Abstract
Sex steroids negatively regulate B lymphopoiesis in adult mice. Paradoxically, lymphocytes arise during fetal life, when estrogen levels are high and maternal lymphopoiesis is suppressed. Here we demonstrate that embryonic B lymphopoiesis was unaffected by estrogen, but sensitive to glucocorticoids. Both fetal and adult precursors contained glucocorticoid receptor transcripts, but only adult precursors expressed estrogen receptor α and β together with the androgen receptor. Fetal hematopoietic cells did not efficiently acquire functional estrogen receptors after transplantation to irradiated adult mice. Sex steroid receptors were also expressed in a stage- and developmental age-dependent fashion in human precursors. A developmental switch in responsiveness of hematopoietic cells to sex steroids may be essential for formation of the immune system.
Steroid hormones control a wide variety of biological processes, including the development of the immune system. Sex steroids are of particular interest because of findings that B lymphocyte formation is selectively reduced in bone marrow of estrogen-treated adult mice (1, 2). Reciprocally, B lymphopoiesis is abnormally elevated in either castrated male or ovariectomized female mice (3, 4). The same is true of hormone-deficient hypogonadal animals and androgen receptor (AR)-deficient male Tfm/Tfm mice (5–7). Both stromal and hematopoietic cells in marrow express estrogen receptors (ERs) and could represent targets for hormone mediated regulation of lymphocyte formation (6, 8). However, recent studies revealed that purified lymphocyte precursors are directly influenced by estrogen in culture (9). Furthermore, a very discrete population of c-kitHi Sca-1+ CD27+ TdT+ early lymphocyte precursors is selectively depleted in estrogen-treated mice (10). Transplantation studies conducted with ER gene-targeted animals indicate that ERα expressed by lymphocyte precursors is particularly important for hormone-mediated suppression of lymphopoiesis (11).
Sex steroid levels rise during pregnancy, presumably accounting for the marked suppression of B lymphocyte precursors in maternal bone marrow and transient atrophy of the thymus (12–17). The fetus also synthesizes sex steroids, and it is enigmatic that B lineage lymphocytes first appear in substantial numbers in fetal liver, where levels of the estrogens estriol and estetrol are particularly high (18–20). However, previous findings that lymphohematopoietic cells in fetal and adult tissues have some different properties provide a possible explanation for this enigma (21–25). Indeed, we now show that this extends to the expression of functional estrogen receptors. We developed methods for isolation and culture of rare lymphocyte precursors and used them to determine that adult but not fetal precursors are estrogen sensitive. Furthermore, analysis of transcripts corresponding to various sex steroid receptors revealed that they are expressed in a discrete age- and stage-dependent fashion. Practical consequences of this developmental switch include protection of the fetus from estrogen-like compounds. Additionally, fetal lymphocyte precursors did not efficiently acquire adult properties after transplantation to adult mice, raising questions about the origins of stem cells in adult marrow.
Materials and Methods
Animals.
The congenic strains of mice, C57BL/6J (CD45.2 alloantigen), C57BL/6SJL (CD45.1 alloantigen), and recombination activating gene 1 (RAG-1)-deficient mice (CD45.2 alloantigen) were purchased from The Jackson Laboratory and maintained in our laboratory animal facility. Embryonic staging was based on the appearance of vaginal plugs after overnight mating, and 15 days postcoitus (dpc) embryos were used for most experiments.
Human Cell Sources.
Cord blood samples were obtained from placentas of healthy newborns collected at the Oklahoma University Hospital (Oklahoma City). Adult bone marrow samples were collected from patients that were undergoing hip replacement surgery at the Bone and Joint Hospital (Oklahoma City). An Institutional Review Committee approved all procedures involving human cells.
Fetal Liver Organ Cultures (FLOC).
We used a method that was originally described by Owen et al. (26) and modified by Ceredig et al. (27). Briefly, small pieces of fetal liver were put on nitrocellulose filters (Millipore) supported with gelatin sponges (Gelfoam; Amersham Pharmacia and Upjohn) and cultured in 24-well flat-bottom culture plates (Costar) containing DMEM-based medium supplemented with 5 × 10−5 M 2-mercaptoethanol/1× nonessential amino acids (GIBCO/BRL)/0.03% Primatone (Quest International, Naarden, The Netherlands)/2% FCS. Fragments were incubated for 6 days in a humidified CO2 incubator at 37°C with or without hormones or reagents. Fetal liver cells recovered from culture were dispersed by pipetting in staining buffer (phosphate-buffered salt solution with 3% FCS and 0.1% sodium azide). The fetal liver cells were transferred to new tubes and then stained with FITC-conjugated goat anti-mouse IgM (Zymed) and allophycocyanin-conjugated rat anti-mouse CD45R (PharMingen).
Cell Sorting and Flow Cytometry.
Bone marrow cells and fetal liver cells were harvested and enriched for lineage-negative cells by incubation with antibodies to lineage markers, anti-Gr-1 (Ly-6G; RB6–8C5) and anti-CD11b/Mac-1 (M1/70) for myeloid cells, anti-CD19 (1D3) and anti-CD45R/B220 (RA3/6B2) for B lineage cells and Ter-119 for erythroid cells, followed by negative selection with the MACS cell separation system (Miltenyi Biotec, Auburn, CA). These partially lineage-depleted cells were further stained with FITC-rat anti-mouse lineage markers (Gr-1, Mac-1, CD2, CD3, and CD8), phycoerythrin (PE)-anti-mouse lineage markers (Ter-119 and CD45R) and allophycocyanin-rat anti-mouse c-kit. Lineage-positive cells were electronically gated out and lineage negative fractions were sorted as c-kitHi or c-kitLo cells on the MoFlo (Cytomation, Ft. Collins, CO). In adoptive transfer experiments, PE-anti-Sca-1antibody (Ly6A/E; E13–161.7) was used for sorting. In this case, PE-anti-Ter-119 was eliminated, and FITC-anti-CD45R was used instead of PE-conjugated antibody. All mAbs were purchased from PharMingen. Freshly isolated human bone marrow and cord blood cells were enriched to CD34+ or CD34− fractions by positive and negative selection using the MACS separation system. Enriched cells were stained with FITC-anti-CD34 (HPCA-2) from Becton Dickinson, then sorted into CD34+ and CD34− populations on the MoFlo. FITC-anti-CD11b/Mac-1 (PharMingen), PE-anti-CD19 (PharMingen), and PE-anti IgD (Southern Biotechnology Associates) were used for mouse cell phenotype determination. Flow cytometry was performed on FACScalibur (Becton Dickinson) and the data were analyzed with flojo software (Treestar, San Carlos, CA)
Cell Culture.
Details of stromal cell-free, serum-free culture of early lymphocyte precursors are described elsewhere (9). Briefly, sorted cells were cultured with X-VIVO15 medium (BioWhittaker) containing 1% detoxified BSA (Stem Cell Technologies, Vancouver, BC, Canada), 2 mM l-glutamine, 5 × 10−5 M 2-mercaptoethanol, 100 units/ml penicillin, and 100 mg/ml streptomycin. Recombinant mouse stem cell factor (20 ng/ml), Flk2/Flt3 ligand (100 ng/ml) (R&D Systems) and recombinant mouse IL-7 (1 ng/ml) (Endogen, Cambridge, MA) were included to drive B lymphoid lineage differentiation. 17β-Estradiol and dexamethasone used for cell culture were from Sigma.
Reverse Transcriptase (RT)-PCR Analysis of Hormone Receptor Expression.
The mRNAs were isolated from sorted cells by using MicroPoly(A) Pure (Ambion, Austin, TX) and converted to cDNA with Moloney murine leukemia virus RT (GIBCO/BRL). The PCR was conducted by using combination with ampli-Taq DNA polymerase (Takara, Shiga, Japan) and TaqStart antibody (CLONTECH) at three different cycles to confirm the reaction is in the exponential phase. To compare the relative expression level of receptors, serial 5-fold dilutions of cDNA were amplified at appropriate cycles. In the case of no amplification, secondary PCR was performed by using 2 μl for 20-μl reaction volume from first reaction as a template to confirm the lack of transcripts. Anti-Taq antibody was inactivated by heating at 95°C for 7 min before amplification carried out as follows: 15 s at 94°C, 15 s at 57°C, and 30 s at 72°C. The following gene specific primers were used: 5′-GACCAGATGGTCAGTGCCTT-3′ and 5′-ACTCGAGAAGGTGGACCTGA-3′ for mouse ERα (28); 5′-CAGTAACAAGGGCATGGAAC-3′ and 5′-GTACATGTCCCACTTCTGAC-3′ for mouse ERβ (28); 5′-CCATCCAAGACCTATCGAGG-3′ and 5′-TGAGTCATCCTGATCTGGAG-3′ for mouse AR (29); 5′-TGCTATGCTTTGCTCCTGATCTG and 5′-TGTCAGTTGATAAAACCGCTGCC-3′ for mouse glucocorticoid receptor (GR; ref. 30); 5′-AGACATGAGAGCTGCCAACC-3′ and 5′-GCCAGGCACATTCTAGAAGG-3′ for human ERα (31); 5′-TCACATCTGTATGCGGAACC-3′ and 5′-CGTAACACTTCCGAAGTCGG-3′ for human ERβ (31); 5′-ATGGCTGTCATTCAGTACTCCTGGA-3′ and 5′-AGATGGGCTTGACTTTCCCAGAAAG-3′ for human AR (32); 5′-TCGACCAGTGTTCCAGAGAAC-3′ and 5′-TTTCGGAACCAACGGGAATTG-3′ for human GR (33); and 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′ for mouse and human β-actin (33). PCR products were resolved on 2% agarose gels and visualized by ethidium bromide staining. Corresponding PCR products were excised and validated by sequencing.
Fetal Liver Cell Transplantation.
Purified lineage marker negative (Lin−) c-kitHi Sca-1+ hematopoietic progenitor cells (1 × 104) from 15-dpc fetal liver of C57BL/6 SJL mice (CD45.1) were transferred intravenously into irradiated (500 rad) RAG-1-deficient mice (CD45.2). Four weeks after transplantation, bone marrow cells were harvested from these mice. Biotin-anti-CD45.1 (Ly5.1; A20) and CD45.2 (Ly5.2; 104) mouse mAbs were used in combination with streptavidin-RED613 (GIBCO/BRL) to distinguish donor cells from recipient cells. The donor fetal liver type Lin− c-kitHi Sca-1+ CD45.1+ cells and recipient bone marrow-type Lin− c-kitHi Sca-1+ CD45.1− were both recovered by sorting. To see the effect of estrogen in reconstituted RAG-1-deficient mice with purified Lin− c-kitHi Sca-1+ hematopoietic progenitor cells from 8-week-old C57BL/6SJL adult bone marrow or 15-dpc fetal liver, pellets released 17β-estradiol constantly at 0.1 mg per 21 days (Innovative Research of America) were implanted s.c. under anesthesia 1 day before transplantation, and additional pellets were injected 21 days later to maintain high hormone levels.
Results and Discussion
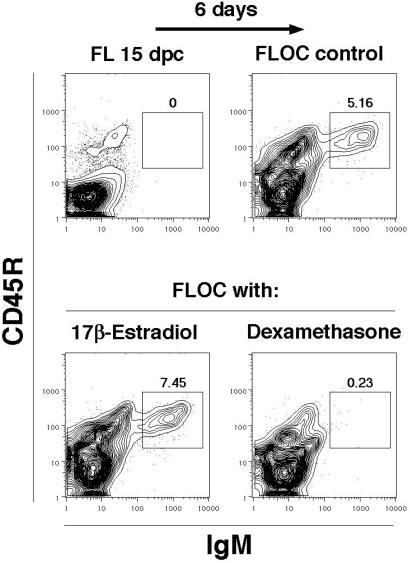
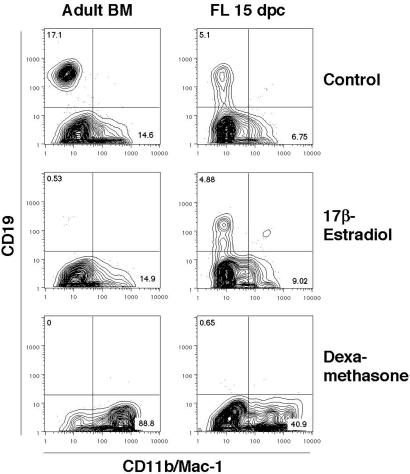
We conducted initial experiments with FLOC that provided a natural three-dimensional environment and supported all stages of lymphopoiesis (26, 27). Formation of CD45R+ surface IgM+ B cells during 6 days of FLOC was unaffected by addition of up to 10−4 M 17β-estradiol (Fig. 1). The lowest dose used was sufficient to suppress B lymphopoiesis in cultures initiated with lymphocyte precursors taken from adult bone marrow (ref. 9 and Fig. 2 Middle). In contrast, dexamethasone or hydrocortisone completely prevented B lymphocyte formation in FLOC (Fig. 1 and data not shown). It seemed unlikely that poor diffusion accounted for the resistance of fetal B lymphopoiesis to estrogen, because the higher molecular weight glucocorticoids were effective. Alternatively, fetal liver might contain enzymes or cells capable of selectively inactivating sex steroids. To test this possibility, we isolated progenitor cells from adult bone marrow or 15-dpc fetal liver and compared their hormone sensitivities under defined stromal cell-free, serum-free conditions (Fig. 2). As previously shown, the Lin− c-kitLo fraction of bone marrow is highly enriched for lymphocyte precursors (9, 34, 35). Generation of CD19+ B lineage lymphocytes from these cells was selectively suppressed by the addition of estrogen, whereas CD11b/Mac-1+ myeloid lineage cells were unaffected. However, estrogen had no effect on production of B lineage lymphocytes in cultures initiated with 15-dpc fetal liver precursors (Fig. 2). B lymphopoiesis was completely suppressed in cultures of fetal or adult cells when dexamethasone was present and proportions of myeloid lineage cells were elevated 2.1- to 3.1-fold relative to control cultures. These experiments demonstrate a remarkable difference in the intrinsic, lineage-specific sensitivities of fetal and adult lymphocyte precursors to estrogen.
Figure 1.
B lymphopoiesis in FLOC is unaffected by estrogen. Fragments of 15-dpc fetal liver (FL) from C57BL/6J mice were cultured for 6 days in the presence of medium alone, 17β-estradiol (10−4 M), or dexamethasone (10−8 M). Flow cytometry was performed on the recovered cells, as well as suspensions of the initial fetal liver with the indicated mAbs. The regions of the contour plots containing newly formed B cells are marked with boxes (percentages are indicated above the boxes). Before culture, fetal liver lacked CD45R+ IgM+ B cells (Upper Left), but these cells emerged during 6 days of organ culture (Upper Right, 0.16 ± 0.01 × 106 B cells per fragment). In three independent experiments, B cell production was unaffected by inclusion of estrogen (Lower Left, 0.18 ± 0.01 × 106 B cells per fragment), whereas it was always totally suppressed by the synthetic glucocorticoid dexamethasone (Lower Right, <0.01 × 106 B cells per fragment).
Figure 2.
Progenitors from fetal liver, but not adult marrow, generate B lineage cells in the presence of estrogen. Bone marrow (BM) cells from 8-week-old C57BL/6J mice and fetal liver (FL) cells from 15 dpc embryos were harvested, enriched for lineage-negative cells, and sorted as Lin− c-kitLo cells on the MoFlo. These highly enriched precursors were then cultured for 7 days under stromal cell-free, serum-free conditions in the presence of medium alone, 17β-estradiol (10−8 M) or dexamethasone (10−8 M). Recovered cells were evaluated by flow cytometry. Lymphoid progenitors from either bone marrow or fetal liver gave rise to CD19+ B lineage cells in stromal-cell-free, serum-free cultures (Top, 10.7 ± 0.26 × 103 CD19+ cells per marrow precursor culture, and 1.84 ± 0.16 × 103 CD19+ cells per fetal precursor culture). In two independent experiments, estrogen blocked B lineage differentiation from adult (Left Middle, 0.17 ± 0.03 × 103 CD19+ cells per marrow precursor culture), but not fetal (Right Middle, 2.26 ± 0.14 × 103 CD19+ cells per fetal precursor culture) progenitors. The synthetic glucocorticoid dexamethasone totally blocked generation of CD19+ lymphocytes and induced CD11b/Mac-1+ myeloid lineage cells regardless of the source of precursors (Bottom, < 0.07 × 103 CD19+ cells recovered per culture).
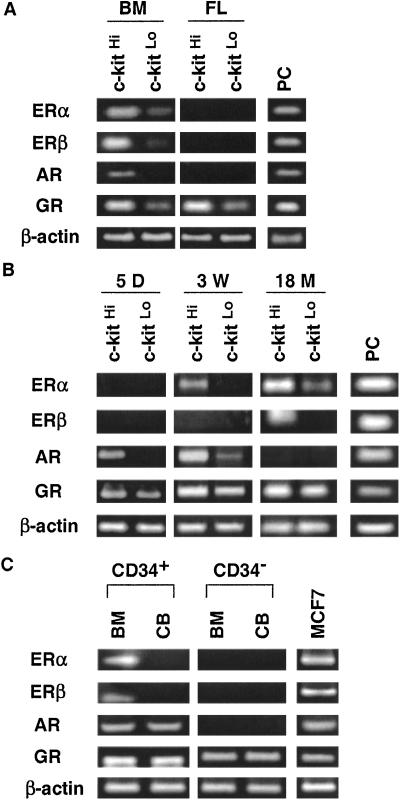
Little information was available about expression of steroid hormone receptors on lymphohematopoietic cells, and an explanation was sought for the hyposensitivity of progenitors in fetal liver. We used RT-PCR to detect transcripts for hormone receptors (Fig. 3A). The Lin− fractions of adult marrow and fetal liver were isolated and further separated on the basis of c-kit density because this growth factor receptor is progressively down-regulated as differentiation proceeds (34). Four hormone receptor genes were transcribed by the early Lin− c-kitHi precursor fraction of adult bone marrow. ERα, ERβ, and GR transcripts were reduced, but still detectable, in the Lin− c-kitLo fraction of adult marrow, whereas mRNA for AR was found only in the less-differentiated Lin− c-kitHi fraction (Fig. 3A). In contrast, fetal-liver-derived precursors expressed only GR and sex steroid receptor transcripts were undetectable.
Figure 3.
Receptors for sex steroids are not acquired by hematopoietic cells until after birth. (A) Lin− c-kitHi or Lin− c-kitLo cells were sorted from 8-week-old adult mice bone marrow (BM) or 15-dpc fetal liver (FL). Transcripts corresponding to hormone receptors were then examined by RT-PCR. RNAs from uterus for ERs, kidney for AR, and 70Z/3 pre-B cells for GR were used as positive controls (PC). (B) Lin− c-kitHi or Lin− c-kitLo cells were sorted from bone marrow of 5-day-old (5 D), 3-week-old (3 W), or 18-month-old (18 M) mice. The expression of hormone receptors was examined by RT-PCR. (C) Freshly isolated human bone marrow (BM) and cord blood (CB) cells were sorted into CD34+ and CD34− populations. Hormone-receptor expression was examined by RT-PCR. RNA from the MCF7 human breast cancer cell line served as a positive control.
The developmental dependence of steroid hormone receptor expression was then more carefully examined, using bone marrow cells from mice of different ages (Fig. 3B). GR transcripts were detected in all samples, and AR mRNA was found in the Lin− c-kitHi marrow fraction isolated from 5-day-old mice. ERs were acquired more slowly in development, with ERα transcripts being detectable in samples from 3-week-old mice and ERβ mRNA still later. These findings indicate that hormone-receptor expression in lymphohematopoietic precursors is highly regulated with respect to developmental age as well as differentiation stage. One study found weak estrogen-binding activity in murine fetal liver separated by density centrifugation, and fetal thymic atrophy resulted from maternal treatment with the synthetic estrogen diethylstilbestrol (36). Others found that fetal thymocytes in organ fragment cultures were sensitive to high-dose exposure to estrogen (37). However, our findings with B cell precursors in fetal liver and developing bones are most compatible with those reported by Barr et al. (38), who found fetal thymic organ cultures to be estrogen resistant and glucocorticoid sensitive, whereas ER expression in the thymus was markedly age dependent. Therefore, the lack of hormone receptors and resulting insensitivity to estrogen may allow T and B lymphoid lineages to emerge during embryonic development when high hormone levels suppress maternal lymphopoiesis.
It has long been known that lymphocyte precursors in adult marrow are sensitive to glucocorticoids (39), and we now report that fetal cells express functional receptors for this class of hormones. However, the situation is quite different from sex steroids. Placental cells have an active 11β-hydroxysteroid dehydrogenase that catalyzes the conversion of most of the biological active cortisol to inactive cortisone and of prednisolone to its inactive analog prednisone. This catalysis is but one mechanism that may protect the fetus from high maternal cortisol levels (40). Fetal plasma concentrations of prednisolone are only 10% of those for the mother (41). Embryonic tissues also contain large amounts of C11-hydroxysteroid dehydrogenase, which converts active cortisol to inactive cortisone (42). At birth, fetal cortisol levels are only one-third or less of the maternal values (43). Some synthetic steroids, such as dexamethasone, cross the placenta essentially unchanged, a fact that can even be exploited therapeutically (44). Indeed, we found that precursors in fetal liver lost the ability to produce B cells in cultures when pregnant mice were treated with dexamethasone for 24 h (unpublished data). We conclude that different mechanisms are used to protect the developing immune system from sex steroids and glucocorticoids.
Species differences in hormone-receptor expression and function are possible, and we investigated whether our experimental animal findings were applicable to humans. As in the mouse, ER and AR transcripts were detectable in relatively undifferentiated human cells isolated from adult bone marrow and identified on the basis of CD34 expression (Fig. 3C). Only GR mRNA was found in the more mature CD34− cells obtained from the same specimens. We also investigated hormone-receptor expression by RT-PCR in umbilical cord blood cells, as these are being increasingly used as a source of stem cells for transplantation (45) and should be representative of the human neonatal period (Fig. 3C). It is interesting that although GR transcripts were again easily detected in all cord blood fractions, AR mRNA was found only in CD34+ cells, and neither of the ERs was expressed. This pattern is similar to that found in 5-day-old murine bone marrow. With the exception of established cell lines, little information was available about ER expression by human hematopoietic cells (46–48). However, consistent with our results, one study found no transcripts for ERα in unfractionated, mid-gestational fetal liver (49). We conclude that human lymphohematopoietic cells also express steroid hormone receptors in an age and differentiation stage-dependent fashion.
Although many studies have revealed differences in embryonic and adult blood cell formation (21–25), it is believed that bone marrow is colonized by stem cells during late embryonic development. Thus, we expected that environmental conditions would dictate an adult pattern of steroid hormone receptor expression. However, this was not the case in a RT-PCR analysis of lymphohematopoietic cells recovered from irradiated adult mice transplanted 4 weeks earlier with Lin− c-kitHi Sca-1+ stem cells from 15-dpc fetal liver (Fig. 4A). Only GR transcripts were detectable in donor-type Lin− c-kitHi Sca-1+ CD45.1+ cells. Lin− c-kitHi Sca-1+ fractions of adult or fetal tissues were then transferred into adult untreated mice or animals containing time-release estrogen pellets (Fig. 4B). Donor-type CD45.1+ cells in the recipient spleens were identified by flow cytometry and analyzed for B lymphocyte lineage development. Hormone treatment almost completely blocked B lymphocyte formation when the donor cells were of adult origin. In contrast, B lymphopoiesis initiated with transplanted fetal cells was reduced only by an average of 50%. These results suggest that fetal hematopoietic cells do not rapidly acquire functional ERs during residence within adult bone marrow. They also raise the provocative possibility that stem cells in fetal liver are intrinsically different from those that colonize the marrow of developing bones. We note that patterns of hematopoietic regeneration in patients transplanted with stem cells from human cord blood are not identical to those receiving adult marrow (50, 51). Our experiments show that hormones have the potential for modulating lymphocyte recovery in transplant recipients. Estrogen and glucocorticoid levels could be informative indices of success, and possibly therapeutically manipulated to improve immunological recovery.
Figure 4.
Fetal hematopoietic cells do not express hormone receptors after transfer to adult mice. (A) Purified Lin− c-kitHi Sca-1+ hematopoietic progenitor cells from 15-dpc fetal liver were transferred into irradiated RAG-1-deficient mice. Four weeks after transfer, bone marrow cells were harvested and sorted into fetal-liver-derived Lin− c-kitHi Sca-1+ CD45.1+ and adult recipient Lin− c-kitHi Sca-1+ CD45.1− populations. The expression of hormone receptors was then examined by RT-PCR. PC, positive control. (B) We used an identical experimental design to recover spleen cell suspensions from mice transplanted 4 weeks earlier with Lin− c-kitHi Sca-1+ cells isolated from adult bone marrow or fetal liver, and we examined the cells by flow cytometry. Some of the recipients were hormone-treated, and donor CD45.1+ cells were gated as shown for analysis with the indicated antibodies. (Upper) the engraftment and differentiation of donor Lin− c-kitHi Sca-1+ CD45.1+ cells. B cells bearing various levels of IgM and IgD, representing various stages of maturity, were present in recipient spleens regardless of transplant source. (Lower) The remarkable estrogen resistance of fetal liver as compared with adult bone marrow-derived precursors.
In summary, these observations further our understanding of fetal immune system development and the extent to which it may be influenced by estrogen-like compounds. The finding that androgen receptors are acquired at an earlier age than estrogen receptors could conceivably be important in understanding the gender bias that exists for some diseases. There are remarkable similarities in patterns of hormone receptor expression in humans and mice, like placental hormone biosynthesis (52), validating experimental models for addressing many important questions, among them issues relating to adult, as contrasted with embryonic sources of stem cells, with potential for regenerating many tissue types (53).
Acknowledgments
We thank K. Garrett, R. Meka, S. Gregory, J. Henthorn, and V. Dandapani for technical assistance. Drs. M. Coggeshall, X.-H. Sun, L. Thompson, and J. Owen provided valuable comments on the manuscript. This work was supported by National Institutes of Health Grants AI20069 and NIH-1-P20-RR15577.
Abbreviations
- ER
estrogen receptor
- AR
androgen receptor
- GR
glucocorticoid receptor
- FLOC
fetal liver organ cultures
- dpc
days postcoitus
- Lin
lineage marker
- RT
reverse transcriptase
- RAG-1
recombination activating gene 1
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Medina K L, Kincade P W. Proc Natl Acad Sci USA. 1994;91:5382–5386. doi: 10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medina K L, Strasser A, Kincade P W. Blood. 2000;95:2059–2067. [PubMed] [Google Scholar]
- 3.Wilson C A, Mrose S A, Thomas D W. Blood. 1995;85:1535–1539. [PubMed] [Google Scholar]
- 4.Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T. J Clin Invest. 1994;94:1090–1097. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smithson G, Beamer W G, Shultz K L, Christianson S W, Shultz L D, Kincade P W. J Exp Med. 1994;180:717–720. doi: 10.1084/jem.180.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smithson G, Couse J F, Lubahn D B, Korach K S, Kincade P W. J Immunol. 1998;161:27–34. [PubMed] [Google Scholar]
- 7.Ellis T M, Moser M T, Le P T, Flanigan R C, Kwon E D. Int Immunol. 2001;13:553–558. doi: 10.1093/intimm/13.4.553. [DOI] [PubMed] [Google Scholar]
- 8.Smithson G, Medina K, Ponting I, Kincade P W. J Immunol. 1995;155:3409–3417. [PubMed] [Google Scholar]
- 9.Kouro T, Medina K L, Oritani K, Kincade P W. Blood. 2001;97:2708–2715. doi: 10.1182/blood.v97.9.2708. [DOI] [PubMed] [Google Scholar]
- 10.Medina K L, Garrett K P, Thompson L F, Rossi M I, Payne K J, Kincade P W. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 11.Thurmond T S, Murante F G, Staples J E, Silverstone A E, Korach K S, Gasiewicz T A. Endocrinology. 2000;141:2309–2318. doi: 10.1210/endo.141.7.7560. [DOI] [PubMed] [Google Scholar]
- 12.Turnbull A C, Patten P T, Flint A P, Keirse M J, Jeremy J Y, Anderson A B. Lancet. 1974;i:101–103. doi: 10.1016/s0140-6736(74)92337-x. [DOI] [PubMed] [Google Scholar]
- 13.Jacquet P, Gerber G B, Leonard A, Maes J. Experientia. 1977;33:1375–1377. doi: 10.1007/BF01920190. [DOI] [PubMed] [Google Scholar]
- 14.Wilson M, Morganti A A, Zervoudakis I, Letcher R L, Romney B M, Von Oeyon P, Papera S, Sealey J E, Laragh J H. Am J Med. 1980;68:97–104. doi: 10.1016/0002-9343(80)90178-3. [DOI] [PubMed] [Google Scholar]
- 15.Medina K L, Smithson G, Kincade P W. J Exp Med. 1993;178:1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phuc L H, Papiernik M, Berrih S, Duval D. Clin Exp Immunol. 1981;44:247–252. [PMC free article] [PubMed] [Google Scholar]
- 17.Phuc L H, Papiernik M, Dardenne M. Clin Exp Immunol. 1981;44:253–261. [PMC free article] [PubMed] [Google Scholar]
- 18.Strasser A, Rolink A, Melchers F. J Exp Med. 1989;170:1973–1986. doi: 10.1084/jem.170.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tulchinsky D. J Clin Endocrinol Metab. 1973;36:1079–1087. doi: 10.1210/jcem-36-6-1079. [DOI] [PubMed] [Google Scholar]
- 20.Tulchinsky D, Frigoletto F D, Jr, Ryan K J, Fishman J. J Clin Endocrinol Metab. 1975;40:560–567. doi: 10.1210/jcem-40-4-560. [DOI] [PubMed] [Google Scholar]
- 21.Landreth K S, Kincade P W, Lee G, Medlock E S. J Immunol. 1983;131:572–580. [PubMed] [Google Scholar]
- 22.Hardy R R, Hayakawa K. Proc Natl Acad Sci USA. 1991;88:11550–11554. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dzierzak E, Medvinsky A. In: Molecular Biology of B Cell and T Cell Development. Monroe J G, Rothenberg E V, editors. Totowa, NJ: Humana; 1998. pp. 3–25. [Google Scholar]
- 24.Wang J H, Nichogiannopoulou A, Wu L, Sun L, Sharpe A H, Bigby M, Georgopoulos K. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Tajima F, Ogawa M. Exp Hematol. 2000;28:1269–1273. doi: 10.1016/s0301-472x(00)00535-x. [DOI] [PubMed] [Google Scholar]
- 26.Owen J J, Cooper M D, Raff M C. Nature (London) 1974;249:361–363. doi: 10.1038/249361a0. [DOI] [PubMed] [Google Scholar]
- 27.Ceredig R, ten Boekel E, Rolink A, Melchers F, Andersson J. Int Immunol. 1998;10:49–59. doi: 10.1093/intimm/10.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Tachibana M, Kobayashi Y, Kasukabe T, Kawajiri K, Matsushima Y. Invest Ophthalmol Visual Sci. 2000;41:64–66. [PubMed] [Google Scholar]
- 29.Tachibana M, Kasukabe T, Kobayashi Y, Suzuki T, Kinoshita S, Matsushima Y. Invest Ophthalmol Visual Sci. 2000;41:668–670. [PubMed] [Google Scholar]
- 30.Abbott B D, Schmid J E, Brown J G, Wood C R, White R D, Buckalew A R, Held G A. Toxicol Sci. 1999;47:76–85. doi: 10.1093/toxsci/47.1.76. [DOI] [PubMed] [Google Scholar]
- 31.Kurebayashi J, Otsuki T, Kunisue H, Tanaka K, Yamamoto S, Sonoo H. Clin Cancer Res. 2000;6:512–518. [PubMed] [Google Scholar]
- 32.McCrohon J A, Death A K, Nakhla S, Jessup W, Handelsman D J, Stanley K K, Celermajer D S. Circulation. 2000;101:224–226. doi: 10.1161/01.cir.101.3.224. [DOI] [PubMed] [Google Scholar]
- 33.Bland R, Worker C A, Noble B S, Eyre L J, Bujalska I J, Sheppard M C, Stewart P M, Hewison M. J Endocrinol. 1999;161:455–464. doi: 10.1677/joe.0.1610455. [DOI] [PubMed] [Google Scholar]
- 34.Payne K J, Medina K L, Kincade P W. Blood. 1999;94:713–723. [PubMed] [Google Scholar]
- 35.Tudor K S, Payne K J, Yamashita Y, Kincade P W. Immunity. 2000;12:335–345. doi: 10.1016/s1074-7613(00)80186-7. [DOI] [PubMed] [Google Scholar]
- 36.Holladay S D, Blaylock B L, Comment C E, Heindel J J, Fox W M, Korach K S, Luster M I. Cell Immunol. 1993;152:131–142. doi: 10.1006/cimm.1993.1273. [DOI] [PubMed] [Google Scholar]
- 37.Rijhsinghani A, Bhatia S K, Kantamneni L, Schlueter A, Waldschmidt T J. Am J Reprod Immunol. 1997;37:384–390. doi: 10.1111/j.1600-0897.1997.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 38.Barr I G, Pyke K W, Pearce P, Toh B H, Funder J W. J Immunol. 1984;132:1095–1099. [PubMed] [Google Scholar]
- 39.Merino R, Ding L, Veis D J, Korsmeyer S J, Nunez G. EMBO J. 1994;13:683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy B E, Clark S J, Donald I R, Pinsky M, Vedady D. Am J Obstet Gynecol. 1974;118:538–541. doi: 10.1016/s0002-9378(16)33697-3. [DOI] [PubMed] [Google Scholar]
- 41.Beitins I Z, Bayard F, Ances I G, Kowarski A, Migeon C J. J Pediatr. 1972;81:936–945. doi: 10.1016/s0022-3476(72)80547-x. [DOI] [PubMed] [Google Scholar]
- 42.Murphy B E. J Steroid Biochem. 1981;14:811–817. doi: 10.1016/0022-4731(81)90226-0. [DOI] [PubMed] [Google Scholar]
- 43.Campbell A L, Murphy B E. J Clin Endocrinol Metab. 1977;45:435–440. doi: 10.1210/jcem-45-3-435. [DOI] [PubMed] [Google Scholar]
- 44.Blanford A T, Murphy B E. Am J Obstet Gynecol. 1977;127:264–267. doi: 10.1016/0002-9378(77)90466-5. [DOI] [PubMed] [Google Scholar]
- 45.Gluckman E. Exp Hematol. 2000;28:1197–1205. doi: 10.1016/s0301-472x(00)00540-3. [DOI] [PubMed] [Google Scholar]
- 46.Danel L, Menouni M, Cohen J H, Magaud J P, Lenoir G, Revillard J P, Saez S. Leuk Res. 1985;9:1373–1378. doi: 10.1016/0145-2126(85)90125-0. [DOI] [PubMed] [Google Scholar]
- 47.Suenaga R, Evans M J, Mitamura K, Rider V, Abdou N I. J Rheumatol. 1998;25:1305–1312. [PubMed] [Google Scholar]
- 48.Rider V, Jones S R, Evans M, Abdou N I. Clin Immunol. 2000;95:124–134. doi: 10.1006/clim.2000.4844. [DOI] [PubMed] [Google Scholar]
- 49.Brandenberger A W, Tee M K, Lee J Y, Chao V, Jaffe R B. J Clin Endocrinol Metab. 1997;82:3509–3512. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 50.Barker J N, Davies S M, DeFor T, Ramsay N K, Weisdorf D J, Wagner J E. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 51.Rocha V, Cornish J, Sievers E L, Filipovich A, Locatelli F, Peters C, Remberger M, Michel G, Arcese W, Dallorso S, et al. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 52.Albrecht E D, Pepe G J. Endocr Rev. 1990;11:124–150. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- 53.Krause D S, Theise N D, Collector M I, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis S J. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]