Abstract
OBJECTIVE
Nephrotic syndrome (NS) is a kidney disease known to adversely impact health-related quality of life (HRQOL). Patient-reported outcome (PRO) measures are commonly used to characterize HRQOL and the patient disease experience. This study aims to improve the interpretability and clinical utility of the Patient-Reported Outcomes Measurement Information System® (PROMIS®) by identifying distinct meaningful HRQOL profiles in children and adults with NS.
METHODS
Patients were from two prospective NS cohort studies (PROMIS-II: 121 children; NEPTUNE: 40 children and 219 adults) with data from six PROMIS domains. Latent Profile Analysis was used to identify subgroups of patients based on PROMIS score patterns. A 3-step analysis of latent profile predictors was used to determine how clinical parameters predicted HRQOL profile membership.
RESULTS
We identified three HRQOL profiles (Good, Average, and Poor) with strong indicators of membership classification (entropy>0.86). Complete proteinuria remission, reduction in symptoms, and shorter disease duration, were significant predictors of better HRQOL profile membership.
CONCLUSIONS
Patients with NS can be classified by HRQOL into clinically meaningful categories. Integrating this approach into clinic may help in the identification of individuals with poor HRQOL will help clinicians better manage their symptoms and researchers study the causes and possible interventions for these patients. PROMIS HRQOL profiles were reproducible in replication cohorts.
INTRODUCTION
Nephrotic syndrome (NS) is characterized by relapsing and remitting episodes of proteinuria, hypoalbuminemia, and edema (Gipson et al., 2009). Disease complications and therapies may include an increased risk for severe infections, sleep disturbances, mood swings, as well as frequent hospitalizations, and end-stage kidney disease (Hodson & Alexander, 2008; Hoyer, Vester, & Ulrich Becker, 2008). Both the natural disease course and immunosuppressive treatments of NS are related to poorer health-related quality of life (HRQOL) (Perrone, Coons, Cavanaugh, Finkelstein, & Meyer, 2013), i.e., the impact that a disease has on mental, physical and/or social well-being (D. F. Cella, 1995). This is especially important given that HRQOL is increasingly recognized as a patient centered marker of clinical efficacy. (Beitz, 1999; Beitz, Gnecco, & Justice, 1996; FDA.GOV)
Patient-reported outcomes (PRO) measures have become an accepted method to assess HRQOL and now PROs serve as accepted study endpoints for clinical trials (Murthy & Wood, 2015). In addition to the clinical measures of disease activity, PRO assessment can assist in understanding the patient experience and function to improve clinical care and trials (Gipson et al., 2013). Although PROs have become accepted endpoints, continuous PRO scores are difficult to interpret in clinical practice and more work is needed to define specific categories of high and low HRQOL to focus care and research.
The Patient-Reported Outcomes Measurement Information System® (PROMIS®) provides a dynamic assessment of HRQOL for both adults and children (www.HealthMeasures.net). PROMIS was designed for use across clinical diseases, and is comprised of a broad range of measures of mental, physical and social aspects of HRQOL. Furthermore, a significant amount of work has been done to validate PROMIS pediatric measures in children with kidney disease. Specifically, PROMIS measures are sensitive to markers of disease activity in children with NS and chronic kidney disease (CKD) in cross-sectional investigations (Buckner et al., 2014; Selewski et al., 2014; Selewski et al., 2015). Convergent validity has previously been established through significant correlations between the PROMIS and PedsQL instruments (Selewski, et al., 2015). PROMIS domains have demonstrated both high test-retest reliability (0.727-0.883) and Cronbach’s alpha (0.906-0.991) (Bartlett et al., 2015). These domains also show measurement invariance by age and gender (Kim, Chung, Amtmann, Revicki, & Cook, 2013). Despite this work, there remain knowledge gaps that limit full adoption of these measures in clinical and research arenas.
An important step in increasing the clinical utilization of PRO instruments is to improve the interpretability of PRO measures by providing clinicians and researchers the ability to identify NS patients at risk for poor HRQOL or those who would potentially benefit from intervention. For example, PRO measurements such as PROMIS generally yield numeric values, often between 0 and 100, indicating their HRQOL. These continuous values may be practical for researchers, but could be difficult for clinicians to interpret and guide patient care. For example, researchers can use these detailed PRO scores in statistical models measuring within-patient change in PRO over time. However, it is harder for clinicians to act on these continuous numeric values without clear categories of poor or good PRO, and clinicians may benefit from tools that summarize PRO scores into clinically distinct categories.
In order for PROs to be clinically meaningful, it is important to have clear methods of determining if a patient has good, average, or poor HRQOL. To date there is limited data on establishing meaningful clinical profiles for PROMIS measures in other clinical populations. For example, clinical profiles have been developed for PROMIS in rheumatology (Nagaraja et al., 2018) and juvenile idiopathic arthritis (Morgan et al., 2017). This work has employed a benchmarking approach to establish clinically meaningful cut-points (i.e., an adaptation of a bookmark standard-setting procedure in which clinical vignettes are reviewed by clinical experts in order to derive consensus-driven cut scores).
An alternative method for developing clinically meaningful profiles is to use a latent profile analysis (LPA). Briefly, LPA is a type of latent measurement model which uses observed continuous variables to assign patients into categories of an unobserved, or “latent”, variable (Heinen, 1993; Lazarsfeld & Henry, (1968).; B. O. Muthen, 2002). These “latent categories” are referred to as profiles. Previous studies have used LPA to identify patterns of HRQOL scores and the association between categories of HRQOL severity and co-occurring symptoms and functional impairments (Buckner, et al., 2014; Fenton, Grey, Armstrong, McCarroll, & Von Gruenigen, 2013). An advantage of using an LPA is that it categorizes patients into discrete groups based on their common HRQOL experiences that can be further evaluated to determine what clinical and patient characteristics are associated with each group (HRQOL profile). LPA has been successfully used with PROMIS measures in children with cancer and adults with chronic pelvic pain (Buckner, et al., 2014; Fenton, Grey, Armstrong, et al., 2013; Fenton, Grey, Reichenbach, McCarroll, & Von Gruenigen, 2013). In each of these analyses researchers identified clinical characteristics that placed patients at risk of membership in Poor HRQOL profiles. LPA has not yet been performed in patients with NS and represents an important step in the clinical deployment of the PROMIS instrument.
The primary goal of this study is to use an LPA to define clinically distinct HRQOL profiles in patients with NS. In clinical practice, this technique could be used to summarize information from multiple PRO instruments and provide a summary HRQOL to the clinician. We hypothesized that meaningful HRQOL profiles can be identified for NS and that profile membership will change over time in response to changes in disease status.
METHODS
Study design and participants
The data in this analysis come from two longitudinal cohorts of NS patients, PROMIS-II and NEPTUNE (Gadegbeku et al., 2013; Selewski, et al., 2015). Each participating center of the two cohort studies obtained Institutional Review Board (IRB) approval, and informed consent was obtained from all individual participants included in the studies (see Author Note for complete list of sites). In both PROMIS II and NEPTUNE, patients were consented at their pediatric nephrology visit. The consent was thoroughly explained in language such that the child was able to understand. All questions from the parent(s) and child were answered, and children over the age of 10 were asked to provide either verbal or written assent to participate in the study, depending on their ability level. In the PROMIS II study, we also employed an online consent, where parents were asked to check either “I accept” or “I do not accept”. The consent was then verbally verified when the study coordinator called to confirm eligibility. In the case of online consent, a waiver of assent was obtained from the University of Michigan IRB.
The PROMIS-II study enrolled children age 8-17 years old with active NS across 14 sites in the United States of America (USA) and Canada (12 from the Midwest Pediatric Nephrology Consortium and 2 additional participating centers in the USA). The inclusion criteria and study design have been previously described (Gadegbeku, et al., 2013; Selewski, et al., 2015). Patients completed three visits with PROMIS assessments, one at baseline and two at follow-up visits within a year after their initial visit. The first follow-up visit occurred either once the patient reached remission of proteinuria or three months after their initial visit if remission was not achieved. The second follow-up visit occurred 12 months after the initial visit.
NEPTUNE patients were enrolled at the time of first clinically indicated kidney biopsy. The details of the NEPTUNE study have been previously published (Gadegbeku, et al., 2013). After biopsy, patients returned for a baseline visit and then follow-up visits every four months for the first year and every six months thereafter. The NEPTUNE study sample selected for this analysis included children age 8-17 years and for the adult sample ages ≥ 18 years with at least three PROMIS assessments collected.
The PROMIS-II cohort was used to initially identify and develop the latent profiles. Independent profile development was then performed on the NEPTUNE pediatric cohort and adult cohorts.
PRO Measures
We examined the following child and adult PROMIS domains: Fatigue, Pain Interference, Anxiety and Depression. Each of these four domains used different questions for children and adults. In children, we also examined Mobility, and Peer Relationships, and in adults we examined Physical Function and Satisfaction with Social Roles and Activities. PROMIS measures were limited to the PROMIS domains available and validated in children with nephrotic syndrome at the launch of the PROMIS-II and NEPTUNE studies. As there were little data on PRO in adults with nephrotic syndrome at that time, similar and comparable PROMIS adult domains were selected among existing instruments We used the PROMIS Assessment Center online interface with a computer adaptive test design (www.assessmentcenter.net) to administer questionnaires and collect patient-reported data. Each item was asked in reference to “In the past 7 days.” Responses included five options ranging from “never” to “almost always” in the majority of domains and from “with no trouble” to “not able to do” for the physical functioning measures. Each domain generates a T-score as an aggregate score of multiple questionnaire items with a mean of 50 and a standard deviation of 10. For the adult measures, T-scores are relative to the general population while calibration samples included both members of the general population and from clinical settings (D. Cella et al., 2010; DeWitt et al., 2011; Hinds et al., 2013; Irwin et al., 2012; Liu et al., 2010; Rothrock et al., 2010; Varni et al., 2010). A higher score indicates higher levels of the domain consistent with the measure’s name. For example, a higher Physical Functioning score indicates better physical functioning (e.g., better outcome) while a higher Anxiety score indicates higher anxiety (e.g., worse outcome).
Outcomes
HRQOL latent profiles were the outcomes of interest. We hypothesized predictors of HRQOL profile membership included proteinuria remission status, edema, number of symptoms, number of medications, health care utilization, and co-existing conditions.
Statistical analysis
LPA profile selection
LPA (Heinen, 1993; Lazarsfeld & Henry, (1968).; B. O. Muthen, 2002), a posterior membership probability modeling approach, was used to identify categorical subgroups of NS patients based on their scores on six PROMIS HRQOL domains. First, the optimal number of latent profiles was selected by running a series of models with an increasing number of latent profiles. The optimal model was selected using a combination of empirical model fit indices and profile interpretability using the following selection indices: Akaike, Bayesian, and adjusted Bayesian information criterion; Lo-Mendell-Rubin likelihood ratio test; adjusted Lo-Mendell-Rubin likelihood ratio test; and the bootstrap likelihood ratio test (Lo, Mendell, & Rubin, 2001; McCutcheon, 1987). The quality of profile assignment was examined using entropy statistic and the average posterior probability of profile membership stratified by assignment to the most likely latent profile. Model selection was done first using the PROMIS-II cohort and then model selection was repeated separately for the NEPTUNE pediatric and adult samples for confirmation. NEPTUNE samples were not used to formally validate or confirm the exact model used in the PROMIS sample and are instead used as an independent derivation cohort. Additionally, we explored using an LPA with a combined PROMIS-II and NEPTUNE pediatric sample.
Predictors of profile membership
Longitudinal predictors of profile membership were tested in the PROMIS-II cohort using the number of profiles favored from the exploratory, cross-sectional LPA analyses. LPA analyses were repeated on all 314 study visits across 121 patients. Equality restrictions were imposed on the means and variances of each profile to ensure all profiles were defined identically at each study visit and to match the baseline LPA assessment. All multinomial logistic regressions of class membership used a 3-step approach for latent profile predictors. In this approach, the latent categorical variable is used as the distal outcome. First, the most likely profile membership of each assessment are obtained from the posterior probabilities of the LPA along with the uncertainty rate. For example, these models do not assign participants to profiles with complete certainty, and the specific outcome is the probability the observation belongs to each of the profiles. 3-step LPA first classifies observations and then analyzes the most likely membership together with covariates and accounts for measurement error in the most likely assigned latent profile (Asparouhov & Muthen, 2014; Vermunt, 2010).
The following covariates were tested: disease duration (≥6 months vs. <6 months), steroid exposur, other immunosuppressive therapy exposure (treated vs. untreated at the time of study visit), obesity (based on BMI percentile and BMI at the time of study visit for children and adults respectively), edema (at the time of study visit assessed by clinician assessment), number of symptoms (a list of reportable symptoms are included in Appendix 1), number of other medical conditions (condition list included in Appendix 1), number of medications, health care utilization in the six months before the study visit (any emergency room visits or hospitalization in the 6 months prior to study visit), proteinuria remission status, urine protein: creatinine ratio (UP:C), estimated glomerular filtration rate (eGFR), and serum albumin. eGFR was estimated using the CKD-Epi equation for adults age ≥18 years and the CKiD formula for children and represents overall kidney function with lower number representing poorer function (Levey et al., 2009; Schwartz et al., 2009). Any factor that was a significant predictor of profile membership in the univariate analysis at α=0.25 was included for multivariable backward selection. After fitting the multivariable model, the variable with the highest non-significant p-value at α=0.05 was removed and the model was refit. This process was repeated until all remaining variables were significant at α=0.05.
LPAs were conducted using Mplus 8.0, while descriptive statistics and data visualizations were completed using SAS 9.4 (L. K. Muthen & Muthen, 1998-2012; SASInstituteInc, 2014).
RESULTS
Baseline characteristics of each cohort are described in Table 1. The PROMIS-II cohort included 56 incident (disease duration at baseline <30 days) and 65 prevalent (disease duration at baseline ≥30 days) pediatric cases. The NEPTUNE cohort included 87 incident and 172 prevalent cases. The PROMIS-II cohort was more likely to have edema compared to the NEPTUNE pediatric (74% vs. 35%, p<0.01) and adult cohorts (74% vs. 56%, p<0.01). Additionally, the PROMIS-II cohort showed higher degrees of proteinuria than the NEPTUNE pediatric cohort (median urine protein: creatinine ratio [g/g] 5.2 [IQR=2.2, 8.6] vs. 2.6 [1.6, 7.3], p=0.02).
Table 1.
Baseline demographic and clinical characteristics of the PROMIS-II and NEPTUNE cohorts
| PROMIS-II Cohort N=121 |
NEPTUNE Pediatric Cohort N=40 |
NEPTUNE Adult Cohort N=40 |
|
|---|---|---|---|
| Sex | |||
| Male, n (%) | 79 (65) | 24 (60) | 137 (63) |
| Female, n (%) | 42 (35) | 16 (40) | 82 (37) |
| Age (years) | |||
| 8-12, n (%) | 65 (54) | 9 (23) | 0 (0) |
| 13-17, n (%) | 56 (46) | 31 (77) | 0 (0) |
| ≥18, n (%) | 0 (0) | 0 (0) | 219 (100) |
| Race | |||
| Caucasian, n (%) | 64 (53) | 15 (38) | 132 (60) |
| Black/African American, n (%) | 33 (28) | 20 (50) | 31 (14) |
| Asian, n (%) | 14 (12) | 1 (3) | 39 (18) |
| Other, n (%) | 10 (8) | 4 (10) | 17 (8) |
| Hispanic or Latino ethnicity, n (%) | 10 (8) | 7 (18) | 27 (12) |
| Disease duration | |||
| Incident (<30 days), n (%) | 56 (46) | 15 (38) | 72 (33) |
| Prevalent (≥30 days), n (%) | 65 (54) | 25 (62) | 147 (67) |
| Urine protein: creatinine ratio (g/g), median (IQR) | 5.2 (2.2, 8.6) | 2.6 (1.6, 7.3) | 4.1 (1.9, 6.8) |
| eGFR ml/min/1.73m2, median (IQR) | 110 (83, 134) | 88 (69, 108) | 65 (43, 96) |
| Edema present, n (%) | 90 (74) | 14 (35) | 124 (56) |
| Diagnosis | |||
| Minimal change disease, n (%) | 19 (16) | 12 (30) | 28 (13) |
| Focal segmental glomerulosclerosis, n (%) | 19 (16) | 18 (45) | 69 (32) |
| Membranous nephropathy, n (%) | 1 (1) | 1 (3) | 57 (26) |
| Other glomerular disease, n (%) | 16 (13) | 9 (23) | 65 (30) |
| Nephrotic Syndrome, not otherwise classified*, n (%) | 66 (55) | 0 (0) | 0 (0) |
Counts presented as n (%), Continuous variable presented as Median (Interquartile range)
In children, nephrotic syndrome is commonly treated based on clinical signs in the absence of a kidney biopsy informed diagnosis
PROMIS Scores
Baseline PROMIS HRQOL score distributions are shown for the different cohorts in Figure 1. While the median domain scores for most domains were near the standardized mean of 50, there was a wide range of scores crossing 1 (+/- 10 points) and 2 (+/- 20 points) standard deviations distance from the standardized mean.
Figure 1.
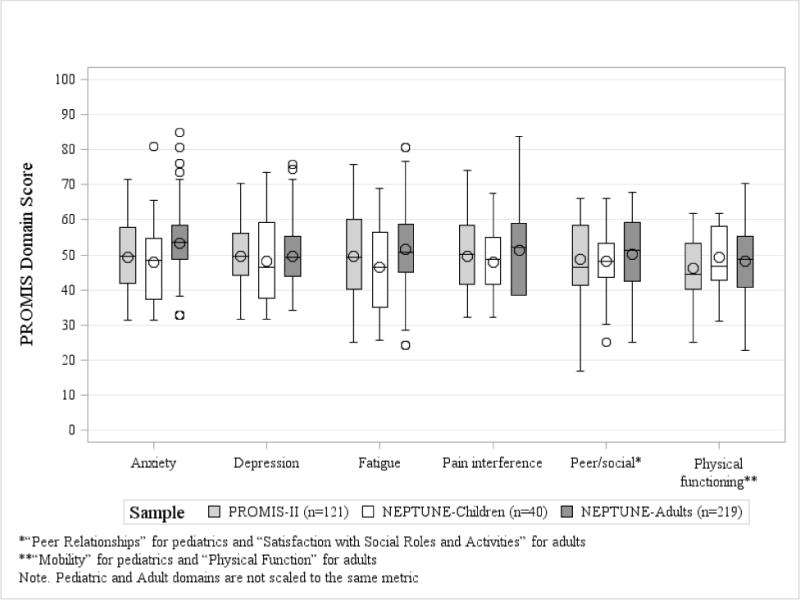
Baseline PROMIS domain scores the PROMIS-II and NEPTUNE cohorts
Latent Profile Selection
Model selection indices used to determine the proper number of latent profiles from each cohort are presented in Table 2. Better model fit is indicated by lower akaike, Bayesian, and adjusted Bayesian information criteria, higher entropy values, and significant p-values for the Vuong-Lo-Mendell-Rubin, adjusted Vuong-Lo-Mendell-Rubin, and parametric bootstrapped likelihood ratio tests; bolded values in Table 2 indicate better values for each of these characteristics. Four of the seven tests in the PROMIS-II cohort (the Bayesian information criterion, Vuong-Lo-Mendell-Rubin likelihood ratio test, adjusted Vuong-Lo-Mendell-Rubin likelihood ratio test, and entropy statistic) favored the three profile model. Four of the seven tests also favored the three profile model in the NEPTUNE pediatric and adult cohorts as well. While certain tests favored a two or four profile model, the majority of tests across each sample indicated strongest fit with the three profile model. Model interpretability and usefulness also favored a three profile solution which we labeled as “Good”, “Average”, and “Poor” HRQOL.
Table 2.
Model selection indices comparing 1, 2, 3 and 4 PRO latent profile models
| AIC | BIC | ABIC | LMR LR | ALMR LR | BLRT | Entropy | |
|---|---|---|---|---|---|---|---|
| PROMIS-II Cohort (n=121)
| |||||||
| Number of profiles | |||||||
| 1-Profile solution | 5494 | 5528 | 5490 | --- | --- | --- | --- |
| 2-Profile solution | 5287 | 5340 | 5280 | <0.01 | <0.01 | <0.01 | 0.87 |
| 3-Profile solution | 5237 | 5310 | 5228 | <0.01 | <0.01 | <0.01 | 0.87 |
| 4-Profile solution | 5223 | 5315 | 5211 | 0.62 | 0.63 | <0.01 | 0.85 |
|
| |||||||
| NEPTUNE Pediatric Cohort (n=40)
| |||||||
| Number of profiles | |||||||
| 1-Profile solution | 2490 | 2515 | 2477 | --- | --- | --- | --- |
| 2-Profile solution | 2379 | 2417 | 2357 | <0.01 | <0.01 | <0.01 | 0.91 |
| 3-Profile solution | 2346 | 2398 | 2316 | 0.39 | 0.40 | <0.01 | 0.91 |
| 4-Profile solution | 2337 | 2403 | 2326 | 0.26 | 0.26 | 0.06 | 0.89 |
|
| |||||||
| NEPTUNE Adult Cohort (n=219)
| |||||||
| Number of profiles | |||||||
| 1-Profile solution | 11337 | 11379 | 11341 | --- | --- | --- | --- |
| 2-Profile solution | 10765 | 10832 | 10771 | 0.02 | 0.02 | <0.01 | 0.85 |
| 3-Profile solution | 10548 | 10640 | 10557 | <0.01 | <0.01 | <0.01 | 0.88 |
| 4-Profile solution | 10504 | 10621 | 10566 | 0.67 | 0.68 | <0.01 | 0.86 |
|
| |||||||
| PROMIS-II and NEPTUNE Pediatric Combined Cohort (n=161)
| |||||||
| Number of profiles | |||||||
| 1-Profile solution | 7308 | 7345 | 7307 | --- | --- | --- | --- |
| 2-Profile solution | 6999 | 7057 | 6997 | <0.01 | <0.01 | <0.01 | 0.88 |
| 3-Profile solution | 6919 | 6999 | 6917 | <0.01 | <0.01 | <0.01 | 0.89 |
| 4-Profile solution | 6972 | 7004 | 6899 | 0.35 | 0.36 | <0.01 | 0.84 |
AIC=Akaike information criterion; BIC=Bayesian information criterion; ABIC=Sample-size adjusted Bayesian information criterion; LMR LR= Vuong-Lo-Mendell-Rubin likelihood ratio test; AMR LR= Adjusted Vuong-Lo-Mendell-Rubin likelihood ratio test; BLRT=Parametric bootstrapped likelihood ratio test. Better model fit is indicated by lower akaike, Bayesian, and adjusted Bayesian information criteria, higher entropy values, and significant p-values for the Vuong-Lo-Mendell-Rubin, adjusted Vuong-Lo-Mendell-Rubin, and parametric bootstrapped likelihood ratio tests; bolding indicates better values for each of these characteristics
The mean PROMIS HRQOL scores by each of the three latent profiles in the PROMIS-II cohort are shown in Figure 2. The three profile model clearly sorted patients into the three profiles, Good, Average, and Poor levels of HRQOL with the Average profile being the most populous (20%, 49%, and 31% of PROMIS-II participants, respectively). The Average profile had mean domain scores close to the reference value of 50, while the Poor and Good profiles tended to have mean scores shifted by at least 1 standard deviation from the average profile (score shift was in the expected direction for all PROMIS measures).
Figure 2. PROMIS domain scores of three latent profiles from by cohort. Mean and 95% confidence intervals are plotted.
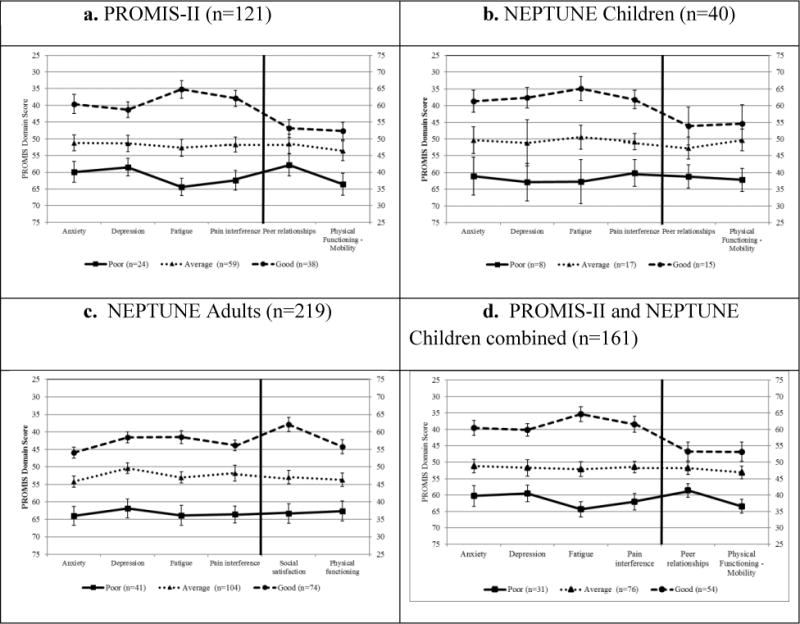
*Anxiety, Depression, Fatigue, and Pain interference are plotted using the left Y-axis; Peer relationships and Physical functioning – mobility are plotted using the right Y-axis
Following identification of the latent profiles, the findings were replicated in independent pediatric and adult NS samples. The pediatric and adult NEPTUNE cohorts also favored the three profile solution. Entropy statistics were equally strong in the pediatric (0.91) and adult (0.88) NEPTUNE samples compared to the PROMIS-II cohort (0.87). Posterior assignment probabilities for each sample are presented in Table 3. All probabilities were ≥0.90 and indicated very strong membership classification. For example, the posterior classification probabilities indicated that a PROMIS-II patient was assigned to the Good HRQOL profile, with 96% certainty according to the model.
Table 3.
Latent profile assignment probabilities using the three-category profile LPA models in the PROMIS-II and NEPTUNE cohorts
| PROMIS-II Cohort (n=121) | |||
|---|---|---|---|
| Poor | Average | Good | |
| Poor (n=24) | 0.90 | 0.10 | 0.00 |
| Average(n=59) | 0.04 | 0.94 | 0.02 |
| Good (n=38) | 0.00 | 0.04 | 0.96 |
|
| |||
| NEPTUNE Pediatric Cohort (n=40) | |||
| Poor | Average | Good | |
|
| |||
| Poor (n=8) | 0.91 | 0.09 | 0.00 |
| Average (n=17) | 0.01 | 0.98 | 0.01 |
| Good (n=15) | 0.00 | 0.01 | 0.99 |
|
| |||
| NEPTUNE Adult Cohort (n=219) | |||
| Poor | Average | Good | |
|
| |||
| Poor (n=41) | 0.99 | 0.01 | 0.00 |
| Average (n=104) | 0.04 | 0.93 | 0.03 |
| Good (n=74) | 0.00 | 0.04 | 0.96 |
|
| |||
| PROMIS-II and NEPTUNE Pediatric Combined Cohort (n=161) | |||
|
| |||
| Poor | Average | Good | |
| Poor (n=31) | 0.92 | 0.08 | 0.00 |
| Average (n=76) | 0.04 | 0.94 | 0.02 |
| Good (n=54) | 0.00 | 0.04 | 0.96 |
Note. Bolding indicates agreement with prediction and assignment in each sample.
The PROMIS-II and NEPTUNE cohorts all showed strong separation among profiles by symptom severity (Figure 2a-2d). Both pediatric cohorts showed very similar mean domain scores (Figures 2a and 2b) as did the combined pediatric cohort (Figure 2d). Peer relationships did not discriminate profile membership as strongly as other domains for children. This is seen by the overlapping 95% confidence intervals for the PROMIS-II and pediatric NEPTUNE samples. However, among the adult NEPTUNE sample, there was strong separation in mean scores across all domains between the three latent profiles (Figure 2c).
Predicting Profile Membership
The unadjusted and adjusted multinomial logistic regression results predicting latent profile membership are shown in Table 4. Significant unadjusted (i.e., univariate) predictors of profile membership included disease duration, edema, obesity, health care utilization, number of symptoms, number of medical conditions, number of medications, proteinuria remission, and serum albumin. The final adjusted model determined that disease duration, number of symptoms, and proteinuria remission were each independently associated with profile membership. A higher number of symptoms was associated with a decreased likelihood of belonging to the Good HRQOL (OR=0.7 [95%CI=0.6, 0.8]) profile compared to Poor HRQOL profile. When a patient reached complete remission, they were more likely to be in the Good than Poor profile (OR=5.6 [95%CI=2.0, 15.3]). Prevalent patients were also less likely to belong to the Good compared to the Poor profile (OR=0.3 [95%CI=0.1, 0.7]).
Table 4.
Longitudinal predictors of latent profile membership in the PROMIS-II from unadjusted and adjusted multinomial logistic regression models
| Unadjusted Models | Final Multivariable Adjusted Model | |||||
|---|---|---|---|---|---|---|
| ORGood | ORAverage | ORPoor | ORGood | ORAverage | ORPoor | |
| Prevalent | 0.3 (0.1, 0.8) | 0.9 (0.4, 2.2) | REF | 0.3 (0.1, 0.7) | 0.9 (0.4, 2.1) | REF |
| Steroid exposure | 1.1 (0.5, 2.4) | 0.9 (0.4, 2.0) | REF | |||
| Other therapy | 0.1 (0.1, 0.8) | 1.1 (0.3, 4.0) | REF | |||
| Obese | 0.4 (0.1, 0.9) | 0.5 (0.2, 1.3) | REF | |||
| Edema | 0.3 (0.2, 0.8) | 0.9 (0.4, 1.9) | REF | |||
| Number of symptoms | 0.6 (0.5, 0.7) | 0.8 (0.7, 0.9) | REF | 0.7 (0.6, 0.8) | 0.9 (0.7, 1.0) | REF |
| Number of medical conditions | 0.7 (0.6, 0.9) | 0.8 (0.7, 0.9) | REF | |||
| Number of medications | 0.7 (0.6, 0.9) | 0.8 (0.7, 0.9) | REF | |||
| ER/hospital visits | 0.5 (0.2, 1.1) | 0.4 (0.2, 0.9) | REF | |||
| Proteinuria remission | 7.8 (3.0, 20.7) | 2.8 (1.1, 7.0) | REF | 5.6 (2.0, 15.3) | 2.6 (1.0, 6.7) | REF |
| eGFR ml/min/1.73m2 (per 30) | 1.1 (0.7, 1.7) | 0.9 (0.5, 1.5) | REF | |||
| UP:C g/g (per 1) | 0.9 (0.9, 1.0) | 1.0 (0.9, 1.0) | REF | |||
| Serum Albumin g/dL (per 1) | 1.5 (1.0, 2.1) | 1.0 (0.7, 1.5) | REF | |||
Analysis was limited to 121 individuals (314 observations); Maternal education=maternal education≥ college degree; obese=BMI percentile>95th percentile; ER/hospital visits=any emergency room or hospital visits 6th months before the baseline visit or before the previous visit for follow-up visits; UP:C=urine protein:creatinine ratio
Note. Bolding indicates p<0.05
Change in Profile Membership Over Time
Transitions between profile membership over time for the 121 PROMIS-II patients with complete PRO data at all visits are described in Table 5. Between visit 1 and 2 (median of 3 months apart), 57% of the 107 patients with visits 1 and 2 were estimated to remain in the same profile while the remaining 43% transitioned. Patients in the Poor profile were more likely to transition: 80% (28/35) of those in Good profile at visit 1 remained in that profile compared to 48% (25/52) of Average remaining in Average, and 40% (8/20) of Poor remaining in the Poor profile. A similar trend was found for transitions between visit 2 and 3.
Table 5.
Transitions between latent profiles among PROMIS-II patients. Class counts and proportions based on most likely class pattern.
| Visit 2 Profile | ||||
|---|---|---|---|---|
| Visit 1 Profile | Poor (n=12) n (row %) |
Average (n=36) n (row %) |
Good (n=59) n (row %) |
Missed Visit (n=14) n (row %) |
| Poor (n=24) | 8 (33) | 4 (17) | 8 (33) | 4 (17) |
| Average(n=59) | 4 (7) | 25 (42) | 23 (39) | 7 (12) |
| Good (n=38) | 0 (0) | 7 (18) | 28 (74) | 3 (8) |
|
| ||||
| Visit 3 Profile | ||||
| Visit 2 Profile | Poor (n=9) n row (%) |
Average (n=31) n (row %) |
Good (n=46) n (row %) |
Missed Visit (n=35) n (row %) |
|
| ||||
| Poor (n=12) | 1 (8) | 5 (42) | 5 (42) | 1 (8) |
| Average(n=36) | 9 (25) | 14 (39) | 1 (3) | 12 (33) |
| Good (n=59) | 33 (56) | 12 (20) | 2 (3) | 12 (20) |
| Missed Visit (n=14) | 3 (7) | 0 (0) | 1 (7) | 10 (71) |
Changes in latent profile were not always associated with significant changes in all six PROMIS domain scores. For example, one patient who transitioned from Poor to Good had large improvements in Anxiety (−20.2), Fatigue (−27.8) and Mobility (18.3), and minimal change in Depression (−1.0) and Pain Interference (0.3), whereas another patient had large improvements in Depression (−17.1) and Pain Interference (−19.9), but minimal change in Mobility (2.4).
DISCUSSION
PRO measures provide clinicians and researchers the ability to understand and track the impact that chronic conditions, such as NS, and their treatments, have on patients’ HRQOL. NS represents a chronic condition in which a significant amount of work has been done to validate and apply PRO measures (Gipson, et al., 2013; Gipson et al., 2011; Selewski, et al., 2014; Selewski, et al., 2015). Adoption of PRO measures into clinical or research arenas as endpoints requires both validation and the ability to clinically interpret HRQOL scores. Our study shows that clinically meaningful subgroups of patients can be identified using HRQOL data from PROMIS. Specifically, three distinct profile groups were supported by the data and included patients with NS with “Poor,” “Average,” or “Good” HRQOL. These subgroups were predicted by disease characteristics including proteinuria remission, disease duration, and symptom burden.
In chronic disease management, clinicians recognize that each patient’s perception and experience of disease is unique and, as a result, identifying disease characteristics that impact PRO in chronic disease can be challenging. Designing processes for reporting results and identifying aids to facilitate score interpretation are integral steps to the incorporation of PRO measures into clinical care (Snyder et al., 2012). We envision these latent profiles being used in practice to stratify patients into clinically actionable groups. For example, patients in the Poor HRQOL profile might be stronger candidates for targeted interventions and require additional resources. Nephrologists already measure and interpret clinically meaningful strata of other laboratory markers of disease activity and progression such as eGFR, serum albumin, and UP:C when treating patients with NS, and we expect patient-reported markers may reach the same level of utility.
Some practices have incorporated other HRQOL measures into electronic medical health records (Carlson, Waller, Groff, Zhong, & Bultz, 2012; Clark, Bardwell, Arsenault, DeTeresa, & Loscalzo, 2009; DeWalt et al., 2015; Wagner et al., 2015). HRQOL has been identified as a significant predictor of hospital readmission, (Abernethy et al., 2009; Johnston et al., 2015; M. Kopp, Kaur, & Hanson, 2015), and LPA may prove useful to improve the integration of HRQOL results into a simple indicator for use in clinical practice.
This study offers two potential applications for clinicians interested in identifying patients with poor HRQOL. The first is minor: our final multivariable model indicated that number of symptoms, disease duration, and proteinuria remission were the strongest independent predictors of HRQOL, and that other tested covariates, such as steroid use or obesity, were not significant after adjustment for number of symptoms. While unsurprising, this finding emphasizes the importance of symptom management in HRQOL. A broader p of these results are the implications of using this type of latent variable model in clinic. In our experience, nephrologists struggle to use several different HRQOL measurements on continuous uncertain scales. However, if all patients within a clinic completed the same domain assessments, an LPA could be fit on all data available to that practice and inform the clinician if this patient has “Good”, “Average”, or “Poor” HRQOL. The domain scores themselves would of course be available as well. This approach could be improved by pooling data from many nephrology clinics, and perhaps the study data collected in these studies. This would give the clinician the option of creating the profiles referent to all pooled data, or just the patients seen in their clinic.
While this study offers important contributions to the NS literature utilizing PROs, it is also important to acknowledge several study limitations. First, the adult and pediatric PROMIS physical functioning and social functioning item banks are not perfectly aligned in their content and structure. While the Pain Interference, Fatigue, Depression and Anxiety domains are intended to measure the same construct with slightly different questions for pediatric and adult participants, the differences in the pediatric Peer relationships domain and the adult Social functioning domains reflect differences in content greater than differently worded questions. Consequently, the NEPTUNE pediatric and adult samples were given slightly different domains (i.e., Peer relationships vs. Social functioning, and Physical functioning – mobility vs. Physical functioning). This difference did not prevent the successful segregation of PRO profiles, and both the adult and pediatric domains favored a three profile model. Second, although parent proxy forms are now available for measuring HRQOL in children under 8 years old, these were not at the time this study was implemented. The PROMIS domains used were also limited to those available at the time the studies began. Additionally, this study was limited to English speaking participants as the PROMIS instruments had not yet been translated and validated for other languages at the time of data collection.
With respect to the methods used, latent variable analyses such as latent profile models, traditionally require a minimum of 200 subjects or 10 subjects per observed variable to guarantee stability in the model estimates (Bollen, 1989; Tein, Coxe, & Cham, 2013). Both of our pediatric samples do not meet the 200 subject requirement, and while our assignment probabilities for these models appear strong (≥0.9), we still highlight this as a limitation to the generalizability and stability of our results. Another methodological problem related to our sample size was our inability to power and fit latent transition analysis (Lanza & Collins, 2008) given there were only 121 PROMIS-II patients, and ony 82 PROMIS-II patients with complete data at all study visits.
Despite these limitations, this study also exhibits several strengths. First, this analysis included an independent comparison samples including both children and adults. Second, the multi-center participation in PROMIS-II and NEPTUNE supports the generalizability of findings to the US and Canada. Finally, this is one of the only longitudinal analyses on PRO in patients with NS.
PROMIS can be used to reliably classify both children and adults with NS into three distinct clinically interpretable and meaningful HRQOL profiles. In addition, HRQOL profile membership was responsive to markers of NS disease status. Taken together, these findings provide support for the clinical utility of PROs (i.e., PROMIS) in NS and represent a critical step in the mobilization of HRQOL measures into the clinical and research realms.
Supplementary Material
Acknowledgments
The investigators are indebted to the children and families who graciously participated in these studies.
PROMIS® was funded with cooperative agreements from the National Institutes of Health (NIH) Common Fund Initiative (Northwestern University, PI: David Cella, PhD, U54AR057951, U01AR052177; Northwestern University, PI: Richard C. Gershon, PhD, U54AR057943; American Institutes for Research, PI: Susan (San) D. Keller, PhD, U54AR057926; State University of New York, Stony Brook, PIs: Joan E. Broderick, PhD and Arthur A. Stone, PhD, U01AR057948, U01AR052170; University of Washington, Seattle, PIs: Heidi M. Crane, MD, MPH, Paul K. Crane, MD, MPH, and Donald L. Patrick, PhD, U01AR057954; University of Washington, Seattle, PI: Dagmar Amtmann, PhD, U01AR052171; University of North Carolina, Chapel Hill, PI: Harry A. Guess, MD, PhD (deceased), Darren A. DeWalt, MD, MPH, U01AR052181; Children’s Hospital of Philadelphia, PI: Christopher B. Forrest, MD, PhD, U01AR057956; Stanford University, PI: James F. Fries, MD, U01AR052158; Boston University, PIs: Alan Jette, PT, PhD, Stephen M. Haley, PhD (deceased), and David Scott Tulsky, PhD (University of Michigan, Ann Arbor), U01AR057929; University of California, Los Angeles, PIs: Dinesh Khanna, MD (University of Michigan, Ann Arbor) and Brennan Spiegel, MD, MSHS, U01AR057936; University of Pittsburgh, PI: Paul A. Pilkonis, PhD, U01AR052155; Georgetown University, PIs: Carol. M. Moinpour, PhD (Fred Hutchinson Cancer Research Center, Seattle) and Arnold L. Potosky, PhD, U01AR057971; Children’s Hospital Medical Center, Cincinnati, PI: Esi M. Morgan DeWitt, MD, MSCE, U01AR057940; University of Maryland, Baltimore, PI: Lisa M. Shulman, MD, U01AR057967; and Duke University, PI: Kevin P. Weinfurt, PhD, U01AR052186). NIH Science Officers on this project have included Deborah Ader, PhD, Vanessa Ameen, MD (deceased), Susan Czajkowski, PhD, Basil Eldadah, MD, PhD, Lawrence Fine, MD, DrPH, Lawrence Fox, MD, PhD, Lynne Haverkos, MD, MPH, Thomas Hilton, PhD, Laura Lee Johnson, PhD, Michael Kozak, PhD, Peter Lyster, PhD, Donald Mattison, MD, Claudia Moy, PhD, Louis Quatrano, PhD, Bryce Reeve, PhD, William Riley, PhD, Peter Scheidt, MD, Ashley Wilder Smith, PhD, MPH, Susana Serrate-Sztein, MD, William Phillip Tonkins, DrPH, Ellen Werner, PhD, Tisha Wiley, PhD, and James Witter, MD, PhD. The contents of this article uses data developed under PROMIS. These contents do not necessarily represent an endorsement by the US Federal Government or PROMIS. See www.nihpromis.org for additional information on the PROMIS® initiative.
The Nephrotic Syndrome Study Network Consortium (NEPTUNE); U54-DK-083912, is a part of the National Center for Advancing Translational Sciences (NCATS) Rare Disease Clinical Research Network (RDCRN), supported through a collaboration between the Office of Rare Diseases Research (ORDR), NCATS, and the National Institute of Diabetes, Digestive, and Kidney Diseases. RDCRN is an initiative of ORDR, NCATS. Additional funding and/or programmatic support for this project has also been provided by the University of Michigan, NephCure Kidney International, and the Halpin Foundation.
References
- Abernethy AP, Herndon JE, 2nd, Wheeler JL, Day JM, Hood L, Patwardhan M, Lyerly HK. Feasibility and acceptability to patients of a longitudinal system for evaluating cancer-related symptoms and quality of life: pilot study of an e/Tablet data-collection system in academic oncology. J Pain Symptom Manage. 2009;37(6):1027–1038. doi: 10.1016/j.jpainsymman.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Asparouhov T, Muthen B. Auxiliary Variables in Mixture Modeling: Three-Step Approaches Using Mplus. Structural Equation Modeling-a Multidisciplinary Journal. 2014;21(3):329–341. doi: 10.1080/10705511.2014.915181. [DOI] [Google Scholar]
- Bartlett SJ, Orbai AM, Duncan T, DeLeon E, Ruffing V, Clegg-Smith K, Bingham CO., 3rd Reliability and Validity of Selected PROMIS Measures in People with Rheumatoid Arthritis. PLoS One. 2015;10(9):e0138543. doi: 10.1371/journal.pone.0138543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz J. Quality-of-life end points in oncology drug trials. Oncology (Williston Park) 1999;13(10):1439–1442. discussion 1442 passim. [PubMed] [Google Scholar]
- Beitz J, Gnecco C, Justice R. Quality-of-life end points in cancer clinical trials: the U.S. Food and Drug Administration perspective. J Natl Cancer Inst Monogr. 1996;(20):7–9. [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Buckner TW, Wang J, DeWalt DA, Jacobs S, Reeve BB, Hinds PS. Patterns of symptoms and functional impairments in children with cancer. Pediatr Blood Cancer. 2014;61(7):1282–1288. doi: 10.1002/pbc.25029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Waller A, Groff SL, Zhong L, Bultz BD. Online screening for distress, the 6th vital sign, in newly diagnosed oncology outpatients: randomised controlled trial of computerised vs personalised triage. Br J Cancer. 2012;107(4):617–625. doi: 10.1038/bjc.2012.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Hays R. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. doi: 10.1016/j.jclinepi.2010.04.011S0895-4356(10)00173-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella DF. Measuring quality of life in palliative care. Seminars in oncology. 1995;22(2 Suppl 3):73–81. [PubMed] [Google Scholar]
- Clark K, Bardwell WA, Arsenault T, DeTeresa R, Loscalzo M. Implementing touch-screen technology to enhance recognition of distress. Psychooncology. 2009;18(8):822–830. doi: 10.1002/pon.1509. [DOI] [PubMed] [Google Scholar]
- DeWalt DA, Gross HE, Gipson DS, Selewski DT, DeWitt EM, Dampier CD, Varni JW. PROMIS((R)) pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Qual Life Res. 2015;24(9):2195–2208. doi: 10.1007/s11136-015-0953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt EM, Stucky BD, Thissen D, Irwin DE, Langer M, Varni JW, Dewalt DA. Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: built using item response theory. J Clin Epidemiol. 2011;64(7):794–804. doi: 10.1016/j.jclinepi.2010.10.012. doi: 10.1016/j.jclinepi.2010.10.012S0895-4356(10)00364-1 [pii]FDA.GOV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton BW, Grey SF, Armstrong A, McCarroll M, Von Gruenigen V. Latent profile analysis of pelvic floor muscle pain in patients with chronic pelvic pain. Minerva Ginecol. 2013;65(1):69–78. [PubMed] [Google Scholar]
- Fenton BW, Grey SF, Reichenbach M, McCarroll M, Von Gruenigen V. Phenotyping chronic pelvic pain based on latent class modeling of physical examination. Pain Res Treat. 2013;2013:891301. doi: 10.1155/2013/891301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Kretzler M. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83(4):749–756. doi: 10.1038/ki.2012.428. doi: 10.1038/ki.2012.428ki2012428 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson DS, Massengill SF, Yao L, Nagaraj S, Smoyer WE, Mahan JD, Greenbaum LA. Management of childhood onset nephrotic syndrome. Pediatrics. 2009;124(2):747–757. doi: 10.1542/peds.2008-1559. doi: 10.1542/peds.2008-1559peds.2008-1559 [pii] [DOI] [PubMed] [Google Scholar]
- Gipson DS, Selewski DT, Massengill SF, Wickman L, Messer KL, Herreshoff E, DeWalt DA. Gaining the PROMIS perspective from children with nephrotic syndrome: a Midwest pediatric nephrology consortium study. Health Qual Life Outcomes. 2013;11:30. doi: 10.1186/1477-7525-11-30. doi: 10.1186/1477-7525-11-301477-7525-11-30 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, Friedman AL. Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int. 2011;79(6):678–685. doi: 10.1038/ki.2010.485. doi: 10.1038/ki.2010.485ki2010485 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen T. Discrete latent variable models. Tilburg, The Netherlands: Tilburg University Press; 1993. [Google Scholar]
- Hinds PS, Nuss SL, Ruccione KS, Withycombe JS, Jacobs S, DeLuca H, DeWalt DA. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer. 2013;60(3):402–408. doi: 10.1002/pbc.24233. [DOI] [PubMed] [Google Scholar]
- Hodson EM, Alexander SI. Evaluation and management of steroid-sensitive nephrotic syndrome. Curr Opin Pediatr. 2008;20(2):145–150. doi: 10.1097/MOP.0b013e3282f4307a. doi: 10.1097/MOP.0b013e3282f4307a00008480-200804000-00008 [pii] [DOI] [PubMed] [Google Scholar]
- Hoyer PF, Vester U, Ulrich Becker J. Steroid Resistant Nephrotic Syndrome. First. Philadelphia, PA: Mosby, Inc; 2008. [Google Scholar]
- Irwin DE, Stucky BD, Langer MM, Thissen D, DeWitt EM, Lai JS, DeWalt DA. PROMIS Pediatric Anger Scale: an item response theory analysis. Qual Life Res. 2012;21(4):697–706. doi: 10.1007/s11136-011-9969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston KL, Lawrence SM, Dodds NE, Yu L, Daley DC, Pilkonis PA. Evaluating PROMIS instruments and methods for patient-centered outcomes research: Patient and provider voices in a substance use treatment setting. Qual Life Res. 2015 doi: 10.1007/s11136-015-1131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chung H, Amtmann D, Revicki DA, Cook KF. Measurement invariance of the PROMIS pain interference item bank across community and clinical samples. Qual Life Res. 2013;22(3):501–507. doi: 10.1007/s11136-012-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Collins LM. A new SAS procedure for latent transition analysis: Transitions in dating and sexual risk behavior. Developmental Psychology. 2008;44(2):446–456. doi: 10.1037/0012-1649.44.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarsfeld PF, Henry NW. Latent structure analysis. Boston: Houghton Mifflin; 1968. [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. doi: 150/9/604 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Cella D, Gershon R, Shen J, Morales LS, Riley W, Hays RD. Representativeness of the Patient-Reported Outcomes Measurement Information System Internet panel. J Clin Epidemiol. 2010;63(11):1169–1178. doi: 10.1016/j.jclinepi.2009.11.021. doi: 10.1016/j.jclinepi.2009.11.021S0895-4356(10)00172-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YT, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. doi: 10.1093/biomet/88.3.767. [DOI] [Google Scholar]
- M KH, Kopp L, Kaur N, Hanson BJ. A facile method for simultaneously measuring neuronal cell viability and neurite outgrowth. Curr Chem Genom Transl Med. 2015;9:6–16. doi: 10.2174/2213988501509010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon A. Latent class analysis. Newbury Park: Sage Publications; 1987. [Google Scholar]
- Morgan EM, Mara CA, Huang B, Barnett K, Carle AC, Farrell JE, Cook KF. Establishing clinical meaning and defining important differences for Patient-Reported Outcomes Measurement Information System (PROMIS((R))) measures in juvenile idiopathic arthritis using standard setting with patients, parents, and providers. Qual Life Res. 2017;26(3):565–586. doi: 10.1007/s11136-016-1468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy HS, Wood WA. The Value of Patient Reported Outcomes and Other Patient-Generated Health Data in Clinical Hematology. Curr Hematol Malig Rep. 2015;10(3):213–224. doi: 10.1007/s11899-015-0261-6. [DOI] [PubMed] [Google Scholar]
- Muthen BO. Beyond SEM: General Latent Variable Modeling. Behaviormetrika. 2002;29(1):81–117. [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide (Seventh Edition ed) Los Angeles, CA: Muthén & Muthén; 1998-2012. [Google Scholar]
- Nagaraja V, Mara C, Khanna PP, Namas R, Young A, Fox DA, Khanna D. Establishing clinical severity for PROMIS((R)) measures in adult patients with rheumatic diseases. Qual Life Res. 2018;27(3):755–764. doi: 10.1007/s11136-017-1709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone RD, Coons SJ, Cavanaugh K, Finkelstein F, Meyer KB. Patient-reported outcomes in clinical trials of CKD-related therapies: report of a symposium sponsored by the national kidney foundation and the U.S. Food and Drug Administration. American Journal of Kidney Diseases. 2013;62(6):1046–1057. doi: 10.1053/j.ajkd.2013.07.004. doi: 10.1053/j.ajkd.2013.07.004S0272-6386(13)01074-3 [pii] [DOI] [PubMed] [Google Scholar]
- Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2010;63(11):1195–1204. doi: 10.1016/j.jclinepi.2010.04.012. doi: 10.1016/j.jclinepi.2010.04.012S0895-4356(10)00174-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASInstituteInc. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc; 2014. [Google Scholar]
- Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. doi: 10.1681/ASN.2008030287ASN.2008030287 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selewski DT, Massengill SF, Troost JP, Wickman L, Messer KL, Herreshoff E, Gipson DS. Gaining the Patient Reported Outcomes Measurement Information System (PROMIS) perspective in chronic kidney disease: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2014;29(12):2347–2356. doi: 10.1007/s00467-014-2858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selewski DT, Troost JP, Massengill SF, Gbadegesin RA, Greenbaum LA, Shatat IF, Gipson DS. The impact of disease duration on quality of life in children with nephrotic syndrome: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2015 doi: 10.1007/s00467-015-3074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY, Santana M. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res. 2012;21(8):1305–1314. doi: 10.1007/s11136-011-0054-x. [DOI] [PubMed] [Google Scholar]
- Tein JY, Coxe S, Cham H. Statistical Power to Detect the Correct Number of Classes in Latent Profile Analysis. Struct Equ Modeling. 2013;20(4):640–657. doi: 10.1080/10705511.2013.824781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW, Stucky BD, Thissen D, Dewitt EM, Irwin DE, Lai JS, Dewalt DA. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J Pain. 2010;11(11):1109–1119. doi: 10.1016/j.jpain.2010.02.005. doi: 10.1016/j.jpain.2010.02.005S1526-5900(10)00326-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt JK. Latent Class Modeling with Covariates: Two Improved Three-Step Approaches. Political Analysis. 2010;18(4):450–469. doi: 10.1093/pan/mpq025. [DOI] [Google Scholar]
- Wagner LI, Schink J, Bass M, Patel S, Diaz MV, Rothrock N, Cella D. Bringing PROMIS to practice: brief and precise symptom screening in ambulatory cancer care. Cancer. 2015;121(6):927–934. doi: 10.1002/cncr.29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


