Abstract
Objective:
Craniopharyngioma survivors experience cognitive deficits that negatively impact quality of life. Aerobic fitness is associated with cognitive benefits in typically developing children and physical exercise promotes recovery following brain injury. Accordingly, we investigated cognitive and neural correlates of aerobic fitness in a sample of craniopharyngioma patients.
Method:
Patients treated for craniopharyngioma [N=104; 10.0±4.6 years; 48% Male] participated in fitness, cognitive and fMRI (n=51) assessments following surgery but prior to proton radiation therapy.
Results:
Patients demonstrated impaired aerobic fitness [peak oxygen uptake (PKVO2) = 23.9±7.1; 41% impaired (i.e., 1.5 SD < normative mean)], motor proficiency [Bruininks-Oseretsky (BOT2) = 38.6±9.0; 28% impaired] and executive functions (e.g., WISC-IV Working Memory Index (WMI) = 96.0±15.3; 11% impaired). PKVO2 correlated with better executive functions (e.g., WISC-IV WMI r=.27, p=.02) and academic performance (WJ-III Calculation r=.24, p=.04). BOT2 correlated with better attention (e.g., CPT-II omissions r=.26, p=.04) and executive functions (e.g., WISC-IV WMI r=.32, p=.01). Areas of robust neural activation during an n-back task included superior parietal lobule, dorsolateral prefrontal cortex, middle and superior frontal gyri (p<.05, corrected). Higher network activation was associated with better working memory task performance and better BOT2 (p<.001).
Conclusions:
Prior to adjuvant therapy, children with craniopharyngioma demonstrate significantly reduced aerobic fitness, motor proficiency and working memory. Better aerobic fitness and motor proficiency are associated with better attention and executive functions, as well as greater activation of a well established working memory network. These findings may help explain differential risk/resiliency with respect to acute cognitive changes that may portend cognitive late effects.
Keywords: pediatric, brain tumor, cognitive late effects, fMRI, acquired brain injury, exercise
Childhood brain tumor survivors are at significant risk for cognitive impairments that are associated with decreased academic and vocational attainment, as well as a reduced quality of life (Castellino, Ullrich, Whelen, & Lange, 2014; Mitby et al., 2003; Mostow, Byrne, Connelly, & Mulvihill, 1991; Mulhern & Butler, 2004; Mulhern, Merchant, Gajjar, Reddick, & Kun, 2004). With a growing survivor population, efforts to improve long-term cognitive outcomes become imperative. Longitudinal trials of children treated for brain tumors reveal declines on global cognitive measures that reflect a failure to learn new skills at the same rate as healthy peers (Mulhern et al., 2004; Palmer et al., 2001). Emerging research suggests impairments in attention, working memory and processing speed may be proximal contributors to these global declines (e.g., Castellino et al., 2014; Krull, Hardy, Kahalley, Schuitema, & Kesler, 2018; Schatz, Kramer, Ablin, & Matthay, 2000; Ullrich & Embry, 2012). Risk factors for cognitive deficits have most consistently included younger age at treatment, longer time since treatment, higher treatment intensity, female gender and complicating medical factors (e.g., stroke or meningitis; Castellino et al., 2014; Krull et al., 2018; Mulhern et al., 2004; Ullrich & Embry, 2012). Even after accounting for these risk factors, there is variability in cognitive outcomes among brain tumor survivors that suggests the presence of protective factors for some. The greater childhood acquired brain injury literature suggests protective factors might include genetic factors (e.g., allelic variants of apolipoprotein E), pre-injury factors (e.g., higher premorbid cognitive ability or better behavioral adjustment) and contextual factors (e.g., higher socioeconomic status or better family functioning; see Yeates, Levin, & Ponsford, 2017).
Craniopharyngioma is a slow growing, extra-axial tumor located in the sella/suprasellar brain region. While craniopharyngiomas are rare, 30–50% are diagnosed during childhood, with a peak onset between 5 and 14 years of age (Muller et al., 2011; Ozyrut et al., 2014). Prognosis is very good with overall survival rates greater than 80% at ten years post diagnosis (Poretti, Grotzer, Ribi, Schonle, & Boltshauser, 2004). While craniopharyngiomas are benign in histology, they can be difficult to manage medically given their neuroanatomical location. Long-term morbidity is associated with tumor and treatment effects on surrounding structures including: endocrinopathies, sleep disturbance and decreased fitness due to hypothalamic/ pituitary damage; visual disturbance due to mass effect on the optic chiasm; and memory impairment due to disruption of Papez circuit including the mammillary bodies (Fournier-Goodnight et al., 2017; Muller et al., 2011; Ozyurt et al., 2014). Of particular relevance to the current study, prior research has shown a significant proportion of children surgically treated for craniopharyngioma have aerobic fitness lower than healthy peers, even when appropriately treated with growth hormone replacement (Piquel et al., 2012). Consensus is lacking regarding the optimal treatment approach for pediatric craniopharyngioma, with some medical teams preferring gross total resection and others preferring limited surgery followed by radiation therapy (Muller, 2017). While surgery and/or radiation therapy are often required for disease control, both are associated with cognitive risk (Poretti et al., 2004). Typically, survivors of childhood craniopharyngioma demonstrate intact intellectual functioning with deficits noted in sustained attention (Fjalldal et al., 2013; Ozyurt et al., 2014), processing speed (Fjalldal et al., 2013; Waber et al., 2006), and learning and memory (Fjalldal et al., 2013; Ondruch, Maryniak, Kropiwnicki, Roszkowski, & Daszkiewicz, 2011; Ozyurt et al., 2014). Poorer performance has been associated with hypothalamic involvement of the tumor (Fjalldal et al., 2013; Ozyurt et al., 2014;), hydrocephalus, cerebrospinal fluid diversion procedures, and younger age at irradiation (Di Pinto, Conklin, Li, & Merchant, 2012).
The medical benefits of aerobic exercise are well established for the general population including decreased risk for metabolic, cardiovascular and metastatic disease (Powell & Blair, 1994). Cognitive benefits of exercise are emerging with evidence to suggest improvements in learning and memory (Winter et al., 2007), delayed age-related memory decline or neurodegenerative disease (Friedland et al., 2001; Laurin, Verreault, Lindsay, MacPherson, & Rockwood, 2001), and promotion of recovery following acquired brain injury (Devine & Zafonte, 2009). Research using murine models has provided clues regarding underlying mechanisms for exercise-related cognitive benefits including neurogenesis, synaptic plasticity, angiogenesis and enhanced neurotransmitter function (Farmer et al., 2004; Chaouloff, 1989; van Praag, Christie, Sejnnowski, & Gage, 1999).
Correlational studies indicate children of higher aerobic fitness outperform lower fit children on cognitive, academic and real world problem solving tasks (Chaddock, Hillman, et al., 2012; Fedewa & Ahn, 2011; Pontifex et al., 2011). Further, higher fit children may have larger regional brain volumes in the basal ganglia (Chaddock, Erickson, Prakash, VanPatter, et al., 2010) and hippocampus (Chaddock, Erickson, Prakash, Kim, et al., 2010), which could mediate the relationship between fitness level and learning. An association in children between aerobic fitness and greater efficiency of neural networks underlying executive functions has also been revealed by fMRI studies (Chaddock, Erickson, et al., 2012; Voss et al., 2011). There are relatively few exercise intervention studies with children, with most targeting obesity and some revealing cognitive benefits (Castelli, Hillman, Hirsch, Hirsch & Drollette, 2011; Davis et al., 2007; Davis et al., 2011; Donnelly et al., 2009). For example, Davis and colleagues conducted a randomized controlled trial of school age children and found an effect of daily exercise on executive function and math achievement. There was also evidence of increased neural activity in bilateral prefrontal cortices in the exercise group compared to controls (Davis et al., 2007; Davis et al., 2011). Multiple studies suggest aerobic exercise may influence executive functions in particular (e.g., Chaddock, Erickson, et al., 2012; Chaddock, Hillman, et al., 2012; Davis et al., 2007; Davis et al., 2011).
In the current study, we evaluated a group of children diagnosed with craniopharyngioma following surgical resection but prior to adjuvant radiation therapy. Our primary goal was to investigate cognitive and neural correlates of aerobic fitness in order to detect differential risk/resiliency for acute cognitive changes that might portend late effects and identify targets for intervention. Based on the existing literature, we hypothesized: children would have significantly reduced aerobic fitness and motor proficiency at this point in treatment; children with higher fitness would exhibit better executive functioning (i.e., sustained attention, working memory, verbal fluency and behavioral inhibition); and better performance on working memory measures would correlate with greater activation of related neural networks as revealed by fMRI.
Method
Participants
All study participants were patients enrolled on a phase II trial of limited surgery and proton therapy (PT), or observation after radical resection (RT2CR; NCT01419067). Participants were infants through 21 years of age, newly diagnosed with craniopharyngioma by histology, cytology or neuroimaging. Children were excluded from participation for a history of treatment with fractionated radiation therapy, intracystic P-32, intracystic bleomycin or radiosurgery, and if pregnant. Those under two years of age, with limited English proficiency, premorbid neurological (e.g., traumatic brain injury) or neurodevelopmental (e.g., Down syndrome) conditions did not receive protocol-based cognitive evaluations. This study was conducted at St. Jude Children’s Research Hospital between August 2011 and May 2016 as approved by the Institutional Review Board; the study was completed in accordance with the Helsinki Declaration and written informed consent was obtained prior to participation.
Procedure
Cognitive Assessment.
Patients participated in baseline cognitive assessments after surgery but prior to initiation of PT. All utilized cognitive measures have age-specific norms from representative standardization samples, and demonstrated reliability and validity. Intellectual ability was assessed using an 8 subtest pro-rated IQ from an age appropriate Wechsler scale [Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003) for children age 6 to 16 years; Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV; Wechsler 2008) for children ≥ 17 years]. These measures provide an age-standardized score with mean of 100 and standard deviation of 15. Based on the existing aerobic fitness and exercise literature, additional cognitive measures were selected for this study from the larger protocol battery to assess attention, executive functions and academic skills.
The Conners’ Continuous Performance Test-Second Edition (CPT-II; Conners, 2004) is a computerized measure of sustained attention. Letters are presented one at a time on a computer screen and the participant presses the space bar as quickly and accurately as possible for any letter except the letter “X”. The CPT-II program computes an omission score (index of inattention), commission score (index of impulsivity), and hit reaction time (index of processing speed). Scores are age-standardized T-scores with mean of 50 and standard deviation of 10.
Selected executive function measures included the Working Memory Index (WMI) from the age appropriate Wechsler Scale, comprised of the Digit Span and Letter-Number Sequencing subtests. Digit Span includes Digit Span Forward (participant repeats digits verbatim) and Digit Span Backward (participant repeats digits in reverse order). For Letter-Number Sequencing, the examiner presents sequences of numbers and letters after which the participant repeats the numbers in ascending order followed by the letters in alphabetical order. Retrieval Fluency from the Woodcock-Johnson Test of Cognitive Abilities, Third Edition (WJ-III; Woodcock, McGrew & Mather, 2001) was selected as a measure of verbal fluency that requires the participant to name as many exemplars of a provided category as they can in one minute. The Color-Word Interference Test from the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan & Kramer, 2001) was selected as a measure of behavioral inhibition. For this task, the participant must inhibit a pre-potent response (reading a color name) for a more challenging response (naming the incongruent ink color of the color name). Finally, parents completed the Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000), a parent questionnaire consisting of 86 items rated as occurring “never, sometimes or often”. The Working Memory (WM) Scale, Behavior Regulation and Metacognitive Indices (BRI/MCI), and the Global Executive Composite (GEC) were chosen as scores of interest. All scores are age standardized. The Wechsler WMI and WJ-III Retrieval Fluency have a mean of 100 and standard deviation of 15. The D-KEFS Color Word Interference has a mean of 10 and standard deviation of 3. The BRIEF has a mean of 50 and standard deviation of 10, with higher scores indicative of more problems.
To assess academic skills, we selected two subtests from the Woodcock Johnson Tests of Academic Achievement- Third Edition (WJ-III; Woodcock, McGrew & Mather, 2001). The Letter-Word Identification subtest requires basic letter recognition followed by single word reading. The Calculation subtest requires completion of simple to complex mathematical computations based on age. Both of these subtests produce an age standardized score with a mean of 100 and standard deviation of 15.
Fitness Assessment.
The Bruinicks-Oseretsky Test of Motor Proficiency, Second Edition (BOT2; Bruininks & Bruininks, 2005) was administered by an exercise physiologist as a norm-referenced measure of gross and fine motor skills. The motor composite score from this measure captures fine motor control, manual coordination, body coordination, strength and agility. The score is age standardized with a mean of 50 and a standard deviation of 10. Internal consistency reliability (.95-.96) and inter-rater reliability (.98) coefficients are extremely high for the study age range. Criterion oriented validity has also been demonstrated with a high correlation between the BOT2 and the Peabody Developmental Motor Scales (.73).
Peak oxygen uptake (PKVO2) was calculated as a measure of aerobic fitness. Participants completed a graded maximal exercise test using a cycle ergometer. During this test, concentrations of oxygen and carbon dioxide of inhaled and exhaled air were measured using a MedgraphicsUltima Cardio 02® metabolic testing system that collects breath-by-breath measurements via a face mask. PKVO2 represents oxygen consumption when the participant can go no further. It is measured in ml/kg/min, accounting for body size. Average time on the cycle ergometer was 7 minutes, 51 seconds and correlated with age (r=.63, p<.001). Gender- and body size-based norms were used to derive predicted PKVO2 scores for comparison with patient performance (James et al., 1980).
Neuroimaging.
The fMRI examination was designed to explore neural correlates of WM performance in relation to fitness indicators. Neuroimaging was completed during baseline, prior to PT. Before scanning, participants watched a presentation about scanning procedures, practiced fMRI tasks and tried the manual response mechanism. All scans were completed on 3 Tesla Magnets (Trio and Skyra models, Siemens Medical Systems). Conventional MRIs were used to identify morphologic abnormalities, facilitate spatial normalization of brain images, and visualize fMRI results. Whole brain functional images were acquired with T2*-weighted EPI pulse sequences (TR= 2 s, TE= 30 ms, FOV= 192 mm, matrix= 64 × 64, bandwidth= 2055 Hz/pixel, 32 slices, slice thickness= 3.5 mm).
During fMRI, participants completed a sensorimotor task (visual, auditory, and motor stimulation) and a verbal n-back working memory task used in prior studies with healthy adults (Jacola et al., 2015). During the sensorimotor survey, participants viewed an alternating checkerboard, heard a series of ascending and descending tones, and were asked to tap their fingers while the checkerboard was visible. Sensory blocks were interleaved with periods of rest with a simple fixation cross (Zou et al., 2011). For the n-back working memory task, participants viewed a continuous stream of phonologically distinct letters presented one at a time on a computer screen. Participants were instructed to respond when the currently presented letter was identical to the letter presented at the specified interval (1- or 2-back). There was a 0-back control condition during which participants always responded to a pre-determined letter (e.g., X). Stimuli were presented at the back of the magnet with an LCD projector and viewed via a mirror on the head coil.
Data Analytic Plan
To characterize the sample, descriptive statistics of demographic and clinical variables were calculated. One-sample Z-tests were used to investigate how the sample differed from the normative population at baseline on measures of cognition, aerobic fitness and motor proficiency. Given there is no large scale PKVO2 normative data for computation of a Z-test, a one sample t-test was used to compare observed performance to predicted performance using body size and gender-based norms (James et al., 1980). Correlations were conducted to examine relationships among clinical, cognitive, aerobic fitness and motor proficiency measures. All p-values were two-sided. Analyses were implemented in SAS 9.4.
Neuroimaging analysis targeted three areas: 1) patterns of activation during completion of a sensorimotor survey and the n-back working memory task to establish relative rest and task-related activity in this sample, 2) associations of neural activation and n-back accuracy to identify activation patterns associated with better working memory performance, and 3) associations of neural activation and fitness/motor proficiency indicators to identify activation patterns associated with higher fitness. Functional images were analyzed with Statistical Parametric Mapping (SPM) via a 2-level analysis. In the first-level analysis, data were analyzed according to a fixed-effect generalized linear model with task-induced activity represented by a boxcar function convolved with canonical hemodynamic response function. Contrasts selecting for activation of interest were set in a model and contrast images from each participant were used as a variable in a second-level, random-effects analysis. For the n-back task, the contrast of interest was 2-back > 0-back given anticipation of greater variability in performance on the 2-back condition that would optimize sensitivity to performance-related effects.
Results
Demographic and Clinical Characteristics
Sample characteristics are reported in Table 1. The group was on average 10 years of age, balanced by gender (48% male) and largely Caucasian (65%). The majority of patients underwent tumor resection (72%). Prior to baseline study assessments, patients underwent an average of 1.5 cranial surgical procedures (median = 1; range 0 – 8), with 33% requiring cerebral spinal fluid diversion and 53% experiencing diabetes insipidus (DI). Based on body mass index (BMI), 24% of the sample was overweight (BMI ≥ 25) and 15% were obese (BMI ≥ 30).
Table 1.
Demographic and Clinical Characteristics
| n | (%) | ||
|---|---|---|---|
| Gender | |||
| Male | 50 | 48.1 | |
| Female | 54 | 51.9 | |
| Race | |||
| Caucasian | 68 | 65.4 | |
| African American | 16 | 15.4 | |
| Asian | 4 | 3.8 | |
| Othera | 16 | 15.4 | |
| Extent of Surgical Resection | |||
| No surgery | 12 | 11.5 | |
| Catheter only | 17 | 16.4 | |
| Resection | 75 | 72.1 | |
| Cerebral Spinal Fluid Diversionb | |||
| No | 70 | 67.3 | |
| Yes | 34 | 32.7 | |
| Diabetes Insipidusc | |||
| No | 49 | 47.1 | |
| Yes | 55 | 52.9 | |
| n | M (SD) | Range | |
| Age at Baseline (Years) | 104 | 10.0 (4.6) | 1.0 – 20.2d |
| Number of Surgeries | 104 | 1.5 (1.2) | 0 – 8 |
| Time Since Surgery (Months)e | 92 | 6.5 (10.3) | 0.2 – 50.3 |
| Body Mass Index | 95 | 22.6 (6.2) | 14.3 – 44.8 |
| PKVO2 | 88 | 23.9 (7.1) | 8.3 – 43.1 |
| BOT2f | 86 | 38.6 (9.0) | 20 – 61 |
| Wechsler IQg | 93 | 100.8 (16.0) | 56 – 130 |
Other race was composed of participants with race marked as “unknown”.
Includes patients who received either ventriculoperitoneal shunting or endoscopic third ventriculostomy.
Determined based on whether patient was prescribed desmopressin prior to baseline cognitive evaluation.
Cognitive data was not gathered for children under 2 years (n= 2).
Does not include patients who did not have surgery.
Bruinicks-Oseretsky Test of Motor Proficiency, Second Edition; age standardized with a mean of 50 ± 10.
8 subtest pro-rated IQ score from the age-appropriate Wechsler measures; age standardized with mean of 100 ± 15.
Cognitive Performance, Motor Proficiency and Aerobic Fitness
Performance of this sample on the full cognitive battery administered at baseline has been previously reported (Fournier-Goodnight et al., 2017). For the smaller set of cognitive variables selected for this study, one sample Z-tests indicate the sample performed significantly worse than normative expectations on measures of attention regulation (CPT-II omissions- 52.4 ± 14.4, p= 0.049, effect size [Cohen’s d]= 0.240), working memory (Wechsler WMI- 96.0 ± 15.3, p= 0.016, d= 0.267; BRIEF WM scale- 52.6 ± 11.7, p= 0.012, d= 0.255) and verbal fluency (W-JIII Retrieval Fluency- 95.0 ± 15.8, p= 0.002, d= 0.336). However, they were significantly less impulsive than normative expectations (CPT-II commissions- 46.8 ± 10.1, p= 0.009, d= 0.318). All other investigated cognitive variables were within the range of normative expectations. One-sample Z-tests indicated the group demonstrated significantly worse motor skills than normative expectations on the BOT2 (38.6 ± 9.0, p< 0.001, d= 1.138). A one sample t-test revealed significantly worse aerobic fitness than normative expectations as measured by PKV02 (−11.54 ± 8.22, p< 0.001, d= 1.403).
Correlations among demographic/clinical variables with cognitive and fitness measures indicate that older age was significantly associated with higher PKVO2. Female gender was significantly associated with developing DI and worse PKVO2. A higher number of surgical procedures was significantly associated with developing DI, worse PKVO2 and worse BOT2 performance. A greater extent of surgical resection was significantly associated with developing DI, worse PKVO2 and worse attention regulation (CPT-II Omissions, CPT-II Commissions). DI was associated with worse PKVO2 and BOT2. Higher BMI was associated with worse PKVO2. PKVO2 and BOT2 were significantly correlated (n= 82, r= 0.43, p< 0.001). See Table 2.
Table 2.
Correlations among Demographic/Clinical Factors with Cognitive and Fitness Measures
| Measures | Na | Age | Gender | BMI | Number of Surgeries | Extent of Resection | DI |
|---|---|---|---|---|---|---|---|
| Wechsler IQ | 93 | −.01 | −.14 | .03 | −.06 | −.14 | .11 |
| CPT-II Omissions | 67 | −.09 | .22 | −.09 | .15 | .26* | .10 |
| CPT-II Commissions | 67 | −.05 | .07 | −.02 | .15 | .26* | .24 |
| CPT-II Hit RT | 67 | .06 | .17 | −.07 | −.04 | −.03 | −.03 |
| Wechsler WMI | 81 | −.06 | −.13 | −.04 | −.09 | −.08 | .06 |
| WJ-III Retrieval Fluency | 87 | −.10 | .06 | −.05 | −.15 | −.16 | −.01 |
| D-KEFS CWI | 52 | −.20 | −.17 | −.03 | .02 | −.01 | −.05 |
| BRIEF WM | 98 | −.15 | −.01 | −.01 | .08 | .02 | 0.17 |
| BRIEF MCI | 78 | −.07 | −.03 | −.02 | .06 | .11 | .18 |
| BRIEF BRI | 79 | −.06 | −.10 | .14 | .22 | −.06 | .18 |
| BRIEF GEC | 97 | −.17 | −.09 | .00 | .14 | .02 | .18 |
| WJ-III LWI | 76 | .01 | −.12 | .05 | .07 | .00 | .12 |
| WJ-III Calculation | 74 | .08 | −.12 | .20 | .06 | .03 | .16 |
| BOT2 | 86 | −.03 | −.06 | −.14 | −.42*** | −.18 | −.23* |
| PKVO2 | 88 | .29** | −.33** | −.24* | −.34** | −.26* | −.28** |
| DI | 104 | −.10 | .21* | .10 | .28** | .42*** | ----- |
Sample size for correlations is decided by sample size for cognitive measures except for BMI (n=95); Age, Gender, Number of Surgeries, Extent of Resection and DI have a sample size of 104. Note.> Pearson correlations computed to assess association for two continuous variables, Spearman for an ordinal and continuous variable or binary variable, and Point-biserial for a binary and continuous variable. Coding for correlations: Gender- Male= 1, Female= 2; Extent of Resection- No Surgery= 1, Catheter Only= 2, Resection= 3; DI- No=0, Yes=1 BMI Body Mass Index, DI Diabetes Insipidus, IQ Intelligence Quotient, CPT-II Conners’ Continuous Performance Test, Second Edition, RT Reaction Time, WMI Working Memory Index, WJ-III Woodcock Johnson, Third Edition, D-KEFS Delis-Kaplan Executive Function System, CWI Color-Word Interference, BRIEF Behavior Rating Inventory of Executive Function, WM Working Memory, MCI Metacognitive Index, BRI Behavior Regulation Index, GEC Global Executive Composite, LWI Letter-Word Identification, BOT2 Bruinicks-Oseretsky Test of Motor Proficiency, Second Edition
p <.001,
p <.01,
p <.05
Correlations among fitness indicators and cognitive measures indicated better PKVO2 was significantly associated with better working memory (Wechsler WMI), verbal fluency (WJ-III Retrieval Fluency) and mathematics (WJ-III Calculation). Better BOT2 performance was associated with higher IQ (Wechsler IQ), better attention regulation (CPT-II Omissions; CPT- Hit RT), better working memory (Wechsler WMI), better verbal fluency (WJ-III Retrieval Fluency) and better parent reported executive functions (BRIEF MCI, BRIEF BRI). When accounting for multiple comparisons using the False Discovery Rate (Benjamini & Hochberg, 1995), within measures, two of the three significant correlations between PKVO2 and cognitive variables remained significant and five of the seven significant correlations between BOT2 and cognitive variables remained significant. See Table 3 for a summary of correlations.
Table 3.
Correlations among Fitness Indicators and Cognitive Measures
| PKVO2 | BOT2 | |||
|---|---|---|---|---|
| n | r | n | r | |
| Wechsler IQ | 82 | .20 | 78 | .23*a |
| CPT-II Omissions | 65 | −.20 | 63 | −.26* |
| CPT-II Commissions | 65 | −.06 | 63 | −.17 |
| CPT-II Hit RT | 65 | .00 | 63 | −.25* |
| Wechsler WMI | 76 | .27*a | 74 | .32**a |
| WJ-III Retrieval Fluency | 83 | .23*a | 80 | .32**a |
| D-KEFS CWI | 51 | .14 | 50 | .27 |
| BRIEF WM | 85 | −.05 | 82 | −.20 |
| BRIEF MCI | 74 | .00 | 72 | −.28*a |
| BRIEF BRI | 75 | −.06 | 72 | −.35**a |
| BRIEF GEC | 84 | −.07 | 82 | −.19 |
| WJ-III LWI | 73 | .13 | 70 | .12 |
| WJ-III Calculation | 71 | .24* | 68 | .19 |
Note. Pearson correlations computed to assess association for two continuous variables IQ Intelligence Quotient, CPT-II Conners’ Continuous Performance Test, Second Edition, RT Reaction Time, WMI Working Memory Index, WJ-III Woodcock Johnson, Third Edition, D-KEFS Delis-Kaplan Executive Function System, CWI Color-Word Interference, BRIEF Behavior Rating Inventory of Executive Function, WM Working Memory, MCI Metacognitive Index, BRI Behavior Regulation Index, GEC Global Executive Composite, LWI Letter-Word Identification, BOT2 Bruinicks-Oseretsky Test of Motor Proficiency, Second Edition.
p <.01,
p <.05,
Statistical results remained significant after accounting for multiple comparisons using the False Discovery Rate within measures.
Neuroimaging Findings
Fifty-one of the 104 study participants participated in fMRI examination with usable data. Reasons for not participating included: less than 8 years of age (n= 38), anxiety/ claustrophobia (n= 3), vision impairment (n= 6) and patient refusal (n= 2). Data for an additional four patients were excluded due to shunt artifact (n= 2), braces artifact (n= 1) and poor slice positioning (n= 1). Comparison of those with and without useable fMRI exams revealed the group with exams was significantly older (13.1 ± 3.2 vs. 7.0 ± 3.5, p< 0.001), consistent with greater challenges conducting non-sedated MRI exams with younger children. There were no significant differences between groups in gender, race, and rate of surgical resection, use of CSF diversion or DI status.
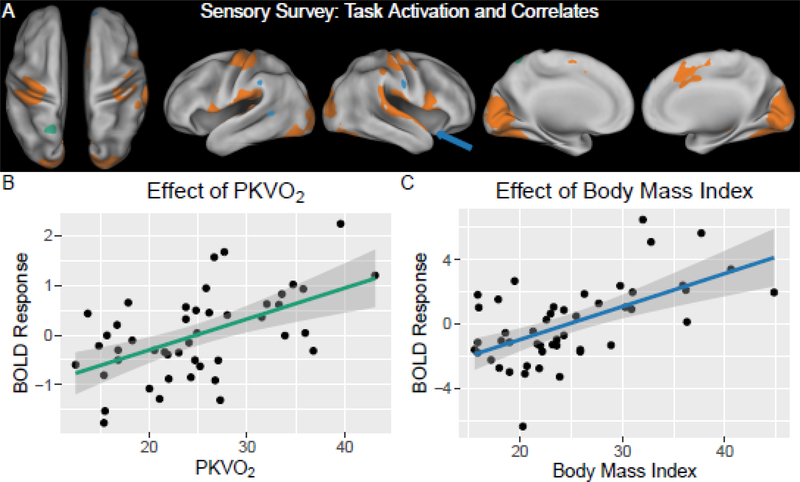
The sensorimotor survey task revealed a pattern of brain activity consistent with known sensory and motor cortex (Table 4; Figure 1). Robust activation was detected along the calcarine sulcus and extended into the fusiform, lingual and middle occipital gyri known for vision processing. Task activation was also located in the superior temporal gyrus and pre-central gyrus known for primary auditory and motor processing, respectively. Additional activation was detected in cingulum cortex known to be associated with attention (Ogg et al., 2008). Activity in part of the left precuneus was positively associated with higher PKVO2 (p< 0.05, FWE corrected). Additionally, higher brain activation in right insula, left superior temporal and supramarginal areas was associated with higher BMI (p< 0.05, FWE corrected). There were no associations between activity during the sensorimotor survey and BOT2 performance, gender or DI status.
Table 4.
fMRI Activation Coordinates
| Area | Peak T-value | X | Y | Z | Cluster Size (voxels) |
|---|---|---|---|---|---|
| Sensorimotor Survey | |||||
| Task | |||||
| Lingual Gyrus | 16.16 | −14 | −90 | −14 | 12,232 |
| Lingual Gyrus | 15.66 | 12 | −94 | −6 | |
| Calcarine Sulcus | 15.24 | 2 | −88 | −6 | |
| Precentral Gyrus | 12.12 | −34 | −26 | 56 | 4442 |
| Precentral Gyrus | 11.35 | −38 | −22 | 62 | |
| Postcentral Gyrus | 10.67 | −46 | −22 | 60 | |
| Precentral Gyrus | 11.26 | 36 | −24 | 60 | 1173 |
| Inferior Parietal Lobule | 8.26 | 54 | −34 | 54 | |
| Precentral Gyrus | 8.21 | 50 | 0 | 52 | |
| Temporal Pole | 10.81 | 54 | 14 | −4 | 3471 |
| Temporal Pole | 10.48 | 56 | 6 | 0 | |
| Superior Temporal Gyrus | 9.55 | 64 | −14 | 8 | |
| Mid-Cingulate Area | 8.42 | 4 | 16 | 44 | 980 |
| Supplemental Motor Area | 8.41 | 0 | −4 | 56 | |
| Supplemental Motor Area | 7.78 | 2 | 4 | 48 | |
| PKVO2 | |||||
| Precuneus | 4.28 | −2 | −54 | 68 | 74 |
| Body Mass Index | |||||
| Insula | 4.94 | 48 | 16 | −12 | 320 |
| Post Central Gyrus | 4.31 | 60 | −14 | 36 | 161 |
| Supramarginal Gyrus | 4.38 | −58 | −42 | 36 | 113 |
| Middle Temporal Gyrus | 4.34 | −56 | −52 | 6 | 403 |
| N-Back | |||||
| Task | |||||
| Superior Frontal Gyrus | 7.8 | 26 | 4 | 64 | 969 |
| Precuneus | 7.33 | −12 | −70 | 58 | 5398 |
| Precuneus | 6.76 | 12 | −68 | 56 | |
| Inferior Parietal Lobule | 6.56 | −42 | −46 | 48 | |
| Middle Frontal Gyrus | 7.26 | −26 | 6 | 62 | 1453 |
| Precentral Gyrus | 4.12 | −36 | −2 | 42 | |
| Precentral Gyrus | 3.90 | −44 | 2 | 46 | |
| Supplementary Motor Area | 6.21 | 0 | 18 | 52 | 703 |
| Middle Frontal Gyrus | 4.68 | −36 | 56 | 18 | 165 |
| Middle Frontal Gyrus | 4.22 | −36 | 56 | 8 | |
| Middle Frontal Gyrus, Orbital | 3.66 | −44 | 48 | 0 | |
| Commission Errors | |||||
| Middle Occipital Gyrus | 5.54 | 30 | −62 | 38 | 1556 |
| Inferior Parietal Lobule | 5.29 | 32 | −44 | 42 | |
| Angular Gyrus | 4.56 | 26 | −58 | 42 | |
| Inferior Parietal Lobule | 5.50 | −30 | −44 | 52 | 2644 |
| Superior Parietal Lobule | 5.45 | −28 | −58 | 52 | |
| Inferior Parietal Lobule | 5.20 | −26 | −58 | 42 | |
| BOT2 score* | |||||
| Precuneus | 5.08 | −14 | −64 | 50 | 2051 |
| Superior Parietal Lobule | 4.22 | −14 | −64 | 56 | |
| Superior Parietal Lobule | 4.08 | −18 | −58 | 52 | |
| Precentral Gyrus | 4.74 | −34 | −2 | 38 | 863 |
| Frontal Middle Gyrus | 4.49 | −26 | −2 | 52 | |
| Superior Frontal Gyrus | 3.79 | −20 | 8 | 64 | |
| Middle Frontal Gyrus | 4.60 | 28 | 12 | 58 | 2787 |
| Insula | 4.32 | 36 | 28 | 32 | |
| Inferior Frontal Gyrus, Opercularis | 4.21 | 40 | 18 | 8 | |
Note. All results shown are p< 0.05 FWE corrected for multiple comparisons. All results are controlled for age except for BOT2 scores, which fall below FWE threshold with age in the model, but clusters were consistent across all models evaluated.
Figure 1. FMRI Findings during Sensorimotor Survey Task.
(A) Orange overlay shows brain activation during visual, auditory and motor stimulation (contrast: task > rest in a random-effects group analysis; p < 0.05 with family-wise error [FWE] correction, minimum cluster size 200 voxels). Green overlay denotes an area where activity was positively associated with aerobic capacity (regression: task > rest versus PKVO2; p< 0.05 with FWE correction, minimum cluster size 50 voxels). Blue overlay shows areas were brain activity was positively associated body mass index (regression: task > rest versus body mass index measurements; p< 0.05 with FWE correction, minimum cluster size 50). (B) Scatter plot of activity versus PKVO2 at the cluster located in the left precuneas. (C) Scatter plot of activity versus BMI at the cluster in the right insula/temporal pole, identified by the arrow in (A).
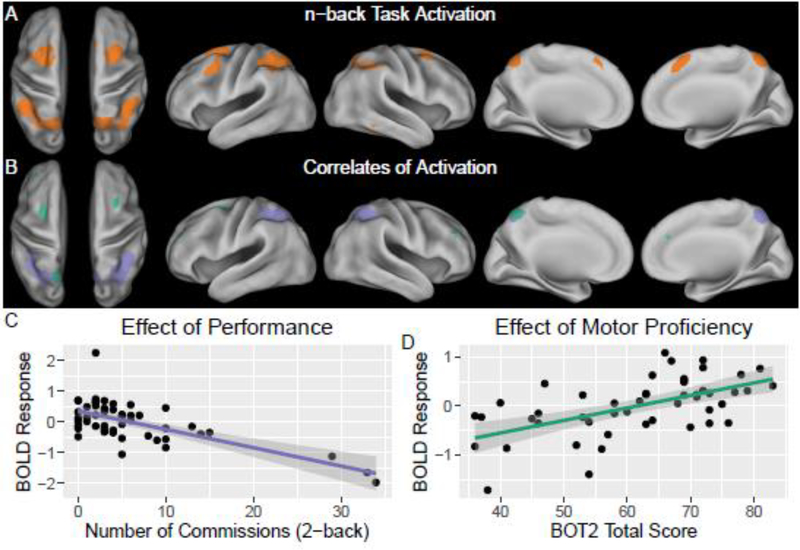
The pattern of activation in the n-back task (Table 4; Figure 2) was consistent with the neuroimaging literature (Nee & Brown, 2013; Owen, McMillan, Laird, & Bullmore, 2005). Analysis identified robust activation in bilateral superior parietal lobule, dorsolateral prefrontal cortex, and middle and superior frontal gyri (p< 0.05, FWE corrected). Decreased activation in posterior regions within the working memory network were associated with increasing commission errors (p< 0.05, FWE corrected). Activity in anterior areas within the working memory network were positively associated with BOT2 scores (p< 0.05, univariate analysis, FWE corrected). All associations controlled for age except the BOT2 association falls just below significance (p< 0.1, FWE corrected) with age in the model. However, this sub-threshold pattern was consistent across all models tested. There were no associations between activity during the n-back task and PKVO2, gender or DI status.
Figure 2. FMRI Findings during N-back Working Memory Task.
(A) Orange overlay shows brain activation during n-back task (contrast: 2-back > 0-back in a random-effects group analysis; p< 0.05 with family-wise error [FWE] correction, minimum cluster size 200 voxels). (B) Neural correlates of performance and motor proficiency. Light purple denotes areas where activity was negatively associated with commission errors during the task (regression: 2-back > 0-back versus commission errors during the 2-back task; p< 0.05 with FWE correction, minimum cluster size 200 voxels). Green overlay shows areas were brain activity was positively associated to motor proficiency (univariate regression: 2-back > 0-back versus BOT2 total scores; p< 0.05 with FWE correction, minimum cluster size 200; clusters drop just below FWE significance (P ~ 0.1) with age in the model but demonstrate consistency across all models examined). (C) Scatter plot of activity versus commission errors at a sub-cluster in the right superior parietal lobule. (D) Scatter plot of activity versus BOT2 scores at cluster in the left superior frontal gyrus.
Discussion
Consistent with our first hypothesis, study findings indicate children with craniopharyngioma demonstrate worse than expected performance prior to adjuvant therapy not only in cognitive abilities (sustained attention, working memory, and verbal fluency) but also motor proficiency and aerobic fitness. Interestingly, better motor proficiency and aerobic fitness were associated with better attention regulation, executive functions and academic performance. Further, better motor proficiency and better working memory performance were associated with greater activation of frontal and parietal areas, respectively, of a well established fMRI working memory network. Of note, female participants were more likely to develop endocrinopathies and to have worse aerobic fitness. More surgical procedures and greater extent of surgery were also associated with endocrinopathies and worse fitness, as well as worse performance on attention measures.
Given the suprasellar location of craniopharyngioma, it is not surprising that tumor mass effect and/or surgical intervention could result in endocrinopathies and physical deconditioning prior to adjuvant, cancer-directed, therapy. That said, the group as a whole demonstrated significantly compromised aerobic fitness (41% impaired), with enough fitness variability to investigate associations with outcomes of interest. Consistent with our second hypothesis, better motor proficiency and aerobic fitness were associated with better executive functions. This is a novel finding for pediatric brain tumor patients, particularly this early in treatment, but in keeping with correlational studies that indicate children of higher aerobic fitness outperform lower fit children on executive function and academic tasks (Chaddock, Hillman, et al., 2012; Fedewa & Ahn, 2011; Pontifex et al., 2011). These findings may help to explain differential risk/resiliency with respect to acute cognitive changes that may portend late effects among childhood brain tumor survivors. A more active premorbid lifestyle may enhance aerobic fitness that confers cognitive resilience. Alternatively, aerobic and cognitive fitness may share a genetic or biological resiliency with respect to acquired brain injury in keeping with cognitive reserve theories (Bigler & Stern, 2015; Mathias & Wheaton, 2015). It is possible that socioeconomic status could account for some of the shared variance, as children who come from families with greater opportunities for fitness-related activities may also have parents of higher cognitive functioning/education that in turn pass on and foster better executive functions (Ombrellaro et al., 2018). The pediatric oncology literature has also shown males are at less risk for cognitive late effects (e.g., Ris, Packer, Goldwein, Jones-Wallace, & Boyett, 2001), with the current study showing males had less risk for endocrinopathies and better aerobic fitness early in treatment that perhaps serves as a buffer against emergence of cognitive late effects. Further, more aggressive surgical approach was associated with greater endocrinopathies and less aerobic fitness. There is some evidence in the literature that aggressive surgical approach to craniopharyngioma has greater cognitive consequence (Fournier-Goodnight, et al., 2017; Merchant et al., 2006) such that endocrinopathies and/or reduced aerobic fitness might serve as a moderator of cognitive risk.
Performance during fMRI exams revealed aerobic fitness was related to response accuracy and neural activation, as well as motor fitness. During a sensorimotor survey, activation patterns were consistent with primary sensory (visual and auditory) and motor cortex. Interestingly, increased activation in the left precuneus (part of the dorsal attention network) was associated with higher PKVO2, suggesting those of higher fitness might be more engaged in a task of lower cognitive demand. Further, higher activity in insula, superior temporal and supramarginal areas was associated with increased BMI. Activation in the insula area has been associated with plasma levels of ghrelin, the gut hormone promoting appetite, possibly suggesting those with greater activity might have higher appetitive drives that could contribute to weight gain or increased BMI (Zanchi et al., 2017).
During the n-back task, activation patterns were consistent with a well established working memory network including robust activation in bilateral superior parietal lobule, dorsolateral prefrontal cortex, and middle and superior frontal gyri (Nee & Brown, 2013; Owen et al., 2005). Greater activation of posterior regions within this working memory network were associated with fewer commission errors, or more accurate performance. This finding is consistent with findings of Wolfe and colleagues (2013) who conducted a small (n= 9) fMRI study of working memory and its relationship with aerobic fitness in adolescent survivors of pediatric brain tumors (average of 10 years from treatment completion). They found individuals with higher aerobic fitness had better working memory performance on n-back tasks, as well as relatively greater activation of the well established working memory network during the 2-back condition. They interpreted their findings as indicative of more efficient functioning in frontal and parietal brain regions of interest for those of higher cardiorespiratory fitness (Wolfe et al., 2013). In the current study, greater activation (2-back > 0-back) of anterior and medial parietal regions within this working memory network were associated with better motor proficiency (BOT2). Activation of this subnetwork (green in Figure 2) connects motor proficiency to a focal subset (precuneus) of the extensive parietal areas associated with better n-back task performance (purple in Figure 2). The BOT2-associated precuneus node is adjacent to the PKVO2-associated precuneus node detected in the sensorimotor survey (green in Figure 1), and both are within the dorsal attention network (Yeo et al., 2011). The imaging findings identify important associations among patterns of brain activation, fitness and executive functions and may provide clues to the neural mechanisms by which fitness and exercise affect cognitive function.
There is increasing appreciation for the role of aerobic exercise in preserving cognitive functioning and/or enhancing cognitive recovery, with candidate mechanisms identified through animal studies. Murine models have shown aerobic exercise improves brain structure through neurogenesis, synaptogenesis and angiogenesis, as well as increased production of neurochemicals (e.g., brain-derived neurotrophic factor) that promote growth, survival and repair of neurons (for review, see Voss et al., 2011). Multiple animal studies have shown voluntary exercise is associated with neurogenesis in the hippocampus, specifically, that correlates with improved performance on spatial memory tasks (Devine & Zafonte, 2009). Decreased hippocampal volumes, as well as reductions in normal appearing white matter, have been reported among childhood brain tumor survivors who underwent radiation therapy (e.g., Riggs et al., 2014; Reddick et al., 2005). Of note, the hippocampus and cerebral white matter are also two primary brain regions in humans where neural stem cells are located (Ming & Song, 2011). These findings suggest aerobic training interventions may be beneficial for improving both medical (e.g., cardiopulmonary) and cognitive outcomes among pediatric oncology patients.
Devine and Zafonte (2009) conducted a review in which they found three out of four studies assessing cognitive benefits of exercise in individuals with acquired brain injuries showed better cognitive performance of those who exercised relative to those with brain injuries who did not exercise. The small number of exercise intervention studies conducted with children with cancer provide early evidence for feasibility and safety, as well as improvements in cardiopulmonary fitness, strength and fatigue (Huang & Ness, 2011). We are aware of only one exercise trial that measured cognitive outcomes in this population. Mabbott and colleagues conducted a controlled, cross over exercise trial (12-week structured aerobic exercise program with target heart rate monitoring) with 28 children who were long term survivors of brain tumors treated with cranial irradiation (Riggs et al., 2017). They found exercise-related reductions in reaction time across varied cognitive tasks that were predicted by white matter (fractional anisotropy), as well as exercise-related increases in hippocampal volume. This novel study demonstrated that exercise training can have beneficial impact on brain structure of childhood brain tumor survivors thus extending the findings of exercise-related neuroplasticity from animal models to humans. In the study conducted by Mabbott and colleagues (2017), brain tumor survivors were on average five years post diagnosis; current study findings indicate reduced aerobic fitness early in treatment, potentially suggesting a role for earlier exercise intervention.
This study is not without limitations. Presented analyses were cross-sectional and occurred prior to adjuvant cancer-directed therapy. While it is informative to see how early in treatment these children are experiencing cognitive, motor and aerobic fitness impairments, it will be important to follow them longitudinally to see how they do following radiation therapy. The cross-sectional design also means we cannot assume higher aerobic fitness caused better executive function; the design raises the possibility of a third variable (e.g., socioeconomic status) contributing to the relationship between aerobic fitness and cognitive performance. It will be important for future studies to measure and investigate the contribution of parental education and environmental resources to these relationships. The inclusion of functional neuroimaging in the current study is novel and assists with understanding the contributions of underlying neural activity to associations between fitness and cognitive performance. That said, inclusion of structural imaging would have further strengthened study design. Prior studies have found higher fit children have larger basal ganglia (Chaddock, Erickson, Prkash, VanPatter et al., 2010) and hippocampal volumes (Chaddock, Erickson, Prakash, Kim, et al., 2010), which is of interest given findings of decreased hippocampal volumes in children treated with radiation therapy for brain tumors. As it stands, it is unclear how brain volume differences/white matter connectivity contributed to current findings. Future studies should model the contribution of structural and functional plasticity in relation to aerobic fitness and cognitive performance. Finally, the current study investigated aerobic fitness as a cognitive/neuroimaging correlate but was not an exercise intervention study. Exercise intervention studies with the pediatric oncology population are just emerging with significant questions remaining regarding the best type, duration and timing of intervention. Future work should compare aerobic training with other more direct cognitive interventions established in the literature such as psychostimulant medications (Conklin et al., 2010) or computerized cognitive training (Conklin et al., 2015). The incorporation of neuroimaging into these exercise studies will be instrumental to furthering understanding of exercise-related cognitive resiliency and neuroplasticity.
Acknowledgements
The authors thank the patients and their families who volunteered their time to participate in this study. This work was supported in part by the National Cancer Institute (St. Jude Cancer Center Support [CORE] Grant [P30 CA21765] and the American Lebanese Syrian Associated Charities (ALSAC). Portions of this paper were presented at the annual meeting of the International Neuropsychological Society in February 2015. The authors do not have any conflicts of interest to disclose.
References
- Benjamini Y, Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300. [Google Scholar]
- Bigler ED, Stern Y (2015). Traumatic brain injury and reserve. Handbook of Clinical Neurology, 128, 691–710. doi: 10.106/B978-0-444-63521-1.00043-1 [DOI] [PubMed] [Google Scholar]
- Bruininks RH, Bruininks BD (2005). BOT2 Bruiniks-Oseretsky Test of Motor Proficiency, Second Edition. Circle Pines, MN: AGS Publishing. [Google Scholar]
- Castelli DM, Hillman CH, Hirsch J, Hirsch A, Drollette E (2011). FIT Kids: time in target heart zone and cognitive performance. Preventative Medicine, 1, S55–S59. doi: 10.1016/j.ypmed.2011.01.019 [DOI] [PubMed] [Google Scholar]
- Castellino SM, Ullrich NJ, Whelen MJ, Lange BJ (2014). Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors. Journal of the National Cancer Institute, 106 (8) dju 186. 10.1093/jnci/dju186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, VanPatter M,…Kramer F (2010). A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Research, 1358, 172–183. doi: 10.1016/j.brainres.2010.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, VanPatter M, Voss MW, Pontifex MB, …Kramer AF (2010). Basal ganglia volume is associated with aerobic fitness in preadolescent children. Developmental Neuroscience, 32, 249–256. doi: 10.1159/000316648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Voss MWV, VanPatter M, Pontifex MB,…Kramer AF (2012). A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biological Psychology, 89, 260–268. doi: 10.1016/j.biopsycho.2011.10.017 [DOI] [PubMed] [Google Scholar]
- Chaddock L, Hillman CH, Pontifex MB, Johnson CR, Raine LB, Kramer AF (2012). Childhood aerobic fitness predicts cognitive performance one year later. Journal of Sport Sciences, 30, 421–430. doi: 10.1080/02640414.2011.647706 [DOI] [PubMed] [Google Scholar]
- Chaouloff F (1989). Physical exercise and brain monoamines: a review. Acta Physiologica Scandinavica, 137, 1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x [DOI] [PubMed] [Google Scholar]
- Conklin HM, Ogg RJ, Ashford JM, Scoggins MA, Zou P, Clark KN,…Zhang H (2015). Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: a randomized controlled trial. Journal of Clinical Oncology, 33, 3894–3902. doi: 10.1200/JCO.2015.61.6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Reddick WE, Ashford J, Ogg S, Howard SC, Morris EB, …Khan RB (2010). Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. Journal of Clinical Oncology, 28, 4465–4472. doi: 10.1200/JCO.2010.28.4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK (2004). Conners’ Continuous Performance Test II. San Antonio, TX: Pearson Corporation. [Google Scholar]
- Davis CL, Tomporowski PD, Boyle CA, Waller JL, Miller PH, Naglieri JA, Gregoski ME (2007). Effects of aerobic exercise on overweight children’s cognitive functioning: a randomized controlled trial. Research Quarterly for Exercise and Sport, 78, 510–519. doi: 10.1080/02701367.2007.105999450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, …Naglieri JA (2011). Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychology, 30, 91–98. doi: 10.1037/a0021766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH (2001). Delis-Kaplan Executive Function System. San Antonio, TX: Pearson. [Google Scholar]
- Devine JM, Zafonte DO (2009). Physical exercise and cognitive recovery in acquired brain injury: a review of the literature. Physical Medicine and Rehabilitation, 1, 560–575. doi: 10.1016/j.pmrj.2009.03.015 [DOI] [PubMed] [Google Scholar]
- Di Pinto M, Conklin HM, Li C, Merchant TE (2012). Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. International Journal of Radiation Oncology, Biology, Physics, 84, e363–369. doi: 10.1016/j.ijrobp.2012.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JE, Greene JL, Gibson CA, Smith BK, Washburn RA, Sullivan DK, …Williams SL (2009). Physical activity across the curriculum (PAAC): a randomized controlled trial to promote physical activity and diminish overweight and obesity in elementary school children. Preventative Medicine, 49, 336–341. doi: 10.1016/j.ypmed.2009.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR (2004). Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience, 124, 71–19. doi: 10.1016/j.neuroscience.2003.09.029 [DOI] [PubMed] [Google Scholar]
- Fedewea AL, Ahn S (2011). The effects of physical activity and physical fitness on children’s achievement and cognitive outcomes: a meta-analysis. Research Quarterly for Exercise and Sport, 82, 521–535. doi: 10.1080/02701367.2011.10599785 [DOI] [PubMed] [Google Scholar]
- Fjalldal S, Holmer H, Rylander L, Elfving M, Ekman B, Osterberg K, Erfurth EM (2013). Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. The Journal of Clinical Endocrinology and Metabolism, 98, 3253–3262. doi: 10.1210/jc.2013-2000 [DOI] [PubMed] [Google Scholar]
- Fournier-Goodnight AS, Ashford JM, Merchant TE, Boop RA, Indelicato DJ, Wang L,…Conklin HM (2017). Neurocognitive functioning in pediatric craniopharyngioma: performance before treatment with proton therapy. Journal of Neuro-oncology, 134, 97–105. doi: 10.1007/s11060-017-2492-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Chen CH,…Debanne SM (2001). Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group members. Proceedings of the National Academy of Sciences of the USA, 90, 3440–3445. doi: 10.1073/pnas.061002998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L (2000). Behavior Rating Inventory of Executive Function.> Odessa, FL; Psychological Assessment Resources, Inc. [Google Scholar]
- Huang TT, Ness KK (2011). Exercise interventions in children with cancer: a review. International Journal of Pediatrics, 2011, 1–11. doi: 10.1155/2011/461512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacola LM, Willard VM, Ashford JM, Ogg RJ, Scoggins MA, Jones MM, …Conklin HM (2015). Clinical utility of the n-back task in functional neuroimaging studies of working memory. Journal of Clinical and Experimental Neuropsychology, 36, 875–886. doi: 10.1080/13803395.2014.953039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James FW, Kaplan S, Gluek CJ, Tsay JY, Knight MJ, Sarwar CJ (1980). Responses of normal children and young adults to controlled bicycle exercise. Circulation, 61, 902–912. [DOI] [PubMed] [Google Scholar]
- Krull KR, Hardy KK, Kahalley LS, Schuitema Il, Kesler SR (2018). Neurocognitive outcomes and interventions in long-term survivors of childhood cancer. Journal of Clinical Oncology, 36:2181–2189. doi: 10.1200/JCO.2017.76.4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K (2001). Physical activity and risk of cognitive impairment and dementia in elderly person. Archives of Neurology, 58, 498–504. [DOI] [PubMed] [Google Scholar]
- Mathias JL, Wheaton P (2015). Contribution of brain or biological reserve and cognitive or neural reserve to outcome after TBI: A meta-analysis (prior to 2015). Neuroscience and Biobehavioral Reviews, 55, 573–593. doi: 10.1016/j.neubiorev.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Merchant TE, Kiehna EN, Kun LE, Mulhern RK, Li C, Xiong X, …Sanford RA (2006). Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. Journal of Neurosurgery, 104, 94–102. doi: 10.317/ped.2006.104.2.5 [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron, 70, 687–702. doi: 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitby PA, Robison LL Whitton JA, Zevon MA, Gibbs IC, Tersak JM, …Mertens AC (2003). Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer, 97, 115–126. doi: 10.1002/cncr.11117 [DOI] [PubMed] [Google Scholar]
- Mostow EN, Byrne J, Connelly RR, Mulvihill JJ (1991). Quality of life in long-term survivors of CNS tumors of childhood and adolescence. Journal of Clinical Oncology, 9, 592–599. doi: 10.1200/JCO.1991.9.4.592 [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Butler RW (2004). Neurocognitive sequelae of childhood cancers and their treatment. Pediatric Rehabilitation, 1, 1–14. doi: 10.1080/13638490310001655528 [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE (2004). Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncology, 5, 399–408. doi: 10.1016/S1470-2045(04)01507-4 [DOI] [PubMed] [Google Scholar]
- Muller HL, Gebhardt U, Teske C, Faldum A, Zwiener I, Warmuth-Metz M,…Calaminus G (2011). Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. European Journal of Endocrinology, 134, 97–105. doi: 10.1530/EJE-11-0158 [DOI] [PubMed] [Google Scholar]
- Muller HL (2017). Diagnosis, treatment, clinical course, and prognosis of childhood-onset craniopharyngioma patients. Minerva Endocrinology, 42, 356–375. doi: 10.23736/S0391-1977.17.02615-3 [DOI] [PubMed] [Google Scholar]
- Nee DE, Brown JW (2013). Dissociable frontal-striatal and frontal-parietal networks involved in updating hierarchical contexts in working memory. Cerebral Cortex, 13, 2146–2158. doi: 10.1093/cercor/bhs194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombrellaro KJ, Perumal N, Zeiher J, Hoebel J, Ittermann T, Ewert R, …Finger JD (2018). Socioeconomic correlates and determinants of cardiorespiratory fitness in the general adult population: as systematic review and meta-analysis. Sports Medicine- Open, 4: 1–19. doi: 10.1186/s40798-018-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg RJ, Zou P, Allen DN, Hutchins SB, Dutkiewicz RM, Mulhern RK (2008). Neural correlates of a clinical continuous performance test. Magnetic Resonance Imaging, 26, 504–512. doi: 10.1016/j.mri.2007.09.004 [DOI] [PubMed] [Google Scholar]
- Ondruch A, Maryniak A, Kropiwnicki T, Roszkowski M, Daszkiewicz P (2011). Cognitive and social functioning in children and adolescents after the removal of craniopharyngioma. Child’s Nervous System, 27, 391–397. doi: 10.1007/s00381-010-1301-0 [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E (2005). N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25, 46–59. doi: 10.1002/hbm.20131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyurt J, Thiel CM, Lorenzen A, Gebhardt U, Calaminus G, Warmuth-Metz M, Muller HL (2014). Neuropsychological outcome in patients with childhood craniopharyngioma and hypothalamic involvement. Journal of Pediatrics, 164, 876–881. doi: 10.1016/j.peds.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L,…Mulhern RK (2001). Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. Journal of Clinical Oncology, 19, 2302–2308. doi: 10.1200/JC.2001.19.8.2302 [DOI] [PubMed] [Google Scholar]
- Piquel X, Abraham P, Bouhours-Nouet N,Gatlais F, Durfesne S, Rouleau S, Coutant R (2012). Impaired aerobic exercise adaptation in children and adolescents with craniopharyngioma is associated with hypothalamic involvement. European Journal of Endocrinology, 166, 215–222. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ,…Hillman CH (2011). Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. Journal of Cognitive Neuroscience, 23, 1332–1345. doi: 10.1162/jocn.2010.21528 [DOI] [PubMed] [Google Scholar]
- Poretti A, Grotzer MA, Ribi K, Schonle E, Boltshauser E (2004). Outcome of craniopharyngioma in children: long-term complications and quality of life. Developmental Medicine & Child Neurology, 46, 220–229. [DOI] [PubMed] [Google Scholar]
- Powell KE, Blair S, N. (1994). The public health burdens of sedentary living habits: theoretical but realistic estimates. Medicine and Science in Sports and Exercise, 26, 851–86. [PubMed] [Google Scholar]
- Reddick WE, Glass JO, Palmer SL, Wu S, Gajjar A, Langston JW,…Mulhern RK (2005). Atypical white matter volume development in children following craniospinal irradiation. Neuro-oncology, 7, 12–19. doi: 10.1215/S1152851704000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs L, Bouffet E, Laughlin S, Laperriere N Liu F, Skocic J, …Mabbott DJ (2014). Changes to memory structures in children treated for posterior fossa tumors. Journal of the International Neuropsychological Society, 20, 168–180. doi: 10.1017/S135561771300129X [DOI] [PubMed] [Google Scholar]
- Riggs L, Piscione J, Laughlin S, Cunningham T, Timmons BW, Courneya KS, …Mabbott DJ (2017). Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro-Oncology, 19, 440–450. doi: 10.1093/neuonc/now177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM (2001). Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. Journal of Clinical Oncology, 19, 3470–3466. doi: 10.1200/JCO.2001.19.15.3470 [DOI] [PubMed] [Google Scholar]
- Schatz J, Kramer JH, Ablin A, Matthay KK (2000). Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology, 14, 189–200. [DOI] [PubMed] [Google Scholar]
- Ullrich NJ, Embry L (2012). Neurocognitive dysfunction in survivors of childhood brain tumors. Seminars in Pediatric Neurology, 19, 35–42. doi: 10.1016/j.spen.2012.02.014 [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the USA, 96, 13427–13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Chaddock L, Kim JS, Vanpatter M, Pontifex MB, Raine LB,…Kramer F (2011). Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience, 199, 166–176. doi: 10.1016/j.neuroscience.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber DP, Pomeroy SL, Chiverton AM, Kieran MW, Scott RM, Goumnerova LC, Rivkin MJ (2006). Everyday cognitive function after craniopharyngioma in childhood. Pediatric Neurology, 34, 13–19. doi: 10.1016/j.pediatneurol.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children, Fourth Edition.> San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (2008). Wechsler Adult Intelligence Scale, Fourth Edition.> New York, NY: The Psychological Corporation. [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, …Knecht S (2007). High impact running improves learning. Neurobiology of learning and memory, 87, 597–609. doi: 10.1016/j.nlm.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Wolfe KR, Madan-Swain A, Hunter GR, Reddy AT, Banos J, Kana RK (2013). An fMRI investigation of working memory and its relationship with cardiorespiratory fitness in pediatric posterior fossa tumor survivors who received crania radiation therapy. Pediatric Blood Cancer, 60, 669–675. doi: 10.1002/pbc.24331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N (2001). Woodcock-Johnson III Tests of Cognitive Abilities.> Itasca, IL: Riverside Publishing. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N (2001). Woodcock-Johnson III Tests of Achievement.> Itasca, IL, Riverside Publishing. [Google Scholar]
- Yeates KO, Levin HS, Ponsford J (2017). The neuropsychology of traumatic brain injury: looking back, peering ahead. Journal of the International Neuropsychological Society, 23, 806–817. doi: 10.1017/S1355617717000686 [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, …Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi D, Depoorter A, Egloff L, Haller S, Mahlmann L, Lag UE, …Borgwardt S (2017). The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neuroscience and Biobehavioral Reviews, 80, 457–475. doi: 10.1016/j.neubiorev.2017.06.013 [DOI] [PubMed] [Google Scholar]
- Zou P, Helton KJ, Smeltzer M, Li C, Conklin HM, Gajjar A,…Ogg RJ (2011). Hemodynamic responses to visual stimulation in children with sickle cell anemia. Brain Imaging and Behavior, 5, 295–306. doi: 10.1007/s11682-011-9133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]