ABSTRACT
Background
The types of food in complementary feeding of infants and young children are important for growth and development. Food protein quality, as measured by the Digestible Indispensable Amino Acid Score (DIAAS), requires the determination of true ileal digestibility of indispensable amino acids (IAAs) in children.
Objectives
First, the aim of this study was to measure the true ileal IAA digestibility of 4 (rice, finger millet, mung bean, and hen egg) commonly consumed complementary foods in children aged <2 y using the dual-isotope tracer method. Second, we calculated the DIAAS of complementary feeding diets and their relation to stunting in a representative Indian rural population.
Design
Rice, finger millet, and mung bean were intrinsically labeled with deuterium oxide (2H2O), whereas egg was labeled through oral dosing of hens with a uniformly 2H-labeled amino acid mixture. True ileal IAA digestibility was determined by the dual-isotope tracer technique. The DIAAS of complementary food protein was calculated in children aged 1–3 y from a nationally representative survey to evaluate its relation with stunting.
Results
True ileal IAA digestibility was lowest in mung bean (65.2% ± 7.1%), followed by finger millet (68.4 %± 5.3%) and rice (78.5% ± 3.5%), and was highest for egg (87.4% ± 4.0%). There was a significant inverse correlation of complementary food DIAAS with stunting in survey data (r = −0.66, P = 0.044). The addition of egg or milk to nationally representative complementary diets theoretically improved the DIAAS from 80 to 100.
Conclusions
The true ileal IAA digestibility of 4 foods commonly consumed in complementary diets showed that the DIAAS was associated with stunting and reinforces the importance of including animal source food (ASF) in diets to improve growth. This trial was registered at http://ctri.nic.in/clinicaltrials/login.php as CTRI/2017/02/007921.
Keywords: intrinsic labeling, rice, finger millet, mung bean, egg, dual stable isotope tracer method, Digestible Indispensable Amino Acid Score (DIAAS), animal source food (ASF)
Introduction
The types of food selected for complementary feeding and its timing are critical to ensure the optimal growth and development of young children. The period from 6 to 24 mo of life marks a stage of rapid growth, with additional nutrient requirements imposed by a poor environment, which could magnify the deficiencies of a poor-quality diet. In low- and middle-income countries, survey data consistently show that complementary diets lack diversity, with the consumption of a minimally acceptable diet in children aged 6–23 mo being as low as 3% (1). The recent National Family Health Survey (NFHS) 4 (NFHS-4) in India estimated that only 9.6% of children aged 6–23 mo, breastfed or not breastfed, had a minimally acceptable diet (2). This is important, because linear growth faltering starts as early as 3 mo, tracking until 3 y of age, affects close to 71% of infants by 12 mo (3, 4), and leads to later stunting that is so widely prevalent in India (2).
Because infant and young child feeding (IYCF) indicators have been shown to lack specificity in association with growth (5), there is a need for better indicators with a focus on the quality of complementary diets in addition to their diversity. Protein intake, especially of high-quality proteins, in complementary feeding has been shown to promote growth, mainly through insulin-like growth factor I (IGF-I) production (6). However, linking crude protein inadequacy without a quality filter to growth restriction is difficult, because most surveys show children meeting their daily protein requirements. This has been questioned in a recent review (7), which pointed to the need for better measurements of protein quality, preferably by the recently recommended Digestible Indispensable Amino Acid Score (DIAAS) quality-scoring method (8). This is particularly relevant to complementary feeding diets that are mainly cereal based, where the crude protein intake may appear to be adequate (9, 10).
Recently, a dual-isotope method was developed to measure protein digestibility and DIAAS noninvasively in humans (11). In this study, preliminary investigations found a lower-than-expected digestibility for legume proteins, where the digestibility of the limiting amino acid lysine in chickpea and mung bean was found to be near 60% (11). This is a concern, particularly with its implications for appropriate legume–cereal mixes in complementary feeding. Further data are required on the true ileal indispensable amino acid (IAA) digestibility of other food groups such as cereals and animal-source foods (ASFs), especially in young children, to inform DIAAS calculations of their diets. Therefore, this study aimed first to determine the true ileal IAA digestibility of commonly consumed food groups in children using the recently established dual-isotope tracer method (11). Second, it aimed to evaluate representative Indian complementary feeding diets using the DIAAS for different foods and related these to the prevalence of stunting.
Methods
Intrinsic labeling of rice, finger millet, mung bean, and hen egg
Four specific foods (cereals, millets, legumes, and egg) were chosen for the true ileal IAA digestibility measurements. An intrinsic labeling protocol using deuterium oxide (2H2O; 99.9%; Sercon Ltd.) for rice, finger millet (ragi), and mung bean was conducted in collaboration with the University of Agricultural Sciences, Bangalore, India. The growing conditions for plants in pots, regarding specific soil requirement, fertilizers, and water, were optimized. The protocol for labeling mung bean has been explained in detail elsewhere (11) and is briefly described here. Mung bean (Vigna radiata) was grown in a rainout shelter during the rainy (kharif) season in pots containing 2 plants each and watered gravimetrically with normal water first, followed by 2H2O; a prime dose of 400 mL (25% 2H2O) was timed to seed development, 2 wk after anthesis, followed by pulses of 100 mL (2.5% 2H2O) on days 2, 4, 6, and 8 subsequently. The rice (Oryza sativa) and finger millet (Eleusine coracana) were labeled following the same protocol of prime and pulsed dosing, after anthesis. However, they were grown during the winter (rabi) season, December 2016 to April 2017, in pots containing 2 plants each. In each of these protocols, control plants were also grown (2 pots/protocol). Mature seeds were harvested, dried, and stored for use in human experiments. Subsequently, subsamples of seeds from each crop were milled to a fine flour using a mortar and pestle and analyzed as ethoxycarbonyl ethyl esters for 2H isotopic enrichments using gas chromatography–pyrolysis–isotope ratio mass spectrometry (Delta V Advantage; Thermo Fisher Scientific), as described in detail earlier (11).
Uniformly labeled hen eggs were obtained by daily oral dosing of 2 layer hens (BV-300) with 12 mg U-[2H]–labeled crystalline amino acid mix (>97% purity; DLM-6819-1; Cambridge Isotope Laboratories) for 35 d. A detailed explanation of the dosing protocol and egg processing is published elsewhere (12). The labeled eggs were boiled; white and yolk fractions separated, lyophilized, and pooled; and the egg white analyzed for 2H isotopic enrichment of individual amino acids using liquid chromatography–tandem mass spectrometry (6495 Triple Quadrupole with iFunnel technology; Agilent) following the same analytical procedure as that for the seed samples. The 2H-IAA enrichments of the rice, finger millet, mung bean, and egg-white protein provided in Table 1 were sufficient for conducting human experiments. For the feeding experiment, the whole egg was boiled, shelled, and immediately stored in sterile plastic containers at −80°C until the day of the experiment.
TABLE 1.
2H Enrichments of indispensable amino acids of the 4 test proteins
| Test protein group, parts per million excess | ||||
|---|---|---|---|---|
| Amino acids | Rice | Finger millet | Mung bean | Egg1 |
| Methionine | 102 | 98 | 319 | 681 |
| Phenylalanine | 384 | 349 | 596 | 467 |
| Threonine | 272 | 111 | 155 | 466 |
| Lysine | 282 | 256 | 488 | 2622 |
| Leucine | 366 | 327 | 745 | 773 |
| Isoleucine | 314 | 258 | 622 | 804 |
| Valine | 335 | 292 | 610 | 1834 |
| Mean | 260 | 251 | 505 | 1093 |
1Boiled and lyophilized egg white separated from the yolk fraction, pooled and analyzed for enrichments.
Standardized test meal preparation
First, culturally acceptable test meal preparations were standardized and tested for their acceptability in children who were demographically similar to the study participants. This included the taste of the food and the feasibility of the plateau feeding protocol (see below), which included hourly feeding for 6 h. Once the acceptability of the test meal recipes was established, test meals were prepared to provide one-fourth and one-third of the children's daily energy and protein requirements on the experimental day (13). The different test meals consisted of protein sources from labeled and unlabeled foods, of which the proportion of labeled protein contributed from rice or mung bean was 65% and from labeled finger millet or egg was 50%. The mung bean was prepared in the form of a khichdi (serving as the base recipe), which consisted of unlabeled rice and labeled whole mung bean (soaked overnight for 12 h), in a ratio of 2.5:1, cooked with tomato, onion, and garlic to add flavor. For rice, the same recipe of khichdi was adopted, using labeled rice (unpolished) that was manually dehulled and unlabeled mung bean at a ratio of 7:1. The finger millet was provided as a pancake and served with tomato and onion chutney. For the pancake batter, unlabeled dehusked split black gram was soaked for 4 h and ground to a coarse consistency along with water and the labeled finger millet flour, which had been milled using a coffee grinder. The mixture was then set aside for 10 h overnight to ferment before preparing the pancake. For the egg meal, the khichdi preparation remained the same but did not contain mung bean; instead, labeled whole boiled egg (finely chopped) was administered with the meal.
The meals were prepared fresh on the morning of the experiment and the cooked weight portioned into 9 parts; the priming meal comprised 3 portions, whereas single portions served as mini meals for the plateau feeding protocol (below). To the priming and mini meals, U-13C spirulina whole cells (97%; Cambridge Isotope Laboratories), 10 mg/kg divided and mixed into the portions, was added as a standard protein of known digestibility, which had been measured earlier in comparison with a mixture of crystalline amino acids (11). In the finger millet pancake preparation, because the spirulina could not easily be mixed into the preparation, small pieces of bread, butter, and jam sandwiches were used to deliver the U-13C spirulina, which the children ate before the pancake. These sandwiches provided an additional ∼25 kcal energy/meal, and the finger millet meal was adjusted down for this extra energy and protein. The nutrient composition of the test meals is shown in Table 2.
TABLE 2.
Nutrient composition of the standardized test meal for each test protein group1
| Test protein group | ||||
|---|---|---|---|---|
| Nutrients | Rice (n = 4) | Finger millet2 (n = 6) | Mung bean (n = 4) | Egg (n = 6) |
| Energy, kcal | 196.5 ± 26.8 | 219.6 ± 19.2 | 212.8 ± 28.3 | 203.9 ± 50.9 |
| Protein, g | 4.0 ± 0.5 | 4.7 ± 0.4 | 4.8 ± 0.6 | 4.3 ± 1.1 |
| Carbohydrate, g | 29.7 ± 4.1 | 31.8 ± 2.7 | 26.7 ± 3.6 | 26.0 ± 6.5 |
| Fat, g | 6.5 ± 0.9 | 7.8 ± 0.7 | 9.3 ± 1.2 | 10.8 ± 2.7 |
| Protein-to-energy ratio, % | 8.2 ± 0.0 | 8.6 ± 0.1 | 9.0 ± 0.0 | 8.4 ± 0.0 |
1Values are means ± SDs.
2Note: n = 2 subjects were repeated, one for rice and another for the egg test protein group.
Experimental protocol and analyses
Apparently healthy children were recruited, either from the pediatric well-infant clinic at St. John's Medical College and Hospital or from a nearby urban slum. A minimum sample size of 4 children in each test protein group was required to detect a 15% difference in mean true ileal IAA digestibility between a high-quality animal (egg) and low-quality plant (rice, finger millet, and mung bean) protein at a 1% level of significance with a power of 90%, when using an SD of 4.5% for whole boiled egg digestibility that was determined in an earlier experiment on adults following the same method (12). The children selected were not breastfed, were aged 18–24 mo, and underwent a general medical checkup to exclude chronic or congenital systemic disorders. There was no past medical history suggestive of food allergies or serious illness within 3 mo before the study. Those with diarrheal or vomiting episodes in the previous 2 wk, who were administered antibiotics in the previous 4 wk, or were receiving regular iron supplementation were not eligible for participation. A screening blood sample was collected to test for C-reactive protein (CRP), HIV, hepatitis B, and complete blood counts (ABX-Pentra 60C+). Information on general demographic characteristics and 24-h diet recalls was collected through caregiver interviews. Anthropometric measurements were performed using standard procedures and equipment, weight was measured to the nearest 10 g (Seca 354), and length was measured to the nearest millimeter (Seca 417). Nutritional status indicators, weight-for-age zscores (WAZs), height-for-age zscores (HAZs), and weight-for-height zscores (WHZs) were extracted using the WHO Anthroplus software (version 3.2.2, January 2011). The study was approved by the Institutional Ethical Review Board, and written informed consent was obtained from the caregivers of the participants. Subject screening and enrollment details are provided in Supplemental Figure 1.
On the day of the experiment, which was within 1 wk of the screening blood sample, children came along with their mothers to the metabolic ward at 1000 h. A 2-h postprandial period was ensured before starting the experimental protocol, where mothers were requested to feed the children early in the morning before reaching the facility. The experimental duration was 6 h. Test meals were administered using a plateau feeding protocol, starting with the priming meal along with 13C-bicarbonate (>99%; 3 μmol/kg; Cambridge Isotope Laboratories) followed by a single mini-meal every hour for the next 5 h.
To reduce the stress of venous sampling, the screening blood sample was used as the basal sample for isotopic enrichments of amino acids, whereas 3 plateau samples were collected half-hourly from the fifth to sixth hour, after securing an indwelling venous catheter. The sampling time was selected based on previous experiments (11) that showed a plateau for the isotopic enrichment of plasma amino acids from the fifth hour onward. Whole blood was transferred into EDTA-coated anticoagulant Vacutainers (Becton Dickinson) and centrifuged at 3500 rpm for 10 min at 4°C to separate the plasma, which was placed into aliquots and stored at −80°C until analysis. Plasma samples were deproteinized, purified by cation exchange, and derivatized for IAA analysis by liquid chromatography–tandem mass spectrometry (6495 Triple iFunnel Quadrupole; Agilent) and gas chromatography–pyrolysis–isotope ratio mass spectrometry (Delta V Advantage; Thermo Fisher Scientific) to measure [13C] and [2H] isotopic enrichments as explained elsewhere (11, 12). The mean 2H and 13C enrichments of each amino acid at plateau (5th, 5.5th, and 6th hour of the study protocol) were used for the digestibility calculation. Whole-meal samples, including a control meal that was made in exactly the same way, but without labeled foods, underwent gas phase acid hydrolysis before amino acid analysis; and for each amino acid, the ratios of the plasma and food 2H and 13C enrichments were used to calculate digestibility (11).
The true ileal digestibility percentage for each IAA was calculated using the following equation:
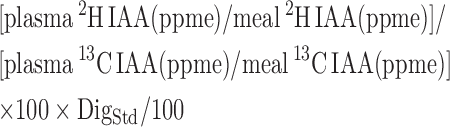 |
(1) |
where DigStd is the true ileal percentage digestibility of IAAs of spirulina protein with reference to crystalline amino acids, and ppme is parts per million excess. The mean DigStd values of spirulina for each amino acid were 84.1%, 95.3%, 82.5%, 77.5%, 86.0%, 84.2%, and 87.1% for methionine, phenylalanine, threonine, lysine, leucine, isoleucine, and valine, respectively. Percentage IAA digestibility was also corrected for the maximum transamination loss of 2H using transamination correction factors as described earlier (11).
DIAAS and Protein Digestibility Corrected Amino Acid Score analysis
Nutrient values of protein and amino acids for foods were taken from the Indian Food-Composition Tables (14). For the calculation of DIAAS, the individual amino acid score (milligrams of amino acid per gram of protein) for each food was calculated and multiplied by its respective true ileal digestibility (obtained from this study) to obtain the true ileal digestible IAA content in the food. For sulfur amino acids (methionine + cysteine) and aromatic amino acids (phenylalanine + tyrosine), the digestibility of cysteine and tyrosine was assumed to be the same as that for methionine and phenylalanine, because earlier studies showed a <6% difference between these amino acids (15–17). The true ileal digestible IAA content adjusted for the total protein content was summed across different foods in the diet to obtain an IAA scoring pattern for the diet in comparison with the requirement score for children aged 1–2 y (8). For the calculation of the Protein Digestibility Corrected Amino Acid Score (PDCAAS), the method defined in the 2007 WHO/FAO/UN University technical report was followed using the reported nitrogen fecal digestibility values in humans for polished rice, millets, beans, milk, and eggs (18).
Analysis of complementary feeding diets in relation to stunting: National Nutrition Monitoring Bureau survey
The relation of stunting with high-quality protein foods in complementary diets was assessed in a representative rural population of children aged 1–3 y from the last available National Nutrition Monitoring Bureau (NNMB) survey of 10 Indian states (19). The food intake was based on a dietary recall at the household level. Seven food groups that contributed significantly to the daily protein intake were selected. These food groups form part of the core IYCF indicators. These were cereals, millets, legumes, milk and milk products, fish, and egg with other flesh foods (meat and poultry); the last 3 food groups were combined to represent ASFs. For the analysis of DIAAS and PDCAAS (described above), the digestibility of one food was selected as representative of each food group; that is, rice for cereals, finger millet for millets, mung bean for legumes, and egg for ASFs (14).
Statistical analysis
To test for differences in IAA digestibility between the protein sources, digestibility values for each IAA across the 4 test proteins were first checked for normality using the Shapiro-Wilk test; because most of the IAA digestibilities were not normally distributed, nonparametric analyses were performed. The Kruskal-Wallis test was performed to determine differences in mean true ileal IAA digestibility between protein groups. Post hoc Bonferroni-adjusted Mann-Whitney U tests were performed to test for differences in mean true ileal digestibility of individual amino acids between the low-quality (rice, finger millet, mung bean) and high-quality (egg) protein groups. Means ± SDs are reported since the median and mean digestibility were very similar (differing by <2%), with low variability. The Spearman correlation was determined to evaluate associations between nutritional status, food groups, and DIAAS across the study participants and the states of India from the NNMB data. The risk of protein inadequacy was calculated by the Estimated Average Requirement cutoff method (20). All tests were considered statistically significant if P < 0.05. All calculations were performed with STATA version 15.1 and in R version 3.5.0.
Results
The children's age, sex, anthropometric measures, nutritional status, and hemoglobin are listed in Table 3. Overall, among the 18 children, 33% were stunted (< −2 SDs for HAZ), 39% were underweight (< −2 SDs for WAZ), and 11% were wasted (< −2 SDs for WHZ), according to the WHO growth standards. More than three-fourths (14 out of 18) of the children were anemic, with a mean ± SD hemoglobin of 9.6 ± 1.4 g/dL. The plasma CRP concentrations were within the normal range, and all children tested negative for HIV and hepatitis B.
TABLE 3.
Demographic and anthropometric characteristics of study participants in each test protein group1
| Test protein group | |||||
|---|---|---|---|---|---|
| Variables | Total (n = 18) | Rice (n = 4) | Finger millet2 (n = 6) | Mung bean (n = 4) | Egg (n = 6) |
| Age, mo | 22.8 ± 2.9 | 21.5 ± 3.8 | 25.1 ± 2.4 | 22.5 ± 3.1 | 22.6 ± 2.2 |
| Boys, n | 11 | 2 | 3 | 4 | 2 |
| Weight, kg | 9.72 ± 1.44 | 9.50 ± 0.97 | 9.51 ± 1.05 | 9.99 ± 1.29 | 9.88 ± 2.25 |
| Length, cm | 81.2 ± 4.0 | 81.6 ± 3.5 | 80.6 ± 1.9 | 82.3 ± 5.8 | 80.3 ± 4.6 |
| Weight-for-age zscore | −1.6 ± 1.1 | −1.5 ± 0.9 | −2.1 ± 1.2 | −1.3 ± 0.5 | −1.4 ± 1.3 |
| Height-for-age zscore | −1.5 ± 1.2 | −0.9 ± 1.2 | −2.2 ± 1.1 | −1.3 ± 1.4 | −1.6 ± 1.0 |
| Weight-for-height zscore | −1.2 ± 0.9 | −1.3 ± 0.6 | −1.3 ± 1.1 | −1.6 ± 0.8 | −0.7 ± 1.3 |
| Hemoglobin, g/dL | 9.6 ± 1.4 | 10.7 ± 1.2 | 10.5 ± 1.5 | 9.4 ± 1.1 | 9.4 ± 1.1 |
1Values are means ± SDs unless otherwise indicated.
2Note: n = 2 subjects were repeated, one for rice and another for the egg test protein group.
True ileal protein digestibility of rice, finger millet, mung bean, and hen egg
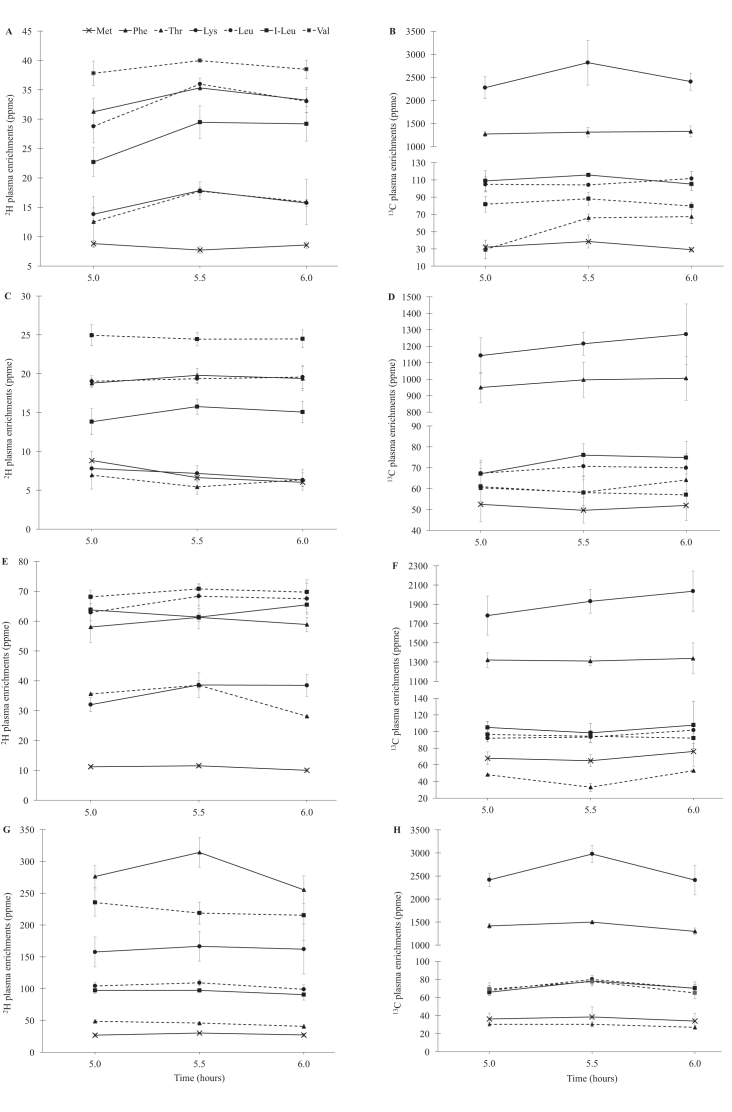
The plateau plasma 2H and 13C isotopic enrichments for each test protein are represented in Figure 1. The mean CVs for 2H and 13C isotopic enrichments at their plateau were 17% and 20%, respectively. The CVs for 2H enrichments ranged from 12% for leucine to 24% for lysine and, for 13C enrichments, ranged from 14% for leucine to 33% for methionine. The mean 2H and 13C IAA enrichments of the meal samples for all the test meals administered are provided in Supplemental Table 1. The mean true ileal IAA digestibilities of rice, finger millet, mung bean, and hen egg are summarized in Table 4. The mean ± SD true ileal IAA digestibility was lowest for mung bean at 65.2% ± 7.1%, followed by 68.4% ± 5.3% for finger millet and 78.5% ± 3.5% for rice, and was the highest at 87.4% ± 4.0% for hen egg. Within each food protein, the IAA with the lowest and highest true ileal digestibility varied. For rice, it ranged from 73% to 84% for threonine and phenylalanine, and for finger millet it ranged from 60% to 75% for methionine and isoleucine, respectively. In mung bean, the lowest digestibility was for methionine (54%) and the highest was for phenylalanine (77%). For egg, the values ranged from 81% for valine to 94% for lysine. The CV of each measured IAA digestibility was low between individuals; for different test proteins, it ranged between 3.8% and 7.6% for valine and methionine in rice, 2.2% and 9.2% for leucine and lysine in mung bean, 4.3% and 10.5% for threonine and phenylalanine in finger millet, and 1.8% and 5.1% for phenylalanine and valine in egg. Overall, compared with egg, the mean true ileal IAA digestibility was significantly lower for rice (P = 0.029), finger millet (P = 0.006), and mung bean (P = 0.029). Between the different foods, the mean individual true ileal IAA digestibility of threonine (P = 0.029) and lysine (P = 0.029) for rice was significantly lower than that for egg; all of the IAA digestibilities for finger millet and mung bean were lower than that for egg (P = 0.006 and P = 0.029 for all IAAs, respectively) (Table4).
FIGURE 1.
Plasma enrichment of IAAs after consumption of different proteins with different subjects in each test protein group, except for 2 subjects who were repeated, one for rice and another for the egg protein group. (A) Plasma appearance of 2H and (B) 13C isotopic enrichments of IAA (ppme) at plateau after consumption of intrinsically labeled rice (n = 4). (C) Plasma appearance of 2H and (D) 13C isotopic enrichments of IAAs (ppme) at plateau after consumption of intrinsically labeled finger millet (n = 6). (E) Plasma appearance of 2H and (F) 13C isotopic enrichments of IAAs (ppme) at plateau after consumption of intrinsically labeled mung bean (n = 4). (G) Plasma appearance of 2H and (H) 13C isotopic enrichments of IAAs (ppme) at plateau after consumption of intrinsically labeled hen egg (n = 6). Plots represent means ± SEs of 2H and 13C isotopic enrichment appearance in plasma. IAA, indispensable amino acid; I-leu, isoleucine; ppme, parts per million excess.
TABLE 4.
True ileal digestibility values of IAAs from mung bean, finger millet, rice and egg in apparently healthy children aged 18–24 mo1
| True ileal digestibility of the test protein group, % | ||||
|---|---|---|---|---|
| Amino acids | Rice (n = 4) | Finger millet2 (n = 6) | Mung bean (n = 4) | Egg (n = 6) |
| Methionine | 79.7 ± 6.1 | 60.1 ± 5.13 | 54.0 ± 4.13 | 85.9 ± 1.8 |
| Phenylalanine | 83.9 ± 3.4 | 69.4 ± 7.33 | 77.2 ± 3.63 | 89.6 ± 1.6 |
| Threonine | 73.4 ± 4.53 | 67.2 ± 2.93 | 61.6 ± 2.13 | 90.1 ± 3.9 |
| Lysine | 78.3 ± 4.13 | 74.5 ± 4.63 | 64.8 ± 6.03 | 93.6 ± 2.1 |
| Leucine | 78.7 ± 3.2 | 67.2 ± 3.93 | 68.0 ± 1.53 | 86.3 ± 3.0 |
| Isoleucine | 80.5 ± 3.3 | 75.4 ± 3.73 | 63.0 ± 6.13 | 85.4 ± 2.2 |
| Valine | 75.2 ± 2.9 | 65.3 ± 3.23 | 68.0 ± 2.33 | 81.2 ± 4.1 |
| Mean IAAs | 78.5 ± 3.53 | 68.4 ± 5.33 | 65.2 ± 7.13 | 87.4 ± 4.0 |
1Values are means ± SDs. IAA, indispensable amino acid.
2Note: n = 2 subjects were repeated, one for rice and another for the egg protein group.
3Mean true ileal IAA or individual IAA digestibility significantly different from the egg protein group.
Table 5 shows a calculation of the DIAAS for the diet of a single child from this study; the same calculation was used for all subjects (for a detailed explanation of the calculation, refer to reference 8). Across subjects, the DIAAS and PDCAAS were 106% ± 14% and 114 ± 14%, respectively, calculated from the lowest value, which was usually lysine. There was no correlation of food groups consumed or DIAAS with nutritional status indicators (HAZ, WAZ, or WHZ) in this small sample of children.
TABLE 5.
Calculation of DIAAS for a mixture of foods consumed by a child1
| Composition2 | True ileal digestibility IAA content in mixture, mg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Food | Weight,g | Protein, g | Thr, mg /g protein | SAAs, mg /g protein | AAAs, mg /g protein | Lys, mg /g protein | Thr | SAAs | AAAs | Lys | DIAAS, % |
| Cereals | 166 | 13 | 31 | 44 | 99 | 36 | 315 | 467 | 1073 | 379 | — |
| Millets | 0 | 0 | 38 | 42 | 91 | 28 | 0 | 0 | 0 | 0 | — |
| Legumes | 48 | 11 | 32 | 20 | 90 | 66 | 213 | 113 | 747 | 460 | — |
| Fish, other flesh foods, and egg | 0 | 0 | 64 | 168 | 103 | 82 | 0 | 0 | 0 | 0 | — |
| Milk and milk products | 287 | 9 | 48 | 37 | 106 | 86 | 404 | 300 | 891 | 757 | — |
| Amino acid score (total for each amino acid/total protein content in the mixture), mg/g protein | — | — | — | — | — | — | 27 | 27 | 82 | 47 | — |
| Digestible IAA reference ratio for mixture | — | — | — | — | — | — | 1.02 | 1.06 | 1.77 | 0.93 | 93 |
1AAA, aromatic amino acid (phenylalanine + tyrosine); DIAAS, Digestible Indispensable Amino Acid Score; IAA, indispensable amino acid; SAA, sulfur amino acid (methionine + cysteine).
2The amino acid profile for each foods was taken from the Indian Food Composition Tables, 2017.
The DIAAS and PDCAAS of the food groups in the complementary diet intakes from the NNMB survey for the 10 Indian states (by state and pooled) are presented in Table 6, along with stunting, underweight, and wasting prevalence. These state-level analyses showed significant inverse correlations between the prevalence of stunting or underweight with the DIAAS of the diet (rs = −0.66, P = 0.044, and rs = −0.76, P = 0.011, respectively). This relation was not adjusted for energy intake, because there was no correlation of energy intake with DIAAS or stunting. In contrast, the DIAAS-corrected protein intake or the DIAAS-corrected protein-energy ratio, had inverse but nonsignificant correlations with stunting. Without correction of protein quality by DIAAS, the risk of dietary crude protein inadequacy in the pooled national diets of children aged 1–3 y was 19%. When corrected for quality by the dietary protein DIAAS value of 80%, the risk increased to 29%. It is possible to improve the dietary quality–protein intake (DIAAS ≥100) by adding milk, eggs, or legumes: this required the addition of 200 g milk, 50 g whole egg, or 45 g legumes to the daily diet. By doing so, the DIAAS of the diet protein increased to 101% and 100% for milk and egg, respectively, but remained at 81% when legumes were added. In turn, the intake of quality protein increased sufficiently to reduce the proportion of children at risk of protein inadequacy to 8%, 7%, and 11%, respectively, when milk, egg, and legumes, in the amounts given above, were added. The energy intake also increased by 146, 74, and 132 kcal, respectively, but did not exceed the energy requirement for this age group, such that the DIAAS-corrected protein-energy ratio of the diet improved from 8.3% to 11.9%, 12.9%, and 11.2% for milk, egg, and legume additions, respectively.
TABLE 6.
Calculated DIAAS and PDCAAS values for the complementary diets of children aged 1–3 y with prevalence of stunting, underweight, and wasting across states and pooled survey data of the NNMB survey1
| States | DIAAS, % | PDCAAS, % | DIAAS corrected protein intake, g | Stunting, % | Underweight, % | Wasting, % |
|---|---|---|---|---|---|---|
| Kerala | 96 | 106 | 15.9 | 31.3 | 22.9 | 13.1 |
| Tamil Nadu | 97 | 107 | 20.5 | 28.1 | 31.9 | 23.6 |
| Karnataka | 72 | 82 | 16.9 | 44.1 | 39.8 | 18.3 |
| Andhra Pradesh | 78 | 100 | 14.6 | 46.8 | 36.2 | 14.9 |
| Maharashtra | 82 | 91 | 15.0 | 51.6 | 36.2 | 12.2 |
| Gujarat | 71 | 80 | 15.4 | 62.5 | 57.7 | 27.3 |
| Madhya Pradesh | 66 | 75 | 15.4 | 58.6 | 57.2 | 31.9 |
| Orissa | 68 | 78 | 13.0 | 53.9 | 43.9 | 17.8 |
| West Bengal | 90 | 99 | 18.6 | 48.4 | 42.6 | 24.2 |
| Uttar Pradesh | 79 | 89 | 21.0 | 59.8 | 51.4 | 26.2 |
| Pooled | 80 | 88 | 17.1 | 49.4 | 42.7 | 20.9 |
1DIAAS, Digestible Indispensable Amino Acid Score; NNMB, National Nutrition Monitoring Bureau; PDCAAS, Protein Digestibility Corrected Amino Acid Score.
Discussion
This study provides true ileal IAA digestibility data for 4 foods, in children aged <2 y, by using the dual-isotope tracer approach. The method reflects digestion and absorption that occur only in the small intestine (terminating in the ileum) and hence provides a measure of true ileal digestibility, because it is not affected by endogenous protein secretion. These true ileal digestibility measurements inform the DIAAS, which has been recommended as a more accurate method for protein-quality assessment in different age groups (8). The mean true ileal IAA digestibilities determined for each test protein in these children were 79% for rice, 68% for finger millet, 65% for mung bean, and 87% for hen egg. Because these were the first data, to our knowledge, on true ileal digestibility of different foods in humans in this age group, comparisons were made with the literature for adult humans and growing pig and rat models. For rice, the true ileal IAA digestibility compared well with the standardized ileal digestibility of broken rice (although the cooking process was not described) in postweaned piglets (15) and with the true ileal digestibility of cooked rice in rats (16), except for methionine and lysine, which were ∼20% lower and higher, respectively, in rats. For finger millet, there are no data in humans, pigs, or rats; comparisons were made with cecectomized cockerels (17), where the mean IAA digestibility of pelleted feed (processing method not mentioned) was higher by ∼10–30% for all amino acids when compared with this study. For mung bean, the individual IAA digestibility in children from this study was ∼20% lower for all amino acids when compared with standardized ileal digestibility of steamed mung bean in growing pigs (21) or dehulled cooked mung bean in rats (16). This could be due to the effect of the food matrix in which the test protein was administered, interaction with the state and health of small intestinal absorption, as well as the antinutritional factors (tannins, phytates, trypsin inhibitor) present in the mung bean variety used, as modified by its processing and preparation. For egg, the true leucine ileal digestibility was slightly lower (86% compared with 91%) than that obtained in adult human ileostomates (22). The mean true ileal IAA digestibility of egg was also found to be close to the value (88% compared with 90%) of the apparent oro-fecal digestibility in humans (23). Overall, it appears that the mean IAA digestibility and specifically lysine digestibility were lower than that defined by oro-fecal methods or from animal models, particularly for rice, legumes, and millets. In addition, it is worth noting that the true ileal digestibilities of egg (88% compared with 89%) and mung bean (66% compared with 58%) in children were comparable with those in adults (where the test protein underwent the same processing but was administered in a different meal matrix), as determined previously using the dual-tracer approach (11, 12).
In an ecological analysis conducted across countries, utilizable protein—that is, total protein intake corrected for digestibility (PDCAAS)—has been shown to have a strong and significant inverse association with stunting prevalence (24). When evaluating the limitations of PDCAAS, a recent expert committee recommended replacing PDCAAS with the more accurate DIAAS metric, which uses true ileal IAA digestibility rather than fecal digestibility (8). This should be determined, most preferably in humans. Indeed, an evaluation of protein quality of mixed complementary diets in different Indian states in national representative data (19) showed that their calculated DIAAS and PDCAAS values were different from each other, by 10–20% (Table6), and lysine was the limiting amino acid. Importantly, the DIAAS of mixed complementary foods significantly and inversely related to the prevalence of stunting and underweight, indicating its utility as an indicator of the protein quality of the diet.
It was also possible to estimate how much extra quality-protein food would be needed in the reported pooled estimate of the national diet of children to improve the DIAAS to ≥100 and subsequently determine the percentage reduction in the population at risk of quality-protein inadequacy. For the population of children aged 1–3 y examined, this can be achieved by increasing the milk intake by 200 g (1 glass) or providing an additional 50 g egg (∼1 egg) or adding 45 g (∼0.5 cup) legumes. It is worth pointing out that the effect of adding an extra quantity of legumes resulted in a cereal-to-legume ratio of ∼2:1, as recommended in the IYCF guidelines (25), but had a negligible effect on the DIAAS of the mixed diet protein, due to its poor methionine and lysine digestibility. Additional legumes might also reduce the absorption of micronutrients due to their high phytate content. Further studies are required to test how the digestibility of legumes can be improved (by dehulling or germination, for instance), to reduce the effect of antinutritional factors. The most effective protein for reducing the risk of inadequacy and for improving the DIAAS was from egg and milk. In economic terms, it was cheaper for egg and legumes, with milk being the most expensive.
To further explore the association of ASFs with stunting at the national level in India, a secondary analysis of a national survey, the NFHS-4, was performed (2). This survey only reports the recall of the type of food group provided to the children (6–59 mo; n = 235,873) in the last 24 h (as an indicator of IYCF) but not their quantity or frequency. In an approach similar to that used for the NNMB data, 3 main food groups were considered: cereals and millets, which included food made from grains and fortified infant foods; pulses and legumes, which included food made from beans, peas, lentils, and nuts; and ASFs, which included meat, fish, poultry, eggs, cheese, yogurt, other milk products, and other fresh milk. The unadjusted and adjusted RRs for stunting were determined in the subgroup of children (1–3 y; n = 8146) who consumed ASFs in addition to cereals, pulses, and other food groups compared with those who did not. Adjustments were made for wealth index (as a continuous score), breastfeeding status, maternal educational level, maternal BMI, and birth weight. There was a significantly reduced risk of stunting (RR = 0.75) that remained significant after adjusting (adjusted RR = 0.90) in children aged 1–3 y when ASFs were consumed. With ∼85% of the population consuming any ASFs in addition to other foods, the population prevented fraction, which is the potential proportion of stunting that was prevented by the protective effect of ASFs, was 9%. This robust, although small, effect of ASFs on stunting reflects findings from other similar analyses (26, 27) and underlines the significance of a simple early-life addition of ASFs in the diet to support growth and development (28–30). It is important to note that, although ASFs had a beneficial impact, this could be attributed to their macro- or micronutrient composition.
The strength of this study lies in the rigorous approach used for true ileal IAA digestibility determination, which was applicable for the DIAAS calculation of common complementary feeding foods. Within the small sample of children studied, some were mildly anemic or undernourished. However, there were no associations between hemoglobin values, HAZ, or WAZ and digestibility. Another limitation was the use of correction factors (Digstd; true ileal percentage digestibility of IAAs of spirulina protein with reference to crystalline amino acids and TCF; Transamination Correction Factor), which were not specifically determined in the subjects in this study, because it was difficult to conduct 2 experiments in the children. The findings from the NNMB analyses should be interpreted with caution, because state mean data were used for modeling the DIAAS and could be subject to ecological fallacy. The NFHS analyses were at the individual level and robust, although no causal relation can be inferred. It is not clear whether these digestibility numbers could be applied to infants who are aged <18 mo, although the effect of age is likely to be small, because the digestibilities of legume and egg protein were similar between the children in this study (<2 y) and adults in a previous study using the same method (11, 12).
In conclusion, this study provides true ileal IAA digestibility of 4 foods commonly eaten in complementary diets, which will inform DIAAS assessments. The association of protein quality (as assessed by DIAAS) with the prevalence of stunting underlines the importance of type of protein, and not just quantity of protein, required to fulfill the nutrient requirements of children. Further studies are required to determine the IAA digestibility of other relevant food groups to build into the expanding database of DIAAS and to evaluate whether environmental stresses, such as parasites or environmental enteric dysfunction, affect protein digestion and amino acid absorption at this age.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Rajashekar Reddy for expert assistance in conducting the intrinsic labeling of plants and the field team, nurses, junior research fellow, and the metabolic kitchen cook (St. John's Research Institute, Bangalore, India) in conducting the human experimental protocols and laboratory assistance for sample preparation and analysis.
The authors’ responsibilities were as follows—AVK, NS, FJ, and TP: designed the research; NS: conducted the human research; MSS and S Kashyap: conducted the plant and egg intrinsic-labeling experiments and analyzed samples with AV and SD; AVK and NS: were involved with calculations and interpretation of results. S Kishore and TT: performed statistical analyses; AVK and NS: wrote the manuscript; AVK: had primary responsibility for final content; and all authors: read and approved the final manuscript. The authors declared no competing financial interests.
Notes
Supported by the Wellcome Trust/DBT India Alliance through the Margdarshi fellowship to AVK, and partly by a grant (prime award number: OPP1133329) from the Bill and Melinda Gates Foundation to FJ and AVK. The research was within the Collaborating Center of the International Atomic Energy Agency at St. John's Medical College.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ASF, animal-source food; DIAAS, Digestible Indispensable Amino Acid Score; IAA, indispensable amino acid; IYCF, infant and young child feeding; HAZ, height-for-age z score; NFHS, National Family Health Survey; NNMB, National Nutrition Monitoring Bureau; PDCAAS, Protein Digestibility Corrected Amino Acid Score; WAZ, weight-for-age zscore; WHZ, weight-for-height zscore
References
- 1. WHO. Indicators for assessing infant and young child feeding practices part 3: country profiles. Geneva (Switzerland): WHO; 2010; [Google Scholar]
- 2. International Institute of Population Sciences. National Family Health Survey (NFHS-4), 2015–16. Mumbai: (India): IIPS; 2017. [Google Scholar]
- 3. Mamidi RS, Shidhaye P, Radhakrishna KV, Babu JJ, Reddy PS. Pattern of growth faltering and recovery in under 5 children in India using WHO growth standards—a study on First and Third National Family Health Survey. Indian Pediatr. 2011;48:855–60. [DOI] [PubMed] [Google Scholar]
- 4. Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3):e473–80. [DOI] [PubMed] [Google Scholar]
- 5. Jones AD, Ickes SB, Smith LE, Mbuya MN, Chasekwa B, Heidkamp RA, Menon P, Zongrone AA, Stoltzfus RJ. World Health Organization infant and young child feeding indicators and their associations with child anthropometry: a synthesis of recent findings. Matern Child Nutr. 2014;10(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uauy R, Kurpad A, Tano-Debrah K, Otoo GE, Aaron GA, Toride Y, Ghosh S. Role of protein and amino acids in infant and young child nutrition: protein and amino acid needs and relationship with child growth. J Nutr Sci Vitaminol. 2015;61(Suppl):S192–4. [DOI] [PubMed] [Google Scholar]
- 7. Arsenault JE, Brown KH. Effects of protein or amino-acid supplementation on the physical growth of young children in low-income countries. Nutr Rev. 2017;75(9):699–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. FAO. Dietary protein quality evaluation in human nutrition. Report of an FAO Expert Consultation. Food and Nutrition Paper No. 92 Rome (Italy): FAO; 2013. [PubMed] [Google Scholar]
- 9. Abeshu MA, Lelisa A, Geleta B. Complementary feeding: review of recommendations, feeding practices, and adequacy of homemade complementary food preparations in developing countries–lessons from Ethiopia. Front Nutr. 2016;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuriyan R, Kurpad AV. Complementary feeding patterns in India. Nutr Metab Cardio Dis. 2012;22(10):799–805. [DOI] [PubMed] [Google Scholar]
- 11. Devi S, Varkey A, Sheshshayee MS, Preston T, Kurpad AV. Measurement of protein digestibility in humans by a dual-tracer method. Am J Clin Nutr. 2018;107(6):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kashyap S, Shivakumar N, Varkey A, Duraisamy R, Thomas T, Preston T, Devi S, Kurpad AV. Ileal digestibility of intrinsically labeled hen's egg and meat protein determined with the dual stable isotope tracer method in Indian adults. Am J Clin Nutr. 2018;108:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nutrient Requirements and Recommended Daily Allowances for Indians. A report of the Expert Group of the Indian Council of Medical Research. Hyderabad (India): Indian Council of Medical Research; 2009. [Google Scholar]
- 14. Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. Indian food composition tables. Hyderabad (India): National Institute of Nutrition, Indian Council of Medical Research, Department of Health Research, Ministry of Health and Family Welfare, Government of India; 2017. [Google Scholar]
- 15. Dadalt JC, Gallardo C, Polycarpo GV, Budiño FE, Rogiewicz A, Berto DA, Neto MT. Ileal amino acid digestibility of broken rice fed to postweaned piglets with or without multicarbohydrase and phytase supplementation. Asian-Australas J Anim Sci. 2016;29(10):1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rutherfurd SM, Bains K, Moughan PJ. Available lysine and digestible amino acid contents of proteinaceous foods of India. Br J Nutr. 2012;108(S2):S59–68. [DOI] [PubMed] [Google Scholar]
- 17. Vasan P, Mandal AB, Dutta N, Maiti SK, Sharma K. Digestibility of amino acids of maize, low tannin sorghum, pearl millet and finger millet in caecectomized roosters. Asian-Australas J Anim Sci. 2008;21(5):701. [Google Scholar]
- 18. WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. Report of a joint WHO/FAO/UNU Expert Consultation. WHO Technical Report Series No. 935. Geneva (Switzerland): WHO;2007. [Google Scholar]
- 19. Diet and Nutritional Status of Rural Population, Prevalence of Hypertension and Diabetes among Adults and Infant and Young Child Feeding Practices. Hyderabad: (India): National Institute of Nutrition, Indian Council of Medical Research, NNMB; 2012. [Google Scholar]
- 20. Otten JJ, , Hellwig JP, Meyers LD, editors. Dietary Reference Intakes: the essential guide to nutrient requirements. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 21. Yin YL, Li TJ, Huang RL, Liu ZQ, Kong XF, Chu WY, Tan BE, Deng D, Kang P, Yin FG. Evaluating standardized ileal digestibility of amino acids in growing pigs. Anim Feed Sci Tech. 2008;140(3-4):385–401. [Google Scholar]
- 22. Evenepoel P, Geypens B, Luypaerts A, Hiele M, Ghoos Y, Rutgeerts P. Digestibility of cooked and raw egg protein in humans as assessed by stable isotope techniques. J Nutr. 1998;128:1716–22. [DOI] [PubMed] [Google Scholar]
- 23. Watts JH, Booker LK, McAfee JW, Graham DC, Jones F Jr. Biological availability of essential amino acids to human subjects: II. Whole egg, milk and cottage cheese. J Nutr. 1959;67:497–508. [DOI] [PubMed] [Google Scholar]
- 24. Ghosh S. Protein quality in the first thousand days of life. Food Nutr Bull. 2016;37(1 Suppl):S14–21. [DOI] [PubMed] [Google Scholar]
- 25. Tiwari S, Bharadva K, Yadav B, Malik S, Gangal P, Banapurmath CR, Zaka-Ur-Rab Z, Deshmukh U, Agrawal RK. Infant and young child feeding guidelines, 2016. Indian Paediatr. 2016;53(8):703–13. [DOI] [PubMed] [Google Scholar]
- 26. Krasevec J, An X, Kumapley R, Bégin F, Frongillo EA. Diet quality and risk of stunting among infants and young children in low‐and middle‐income countries. Matern Child Nutr. 2017;13:e12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Darapheak C, Takano T, Kizuki M, Nakamura K, Seino K. Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. Int Arch Med. 2013;6(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iannotti LL, Lutter CK, Stewart CP, Riofrío CA, Malo C, Reinhart G, Palacios A, Karp C, Chapnick M, Cox K et al.. Eggs in early complementary feeding and child growth: a randomized controlled trial. Pediatrics. 2017:e20163459. [DOI] [PubMed] [Google Scholar]
- 29. Iannotti LL, Lutter CK, Waters WF, Gallegos Riofrío CA, Malo C, Reinhart G, Palacios A, Karp C, Chapnick M, Cox K et al.. Eggs early in complementary feeding increase choline pathway biomarkers and DHA: a randomized controlled trial in Ecuador. Am J Clin Nutr. 2017;106(6):1482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang M, Krebs NF. High protein intake from meat as complementary food increases growth but not adiposity in breastfed infants: a randomized trial. Am J Clin Nutr. 2014;100(5):1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.