Abstract
Purpose: We aimed to investigate the expression dynamics of Aquaporin 1 (AQP1) in cervical cancer and evaluate correlations among AQP1 levels and the clinicopathological features of patients with cervical cancer.
Patients and methods: AQP1 mRNA and protein levels in cervical cancer and adjacent normal tissues were evaluated by quantitative reverse-transcription PCR (qRT-PCR) and western blot. Immunohistochemistry (IHC) for AQP1 was performed with a tissue microarray of cervical cancer (containing 63 cases of squamous cell cervical cancers and 10 normal cervical tissues) to investigate clinicopathological outcomes. Cut-off scores for positive expression of AQP1 were determined by receiver operating characteristic analysis. The χ2 test was used to analyze correlations among AQP1 expression and clinicopathological features of cervical cancer.
Results: The expression of AQP1 was decreased in the majority of cervical cancer tissues by qRT-PCR and western blot analysis. Positive expression of AQP1 was observed in 100% (10/10) of normal cervical tissues and in 42.86% (27/63) of cervical cancer tissues by IHC analysis. The cut-off score for positive expression of AQP1 was determined to be 45% of cancer cells. Decreased expression of AQP1 was correlated with clinicopathological features including; poor pathological grade (P=0.000), late International Federation of Gynecology and Obstetrics stage (P=0.008), and positive lymph nodes (P=0.002).
Conclusion: These data suggest that decreased expression of AQP1 correlated with progressive features in patients with cervical cancer. AQP1 levels may serve as a potential biomarker for the diagnosis of cervical cancer.
Keywords: aquaporin 1, cervical cancer, tissue microarray, immunohistochemistry
Introduction
Cervical cancer is the third most commonly diagnosed cancer and the fourth leading cause of cancer-related deaths in women worldwide. Over 85% of newly diagnosed cases and cancer-related deaths occur in developing countries.1 In recent years, cervical cytology screening has greatly improved the early diagnosis of cervical cancer.2 However, incidence and mortality are still high, worldwide. Hence, there is an urgent need for further study of the molecular mechanisms of tumorigenesis and progression, which may identify new screening tools and biomarkers that facilitate early diagnosis as well as potential predictors of disease progression.
The aquaporins (AQPs) are a family of transmembrane water channel proteins expressed in many fluid-transporting tissues (eg, the glandular epithelia and kidney tubules) as well as in non-fluid-transporting tissues (eg, the epidermis). There are 13 known AQPs found in mammals. Their localization in the plasma membrane is crucial for the regulation of water transfer.3 The first member of the family to be identified was AQP1, which is a membrane protein that controls the permeability of endothelial and epithelial barriers by facilitating water movement across the cell membrane.4 In addition to its basic function, human AQP1 expression is heterogeneous and found in many different human tumors.5,6 Several studies have reported up-regulation of AQP1 in malignancies of various organs and tissues,7 such as glial tumors,8 breast cancer,9 and colorectal cancer.10 In contrast, decreased expression of AQP1 was observed in renal cell carcinoma (RCC),11,12 with a correlation between unfavorable outcomes and lower AQP1 expression in RCC,11,12 intrahepatic cholangiocarcinoma,13 and pleural malignant mesothelioma.14 Despite growing evidence that AQP1 is a crucial regulator of human cancers, its involvement in cervical cancer has not been assessed.
Therefore, in the present study, evaluations were made of AQP1 expression levels in cervical cancers and adjacent normal tissues. We then investigated the relationships between AQP1 expression and various clinicopathological parameters.
Materials and methods
Patients and tissue specimens
For quantitative reverse-transcription PCR (qRT-PCR) and western blot analysis, 18 pairs of fresh squamous cell cervical cancer and adjacent normal cervical tissue were collected from patients between September 2013 and February 2014. Immediately after surgery, the tissues were placed in RNAlater (Ambion, Austin, TX, USA). Following the manufacturer's instruction, the samples were kept submerged in RNAlater for at least 24 hrs at 4°C, and afterwards RNAlater was discarded and samples stored at −80°C until used. For tissue microarray (TMA) construction and immunohistochemistry (IHC) analysis, 73 paraffin-embedded tissues diagnosed between 2010 and 2013 were retrieved. All samples were collected from the Department of Obstetrics and Gynecology, the General Hospital of Guangzhou Military Command (Guangzhou, China). These samples were pathologically diagnosed cases of cervical cancer, having received no prior chemotherapy or radiotherapy before surgery. The age of these 63 patients ranged from 32 to 70 years (median, 44 years). Clinicopathological features of these patients included age at diagnosis, histological grade, clinical stage, and pTNM stage. Tumor clinical stage was according to the Federation International of Gynecology and Obstetrics staging of cervical carcinomas. Written informed consent was obtained from all patients for the use of tissue samples and clinical records. The study protocol was approved by the Ethics Committee of the General Hospital of Guangzhou Military Command, in accordance with the Declaration of Helsinki. Histopathological grading was by experienced pathologists.
qRt-PCR
Total RNA from the 18 pairs of frozen tissue was extracted by homogenization in RNAiso Reagent (Takara, Dalian, China), according to the manufacturer’s protocol. RNA was reverse-transcribed to generate cDNA by using a PrimeScript RT-PCR kit (Takara, Dalian, China). The PCR reaction included 90 ng of cDNA template, 0.4 μM of the forward and reverse primers each, 25 μL of the 2× SYBR Premix Ex Taq™ II (Takara, Dalian, China) buffer, and ddH2O to a total volume of 50 μL; β-actin was used as an internal control. For the AQP1 gene, the forward primer was 5′- ATGGCAACAGAAACCAAGAGACA-3′, and the reverse primer was 5′-TGAGAAGCTGGAAATGAGGGAA-3′. For β-actin, the forward primer was 5′- TGGCACCCAGCACAATGAA-3′, and the reverse primer was 5′-CTAAGTCATAGTCCGCCTAG AAGCA-3′. PCR was performed in an ABI 7500 real-time PCR amplifier (Applied Biosystems, Foster City, USA) with a pre-denaturation step of 95°C for 30 s, followed by 40 cycles with a denaturation step of 95°C for 5 s and an elongation step of 60°C for 34 s. Ct values were acquired using the 7,500 system SDS software (Applied Biosystems, Foster City, USA) with manual thresholds. The 2−△△Ct values were calculated as fold change between paired cervical cancer and normal cervical tissue. To acquire stable results, each PCR reaction was performed in triplicate. The qRT-PCR products were identified by electrophoresis in 2% agarose gels and visualized with UV light after staining with ethidium bromide. A Tanon 1,600 image station (Tanon, Shanghai, China) was used to capture band images.
Western blotting
Total protein from 18 paired cervical cancer and adjacent normal tissues was extracted in radio-immunoprecipitation assay buffer containing 1 mM phenylmethanesulfonyl fluoride. After centrifugation, supernatants were collected and protein concentration determined with a BCA Protein Assay Kit (Beyotime, Haimen, China) at 562 nm with a TECAN Infinite 200 microplate reader (TECAN, Austria). Tissue homogenates (35 μg of protein per sample) were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The protein bands were transferred onto a polyvinylidene difluoride membrane (Millipore, USA) with a Trans-Blot SD semi-dry transfer machine (Bio-Rad, USA). The blots were washed with 1× TBST buffer (10 mM Tris-HCl [pH 7.6], 150 mM NaCl, and 0.05% Tween-20), and membranes were blocked overnight with 5% skim milk in TBST and incubated with primary antibody at room temperature for 2 hrs. Polyclonal rabbit anti-human antibody reactive with AQP1 (Merck Millipore, Temecula, USA, at a dilution of 1:500) and monoclonal rabbit anti-human antibody reactive with β-actin (Cell Signaling Technology, USA, at a dilution of 1:3,000) were used as primary antibodies. The membranes were then washed with 1× TBST, and primary antibodies were detected with horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, USA, at a dilution of 1:5,000). Immunoreactive bands were visualized with a BeyoECL Plus Kit (Beyotime, Haimen, China) and scanned with an Image Station 4000R PRO analyzer (Carestream Health, USA).
TMA construction and IHC
Based on hematoxylin-eosin staining, representative sections of cervical cancer and normal cervical tissue in pre-existing paraffin-embedded tissue blocks were identified. A 1.5 mm diameter cylinder was punched from a representative section of each block and placed into a recipient paraffin block to construct the TMA. The TMA block was then sliced into 5 μm thick multiple sections and mounted on microscope slides for IHC. The TMA consisted of 63 cases of cervical cancer and 10 cases of normal control paraffin-embedded tissue. Clinical characteristics of the patients are summarized in Table 1. TMA slides were dried overnight at 37°C, deparaffinized in xylene, rehydrated through graded alcohol, immersed in 3% hydrogen peroxide for 15 mins to block endogenous peroxidase activity, and antigen-retrieved by microwave heating with sodium citrate buffer (pH 6.0) at 100°C for 20 mins. The slides were pre-incubated with 10% normal goat serum in TBST at room temperature for 30 mins to reduce nonspecific reactions. The primary rabbit anti-AQP1 polyclonal antibody (Merck Millipore, Temecula, USA) was diluted (1:500) with 1× phosphate buffered saline and incubated with the TMA overnight in a humidity chamber at 4°C. The slide was sequentially incubated with a polymer peroxidase labeled secondary antibody (ZSGB-Bio, Beijing, China) for 30 mins at room temperature and then stained with a DAB HRP Color Development Kit (Beyotime, Haimen, China). The sections were counterstained with Mayer’s hematoxylin, dehydrated, and mounted. As a negative control, the primary antibody was replaced with normal murine IgG. A known melanoma expressing high levels of AQP1 was used as a positive control.
Table 1.
Relationship between AQP1 expression and clinicopathological features in cervical cancer
| Clinicopathological feature | All cases | AQP1 expression | Pearson Chi-square | P-value‡ | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Age (years) | |||||
| <44^ | 31 | 13 | 18 | 0.021 | 0.884 |
| ≥44 | 32 | 14 | 18 | ||
| Tumor grade | |||||
| G1 | 11 | 10 | 1 | 18.572 | 0.000 |
| G2 | 33 | 15 | 18 | ||
| G3 | 19 | 2 | 17 | ||
| Clinical stage | |||||
| Ia | 6 | 6 | 0 | 15.609 | 0.008 |
| Ib | 12 | 7 | 5 | ||
| IIa | 3 | 1 | 2 | ||
| IIb | 16 | 8 | 8 | ||
| IIIa | 4 | 1 | 3 | ||
| IIIa | 22 | 4 | 8 | ||
| pT status | |||||
| T1(T1a+T1b) | 27 | 14 | 13 | 3.902 | 0.142 |
| T2(T2a+T2b) | 28 | 12 | 16 | ||
| T3(T3a+T3b) | 8 | 1 | 7 | ||
| pN status | |||||
| N0 | 40 | 23 | 17 | 9.593 | 0.002 |
| N1 | 23 | 4 | 19 | ||
| pM status | |||||
| M0 | 63 | 27 | 36 | N/A | N/A |
| M1 | 0 | 0 | 0 | ||
Notes: ^Median age. ‡P-value are from Chi-square test.
IHC evaluation
Immunoreactivity for the AQP1 protein was scored by a semi-quantitative method as the proportion of positive tumor cells over the total number of tumor cells. Scores were assigned in 5% increments (0%, 5%, …, 100%). The scores were accepted if all three investigators (MW, RS, and JZ) agreed. Otherwise, the values were re-estimated until a consensus was obtained. Conclusions were in complete agreement for 85% of the cases, indicating a high degree of reproducibility.
Selection of cut-off scores
Receiver operating characteristic (ROC) curve analysis was utilized to determine the cut-off score by using the 0.0, 1.0 criterion.15 At different AQP1 scores, the sensitivity and specificity for each outcome were plotted, generating various ROC curves. The score closest to the point with both maximum sensitivity and specificity was selected as the cut-off score. Tumors designated as “negative” for AQP1 were those with values below or equal to the cut-off score, whereas “positive” for AQP1 were values above the cut-off score.16,17 In order to perform ROC curve analysis, the clinicopathological features were dichotomized: age (< median age or ≥ median age), histological grade (low G1 or high G2+G3), clinical stage (Ia~Ib or IIa~IIIb), pT stage (early T1 or moderate and late T2+T3), N stage (N0 [no lymph node involvement], N1+N2 [any lymph node involvement]), M stage (M0 [no distant metastasis], or M1 [distant metastasis]).
Statistical analysis
Statistical analysis was performed with the SPSS statistical software program (standard version 13.0, SPSS, Chicago, IL, USA). ROC curve analysis was used to determine the cut-off score for positive expression of AQP1, and areas under curves (AUCs) were calculated. The relationships among AQP1 protein expression and clinicopathological features of cervical cancer patients were estimated using the χ2 test. P<0.05 was considered statistically significant by two-tailed test.
Results
Expression of AQP1 mRNA and protein in paired cervical cancer and adjacent normal tissue
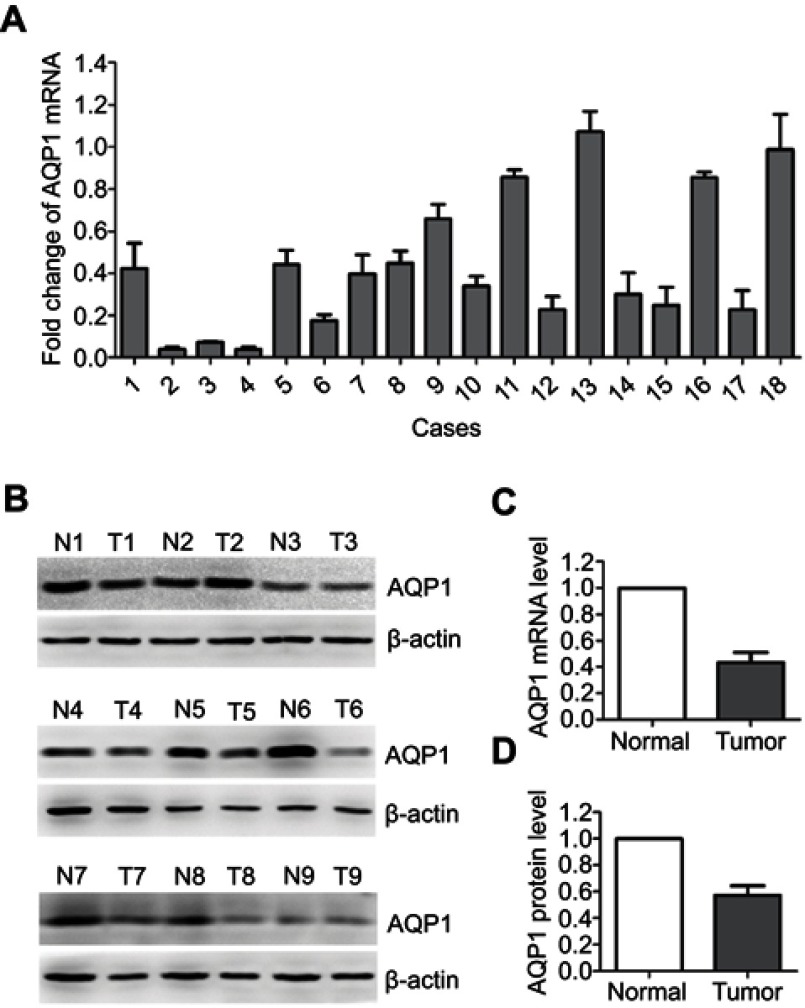
The qRT-PCR results showed that in the majority (17/18) of the sample pairs, fold changes (the 2−△△Ct values) were less than 1 between cervical and adjacent normal cervical tissue (Figure 1A), which indicated that AQP1 mRNA expression was decreased in cervical cancer tissues compared to adjacent normal tissue. Western blot analysis also demonstrated reductions in AQP1 protein for 15 of the 18 cervical cancer tissues compared to their adjacent normal counterparts (representative results are shown in Figure 1B). The mean fold change in AQP1 mRNA expression in cervical cancer tissue compared to adjacent normal tissue was 0.434. Paired t-test showed the difference between the two groups to be statistically significant (P=0.000, Figure 1C). The mean fold change in AQP1 protein level between the cervical cancer tissue and the adjacent normal tissue was 0.428 by semi-quantitative analysis. The difference between the two groups was also statistically significant by paired t-test (P=0.000, Figure 1D).
Figure 1.
qRT-PCR and western blot analysis of AQP1 expression in paired cervical cancer and adjacent normal cervical tissue.
Notes: (A) Fold changes (2−△△Ct values) by qRT-PCR showed a down-regulation of AQP1 mRNA in a majority of cervical cancer cases, when compared with paired normal cervical tissue. Expression levels were normalized for β-actin. (B) Western blotting indicated down-regulation of AQP1 protein in cervical cancer compared to adjacent normal cervical tissue. β-Actin was used as an internal control. T, cervical cancer; N, normal. (C) Significant differences in AQP1 mRNA expression between cervical cancer and adjacent normal cervical tissue (P=0.000). (D) Significant difference in AQP1 protein expression between cervical cancer and adjacent normal cervical tissue (P=0.000). *P<0.05 by paired two-sided t-test.
Abbreviation: qRT-PCR, quantitative reverse-transcription.
Expression levels of AQP1 in cervical cancer tissues by IHC
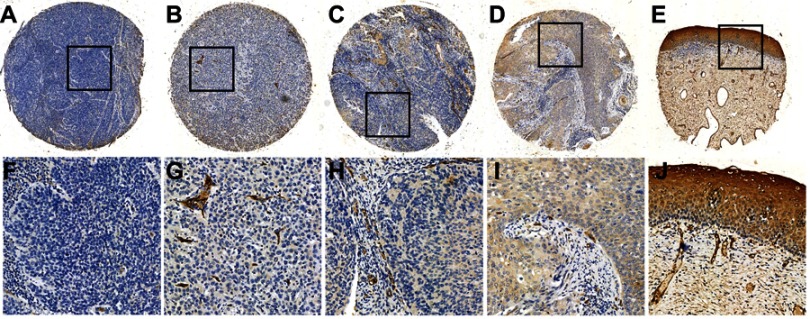
To investigate the AQP1 expression levels in cervical cancer, we examined AQP1 protein expression in 63 cases of cervical cancer and 10 adjacent cervical tissues by IHC and TMA. Immunoreactivity was observed primarily in the membrane of cells, especially in vascular endothelial cells, with occasional yellowish granules in the cytoplasm (Figure 2). Decreased expression of AQP1 was detected in poorly differentiated and advanced stage cervical cancer tissue (Figure 2A) compared to lower grade and stage cervical cancer tissue (Figure 2B, 2C, and 2D). Normal cervical epithelia showed very high AQP1 expression (Figure 2E).
Figure 2.
Expression levels of AQP1 protein in cervical cancer and adjacent normal cervical tissue.
Notes: (A) Negative expression of AQP1 in a cervical cancer case (case 19, grade III), in which none of the tumor cells showed immunoreactivity for the AQP1 protein (×100). (B) Negative AQP1 expression was observed in a cervical cancer sample (case 15, grade II), in which 10% of the tumor cells revealed positive immunostaining for AQP1 in the membrane (×100). (C) Negative AQP1 expression was observed in a cervical cancer sample (case 30, grade II) in which 35% of the tumor cells revealed positive immunostaining for AQP1 in the membrane (×100). (D) Positive AQP1 expression was observed in a cervical cancer sample (case 61, grade I), in which 85% of the tumor cells revealed positive immunostaining for AQP1 in the membrane (×100). (E) Positive expression of AQP1 protein in normal cervical tissue (×100). The lower panels (F–J) indicate higher magnification (×400) of areas in the boxes of (A–E), respectively.
Selection of cut-off scores for AQP1 IHC expression
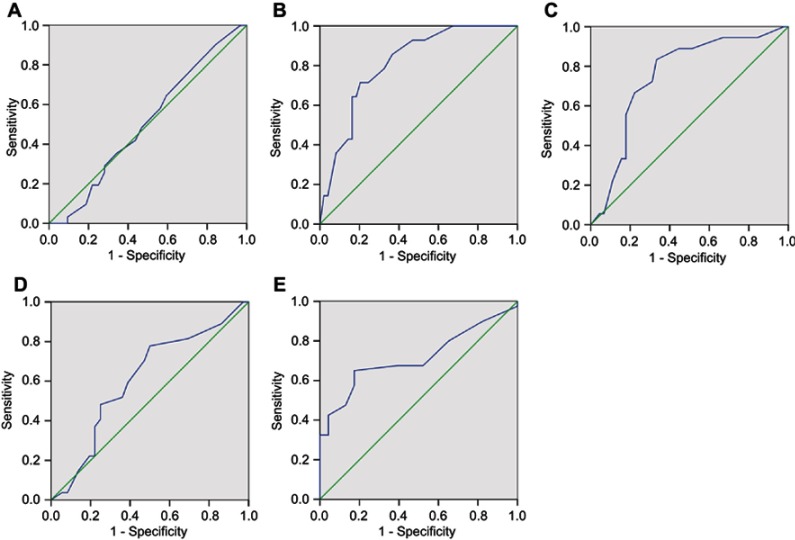
ROC curve analysis was used to determine the cut-off score for expression of AQP1. The ROC for each clinicopathological parameter (Figure 3) clearly identified points on the curves closest to (0.0, 1.0), which were maximal for both sensitivity and specificity for each outcome. Tumors with values below the obtained cut-off score were considered negative for AQP1 protein. The corresponding AUCs and cut-off scores are shown in Figure 3 and Table 2, respectively. Histology grade had the shortest significant distance from the curve to the point (0.0, 1.0). Hence, the cut-off score was determined by histology grade. The cut-off score for positive expression of AQP1 was defined as positive staining for AQP1≥45% of all cancer cells. AQP1 negative expression was observed in 57.14% (36/63) of cervical cancer tissue by IHC analysis. Negative expression of AQP1 has detected in 35/52 (67.3%) of grade G2+ G3 cervical cancers and in 21/45 (46.67%) of stage II + III cervical cancers. Positive expression of AQP1 was observed in 10/10 (100%) of normal cervical tissue, 10/11(90.91%) of grade G1, 6/6 (100%) of stage Ia, and 7/12 (58.3%) of stage Ib cervical cancers (Table 1).
Figure 3.
Receiver operating characteristic (ROC) curves were used to determine the cut-off score for positive AQP1 expression. The sensitivity and specificity for each outcome were plotted, and the areas under ROC curve presented.
Notes: (A) Age, (B) histological grade, (C) clinical stage, (D) pT stage, (E) pN stage.
Table 2.
Area under ROC curve for each clinicopathological feature
| Feature | AUC (95% CI) | P-value |
|---|---|---|
| Age | 0.507 (0.363–0.651) | 0.923 |
| Pathological grade | 0.810 (0.697–0.924) | 0.000 |
| Clinical stage | 0.752 (0.622–0.882) | 0.002 |
| T stage | 0.610 (0.467–0.752) | 0.139 |
| N stage | 0.715 (0.590–0.840) | 0.005 |
| M stage | N/A | N/A |
Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic.
Decreased expression of AQP1 correlates with clinicopathological features of cervical cancer
AQP1 expression in cervical cancer with respect to several standard clinicopathological features is shown in Table 1. The χ2 test showed AQP1 expression to be lower in patients with a poorly differentiated tumor grade (χ2=18.572, P=0.000), advanced clinical stage (χ2=15.609, P=0.008), and in patients with positive lymph nodes (χ2=9.593, P=0.002).
Discussion
AQPs have been reported in at least 13 different tumor cell types,18 with dysregulation observed in tumor cells and vascular endothelial cells. For some tumors, positive correlations have been established between AQP expression and histological grade, metastatic potential, and cancer prognosis. Up-regulation of AQP3 and AQP5 was reported in gastric carcinoma, with the level of expression found to correlate with lymph node metastasis and lympho-vascular invasion.19 Down-regulation of AQPs has been observed for AQP8 in colorectal cancer,20 AQP8 and AQP9 in hepatocellular carcinoma,21 and AQP4 in pleural mesothelioma.22
Despite higher expression in the microvascular, Mobasheri et al,9 reported that AQP1 was heterogeneously expressed in different human tumors and not necessarily expressed in all neoplastic cells as judged by IHC and TMA of prostate, colon, lung, breast, and ovarian cancer. These findings have been confirmed by other reports. Up-regulation of AQP1 was found in glioma, laryngeal cancer, hemangioblastoma, and colorectal cancer,4,23,24,25,26 with clinicopathological feature correlations. Otterbach et al,27 reported that AQP1 was strongly expressed on the membrane of breast cancer cells, with elevated expression significantly associated with poor prognosis. Down-regulation of AQP1 in RCC was reported as a potential prognostic factor for unfavorable outcomes in several cancers. By using different methods, both Takenawa et al, and Huang et al, reported that AQP1 expression was reduced in RCC,12 and that expression levels of AQP1 provided useful prognostic information for patients with RCC. Aishima et al,13 reported that down-regulation of AQP1 correlated with large tumor size, poorly differentiated histology, and positive lymph node metastasis in intrahepatic cholangiocarcinoma. Kao et al,14 suggested that expression of AQP1 by ≥50% of tumor cells was associated with significantly enhanced survival and could be used as an independent prognostic factor for pleural malignant mesothelioma. Interestingly, heterogeneous expression of AQP1 exists among different histopathologic tumor subtypes of RCCs. Huang et al,12 showed that although the majority of RCC subtypes express AQP1 at a lower level than the normal kidney, AQP1 levels were statistically higher in papillary and clear-cell RCCs than in all other subtypes. Median AQP1 expression in papillary RCCs was even higher than that in the normal kidney. Thus, the relationship between AQP1 expression and cancer is complex and requires careful interpretation.
Moreover, AQP1 has been reported to be involved in tumor angiogenesis,28,29 with overexpression of AQP1 in vitro increasing migration and metastasis of certain tumor cell lines.30,31 Another study suggested that AQP1 may serve as a therapeutic target for lung and glial tumors.10 In a recent study, we demonstrated AQP1 to promote cell differentiation of the human erythroleukemia, K562, by inducing the expression of erythroid differentiation related genes.32
The expression level of AQP1 in cervical cancer and its correlation with the clinicopathological features of cervical cancer was poorly recognized. Shi et al,33 found that AQP1 expression significantly increased in the advanced stage, deeper infiltration, metastatic lymph nodes and larger tumor volume in cervical carcinoma in Xinjiang Uygur women of China. Shen et al,34 found that AQP1 showed a higher positivity rate in intraepithelial neoplasia (CIN) than in squamous cervical cancer (SCC) and normal cervical tissues. And there was a significant increase in the expression of AQP1 in stage I than that in stage II of SCC. Herein, qRT-PCR, western blotting, and IHC were used to evaluate expression and immune-localization of AQP1 in patients with cervical cancer. Results showed that AQP1 mRNA and protein levels were decreased in human cervical cancer tissues when compared to corresponding adjacent normal tissues. IHC demonstrated AQP1 protein mainly on the cell membrane of tumor cells and in vascular endothelial cells, with expression reduced in cervical cancer tissue when compared to normal cervical epithelial tissue. AQP1 was found to be associated with tumor status. Decreased expression of AQP1 was significantly correlated to poor pathological grade, late clinical stage, and positive lymph node metastasis. Hence, decreased expression of AQP1 in squamous cell cervical cancer may be related to the process of tumor progression. These findings are consistent with AQP1 expression in RCCs,11,12 in that decreased expression of AQP1 was also observed. AQP1 expression levels in these cancers may be used as a predictive prognostic indicator for these patients. Validation of the levels of AQP1 and other AQPs in cervical cancer requires further large-scale clinical investigations. Detailed investigations into the role of AQP1 in the carcinogenesis and differentiation of cervical cancer are necessary in order to evaluate the use of AQP1 as a biomarker for diagnosis and prognosis of cervical cancer.
Conclusion
This study demonstrates AQP1 expression to be reduced in cervical cancer when compared to adjacent normal tissue. Decreased expression of AQP1 was significantly correlated with poor cancer cell differentiation and unfavorable clinical features in patients with cervical cancer. As such, AQP1 may serve as a potential biomarker for the diagnosis of cervical cancer.
Acknowledgment
The present study was supported by the National Natural Science Foundation of China (grant no. 81672754), the Natural Science Foundation of Guangdong Province, China (grant no. 2015A030313249), the Medical Scientific Research Foundation of Guangdong Province, China (grant no. A2017112), and the Science and Technology Planning Project of Shenzhen Nanshan District (grant no. 2017006).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Jemal A, Bray F, Center MM, et al. A global view on cancer incidence and national levels of the human development index. Int J Cancer. 2016;139:2436–2446. doi: 10.1002/ijc.30382 [DOI] [PubMed] [Google Scholar]
- 2.Sawaya GF, Huchko MJ. Cervical cancer screening. Med Clin North Am. 2017;101:743–753. doi: 10.1016/j.mcna.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown D. The discovery of water channels (Aquaporins). Ann Nutr Metab. 2017;70:37–42. doi: 10.1159/000463061 [DOI] [PubMed] [Google Scholar]
- 4.Tradtrantip L, Jin BJ, Yao X, et al. Aquaporin-targeted therapeutics: state-of-the-field. Adv Exp Med Biol. 2017;969:239–250. doi: 10.1007/978-94-024-1057-0_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribatti D, Ranieri G, Annese T, et al. Aquaporins in cancer. Biochim Biophys Acta. 2014;1840:1550–1553. doi: 10.1016/j.bbagen.2013.09.025 [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos MC, Saadoun S. Key roles of aquaporins in tumor biology. Biochim Biophys Acta. 2015;1848:2576–2583. doi: 10.1016/j.bbamem.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 7.Mobasheri A, Airley R, Hewitt SM, et al. Heterogeneous expression of the aquaporin 1 (AQP1) water channel in tumors of the prostate, breast, ovary, colon and lung: a study using high density multiple human tumor tissue microarrays. Int J Oncol. 2005;26:1149–1158. [PubMed] [Google Scholar]
- 8.Oshio K, Binder DK, Liang Y, et al. Expression of the aquaporin-1 water channel in human glial tumors. Neurosurgery. 2005;56:375–381. discussion −81. [DOI] [PubMed] [Google Scholar]
- 9.Mobasheri A, Barrett-Jolley R. Aquaporin water channels in the mammary gland: from physiology to pathophysiology and neoplasia. J Mammary Gland Biol Neoplasia. 2014;19:91–102. doi: 10.1007/s10911-013-9312-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida T, Hojo S, Sekine S, et al. Expression of aquaporin-1 is a poor prognostic factor for stage II and III colon cancer. Mol Clin Oncol. 2013;1:953–958. doi: 10.3892/mco.2013.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrissey JJ, Mellnick VM, Luo J, et al. Evaluation of urine aquaporin-1 and perilipin-2 concentrations as biomarkers to screen for renal cell carcinoma: a prospective cohort study. JAMA Oncol. 2015;1:204–212. doi: 10.1001/jamaoncol.2015.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Murakami T, Sano F, et al. Expression of aquaporin 1 in primary renal tumors: a prognostic indicator for clear-cell renal cell carcinoma. Eur Urol. 2009;56:690–698. doi: 10.1016/j.eururo.2008.10.014 [DOI] [PubMed] [Google Scholar]
- 13.Aishima S, Kuroda Y, Nishihara Y, et al. Down-regulation of aquaporin-1 in intrahepatic cholangiocarcinoma is related to tumor progression and mucin expression. Hum Pathol. 2007;38:1819–1825. doi: 10.1016/j.humpath.2007.04.016 [DOI] [PubMed] [Google Scholar]
- 14.Kao SC, Armstrong N, Condon B, et al. Aquaporin 1 is an independent prognostic factor in pleural malignant mesothelioma. Cancer. 2012;118:2952–2961. doi: 10.1002/cncr.26497 [DOI] [PubMed] [Google Scholar]
- 15.Cai MY, Zhang B, He WP, et al. Decreased expression of PinX1 protein is correlated with tumor development and is a new independent poor prognostic factor in ovarian carcinoma. Cancer Sci. 2010;101:1543–1549. doi: 10.1111/j.1349-7006.2010.01560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zlobec I, Steele R, Terracciano L, et al. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol. 2007;60:1112–1116. doi: 10.1136/jcp.2006.044537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zablotska IB, Prestage G, de Wit J, et al. The informal use of antiretroviral medications for pre-exposure prophylaxis (PrEP) of HIV among gay men in Australia. J Acquir Immune Defic Syndr. 2013;62:334–338. doi: 10.1097/QAI.0b013e31827e854a [DOI] [PubMed] [Google Scholar]
- 18.Verkman AS, Hara-Chikuma M, Papadopoulos MC. Aquaporins–new players in cancer biology. J Mol Med. 2008;86:523–529. doi: 10.1007/s00109-008-0303-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen L, Zhu Z, Huang Y, et al. Expression profile of multiple aquaporins in human gastric carcinoma and its clinical significance. Biomed Pharmacother. 2010;64:313–318. doi: 10.1016/j.biopha.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 20.Fischer H, Stenling R, Rubio C, Lindblom A. Differential expression of aquaporin 8 in human colonic epithelial cells and colorectal tumors. BMC Physiol. 2001;1:1. doi: 10.1186/1472-6793-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jablonski EM, Mattocks MA, Sokolov E, et al. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007;250:36–46. doi: 10.1016/j.canlet.2006.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roe OD, Anderssen E, Helge E, et al. Genome-wide profile of pleural mesothelioma versus parietal and visceral pleura: the emerging gene portrait of the mesothelioma phenotype. PLoS One. 2009;4:e6554. doi: 10.1371/journal.pone.0006554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saadoun S, Papadopoulos MC, Davies DC, et al. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87:621–623. doi: 10.1038/sj.bjc.6600512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan B, Zhu D, Dong Z, Yang Z. Expression and distribution of aquaporin 1 in laryngeal carcinoma. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;21:269–272. [PubMed] [Google Scholar]
- 25.Chen Y, Tachibana O, Oda M, et al. Increased expression of aquaporin 1 in human hemangioblastomas and its correlation with cyst formation. J Neurooncol. 2006;80:219–225. doi: 10.1007/s11060-005-9057-1 [DOI] [PubMed] [Google Scholar]
- 26.Moon C, Soria JC, Jang SJ, et al. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699–6703. doi: 10.1038/sj.onc.1206762 [DOI] [PubMed] [Google Scholar]
- 27.Otterbach F, Callies R, Adamzik M, et al. Aquaporin 1 (AQP1) expression is a novel characteristic feature of a particularly aggressive subgroup of basal-like breast carcinomas. Breast Cancer Res Treat. 2010;120:67–76. doi: 10.1007/s10549-009-0370-9 [DOI] [PubMed] [Google Scholar]
- 28.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460 [DOI] [PubMed] [Google Scholar]
- 29.Clapp C. Martinez de la Escalera G. Aquaporin-1: a novel promoter of tumor angiogenesis. Trends Endocrinol Metab. 2006;17:1–2. doi: 10.1016/j.tem.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 30.Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–1894. doi: 10.1096/fj.06-5930fje [DOI] [PubMed] [Google Scholar]
- 31.Hoque MO, Soria JC, Woo J, et al. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol. 2006;168:1345–1353. doi: 10.2353/ajpath.2006.050596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei M, Shi R, Zeng J, et al. The over-expression of aquaporin-1 alters erythroid gene expression in human erythroleukemia K562 cells. Tumour Biol. 2015;36:291–302. doi: 10.1007/s13277-014-2614-5 [DOI] [PubMed] [Google Scholar]
- 33.Shi YH, Chen R, Talafu T, et al. Significance and expression of aquaporin 1, 3, 8 in cervical carcinoma in Xinjiang Uygur women of China. Asian Pac J Cancer Prev. 2012;13:1971–1975. [DOI] [PubMed] [Google Scholar]
- 34.Shen Q, Lin W, Luo H, et al. Differential expression of aquaporins in cervical precursor lesions and invasive cervical cancer. Reprod Sci. 2016;23:1551–1558. doi: 10.1177/1933719116646202 [DOI] [PubMed] [Google Scholar]