The effectiveness and risks of longterm opioid therapy for chronic pain: a systematic reviewfor aNational Institutes of Health Pathways to Prevention workshop. Ann Intern Med. 2015;162:276–286. doi: 10.7326/M14-2559 [DOI] [PubMed] [Google Scholar]
- 2.Vowles K, McEntee M, Julnes P, Frohe T, Ney J, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569–576. doi: 10.1097/01.j.pain.0000460357.01998.f1 [DOI] [PubMed] [Google Scholar]
- 3.National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. Available from: https://www.drugabuse.gov/drugs-abuse/opioids/opioid-overdose-crisis. Accessed April27, 2018.
- 4.Prescription opioid overdose data. Atlanta: centers for disease control and prevention; 2016. Available from: https://www.cdc.gov/drugoverdose/data/overdose.html. Accessed April27, 2018.
- 5.Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9–E38. doi: 10.1007/s11606-012 [DOI] [PubMed] [Google Scholar]
- 6.Bohnert A, Ilgen M, Trafton J, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605–612. doi: 10.1097/AJP.0000000000000011 [DOI] [PubMed] [Google Scholar]
- 7.Olsen Y. The CDC guideline on opioid prescribing—rising to the challenge. JAMA. 2016;15:1577–1579. doi: 10.1001/jama.2016.1910 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Opioid data analysis. Available from: https://www.cdc.gov/drugoverdose/data/analysis.html. Accessed May8, 2018.
- 9.Zin CS, Nazar NI, Rahman NS, et al. Trends and patterns of analgesic prescribing in Malaysian public hospitals from 2010 to 2016 : tramadol predominately used. J Pain Res. 2018;11:1959–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adewumi A, Hollingworth S, Maravilla J, Connor J, Alati R. Prescribed dose of opioids and overdose: a systematic review and meta-analysis of unintentional prescription opioid overdose. CNS Drugs. 2018;32(2):101–116. doi: 10.1007/s40263-018-0499-3 [DOI] [PubMed] [Google Scholar]
- 11.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 12.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691. doi: 10.1001/archinternmed.2011.117 [DOI] [PubMed] [Google Scholar]
- 13.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose. Ann Intern Med. 2010;152:85–92. doi: 10.7326/0003-4819-152-2-201001190-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: findings from the National Survey on Drug Use and Health. Addict Behav. 2010;35:1001–1007. doi:10.1016/j.addbeh.2010.06.018. PMID: 20598809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim- B, Jlr I. Sex, gender, and pain: a review of recent clinical experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001.Sex [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell CI, Weisner C, LeResche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100(12):2541–2547. doi: 10.2105/AJPH.2009.180646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes T, Juurlink DN, Dhalla IA, Mailis-Gagnon A, Paterson JM, Mamdani MM. Trends in opioid use and dosing among socio-economically disadvantaged patients. Open Med. 2011;5(1):13–22. [PMC free article] [PubMed] [Google Scholar]
- 19.Serdarevic M, Striley CW, Cottler LB. Sex differences in prescription opioid use. Curr Opin Psychiatry. 2017;30(4):238–246. doi: 10.1097/YCO.0000000000000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Control NC for IP and. CDC compilation of opioid analgesic formulations with morphine milligram equivalent conversion factors; 2013;(01):1–6. Available from: http://www.pdmpassist.org/pdf/BJA_performance_measure_aid_MME_conversion.pdf. Accessed May 8, 2018.
- 21.StataCorp. Stata: Release 13. College Station, TX: StataCorp LLC; 2013. [Google Scholar]
- 22.Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. Sex differences in dose escalation and overdose death during chronic opioid therapy: A population-based cohort study. PLoS One. 2015;10(8):1–11. doi: 10.1371/journal.pone.0134550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87(3):325–334. doi: 10.1016/S0304-3959(00)00296-7 [DOI] [PubMed] [Google Scholar]
- 24.Barnabe C, Besette L, Flanagan C, et al. Sex differences in pain scores and localization in inflammatory arthritis: a systematic review and metaanalysis. J Rheumatol. 2012;39(6):1221–1230. doi: 10.3899/jrheum.111393 [DOI] [PubMed] [Google Scholar]
- 25.Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13(9):1181–1211. doi: 10.1111/j.1526-4637.2012.01467.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han H, Kass PH, Wilsey BL, Li CS. Age, gender, and earlier opioid requirement associations with the rate of dose escalation in long-term opioid therapy. J Opioid Manag. 2013;9(2):129–138. doi: 10.5055/jom.2012.0154 [DOI] [PubMed] [Google Scholar]
- 27.Craft RM, Stratamann J, Bartok R, Walpole T, King S. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacol. 1999;143(1):1–7. doi: 10.1007/s002130050911 [DOI] [PubMed] [Google Scholar]
- 28.Fine P. Pharmacological management of persistent pain in older patients. Clin J Pain. 2004;20(4):220–226. [DOI] [PubMed] [Google Scholar]