Abstract
Purpose: We determined whether electroacupuncture (EA) reduces Netrin-1-induced myelinated primary afferent nerve fiber sprouting in the spinal cord and pain hypersensitivity associated with postherpetic neuralgia (PHN) through activation of μ-opioid receptors.
Methods: PHN was induced by systemic injection of resiniferatoxin (RTX) in rats. Thirty-six days after RTX injection, a μ-opioid receptor antagonist, beta-funaltrexamine (β-FNA) or a κ-opioid receptor antagonist, nor Binaltorphimine (nor-BNI), was injected intrathecally 30 mins before EA, once every other day for 4 times. Mechanical allodynia was tested with von Frey filaments. The protein expression level of Netrin-1 and its receptors (DCC and UNC5H2) were quantified by using western blotting. The myelinated primary afferent nerve fiber sprouting was mapped with the transganglionic tracer cholera toxin B-subunit (CTB).
Results: Treatment with 2 Hz EA at “Huantiao” (GB30) and “Yanglingquan” (GB34) decreased the mechanical allodynia at 22 days and the myelinated primary afferent nerve fiber preternatural sprouting into the lamina II of the spinal dorsal horn at 42 days after RTX injection. Also, treatment with 2 Hz EA reduced the protein levels of DCC and Netrin-1 and promoted the expression of UNC5H2 in the spinal dorsal horn 42 days after RTX injection. Furthermore, the μ-opioid receptor antagonist β-FNA, but not the κ-opioid receptor antagonist nor-BNI, reversed the effect of EA on neuropathic pain caused by RTX. In addition, morphine inhibited the Netrin-1 protein level induced by RTX in SH-SY5Y cells.
Conclusions: Through activation of μ-opioid receptors, treatment with EA reduces the expression level of DCC and Netrin-1 and changes a growth-permissive environment in spinal dorsal horn into an inhibitory environment by increasing UNC5H2, thus decreasing RTX-caused primary afferent nerve sprouting in the spinal dorsal horn and neuropathic pain.
Keywords: postherpetic neuralgia, analgesia, mechanical hyperalgesia, rat, μ-opioid receptors
Introduction
During the last decade, important advances have been made in understanding the mechanisms and treatments of postherpetic neuralgia (PHN).1 However, the efficacy of many treatments remain limited. It is very important to explore thealternative therapies to treat PHN. Clinical studies indicate that electroacupuncture (EA) is effective in relieving neuropathic pain caused by PHN.2,3 In a rat PHN model induced by resiniferatoxin (RTX), EA can reduce tactile allodynia by decreasing the myelinated primary afferent nerve sprouting into the spinal dorsal horn.4
Netrin-1 is an axon guidance factor that contributes to the growth of embryonic spinal cord and cortical neurons.5,6 In the spinal cord of adult rats, Netrin-1 is expressed in neurons and mature oligodendrocytes, which form myelin and is enriched in the axon-myelinating membrane, suggesting that it is related to the growth of myelinated nerve fibers.7 Netrin-1 has two receptors, deleted in colorectal cancer (DCC) and UNC5H. They are expressed on astrocytes,8,9 and are highly expressed in the central nervous system. DCC gene is deleted in most colorectal cancers,10,11 and it was considered as a tumor suppressor gene.10 In addition, DCC is a transmembrane protein of the immunoglobulin10,12 and directs several types of cell motility through regulating synapse function.13 DCC mediates the action of Netrin-1 to attract axons and promotes the growth and elongation of sensory nerve fibers.12,14–16 UNC5H includes four UNC5 homologs in mammals: UNC5H1, UNC5H2, UNC5H3 and UNC5H4.17–19 It has been demonstrated that UNC5H contributes to axon guidance in C. elegans.19,20 Among them, UNC5H2 is the most abundant Netrin-1 receptor expressed in the spinal dorsal horn of adult rats.15 Furthermore, UNC5H mediates the effect of Netrin-1 in repelling axons, which inhibits the growth and extension of sensory nerve fibers.18,21–23 In our previous study, we discovered that RTX upregulates the protein level of Netrin-1 via activation of TRPV1 and increases the protein level of DCC, thus to enhance the myelinated primary afferent nerve sprouting and mechanical allodynia.4
Opioid receptors include μ, δ and κ opioid-receptors. They are G protein-coupled receptors, inhibiting pain transmission.24,25 μ-opioid receptors mediate the pharmacological effects produced by clinically used opioids.26–28 EA produces analgesia in part by activating μ-opioid receptors in the spinal cord.29 However, it remains unknown whether EA down-regulates myelinated primary afferent nerve sprouting and neuropathic pain mediated by Netrin-1 through μ-opioid receptors.
To determine the possible mechanism of EA on inhibiting myelinated primary afferent nerve sprouting and neuropathic pain caused by RTX, we first examined whether EA reduces tactile allodynia, the myelinated primary afferent nerve sprouting and the Netrin-1 protein level through activating μ-opioid receptors in rats treated with RTX. We used beta-funaltrexamine (β-FNA, M.W. 491), an alkylated derivative of naltrexone, which is a highly selective antagonist for the μ-opioid receptors and does not activate kappa- or delta-opioid receptors30,31 or nor-BNI, an antagonist for the κ opioid receptors to observe whether β-FNA or nor-BNI can block the effect of EA. We then determined whether morphine can regulate the protein level of Netrin-1 caused by RTX in SH-SY5Y cells.
Materials and methods
Animal models
Experiments were performed on adult male Sprague–Dawley rats (250–280 g) purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. All protocols in this research were approved by the Animal Care Committee at Huazhong University of Science and Technology and accorded with the ethical guidelines of the International Association for the Study of Pain.32 The rats were housed in plastic cages with a 12 hr light/dark cycle light from 7:00 a.m. to 7:00 p.m. They had free access to water and food. To induce PHN in rats, we followed a previously reported procedure.33 Briefly, each rat in RTX (RTX + NS), RTX + β-FNA, RTX+ nor-BNI, EA + NS (RTX + 2Hz EA + NS), EA + β-FNA (RTX + 2Hz EA + β-FNA), EA+ nor-BNI (RTX + 2Hz EA+ nor-BNI) and sham EA (RTX+ sham EA) groups recived a single intraperitoneal injection of RTX (250 μg/kg, LC Laboratories, Woburn, MA, USA).33 RTX was dissolved in a mixture of 10% ethanol and 10% Tween-80 in sterile 0.9% NaCl (normal saline, NS).34 VEH rats were treated in the same manner with injection of a mixture of 10% ethanol and 10% Tween 80 in sterile 0.9% NaCl (normal saline, NS). Before RTX or VEH injection, the baseline mechanical withdrawal threshold of each rat was measured.
Drug administration
Rats were randomly assigned to VEH (vehicle of RTX), RTX (RTX + NS), RTX + β-FNA, RTX+ nor-BNI, EA + NS (RTX + 2Hz EA + NS), EA + β-FNA (RTX + 2Hz EA + β-FNA), EA+ nor-BNI (RTX + 2Hz EA+ nor-BNI) and sham EA (RTX+ sham EA) treatment groups. B-FNA or nor-BNI (Sigma, St. Louis, MO, USA) was dissolved in sterile 0.9% NaCl (normal saline, NS) at a concentration of 7.5 mg/ml or 2.5 mg/ml, respectively. Each rat in EA + β-FNA or EA+ nor-BNI treatment group was intrathecal injected with 20 nmol starting from 36 days after RTX injection, once every other day for 4 times. Intrathecal injection of β-FNA or nor-BNI was done at 30 min before EA.35 EA + NS group received an equal volume of vehicle (NS) injection. Morphine (Sigma, St. Louis, MO, USA) was dissolved in sterile 0.9% NaCl (normal saline, NS) at a concentration of 64 mg/ml. Morphine was applied to SH-SY5Y cells 24 hr prior to treatment with RTX.
EA treatment
For EA treatment in rats, we followed a previously reported procedure.4 Briefly, in EA + NS group, the rats received EA on the left “Yanglingquan” (GB34) and “Huantiao” (GB30) starting from day 8 to day 42 after RTX injection (6 weeks after RTX injection), once every other day. GB34 and GB30 were chosen because they are effective in improving inflammatory pain and neuropathic pain in rats.4,36,37 Two acupuncture needles were inserted 6–7 mm into two acupoints, which correspond to GB34 and GB30 in humans. GB34 is located at the side of the crus, at the bottom and under the head of fibula in rats; and GB30 lies on the intersection of the outermost 1/3 and the middle 1/3 of the line connecting between the most prominent point of the femur and the sacral hiatus.38 EA (1 mA) was carried out at 2 Hz for 30 min.4 Current was delivered through a Han’s Acupoint Nerve Stimulator (LH202, Huawei Co. Ltd., Beijing, China).4 Each rat was placed in a homemade plastic chamber during EA treatment. The left hinlimb of the rat was stretched out from the hole in the plastic chamber so that the needle can be easily inserted, and the limbs can move freely. The animals kept calm during EA treatment and showed no evidence of distress. For the sham EA group, acupuncture needles were only shallowly inserted into GB34 and GB30 for 30 min without electrical stimulation.
Nociceptive behavioral tests
The mechanical withdrawal threshold was tested by using the “up and down” method.36,39 To test mechanical allodynia, we put rats on a mesh floor. The animals were accommodated to the experimental environment for 30 min before each behavioral test. The behavioral test was carried out 3 times before RTX injection as the baseline of mechanical thresholds and started from day 2 to day 42 after RTX injection once every other day. After an adaptation period of 30 min, a range of standardized von Frey filaments (Stoelting, USA) were used vertically to the plantar surface of right hind paw with enough force to bend the filament for 6 seconds. Quick withdrawal or licking or paw flinching was counted as a positive reaction. The test was carried out two or three times in each rat, and the average value was counted. The interval between the two tests is at least 5 min. The investigators involved in behavioral tests and biochemical assays were blinded to the drug injection throughout the study.
Western blotting
Western blotting test was carried out as previously described.40,41The spinal cord dorsal horn (at L4-L6 levels) mainly receive sensory input from the hindlimb and contributes to synaptic density. 42Because we performed EA and behavioral tests in the hindlimb, we used spinal dorsal horn tissues at the L4-L6 levels to correlate our data. Briefly, the spinal dorsal horn tissues were lysed and homogenized in phenylmethylsulfonyl fluoride and RIPA lysis buffer (Beyotime Biotechnology, Nanjing, China), and centrifuged at 12,000 g at 4 °C for 15 min. The protein concentrations of the supernatant were detected by the Enhanced BCA Protein Assay Kit (Beyotime Biotechnology, China). Then the equal quantity of samples containing loading buffer were separated by 8–12% glycine SDS-polyacrylamide gels for electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were probed with Goat anti-UNC5H2 antibody (1:2,000, R&D Systems, Minneapolis, MN, USA); mouse anti-DCC (1:300, Millipore, Temecula, CA, USA); rabbit anti-Netrin-1 antibody (1:1,000, Abcam, Hong Kong) and mouse anti-β-actin antibody as a loading control (1:5,000; Santa Cruz, Dallas, TX, USA) on a shaker overnight at 4 °C. Then the membranes were incubated with corresponding peroxidase-linked secondary antibodies (Santa Cruz Biotechnology): goat anti-mouse secondary antibody (1:20,000), rabbit anti-goat secondary antibody (1:20,000) and goat anti-rabbit secondary antibody (1:20,000) on a shaker at room temperature for 1 hr. Signal detection of the target protein was detected by enhanced chemiluminescence (ECL Plus Western blotting detection reagents, Pierce, Rockford, IL, USA) system. The optical density of each band in the membranes was measured using the ImageJ analysis software (National Institutes of Health, Bethesda, MD, USA).
SH-SY5Y cells culture
Human neuroblastoma cell lines (SH-SY5Y cells) were acquired from American Type Culture Collection (ATCC, USA). SH-SY5Y cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, USA) including 1× antibiotic-antimycotic solution (including 0.25 µg/ml amphotericin B, 100 U/ml penicillin and 100 µg/ml streptomycin) and 10% fetal bovine serum (FBS, Gibco, USA), and cultured at 37 °C in a constant humidity incubator containing 5% CO2.
After removing the DMEM medium and washing with 0.01M phosphate buffer (PBS, pH 7.4), trypsin (0.25% trypsin contained 0.02% EDTA) preheated to 37 °C was added. The cells were incubated at 37 °C in a humidified incubator with 5% CO2 for 5 min, and the fresh medium containing 10% FBS was then added to terminate the digestion reaction. The medium was transferred to a centrifuge tube, centrifuged at 1,000 rpm for 5 min, and the supernatant was discarded.
SH-SY5Y cell treatments
SH-SY5Y cells were grown in 6-well plates. The cells were stimulated with RTX (80 nM) for 24 hr for western blotting. Furthermore, morphine (1 μM) was added 24 hr prior to treatment with RTX. The protein level of Netrin-1 in each group was detected by western blotting analysis.
Immunofluorescence labeling of myelinated afferent fiber projections to the dorsal horn
To detect the effect of β-FNA on RTX-caused myelinated primary afferent nerve sprouting into the spinal dorsal horn, cholera toxin B-subunit (CTB), a transganglionic tracer, was injected into left sciatic nerve of VEH-, RTX-, EA + NS- and EA + β-FNA-treated rats as described.4 The results were analyzed using immunofluorescence labeling.
In brief, rats were deeply anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and transcardially perfused 4 days after CTB injection. The L4-L6 spinal cord was post fixed in 4% paraformaldehyde for 6–8 hr and dehydrated in 30% sucrose dissolved in 0.1 M phosphate buffer (PBS) at 4 °C for 48 hr. The tissues of spinal cord were sectioned with a cryostat. The thickness of sections was 20 μm. The slides were incubated in blocking buffer (0.2% tween-20 and 5% donkey serum in 0.01M PBS, pH 7.4) at 37 °C for 1 hr, then incubated overnight on a shaker at 4 °C with primary antibodies diluted in 0.01M PBS including 0.3% TritonX-100, 5% donkey serum, and incubated with a secondary antibody at 37 °C for 1 hr. The primary antibodies used were goat anti-CTB (1:250; List Biological Laboratories, Campbell, CA). For secondary antibodies, we used donkey anti-goat IgG conjugated with Dylight 594 (1:500; Jackson ImmunoResearch). We used 6 rats in each group and randomly selected five or six sections from the spinal cord of each rat. All imaging analysis was performed on an Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan) and using Image J software (Bethesda, MD).
Data analysis
Data are presented as means ± SEM. To determine the statistical difference in the behavior data among different time points and different groups, we used two-way ANOVA and Bonferroni’s post hoc test. To determine the statistical difference in the immunofluorescence data, we used one-way ANOVA and Student- Newman-Keus post hoc test. The statistical analysis was performed using GraphPad Prism software. A P-value of less than 0.05 was considered as statistically significant.
Results
MOR contributes to the effects of EA on RTX-induced mechanical allodynia
Intraperitoneal injection of RTX produces long-lasting allodynia starting at 3 weeks and lasts for at least 6 weeks.33 Single acute EA treatment showed no significant analgesic effect, but repeated EA treatments showed a persistent analgesic effect.43,44 For this reason, we conducted our experiments 42 days after RTX injection and applied EA from day 8 to day 42 after RTX injection, once every other day.
There was no difference in the baseline withdrawal threshold between different groups before intraperitoneal injection of RTX. RTX markedly reduced mechanical thresholds 2 days after injection (Figure 1A–C), consistent with our previous results.33 EA was applied to GB34 and GB30 for 30 min, starting from day 8 to day 42 after RTX injection, once every other day. Treatment with 2 Hz EA increased the mechanical thresholds 2 weeks after EA treatment. This effect was sustained for 6 weeks after intraperitoneal injection of RTX (Figure 1B and C). Also, intrathecal injection of β-FNA, but not nor-BNI or normal saline (NS), markedly reversed the effect of 2 Hz EA on the mechanical thresholds (Figure 1B and C).
Figure 1.

Time course of the effect of β-FNA on RTX-caused mechanical allodynia. (A) The paradigm for the time course of the experiment. (B) Time course of mechanical threshold in response to to von Frey filaments in VEH, RTX, RTX + β-FNA, EA + NS, EA + β-FNA and sham EA-treated rats. (C) Time course of mechanical threshold in VEH, RTX, RTX+ nor-BNI, EA + NS, EA+ nor-BNI and sham EA treated rats. EA was applied to the left hindlimb of RTX injected rats for 30 min, starting from day 8 to day 42 after RTX injection, once every other day, as shown by arrows. Intrathecal injection of β-FNA was done 30 min before EA, starting from 36 days after RTX injection, once every other day for 4 times, as indicated by arrowhead. The abbreviations used here are normal saline (NS), vehicle (VEH), RTX + NS (RTX), RTX + 2Hz EA + NS (EA + NS), RTX + 2Hz EA + β-FNA (EA + β-FNA), RTX + 2Hz EA+ nor-BNI (EA+ nor-BNI), RTX+ sham EA (sham EA).Data are expressed as means ± SEM (n=10 rats in each group). *P<0.05, compared with VEH group; #P<0.05, compared with sham EA group; +P<0.05, compared with EA + NS group.
Effect of EA and β-FNA on netrin-1 protein expression in spinal dorsal horn neurons
Netrin-1 is a neuronal guidance factor, expressed in the spinal cord of adult rats23,45,46 and participates in RTX-induced allodynia and myelinated primary afferent nerve sprouting from lamina III-V into lamina II.41 Our previous study has found that intraperitoneal injection of RTX causes abnormal sprouting in the spinal dorsal horn and mechanical allodynia.33 EA may reverse this effect via μ-opioid receptors. To test this hypothesis, we intrathecally injected β-FNA at 30 min before EA treatment and analyzed the expression of Netrin-1 in the dorsal horn of spinal cord by western blotting. RTX markedly increased the protein level of Netrin-1 compared with the VEH group (Figure 2A). EA at 2 Hz markedly decreased the protein level of Netrin-1 compared with the sham EA group. EA plus intrathecal β-FNA injection markedly increased the protein level of Netrin-1 compared with the EA + NS group (Figure 2). However, RTX with intrathecal injection of β-FNA did not affect the Netrin-1 level compared to the RTX group. These results suggest that EA reduces the Netrin-1 protein level in the spinal dorsal horn of RTX rats via MORs.
Figure 2.
Effects of EA and β-FNA on Netrin-1 protein levels in the spinal dorsal horn of RTX rats. (A) Representative gel image shows the protein expression of Netrin-1 in the spinal dorsal horn from VEH-, RTX-, RTX + β-FNA-, EA + NS-, EA + β-FNA- and sham EA-groups of rats. B-actin was used as a loading control. (B) Summary data show the protein levels of Netrin-1 in VEH-, RTX-, RTX + β-FNA-, EA + NS-, EA + β-FNA- and sham EA-treatment. The abbreviations used here are normal saline (NS), vehicle (VEH), RTX + NS (RTX), RTX + 2Hz EA + NS (EA + NS), RTX + 2Hz EA + β-FNA (EA + β-FNA), RTX+ sham EA (sham EA). Data are expressed as means ± SEM (n=6 rats per group). *P<0.05, compared with VEH group; #P<0.05, compared with sham EA group; +P<0.05, compared with EA + NS group.
Effect of EA and β-FNA on DCC protein expression in spinal dorsal horn neurons
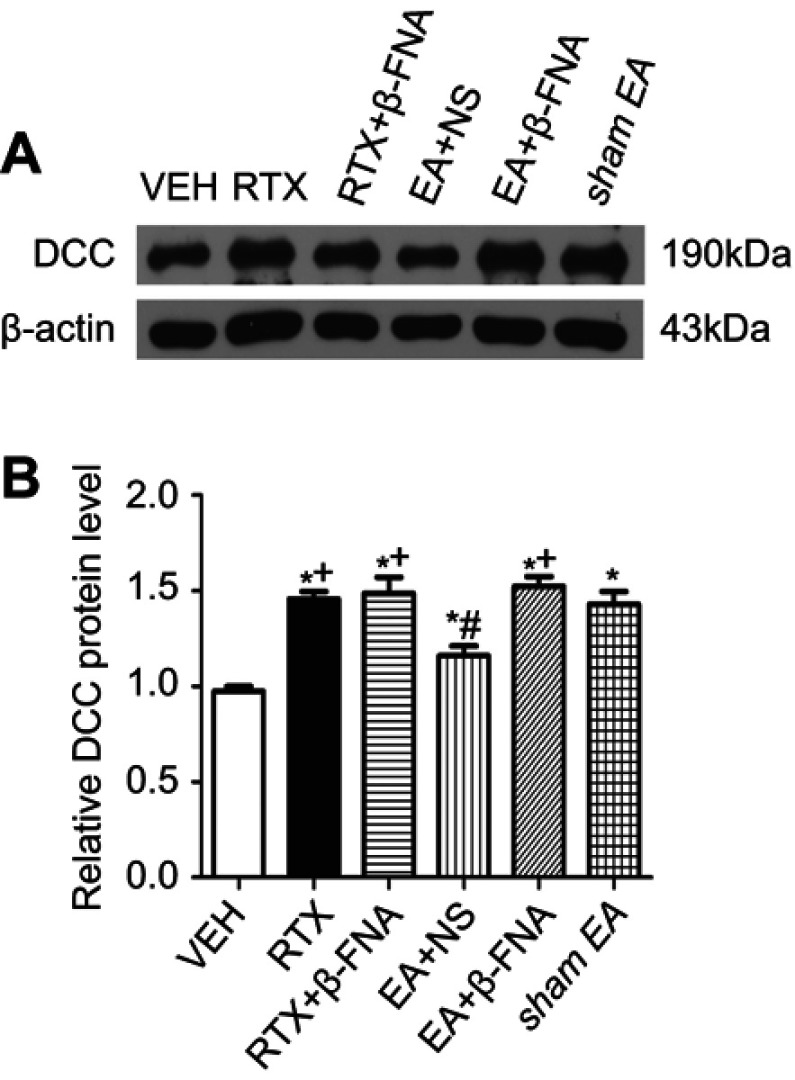
DCC is a receptor of Netrin-1 and mediates the Netrin-1 effect on sensory nerve fibers.12,14,15,47 We determined whether EA and β-FNA can alter the DCC protein level of induced by RTX using western blotting. The DCC level in the RTX group was markedly higher than that in the VEH group, and treatment with 2 Hz EA markedly reduced DCC level compared with the sham EA group. EA with intrathecal injection of β-FNA markedly increased the DCC protein level compared with the EA + NS group (Figure 3). However, RTX with intrathecal injection of β-FNA did not affect the expression of DCC compared with the RTX group.
Figure 3.
Effects of EA and β-FNA on DCC protein levels in the spinal dorsal horn of RTX rats. (A) Representative gel image shows the protein expression of DCC in the spinal dorsal horn obtained from VEH-, RTX-, RTX + β-FNA-, EA + NS-, EA + β-FNA- and sham EA-groups. B-actin was used as a loading control. (B) Summary data show the protein level of DCC in VEH-, RTX-, RTX + β-FNA-, EA + NS-, EA + β-FNA- and sham EA-treated groups. The abbreviations used are normal saline (NS), vehicle (VEH), RTX + NS (RTX), RTX + 2Hz EA + NS (EA + NS), RTX + 2Hz EA + β-FNA (EA + β-FNA), RTX+ sham EA (sham EA). Data are expressed as means ± SEM (n=6 rats per group). *P<0.05, compared with VEH group; #P<0.05, compared with sham EA group; +P<0.05, compared with EA + NS group.
Effect of EA and β-FNA on UNC5H2 protein expression in spinal dorsal horn neurons
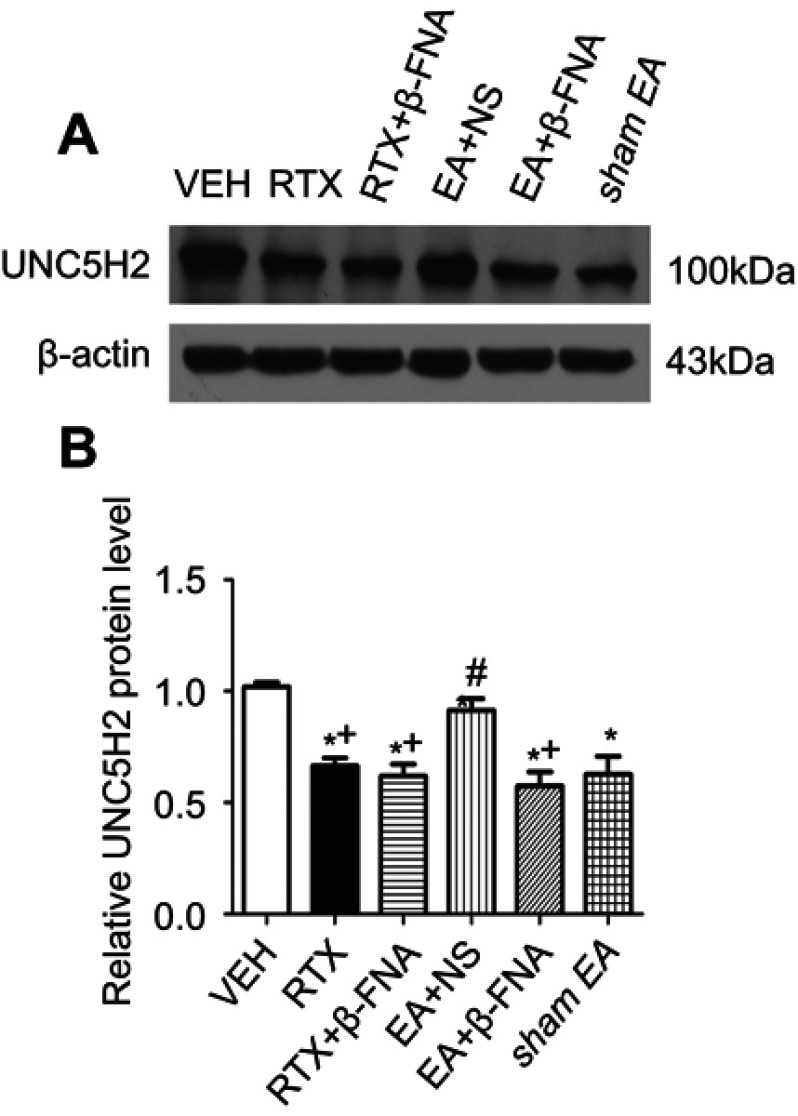
UNC5H2 receptor is highly expressed in the spinal cord of adult rats.23,45,46 We determined whether EA and β-FNA affect the expression of UNC5H2 induced by RTX in the spinal dorsal horn using western blotting. The protein level of UNC5H2 in the RTX group was markedly lower than that in the VEH group, and 2 Hz EA markedly increased its expression level. B-FNA markedly reduced the UNC5H2 protein level compared with the EA + NS group, but did not affect the expression of UNC5H2 compared with the RTX group (Figure 4). These results suggest that EA treatment increases the expression of UNC5H2 in the spinal dorsal horn via MORs.
Figure 4.
Effects of EA and β-FNA on UNC5H2 protein levels in the spinal dorsal horn of RTX rats. (A) Representative gel image shows the protein expression of UNC5H2 in the spinal dorsal horn from VEH-, RTX-, RTX + β-FNA-, EA + NS-, EA + β-FNA- and sham EA-treated rats. B-actin was used as a loading control. (B) Summary data show protein level of UNC5H2 in the VEH-, RTX-, RTX + β-FNA-, EA + NS-, EA + β-FNA- and sham EA-treated groups. The abbreviations used here are normal saline (NS), vehicle (VEH), RTX + NS (RTX), RTX + 2Hz EA + NS (EA + NS), RTX + 2Hz EA + β-FNA (EA + β-FNA), RTX+ sham EA (sham EA). Data are expressed as means ± SEM (n=7 rats per group). *P<0.05, compared with VEH group; #P<0.05, compared with sham EA group; +P<0.05, compared with EA + NS group .
Effect of β-FNA on RTX-induced sprouting of myelinated afferent fibers n the lamina II of spinal dorsal horn
By using the nerve fiber tracer method with cholera toxin B subunit (CTB), we determined the effect of β-FNA on the RTX-induced myelinated nerve fiber sprouting into the lamina II in the spinal cord dorsal horn. In the VEH group, CTB-labeled myelinated nerve fibers were abundant in the III-V layer but not in the I-II layer (Figure 5Aa and B). After RTX administration, CTB-labeled nerve fiber endings were more centrally distributed in the spinal dorsal horn. Compared to the VEH group, the CTB-labeled area of myelinated nerve fibers in lamina II was markedly increased in the RTX group (Figure 5Aa,b and B). Treatment with 2 Hz EA markedly reduced the extension of the CTB-labeled myelinated nerve fibers in lamina II compared with sham EA group (Figure 5Ad,f and B). However, in RTX + β-FNA group, the labeled area of myelinated fibers in lamina II is not different from the RTX group (Figure 5Ab,c and B). These results were consisted with our previous results.4 In addition, compared with EA + NS group, the labeled area of myelinated fibers in lamina II in EA + β-FNA group were markedly increased (Figure 5Ad,e and B). These data suggest that EA inhibits RTX-induced myelinated primary afferent nerve fiber sprouting into the spinal cord lamina II via MORs.
Figure 5.
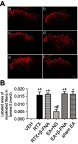
Effect of β-FNA and EA on RTX-caused myelinated primary afferent nerve sprouting into the spinal lamina II. (A) Representative images show CTB-labelled myelinated primary afferent nerve fibers in the spinal dorsal horn of VEH (a), RTX (b), RTX + β-FNA(c), EA + NS (d), EA + β-FNA (e) and sham EA (f) groups. Scale bar, 100 μm. Dotted line shows the partition of laminae II and III in the spinal dorsal horn. Asterisks represent the myelinated primary afferent fiber sprouting into lamina II. (B) Summary data display the area of CTB-labeled afferent terminals into lamina II in different groups. The abbreviations used are normal saline (NS), vehicle (VEH), RTX + NS (RTX), RTX + 2Hz EA + NS (EA + NS), RTX + 2Hz EA + β-FNA (EA + β-FNA), RTX+ sham EA (sham EA). Data are expressed as means ± SEM (n=6 rats/group). *P<0.05, compared with VEH group; #P<0.05, compared with sham EA group; +P<0.05, compared with EA+NS group.
Effect of morphine on the expression of netrin-1 induced by RTX in SH-SY5Y cells
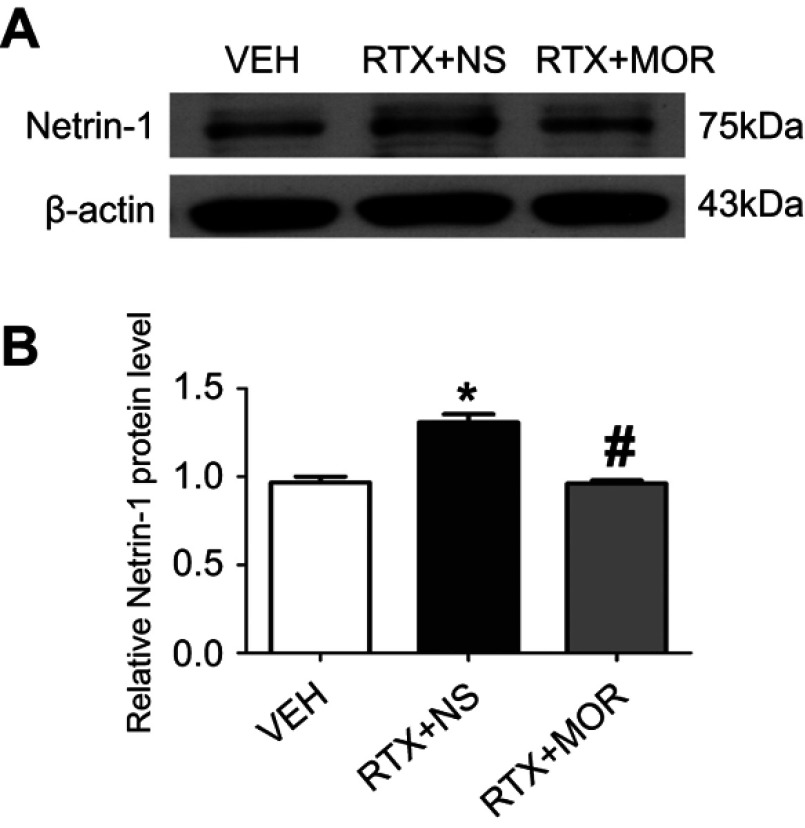
Previous studies have shown that EA increases endogenous opioid peptide levels.48 To determine opioids inhibit the Netrin-1 expression level, morphine was used to simulate the effect of EA. Using human neuroblastoma SH-SY5Y cells, we found that incubation with RTX (80 nM) for 24 hr markedly increased Netrin-1 expression level. Incubation of morphine (1 μM) for 24 hr prior to treatment with RTX markedly reversed the increase of Netrin-1 induced by RTX (Figure 6). The morphine effect is consistent with that of EA in reducing the expression of Netrin-1 through μ-opioid receptors.
Figure 6.
Effect of morphine on Netrin-1 protein levels in SH-SY5Y cells. (A) Representative gel images show the protein expression of Netrin-1 in the SH-SY5Y cells obtained from VEH-, RTX + NS-, and RTX + MOR groups. B-actin was used as a loading control. (B) Summary data show the protein level of Netrin-1 in VEH-, RTX + NS and RTX + MOR groups. The abbreviations used are normal saline (NS), vehicle (VEH), RTX + MOR (RTX + morphine). Data are expressed as means ± SEM. *P<0.05, compared with VEH group; #P<0.05, compared with RTX + NS group (n=6 per group).
Discussion
In this study, we found that EA applied to GB34 and GB30 markedly decreased the tactile allodynia induced by RTX and the myelinated primary afferent nerve sprouting in the spinal dorsal horn caused by RTX injection, also inhibited the Netrin-1and DCC protein level and increased the UNC5H2 protein level in the spinal dorsal horn of RTX-treated rats. Furthermore, the μ-opioid receptors antagonist β-FNA reversed the above effects of EA. In addition, morphine reduced the expression level of RTX-induced up-regulation of Netrin-1 in SH-SY5Y cells. Our results suggest that EA decreases Netrin-1-mediated neuropathic pain and myelinated primary afferent nerve sprouting through μ-opioid receptors.
We used a rat PHN model induced by RTX to investigate the analgesic mechanism of EA and the role of the axon guidance factor Netrin–1 in afferent nerve sprouting. In previous studies, we have found that extensive damage of afferents nerve fibers caused by RTX, which is an ultrapotent agonist of transient receptor potential vanilloid 1 (TRPV1). RTX impairs thermal sensitivity and induces tactile allodynia, which is similar to the clinical symptoms in PHN patients.4,41 Our previous study has indicated that RTX up-regulated the Netrin-1 protein expression in the dorsal horn of spinal cord in RTX-treated rats. Silencing of Netrin-1 remarkable decreased mechanical hyperalgesia and the afferent nerve fiber sprouting into the lamina II of the spinal dorsal horn.41 Netrin-1 thus contributes to the myelinated afferent nerve fiber sprouting into the superficial spinal cord.
Different-frequency EA plus herbal-moxa roll moxibustion is effective in relieving PHN pain in patients.2,3 Our previous study has indicated that EA for 5 weeks can remarkably inhibit myelinated afferent nerve fiber sprouting and improve mechanical allodynia.4 In this study, we found that EA decreased the protein levels of Netrin-1 and its receptor DCC and enhanced the protein expression of UNC5H2. DCC mediates the action of promoting the growth and elongation of sensory nerve fibers,12,14–16 whereas UNC5H2 mediates the function of Netrin-1 in repelling axons, inhibiting the growth and extension of sensory nerve fibers.18,21,22 EA may prevent myelinated afferent nerve fiber sprouting by weakening the effect of Netrin-1 and DCC–mediated attracting axons. In addition, intrathecal injection of the μ-opioid receptor antagonist β-FNA attenuated the EA effect on allodynia and nerve fiber sprouting. This result is consistent with a previous report that opioid receptors are involved in the analgesia effect of EA.29
EA may produce analgesia by promoting the release of endogenous opioid peptides.48 We found that β-FNA can reverse the effect of EA in promoting the protein expression of UNC5H2, inhibiting the protein expression of Netrin-1and DCC in the dorsal horn of spinal cord, and alleviating the myelinated afferent nerve sprouting into the superficial dorsal horn in RTX-treated rats. Anesthetics such as isoflurane inhibit the collapse of growth cone but does not disturb the branch induced by Netrin-1 in the dissociated culture.49 For this reason, in vitro experiment, we used morphine to simulate the effect of EA. A previous study showed that intrathecal injection of morphine or DAMGO significantly increased the withdrawal threshold in the RTX-group.50 In addition, intraperitoneal injection of morphine significantly increased the antinociceptive effect of systemic morphine in RTX-treated rats.50 EA may inhibit the protein expression of Netrin-1 through enhancing the release of opioid peptides and activating μ-opioid receptors at the spinal cord level,51,52 thus inhibiting the protein expression of Netrin-1and DCC in the dorsal horn of spinal cord.
In conclusion, we have demonstrated the critical role of μ-opioid receptor in the effect of EA on decreasing Netrin-1-mediated myelinated afferent nerve fiber sprouting and mechanical allodynia in a PHN model. By activation of μ-opioid receptors, EA downregulates the protein level of DCC and Netrin-1and changes a growth-permissive environment in the spinal dorsal horn into an inhibitory environment by increasing UNC5H2 expression. Netrin-1 may be a target for the EA effect on reducing primary afferent nerve sprouting and allodynia in PHN and other neuropathic pain conditions. This new information improves our understanding of the analgesic mechanism of acupuncture and its clinical use in treating various painful conditions.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No.81804187), the Natural Science Foundation of Jiangsu Province (No.BK20160548), the Major Social Development Project of Zhenjiang city (No.SH2016032)and Doctor startup Fund Program (No. JDFYRC-2015005).
Abbreviation list
B-FNA, beta-funaltrexamine; CTB, cholera toxin B-subunit; DCC, deleted in colorectal cancer; DMEM, dulbecco’s modified eagle medium; EA, electroacupuncture; EDTA, Ethylene Diamine Tetraacetic Acid; FBS, fetal bovine serum; GB30, huantiao; GB34, yanglingquan; MOR, μ-opioid receptors; NS, normal saline; nor-BNI, nor binaltorphimine dihydrochloride; PBS, phosphate buffer; PHN, postherpetic neuralgia; PVDF, polyvinylidene fluoride; RIPA, radio immunoprecipitation assay lysis buffer; RTX, resiniferatoxin; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; SH-SY5Y cells, Human neuroblastoma cell lines; TBS, tris-buffered saline; VEH, vehicle.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Philip A, Thakur R. Post herpetic neuralgia. J Palliat Med. 2011;14(6):765–773. doi: 10.1089/jpm.2011.9685 [DOI] [PubMed] [Google Scholar]
- 2.Grachev Iu V, Kukushkin ML, Sudarikov AP, Zhuravlev VF, Gerasimenko M. Clinical course and treatment of herpetic trigeminal ganglionic neuropathy. Zh Nevrol Psikhiatr Im S S Korsakova. 1998;98(11):4–8. [PubMed] [Google Scholar]
- 3.Wang CY, Fang JQ. [Analysis on therapeutic effect of variable-frequency electroacupuncture combined with herbal-moxa moxibustion for post-zoster neuralgia]. Zhen Ci Yan Jiu. 2012;37(1):64–66. [PubMed] [Google Scholar]
- 4.Wu CH, Lv ZT, Zhao Y, et al. Electroacupuncture improves thermal and mechanical sensitivities in a rat model of postherpetic neuralgia. Mol Pain. 2013;9:18. doi: 10.1186/1744-8069-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards LJ, Koester SE, Tuttle R, O’Leary DD. Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J Neurosci. 1997;17(7):2445–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serafini T, Colamarino SA, Leonardo ED, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87(6):1001–1014. [DOI] [PubMed] [Google Scholar]
- 7.Manitt C, Colicos MA, Thompson KM, Rousselle E, Peterson AC, Kennedy TE. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21(11):3911–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Xu J, Gong J, Shen H, Wang X. Expression of netrin-1 and its receptors, deleted in colorectal cancer and uncoordinated locomotion-5 homolog B, in rat brain following focal cerebral ischemia reperfusion injury. Neural Regen Res. 2013;8(1):64–69. doi: 10.3969/j.issn.1673-5374.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu N, Huang H, Lin F, et al. Effects of treadmill exercise on the expression of netrin-1 and its receptors in rat brain after cerebral ischemia. Neuroscience. 2011;194:349–358. doi: 10.1016/j.neuroscience.2011.07.037 [DOI] [PubMed] [Google Scholar]
- 10.Fearon ER, Cho KR, Nigro JM, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247(4938):49–56. [DOI] [PubMed] [Google Scholar]
- 11.Mehlen P, Fearon ER. Role of the dependence receptor DCC in colorectal cancer pathogenesis. J Clin Oncol. 2004;22(16):3420–3428. doi: 10.1200/JCO.2004.02.019 [DOI] [PubMed] [Google Scholar]
- 12.Keino-Masu K, Masu M, Hinck L, et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87(2):175–185. [DOI] [PubMed] [Google Scholar]
- 13.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138(11):2153–2169. doi: 10.1242/dev.044529 [DOI] [PubMed] [Google Scholar]
- 14.Keeling SL, Gad JM, Cooper HM. Mouse Neogenin, a DCC-like molecule, has four splice variants and is expressed widely in the adult mouse and during embryogenesis. Oncogene. 1997;15(6):691–700. doi: 10.1038/sj.onc.1201225 [DOI] [PubMed] [Google Scholar]
- 15.Meyerhardt JA, Look AT, Bigner SH, Fearon ER. Identification and characterization of neogenin, a DCC-related gene. Oncogene. 1997;14(10):1129–1136. doi: 10.1038/sj.onc.1200935 [DOI] [PubMed] [Google Scholar]
- 16.Fazeli A, Dickinson SL, Hermiston ML, et al. Phenotype of mice lacking functional Deleted In Colorectal Cancer (DCC) gene. Nature. 1997;386(6627):796–804. doi: 10.1038/386796a0 [DOI] [PubMed] [Google Scholar]
- 17.Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386(6627):833–838. doi: 10.1038/386833a0 [DOI] [PubMed] [Google Scholar]
- 18.Engelkamp D. Cloning of three mouse Unc5 genes and their expression patterns at mid-gestation. Mech Dev. 2002;118(1–2):191–197. [DOI] [PubMed] [Google Scholar]
- 19.Thiebault K, Mazelin L, Pays L, et al. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc Natl Acad Sci U S A. 2003;100(7):4173–4178. doi: 10.1073/pnas.0738063100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386(6627):838–842. doi: 10.1038/386838a0 [DOI] [PubMed] [Google Scholar]
- 21.Komatsuzaki K, Dalvin S, Kinane TB. Modulation of G(ialpha(2)) signaling by the axonal guidance molecule UNC5H2. Biochem Biophys Res Commun. 2002;297(4):898–905. [DOI] [PubMed] [Google Scholar]
- 22.Williams ME, Wu SC, McKenna WL, Hinck L. Surface expression of the netrin receptor UNC5H1 is regulated through a protein kinase C-interacting protein/protein kinase-dependent mechanism. J Neurosci. 2003;23(36):11279–11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manitt C, Thompson KM, Kennedy TE. Developmental shift in expression of netrin receptors in the rat spinal cord: predominance of UNC-5 homologues in adulthood. J Neurosci Res. 2004;77(5):690–700. doi: 10.1002/jnr.20199 [DOI] [PubMed] [Google Scholar]
- 24.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120(11):3779–3787. doi: 10.1172/JCI43766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein C, Machelska H. Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacol Rev. 2011;63(4):860–881. doi: 10.1124/pr.110.003145 [DOI] [PubMed] [Google Scholar]
- 26.Matthes HW, Maldonado R, Simonin F, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383(6603):819–823. doi: 10.1038/383819a0 [DOI] [PubMed] [Google Scholar]
- 27.Kieffer BL, Evans CJ. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology. 2009;56 Suppl 1(Suppl 1):205–212. doi: 10.1016/j.neuropharm.2008.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ugur M, Derouiche L, Massotte D. Heteromerization modulates mu opioid receptor functional properties in vivo. Front Pharmacol. 2018;9:1240. doi: 10.3389/fphar.2018.01240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang RX, Wang L, Liu B, et al. Mu opioid receptor-containing neurons mediate electroacupuncture-produced anti-hyperalgesia in rats with hind paw inflammation. Brain Res. 2005;1048(1–2):235–240. doi: 10.1016/j.brainres.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 30.Ward SJ, Fries DS, Larson DL, Portoghese PS, Takemori AE. Opioid receptor binding characteristics of the non-equilibrium mu antagonist, beta-funaltrexamine (beta-FNA). Eur J Pharmacol. 1985;107(3):323–330. [DOI] [PubMed] [Google Scholar]
- 31.Ward SJ, Portoghese PS, Takemori AE. Pharmacological characterization in vivo of the novel opiate, beta-funaltrexamine. J Pharmacol Exp Ther. 1982;220(3):494–498. [PubMed] [Google Scholar]
- 32.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. [DOI] [PubMed] [Google Scholar]
- 33.Pan HL, Khan GM, Alloway KD, Chen SR. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci. 2003;23(7):2911–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience. 2002;114(2):291–299. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Min BI, Na HS, Park DS. Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: mediation by spinal opioid receptors. Brain Res. 2004;998(2):230–236. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Zhang J, Li F, et al. Endogenous anandamide and cannabinoid receptor-2 contribute to electroacupuncture analgesia in rats. J Pain. 2009;10(7):732–739. doi: 10.1016/j.jpain.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 37.Rx Z, Lao L, Wang L, et al. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020(1–2):12–17. doi: 10.1016/j.brainres.2004.05.067 [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Zhang Y, Dai J, Yang J, Gang S. Electroacupuncture (EA) modulates the expression of NMDA receptors in primary sensory neurons in relation to hyperalgesia in rats. Brain Res. 2006;1120(1):46–53. doi: 10.1016/j.brainres.2006.08.077 [DOI] [PubMed] [Google Scholar]
- 39.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- 40.Yuan XC, Wu CH, Gao F, et al. Activation and expression of mu-calpain in dorsal root contributes to RTX-induced mechanical allodynia. Mol Pain. 2017;13:1744806917719169. doi: 10.1177/1744806917719169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu CH, Yuan XC, Gao F, et al. Netrin-1 contributes to myelinated afferent fiber sprouting and neuropathic pain. Mol Neurobiol. 2016;53(8):5640–5651. doi: 10.1007/s12035-015-9482-x [DOI] [PubMed] [Google Scholar]
- 42.Bakkum BW, Henderson CN, Hong SP, Cramer GD. Preliminary morphological evidence that vertebral hypomobility induces synaptic plasticity in the spinal cord. J Manipulative Physiol Ther. 2007;30(5):336–342. doi: 10.1016/j.jmpt.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 43.Mao-Ying QL, Cui KM, Liu Q, et al. Stage-dependent analgesia of electro-acupuncture in a mouse model of cutaneous cancer pain. Eur J Pain. 2006;10(8):689–694. doi: 10.1016/j.ejpain.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Liu SB, Li Q, Wang H, Wang YQ, Mao-Ying QL. Downregulation of spinal G protein-coupled kinase 2 abolished the antiallodynic effect of electroacupuncture. Evid Based Complement Alternat Med. 2015;2015:848603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manitt C, Wang D, Kennedy TE, Howland DR. Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. J Neurosci Res. 2006;84(8):1808–1820. doi: 10.1002/jnr.21070 [DOI] [PubMed] [Google Scholar]
- 46.Wehrle R, Camand E, Chedotal A, Sotelo C, Dusart I. Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. Eur J Neurosci. 2005;22(9):2134–2144. doi: 10.1111/j.1460-9568.2005.04419.x [DOI] [PubMed] [Google Scholar]
- 47.Van Caneghem P, Lapiere CM. [Influence of x-rays on depolymerization of lathyric collagen fibers reconstituted in vitro]. C R Seances Soc Biol Fil. 1975;169(1):242–245. [PubMed] [Google Scholar]
- 48.Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26(1):17–22. [DOI] [PubMed] [Google Scholar]
- 49.Mintz CD, Barrett KM, Smith SC, Benson DL, Harrison NL. Anesthetics interfere with axon guidance in developing mouse neocortical neurons in vitro via a gamma-aminobutyric acid type A receptor mechanism. Anesthesiology. 2013;118(4):825–833. doi: 10.1097/ALN.0b013e318287b850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen SR, Pan HL. Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol. 2006;95(5):3086–3096. doi: 10.1152/jn.01343.2005 [DOI] [PubMed] [Google Scholar]
- 51.Sora I, Takahashi N, Funada M, et al. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997;94(4):1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim HY, Wang J, Lee I, Kim HK, Chung K, Chung JM. Electroacupuncture suppresses capsaicin-induced secondary hyperalgesia through an endogenous spinal opioid mechanism. Pain. 2009;145(3):332–340. doi: 10.1016/j.pain.2009.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]