ABSTRACT
Background
Elevated blood pressure (BP) is a major cause of preventable disease in the United States and around the world. It has been postulated that phosphorus intake may affect BP, with some studies suggesting a direct and others an inverse association.
Objectives
We systematically reviewed the literature on the association of dietary phosphorus with BP in adults and performed a qualitative synthesis.
Methods
We included randomized and nonrandomized behavioral intervention and feeding studies (intervention studies) and prospective observational studies that measured dietary phosphorus intake or urinary phosphorus excretion and BP. We excluded studies of supplements, children, or individuals with major medical conditions. We searched PubMed, Embase, Cochrane Trials, and clinicaltrials.gov on 1 June, 2017 and 22 August, 2018. We assessed studies’ risk of bias in their assessment of phosphorus exposure and BP.
Results
We reviewed 4759 publications and included 14 intervention studies (2497 participants), 3 prospective observational cohorts (17,795 participants), and 2 ongoing trials. No included intervention studies were designed specifically to achieve a phosphorus contrast. Two studies found a significant positive association of dietary phosphorus with systolic BP, 4 a significant inverse association, and 8 no significant association. Four studies found a significant inverse association with diastolic BP and 10 no significant associations. Two cohorts found lower risk of incident hypertension comparing the highest with the lowest quintiles of phosphorus intake and 1 found no significant difference: HR: 0.86 (95% CI: 0.75, 0.98); HR: 0.83 (95% CI: 0.68, 1.02); and HR: 0.75 (95% CI: 0.45, 1.27), respectively.
Conclusions
We found no consistent association between total dietary phosphorus intake and BP in adults in the published literature nor any randomized trials designed to examine this association. This trial was registered at www.crd.york.ac.uk/prospero/ as CRD42017062489.
Keywords: phosphorus, blood pressure, systematic review, urinary excretion, diet
Introduction
Elevated blood pressure (BP) is a major cause of preventable disease in the United States and around the world (1, 2). Nutritional interventions targeting dietary patterns as well as specific nutrients have been shown to reduce BP (2). Available evidence, albeit limited, has suggested that increased total dietary phosphorus intake may be associated with increases (3), decreases (4), or no changes (5) in BP, although mechanistic explanations strongly favor a direct association.
Several studies have suggested increased phosphorus intake is associated with adverse health outcomes related to BP. Supplementing a meal with sodium phosphate was associated with impaired endothelial function (6, 7) and there was a positive association of increased dietary phosphorus intake from all sources and food additives with increased carotid intima-media thickness (8). Other observational studies have found that those who consume higher amounts of dietary phosphorus were at higher (or at least no lower) risk of developing adverse cardiovascular outcomes that are more common in those with higher BP, including greater left ventricular mass, vascular calcification, and all-cause mortality (9–11).
In contrast, several observational studies have found that those who consume higher amounts of dietary phosphorus have significantly lower BP than those who consume lower amounts of dietary phosphorus (4, 12, 13). Potential mechanisms for this are not clear. Habitual high phosphorus intake may result in changes in the expression of sodium phosphorus cotransporters responsible for phosphorus reabsorption in the kidneys, which may, in turn, be involved in overall BP regulation (14, 15). Alternatively, phosphorus occurs as an anion that must be accompanied by a cation; several of the cations that occur with phosphorus [calcium (16) and magnesium (17)] might lower BP. Hence, it is possible that phosphorus is serving as a marker of these other nutrients.
In order to explain the conflicting results found in previous studies, we systematically reviewed the literature to characterize the direction of the association of total dietary phosphorus intake with BP in adults. We included in our systematic review prospective observational studies, randomized behavioral intervention and feeding studies, and nonrandomized behavioral intervention and feeding studies.
Methods
The protocol for our systematic review (Prospero: CRD42017062489; registered 23 May, 2017) was designed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (18). We initially searched on 1 June, 2017 and updated our search on 22 August, 2018. We included randomized and nonrandomized behavioral intervention and feeding studies (intervention studies) and prospective observational studies that measured dietary phosphorus intake or urinary phosphorus excretion and BP.
Study selection
We searched 3 databases of the peer-reviewed literature (PubMed, Embase, and Cochrane Trials) and clinicaltrials.gov for ongoing trials. Our search strategy aimed to identify all studies of humans that estimated dietary intake of phosphorus and BP or hypertension status (Supplemental Methods 1–3). Abstracts were imported into Covidence (Covidence.org) for title and abstract screening and full-text review.
Abstracts and full texts were reviewed independently by 2 members of the review team (STM, ZX, EAH, and SM). The reference lists of included articles, relevant review articles, and relevant cross-sectional studies were searched by STM for relevant articles. Study data were extracted by 1 member of the review team (STM, ZX, EAH, or SM) and checked by a second (STM, ZX, EAH, or SM). Quality assessment was performed by 1 member of the review team (STM, ZX, EAH, or SM) and checked by a second (STM, ZX, EAH, or SM). Any discrepancies between reviewers were resolved by group consensus during periodic meetings.
Inclusion and exclusion criteria
Studies were included if they were original research studies that 1) contained a measure of dietary phosphorus intake or urinary phosphorus excretion and 2) measured BP or reported diagnosis of hypertension or prescription of antihypertensive medication. Studies were excluded if they were studies of dietary supplements, did not have data on adults, were in populations of individuals with major medical conditions (e.g., end stage renal disease), or were cross-sectional in design (no prospective follow-up). In addition, studies were excluded if they were not in languages that were known fluently by the reviewers (English and Chinese) or could not be translated with limited assistance from Google Translate (French, Italian, or Spanish). The full text of all included articles was independently reviewed by STM and either ZX, EAH, or SM. Each article was double reviewed. Disagreements on study design were resolved by a third member of the review team. Review articles were excluded, but their reference lists were searched for potentially relevant articles that were then reviewed. Studies that did not clearly meet exclusion criteria in the title and abstract review stage advanced to full-text review for eligibility. The references of all included articles were searched and relevant articles underwent title and abstract review.
Data extraction
The following information was extracted (when available): sample size, study group, exposure (dietary intake of phosphorus, 24-h urine excretion of phosphorus), outcome (BP, hypertension), P value, z score, statistical test, statistical adjustments, sex distribution, race distribution, age distribution, proportion on BP medication, BMI, and dietary intake data (calories, calcium, sodium, magnesium, potassium, and vitamin D). When necessary, study design articles were used for quality assessment.
Quality assessment
Quality was assessed for 2 domains: the assessment of dietary phosphorus exposure and the assessment of BP. Each domain was classified as having a higher, lower, or unclear risk of bias. Text from each article supporting the assessment was extracted. In addition, the method of assessing phosphorus intake was classified.
To be considered “lower risk” for assessment of dietary phosphorus exposure, studies must have met the following criteria: phosphorus exposure estimated from a chemically analyzed diet with a reported dietary compliance of ≥80% of participants or phosphorus exposure estimated from a 24-h urinary excretion with complete collections (as defined by the study) reported in ≥80% of participants. Studies explicitly not meeting these requirements were considered “higher risk.” Studies not explicitly meeting or failing to meet these requirements were considered “unclear risk.” Studies estimating phosphorus exposure from self-reported intake (24-h dietary recall interview, food diary, or food-frequency questionnaire) linked to a nutrient database were considered “unclear risk.”
To be considered “lower risk” for assessment of BP, studies must have reported blinding of assessors to participant assignment and standard assessment protocol including a rest period before measurement. Studies explicitly not meeting these requirements were considered “higher risk.” Studies not explicitly meeting or failing to meet these requirements were considered “unclear risk.”
Data analysis
In studies reporting BP, the mean difference in the change from baseline to end of study between high and low phosphorus exposure groups was abstracted or calculated from group means. Pooled SEs from group means were used to calculate 95% CIs for studies not reporting mean differences. Mean difference was reported as high compared with low phosphorus exposure group or change in BP per 100-mg increase in phosphorus exposure, based on how the data were analyzed and presented in the individual study. In studies with >2 groups, the mean difference was calculated between the groups with the largest contrast in phosphorus exposure. In studies reporting incident hypertension, the HR comparing between the highest and lowest phosphorus exposure groups was used. We considered performing a meta-analysis, but decided against this after concluding that the studies were few in number and too heterogeneous in terms of design and reporting. Forest plots were produced using R version 3.4.3 (R Project for Statistical Computing) and the forestplot package (19, 20).
Results
Search results and study characteristics
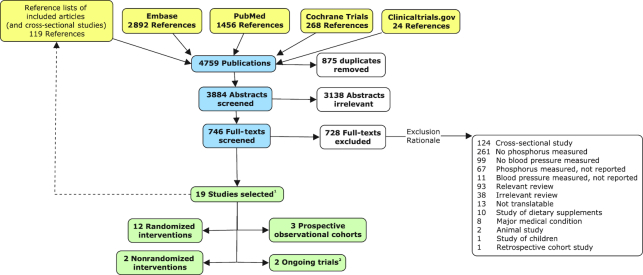
A total of 3884 unique abstracts were screened, 746 full-text articles were reviewed, and 19 studies from 18 full-text articles were included (Figure 1). Of the 19 included studies, 17 were from completed studies and 2 were ongoing trials. In addition, the reference lists of 93 relevant review articles and 119 relevant cross-sectional studies were searched for additional studies.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart. Yellow, source of publication; blue, screening stage; white, excluded publication; green, included study. 119 studies from 18 publications. 2No data to extract.
We included 14 studies of interventions (12 randomized, 2 nonrandomized) including a total of 2497 participants (Table 1; Supplemental Material 1). Studies enrolled between 16 and 810 participants and were 3 wk–37.4 mo in duration. Six studies provided participants with all food (21–26), 2 studies provided dairy products in addition to a self-selected diet (27, 28), and 6 studies provided dietary advice while allowing participants to completely self-select diets (29–34). No studies were designed specifically to achieve a phosphorus contrast.
TABLE 1.
Selected characteristics of included completed studies by study design and exposure type1
| Author (reference) | Study design | Main association of interest in study | Driver of phosphorus contrast | Phosphorus contrast (high–low) | n | Duration | BP inclusion criteria (SBP/DBP, mm Hg) | Special population | Exposure type | Exposure measurement | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Barr et al. (29) | Randomized trial | Milk and BP2 | Dairy | 601 mg/d | 200 | 12 wk | < 170/95 | N/A | Intake | 2 × 3-d food record | SBP/DBP |
| Dugan et al. (30) | Randomized trial | Dairy and BP2 | Dairy | 462 mg/d | 33 | 6 wk | < 140/90 | Metabolic syndrome | Intake | 2 × 5-d food record | SBP/DBP |
| Manios et al. (27) | Randomized trial | Dairy + education and BP2 | Dairy | 346 mg/d | 82 | 5 mo | Not specified | Postmenopausal women | Intake | 1 × 3-d recall | SBP/DBP |
| Zeller et al. (33) | Randomized trial | Protein restriction and renal failure | Reduced protein | Not reported | 35 | 37.4 mo (mean follow-up) | Not specified | Type I diabetes, diabetes nephropathy, diabetes retinopathy with proteinuria, no other renal failure | Intake | ≥1 × 24-h urine | MAP |
| Appel et al. (21) | Randomized trial | Diet pattern and BP | Dairy3 | 143 mg/24h | 459 | 8 wk | < 160/80–95 | N/A | Excretion | 1 × 24-h urine | SBP/DBP |
| Appel et al. (32) | Randomized trial | Behavioral intervention and BP | Dairy3 | 95 mg/24h | 810 | 6 mo | 120–159/80–95 | N/A | Excretion | 1 × 24-h urine | SBP/DBP |
| Funatsu et al. (31) | Randomized trial | Coffee and BP | Dairy (in coffee) | 50 mg/24h | 42 | 10 wk | ≥120/80 | Coffee and alcohol drinkers | Excretion | 1 × 24-h urine | SBP/DBP |
| Hallfrisch et al. (22) | Randomized trial | Whole grains and BP | Grain | 181 mg/24h | 16 | 5 wk | Not specified | Moderately elevated cholesterol | Excretion | 3 × 24-h urine | SBP/DBP |
| Nouvenne et al. (34) | Randomized trial | Low-salt diet and idiopathic hypercalciuria | Other | 63 mg/24h | 210 | 3 mo | SBP > 110 | Idiopathic calcium-oxalate stone formers | Excretion | 1 × 24-h urine | SBP/DBP |
| Sacks et al. (24) | Randomized trial | Diet pattern + sodium and BP | Dairy3 | 179 mg/24h | 412 | 30 d | 120–159/80–95 | N/A | Excretion | 1 × 24-h urine | SBP/DBP |
| Van Beresteijn et al. (28) | Randomized trial | Milk and BP | Dairy | 0.29 mg/mg creatinine | 53 | 7 wk | Normotensive | N/A | Excretion | 1 × 24-h urine | SBP/DBP |
| Nowson et al. (23) | Randomized trial | Diet pattern and BP | Dairy3 | 83 mg/d (intake) 127 mg/24h (excretion) | 94 | 4 wk | ≥120/80 | N/A | Both | 2 × 24-h urine2 × 24-h recall | SBP/DBP |
| Hunt et al. (25) | Nonrandomized trial | Diet pattern and zinc absorption | Vegetarianism | 210 mg/d (intake) 266 mg/24h (excretion) | 21 | 8 wk | Not specified | N/A | Both | 1–14 × 24-h urineChemical analysis | SBP/DBP |
| Marshall et al. (26) | Nonrandomized trial | Diet pattern and BP2 | Other | 616 mg/d | 30 | 3 wk | Normotensive–borderline elevated | N/A | Intake | 1 × 7-d food recordChemical analysis | SBP/DBP |
| Alonso et al. (4) —ARIC | Prospective observational cohort | Identify CVD risk factors | Quintile of phosphorus intake | 773 mg/d | 8208 | 7.1 y | < 140/90 | N/A | Intake | 120-item FFQ | Incident hypertension |
| Alonso et al. (4)—MESA | Prospective observational cohort | Identify CVD risk factors | Quintile of phosphorus intake | 739 mg/d | 2901 | 3.8 y | < 140/90 | N/A | Intake | 66-item FFQ | Incident hypertension |
| Alonso et al. (35) | Prospective observational cohort | Dairy and BP | Quintile of dairy intake | 600 mg/d | 6686 | 27 mo | Normotensive | University students | Intake | 136-item FFQ | Incident hypertension |
ARIC, Atherosclerosis Risk in Communities Study; BP, blood pressure; CVD, cardiovascular disease, DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure, FFQ, food-frequency questionnaire; MAP, mean arterial pressure; MESA, Multi-Ethnic Study of Atherosclerosis; N/A, not applicable; SBP, systolic blood pressure.
And other outcomes.
DASH-style diet.
Of the 5 intervention studies providing all food, 1 estimated dietary phosphorus exposure from chemical analysis of study foods (25) and 4 from urinary phosphorus excretion (21–24). Of the 8 studies allowing partially or completely self-selected diets, 4 estimated dietary phosphorus exposure from urinary phosphorus excretion (28, 31, 32, 34) and 4 from self-reported dietary intake (27, 29, 30, 33).
In 9 of the 14 intervention studies (21, 23, 24, 27–32) (95.5% of the participants), the driver of phosphorus contrast was the dairy content of the diets. Of these 9, 4 studies (21, 23, 24, 32) (77.6% of the participants) were specifically studying a Dietary Approaches to Stop Hypertension (DASH)-style diet pattern rich in low-fat dairy, fruits, and vegetables with lower amounts of fat, cholesterol, and often (but not always) sodium than a typical American diet. One study reported mean arterial BP (33) whereas the others reported systolic blood pressure (SBP) and diastolic blood pressure (DBP).
We included 3 prospective observational cohorts (4, 35), including a total of 17,795 participants. Studies ranged from 2901 to 8208 participants in size and had a mean follow-up time of 27 mo–7.1 y. They all estimated phosphorus intake using food-frequency questionnaires and reported incident hypertension as the outcome.
Assessment of risk of bias
Of the 14 intervention studies included, none were rated as lower risk of bias for both ascertainment of dietary phosphorus exposure and ascertainment of BP (Table 2). Only 1 study (25) was rated as lower risk of bias for ascertainment of dietary phosphorus exposure owing to its use of chemically analyzed food samples. That study compared a lacto-ovo-vegetarian diet with an omnivorous diet, with the phosphorus contrast coming from meat. Only 1 study (23) was rated as higher risk of bias for ascertainment of BP owing to its use of participant-measured BP. Eight studies were rated as unclear risk of bias for ascertainment of BP because they did not mention whether staff were blinded to participants’ assignment (25–28) or did not describe the procedure staff used to measure BP (22, 30, 33, 34). Five studies were rated as lower risk of bias (21, 24, 29, 31, 32). All 3 prospective cohort studies were rated as higher risk of bias for ascertainment of phosphorus exposure owing to their use of self-reported phosphorus intake (4, 35). All 3 were rated as lower risk of bias for ascertainment of BP (4, 35).
TABLE 2.
Assessment of bias for included completed studies1
| Bias in ascertainment of exposure | Bias in ascertainment of outcome | |||
|---|---|---|---|---|
| Author (reference) | Rating | Justification | Rating | Justification |
| Barr et al. (29) | − | Self-reported intake | + | Met requirements |
| Dugan et al. (30) | − | Self-reported intake | ? | Unclear blindingUnclear procedure |
| Manios et al. (27) | − | Self-reported intake | ? | Unclear blinding |
| Zeller et al. (33) | ? | No completeness reported | ? | Unclear blindingUnclear procedure |
| Appel et al. (21) | ? | No completeness reported | + | Met requirements |
| Appel et al.2 (32) | ? | No completeness reported | + | Met requirements |
| Funatsu et al. (31) | ? | No completeness reported | + | Met requirements |
| Hallfrisch et al. (22) | ? | No completeness reported | ? | Unclear blindingUnclear procedure |
| Nouvenne et al. (34) | ? | No completeness reported | ? | Unclear procedure |
| Sacks et al. (24) | ? | No completeness reported | + | Met requirements |
| Van Beresteijn et al. (28) | ? | No completeness reported | ? | Unclear blinding |
| Nowson et al. (23) | − | Self-reported intake | − | Blood pressure measured at home by participants |
| Hunt et al. (25) | + | Chemically analyzed food samples | ? | Unclear blinding |
| Marshall et al. (26) | − | Self-reported intake (baseline only) | ? | Unclear blinding |
| Alonso et al. (4)—ARIC | − | Self-reported intake | + | No interventionStandard procedure |
| Alonso et al. (4)—MESA | − | Self-reported intake | + | No interventionStandard procedure |
| Alonso, 2005 (35) | − | Self-reported intake | + | No interventionStandard procedure |
+, lower risk; ?, unclear risk; −, higher risk. ARIC, Atherosclerosis Risk in Communities Study; MESA, Multi-Ethnic Study of Atherosclerosis.
Writing Group of the PREMIER Collaborative Research Group.
Effect of the intervention on the outcome
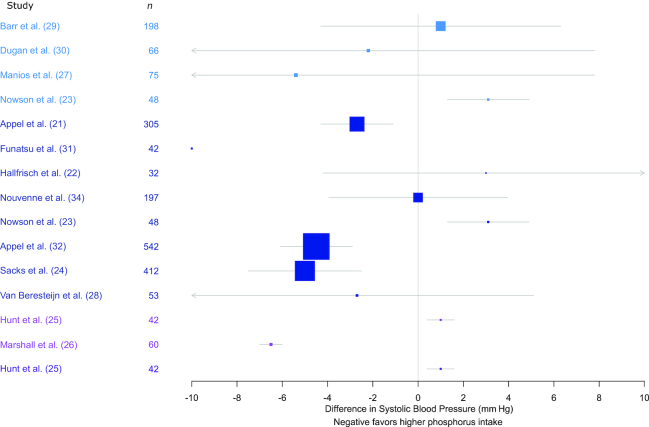
Two studies found a significant positive association between SBP and phosphorus exposure (1.0–3.1-mm Hg higher SBP, high compared with low phosphorus group) (23, 25) (Figure 2). Four studies found a significant negative association between SBP and phosphorus exposure (2.7–6.5-mm Hg lower SBP, high compared with low phosphorus group) (21, 24, 26, 32). Eight studies did not find a significant association between SBP and phosphorus exposure (22, 27–31, 34, 36). None of the included studies found a significant positive association between DBP and phosphorus exposure (Figure 3). Four studies found a significant negative association between DBP and phosphorus exposure (1.9–5.9-mm Hg lower DBP, high compared with low phosphorus group) (21, 24, 26, 32). Ten studies did not find a significant association between DBP and phosphorus exposure (22, 23, 25, 27–31, 34, 36). Three prospective cohort studies were included: the Atherosclerosis Risk in Communities (ARIC) study, Multi-Ethnic Study of Atherosclerosis (MESA), and Seguimiento Universidad de Navarra study. The Seguimiento Universidad de Navarra study found a HR of 0.75 (95% CI: 0.45, 1.27) comparing those in the highest quintile of dairy intake (who also had higher phosphorus intake) with those in the lowest quintile (35).
FIGURE 2.
Mean difference (95% CI) in systolic blood pressure by phosphorus intake group. Study design and phosphorus estimates are as follows: light blue, randomized intervention and dietary intake; dark blue, randomized intervention and urinary excretion; pink, nonrandomized intervention and dietary intake; purple, nonrandomized intervention and urinary excretion.
FIGURE 3.
Mean difference (95% CI) in diastolic blood pressure by phosphorus intake group. Study designs and phosphorus estimates are as follows: light blue, randomized intervention and dietary intake; dark blue, randomized intervention and urinary excretion; pink, nonrandomized intervention and dietary intake; purple, nonrandomized intervention and urinary excretion.
Alonso et al. (4) was the only included study that specifically examined the effect of dietary phosphorus intake on BP. They analyzed individual-level data from both the ARIC and MESA studies and found age-, race-, and sex-adjusted HRs of 0.86 (95% CI: 0.75, 0.98) for incident hypertension among those in the highest quintile of phosphorus intake compared with those in the lowest quintile in ARIC, 0.83 (95% CI: 0.68, 1.02) in MESA, and calculated a pooled HR of 0.85 (95% CI: 0.76, 0.95) (4).
We also included 2 ongoing trials registered on clinicaltrials.gov that will collect data on both dietary phosphorus intake and BP (37, 38). Neither trial is specifically designed to examine the effect of dietary phosphorus intake on BP.
Discussion
In our review of the literature, we did not identify any randomized clinical trials (completed or ongoing) specifically designed to estimate the strength and direction of the association of total dietary phosphorus intake or urinary phosphorus excretion with BP. We identified 3 prospective observational cohort studies with estimates of phosphorus intake and incident hypertension. Of the 17 completed studies included in our review, there was no consistent association between total dietary phosphorus intake and BP. This variability between studies may be due to the drivers of phosphorus contrast or errors in the measurement of phosphorus intake.
Dietary phosphorus contrast between free-living individuals comes from a variety of sources (grains, meat, and dairy accounting for >75% of total intake) (39). Of the interventions we identified, however, dairy intake and a DASH-like diet pattern were the main drivers of higher phosphorus intake. Dairy (especially low-fat dairy) has been found to be negatively associated with BP in several systematic reviews of prospective cohort studies and randomized interventions (40–42). In dairy, phosphorus is primarily in the form of calcium phosphate (43). This makes it difficult, if not impossible, to statistically separate effects of phosphorus from calcium in dairy. DASH-style diets are high in phosphorus, but also in other potentially BP-reducing components. In free-living individuals, high phosphorus intake could reflect a DASH-style diet pattern (rich in low-fat dairy, potassium, and calcium), or a BP-raising diet high in processed meats and snacks. It is not possible to separate the effect of phosphorus intake from other dietary components in these studies (such as calcium or potassium) and the matter is further complicated by potential error in estimating phosphorus intake.
The included studies assessed phosphorus intake through chemical analysis, nutrition databases, or urinary analysis. Each of these methods presents its own limitations. Urinary phosphorus excretion has been proposed as a proxy for phosphorus intake and we used it as such in our study (44–46). However, the dynamic relation between measurements of intake and urinary excretion has not been rigorously characterized. Several studies have found that the association varies within individuals over time, and between individuals by dietary and nondietary factors (47–52). Given that the main drivers of phosphorus contrast in our included studies were dairy intake and a DASH-style diet (rather than a variety of drivers), it is possible that urinary phosphorus excretion was not a valid proxy of phosphorus intake in these studies.
Several of our included studies estimated phosphorus intake using nutrient database estimates of the phosphorus content of foods. The accuracy of nutrient database estimates of phosphorus intake has been shown to vary by the types of food being consumed and whether or not they contain food additives (53–55). This could add error to the estimates of phosphorus intake, reducing the statistical power of these studies. Finally, we found 1 feeding study that assessed phosphorus intake via a chemical analysis of the food samples that were fed to participants. In this study, the change in phosphorus was entirely driven by vegetarian compared with nonvegetarian diets. A recent meta-analysis found vegetarian diets were associated with lower BP than nonvegetarian diets (56). A randomized intervention study to examine the association between total dietary phosphorus intake and BP would need to achieve a large phosphorus contrast while controlling for the intake of specific nutrients and general dietary patterns associated with changes in BP; whether such a trial could be designed is unclear.
Strengths of this review include the search strategy. We used controlled vocabulary in addition to title and abstract search terms, allowing us to find both indexed and nonindexed studies. Our use of double independent abstract and full-text review reduces the likelihood of bias by the authors in the inclusion of studies. We can be fairly confident that, had a study been designed and executed to answer this topic, it would have been captured in our search. This review has several limitations. First, our search was conducted in English and limited to languages that were known fluently by the reviewers (English and Chinese) or could be translated with limited assistance from Google Translate (French, Italian, or Spanish). The reliability of Google Translate was not a concern in the current study, given it was only used for translating specific words (such as phosphorus, BP, and hypertension) to aid in the understanding of abbreviations (for example, the abbreviation for phosphorus in Italian is F for fosforo, not P for phosphorus). No included studies were in a language other than English and 13 studies were excluded for being untranslatable. Second, we were not able to address potential differences by race. Although there are great differences in BP by race, we do not expect that any potential effect of phosphorus on BP would differ by race.
This systematic review highlights both the lack of definitive evidence as to whether total dietary phosphorus intake affects BP and the complexities of answering that question. It is unclear whether a randomized intervention study to examine the association between total dietary phosphorus intake and BP could be designed. It may be more useful (and practical) to study biologically relevant categories of phosphorus-containing ingredients or foods as separate dietary exposures. Although these categories have not been well defined, they may include sodium, calcium, and magnesium phosphate ingredients and foods high in calcium (e.g., dairy) or phytate (e.g., whole grains). In order to provide accurate guidance to the public and the food industry, we must understand the relation of these biologically relevant categories of phosphorus-containing ingredients or foods with BP and other health outcomes.
In summary, in this systematic review, we did not find any randomized trials specifically designed to estimate the strength and direction of the association of total dietary phosphorus intake and urinary phosphorus excretion with BP. We identified 3 prospective observational cohort studies with estimates of phosphorus intake and incident hypertension. Of the 17 completed studies that we identified, there was no consistent association of BP with total dietary phosphorus intake and urinary phosphorus excretion. Rather than studying total dietary phosphorus intake, it may be more useful to study biologically relevant categories of phosphorus-containing ingredients or foods as separate dietary exposures. Although these categories have not been well defined, they may include sodium, calcium, and magnesium phosphate ingredients and foods high in calcium (e.g., dairy) or phytate (e.g., whole grains). Future studies should then assess the relation of these biologically relevant categories of phosphorus-containing ingredients or foods with BP and other health outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Lori Rosman from the Welch Medical Library for her guidance and support in the development of our search strategy. The authors’ responsibilities were as follows—STM, CMR, ES, and LJA: gave input on the design of the systematic review; STM, SM, EAH, and ZX: reviewed abstracts, selected studies, extracted data, and assessed risk of bias; STM: analyzed the data and drafted the manuscript; and all authors: edited the manuscript and read and approved the final version. None of the authors reported a conflict of interest related to the study.
Notes
Supported by NIH/National Heart, Lung, and Blood Institute grant T32 HL007024 (to STM), a grant from the Johns Hopkins Bloomberg School of Public Health Department of Epidemiology Doctoral Research Fund (to STM), National Institute of Diabetes and Digestive and Kidney Diseases Mentored Research Scientist Development Award K01 DK107782 (to CMR), NIH/National Heart, Lung, and Blood Institute grant T32 HL007024 (to EAH), the China Scholarship Council (to ZX), and NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant K24DK106414 (to ES).
Supplemental Methods 1–3 and Supplemental Material 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ARIC, Atherosclerosis Risk in Communities study; BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; MESA, Multi-Ethnic Study of Atherosclerosis; SBP, systolic blood pressure.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ et al.. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. [DOI] [PubMed] [Google Scholar]
- 2. Appel LJ. The effects of dietary factors on blood pressure. Cardiol Clin. 2017;35(2):197–212. [DOI] [PubMed] [Google Scholar]
- 3. Guo W, Li J-Y, King H, Locke FB. Diet and blood nutrient correlations with ischemic heart, hypertensive heart, and stroke mortality in China. Asia Pac J Public Health. 1992;6(4):200–9. [DOI] [PubMed] [Google Scholar]
- 4. Alonso A, Nettleton JA, Ix JH, De Boer IH, Folsom AR, Bidulescu A, Kestenbaum BR, Chambless LE, Jacobs DR. Dietary phosphorus, blood pressure, and incidence of hypertension in the Atherosclerosis Risk In Communities study and the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55(3):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olivo RE, Hale SL, Diamantidis CJ, Bhavsar NA, Tyson CC, Tucker KL, Carithers TC, Kestenbaum B, Muntner P, Tanner RM et al.. Dietary phosphorus and ambulatory blood pressure in African Americans: the Jackson Heart Study. Am J Hypertens. 2018:hpy126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y et al.. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20(7):1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishi T, Shuto E, Ogawa M, Ohya M, Nakanishi M, Masuda M, Katsumoto M, Yamanaka-Okumura H, Sakai T, Takeda E et al.. Excessive dietary phosphorus intake impairs endothelial function in young healthy men: a time- and dose-dependent study. J Med Invest. 2015;62(3–4):167–72. [DOI] [PubMed] [Google Scholar]
- 8. Itkonen ST, Karp HJ, Kemi VE, Kokkonen EM, Saarnio EM, Pekkinen MH, Kärkkäinen MUM, Laitinen EKA, Turanlahti MI, Lamberg-Allardt CJE. Associations among total and food additive phosphorus intake and carotid intima-media thickness – a cross-sectional study in a middle-aged population in southern Finland. Nutr J. 2013;12(PG-94):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamamoto KT, Robinson-Cohen C, de Oliveira MC, Kostina A, Nettleton JA, Ix JH, Nguyen H, Eng J, Lima JAC, Siscovick DS et al.. Dietary phosphorus is associated with greater left ventricular mass. Kidney Int. 2013;83(4):707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takeda E, Yamamoto H, Yamanaka-Okumura H, Taketani Y. Increasing dietary phosphorus intake from food additives: potential for negative impact on bone health. Adv Nutr. 2014;5(1):92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang AR, Lazo M, Appel LJ, Gutiérrez OM, Grams ME, Chang AR, Lazo M, Appel LJ, Gutie OM. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr. 2014;99(2):320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takeda E, Yamamoto H, Yamanaka-Okumura H, Taketani Y. Dietary phosphorus in bone health and quality of life. Nutr Rev. 2012;70(6):311–21. [DOI] [PubMed] [Google Scholar]
- 13. Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, Brown IJ, Zhao L, Stamler J. Dietary phosphorus and blood pressure: international study of macro- and micro-nutrients and blood pressure. Hypertension. 2008;51(3):669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tani Y, Sato T, Yamanaka-Okumura H, Yamamoto H, Arai H, Sawada N, Genjida K, Taketani Y, Takeda E. Effects of prolonged high phosphorus diet on phosphorus and calcium balance in rats. J Clin Biochem Nutr. 2007;40(3):221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rautiainen S, Wang L, Manson JE, Sesso HD. The role of calcium in the prevention of cardiovascular disease—a review of observational studies and randomized clinical trials. Curr Atheroscler Rep. 2013;15(11):362. [DOI] [PubMed] [Google Scholar]
- 17. Jee SH, Miller ER, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: a meta-analysis of randomized clinical trials. Am J Hypertens. 2002;15(8):691–6. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Core Team. R: A Language and Environment for Statistical Computing[Internet]. 2017. Available from: https://www.r-project.org/ (accessed 19 October 2018). [Google Scholar]
- 20. Gordon M, Lumley T. forestplot: Advanced Forest Plot Using “grid” Graphics[Internet]. 2017. Available from: https://cran.r-project.org/package=forestplot (accessed 19 October 2018). [Google Scholar]
- 21. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM et al.. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24. [DOI] [PubMed] [Google Scholar]
- 22. Hallfrisch J, Scholfield DJ, Behall KM. Blood pressure reduced by whole grain diet containing barley or whole wheat and brown rice in moderately hypercholesterolemic men. Nutr Res. 2003;23(12):1631–42. [Google Scholar]
- 23. Nowson CA, Worsley A, Margerison C, Jorna MK, Frame AG, Torres SJ, Godfrey SJ. Blood pressure response to dietary modifications in free-living individuals. J Nutr. 2004;134(9):2322–9. [DOI] [PubMed] [Google Scholar]
- 24. Sacks F, Svetkey L, Vollmer W. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 25. Hunt JR, Matthys LA, Johnson LAK. Zinc absorption, mineral balance, and blood lipids in women consuming controlled lactoovovegetarian and omnivorous diets for 8 wk. Am J Clin Nutr. 1998;67(3):421–30. [DOI] [PubMed] [Google Scholar]
- 26. Marshall MW, Judd JT, Canary JJ. Self-selected vs. controlled diet as a baseline for human studies: effects of nutrient intakes on blood pressure and on constituents of blood and urine. J Am Coll Nutr. 1986;5(4):343–55. [DOI] [PubMed] [Google Scholar]
- 27. Manios Y, Moschonis G, Grammatikaki E, Katsaroli I, Kanelou P, Tanagra S. Nutrition education in postmenopausal women: changes in dietary and cardiovascular indices. Maturitas. 2006;55(4):338–47. [DOI] [PubMed] [Google Scholar]
- 28. Van Beresteijn EC, van Schaik M, Schaafsma G. Milk: does it affect blood pressure? A controlled intervention study. J Intern Med. 1990;228(5):477–82. [DOI] [PubMed] [Google Scholar]
- 29. Barr SI, McCarron DA, Heaney RP, Dawson-Hughes B, Berga SL, Stern JS, Oparil S. Effects of increased consumption of fluid milk on energy and nutrient intake, body weight, and cardiovascular risk factors in healthy older adults. J Am Diet Assoc. 2000;100(7):810–17. [DOI] [PubMed] [Google Scholar]
- 30. Dugan CE, Barona J, Fernandez ML. Increased dairy consumption differentially improves metabolic syndrome markers in male and female adults. Metab Syndr Relat Disord. 2014;12(1):62–9. [DOI] [PubMed] [Google Scholar]
- 31. Funatsu K, Yamashita T, Nakamura H. Effect of coffee intake on blood pressure in male habitual alcohol drinkers. Hypertens Res. 2005;28(6):521–7. [DOI] [PubMed] [Google Scholar]
- 32. Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin P-H, Svetkey LP et al.. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–93. [DOI] [PubMed] [Google Scholar]
- 33. Zeller K, Whittaker E, Sullivan L, Raskin P, Jacobson HR. Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1991;324(2):78–84. [DOI] [PubMed] [Google Scholar]
- 34. Nouvenne A, Meschi T, Prati B, Guerra A, Allegri F, Vezzoli G, Soldati L, Gambaro G, Maggiore U, Borghi L. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: a 3-mo randomized controlled trial. Am J Clin Nutr. 2010;91(3):565–70. [DOI] [PubMed] [Google Scholar]
- 35. Alonso A, Beunza JJ, Delgado-Rodríguez M, Martínez JA, Martínez-González MA. Low-fat dairy consumption and reduced risk of hypertension: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2005;82(5):972–9. [DOI] [PubMed] [Google Scholar]
- 36. van Beresteyn EC, Schaafsma G, de Waard H. Oral calcium and blood pressure: a controlled intervention trial. Am J Clin Nutr. 1986;44(6):883–8. [DOI] [PubMed] [Google Scholar]
- 37. Peterson CM. Effect of time-restricted feeding on fat loss and cardiometabolic risk factors in overweight adults [Internet].clinicaltrials.gov. NCT03459703 2018. Available from: https://clinicaltrials.gov/show/NCT03459703%0A(accessed 19 October 2018). [Google Scholar]
- 38. Aldo FL. Increasing calcium dietary intake helps to control blood presure and body weight [Internet].clinicaltrials.gov. NCT02295852 2014. Available from: https://clinicaltrials.gov/ct2/show/NCT02295852 (accessed 19 October 2018). [Google Scholar]
- 39. McClure ST, Chang AR, Selvin E, Rebholz CM, Appel LJ. Dietary sources of phosphorus among adults in the United States: results from NHANES 2001–2014. Nutrients. 2017;9(2):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ralston RA, Lee JH, Truby H, Palermo CE, Walker KZ. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J Hum Hypertens. 2012;26(1):3–13. [DOI] [PubMed] [Google Scholar]
- 41. McGrane MM, Essery E, Obbagy J, Lyon J, MacNeil P, Spahn J, van Horn L. Dairy consumption, blood pressure, and risk of hypertension: an evidence-based review of recent literature. Curr Cardiovasc Risk Rep. 2011;5(4):287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Drouin-Chartier J-P, Brassard D, Tessier-Grenier M, Côté JA, Labonté M-E, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7(6):1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holt C, Hasnain SS, Hukins DWL. Structure of bovine milk calcium phosphate determined by x-ray absorption spectroscopy. Biochim Biophys Acta. 1982;719(2):299–303. [DOI] [PubMed] [Google Scholar]
- 44. Morimoto Y, Sakuma M, Ohta H, Suzuki A, Matsushita A, Umeda M, Ishikawa M, Taketani Y, Takeda E, Arai H. Estimate of dietary phosphorus intake using 24-h urine collection. J Clin Biochem Nutr. 2014;55(1):62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sakuma M, Morimoto Y, Suzuki Y, Suzuki A, Noda S, Nishino K, Ando S, Ishikawa M, Arai H. Availability of 24-h urine collection method on dietary phosphorus intake estimation. J Clin Biochem Nutr. 2017;60(2):125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trautvetter U, Ditscheid B, Jahreis G, Glei M. Habitual intakes, food sources and excretions of phosphorus and calcium in three German study collectives. Nutrients. 2018;10(2):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kemi VE, Kärkkäinen MUM, Karp HJ, Laitinen KAE, Lamberg-Allardt CJE. Increased calcium intake does not completely counteract the effects of increased phosphorus intake on bone: an acute dose-response study in healthy females. Br J Nutr. 2008;99(4):832–9. [DOI] [PubMed] [Google Scholar]
- 48. Karp HJ, Vaihia KP, Kärkkäinen MUM, Niemistö MJ, Lamberg-Allardt CJE. Acute effects of different phosphorus sources on calcium and bone metabolism in young women: a whole-foods approach. Calcif Tissue Int. 2007;80(4):251–8. [DOI] [PubMed] [Google Scholar]
- 49. Shinozaki N, Murakami K, Asakura K, Uechi K, Kobayashi S, Masayasu S, Sasaki S. Dietary phosphorus intake estimated by 4-day dietary records and two 24-hour urine collections and their associated factors in Japanese adults. Eur J Clin Nutr. 2018;72(4):517–25. [DOI] [PubMed] [Google Scholar]
- 50. St-Jules DE, Jagannathan R, Gutekunst L, Kalantar-Zadeh K, Sevick MA. Examining the proportion of dietary phosphorus from plants, animals, and food additives excreted in urine. J Ren Nutr. 2017;27(2):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Joung H, Jeun BY, Li SJ, Kim J, Woodhouse LR, King JC, Welch RM, Paik HY. Fecal phytate excretion varies with dietary phytate and age in women. J Am Coll Nutr. 2007;26(3):295–302. [DOI] [PubMed] [Google Scholar]
- 52. Delgado-Andrade C, Seiquer I, García MM, Galdó G, Navarro MP. Increased Maillard reaction products intake reduces phosphorus digestibility in male adolescents. Nutrition. 2011;27(1):86–91. [DOI] [PubMed] [Google Scholar]
- 53. Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr. 2007;17(5):350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moser M, White K, Henry B, Oh S, Miller ER, Anderson CA, Benjamin J, Charleston J, Appel LJ, Chang AR. Phosphorus content of popular beverages. Am J Kidney Dis. 2015;65(6):969–71. [DOI] [PubMed] [Google Scholar]
- 55. Krekel C, McClure ST, Chang AR. Improving estimates of phosphorus additive content: manufacturers needed. J Ren Nutr. 2016;26(5):e27–30. [Google Scholar]
- 56. Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A, Okamura T, Miyamoto Y. Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med. 2014;174(4):577–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.