ABSTRACT
Background
Docosahexaenoic acid (DHA) is a long-chain polyunsaturated fatty acid that has been linked to improved vision and cognition in postnatal feeding studies and has been consistently associated with reduction of early preterm birth in prenatal supplementation trials. This is a report of the first long-term follow-up of infants from mothers receiving prenatal DHA supplementation in a US cohort.
Objective
Our objective was to evaluate the efficacy of the prenatal supplementation on both global and granular longitudinal assessments of cognitive and behavioral development.
Methods
In a randomized double-blind clinical trial, mothers received either 600 mg/d of DHA or a placebo beginning at 14.5 weeks of gestation and capsules were provided until delivery. Children from those pregnancies were followed by cognitive and behavioral assessments administered from 10 mo through 6 y of age. From 301 mothers in the initial study, ∼200 infants completed the longitudinal schedule.
Results
Although this intervention had been shown to reduce high-risk pregnancies and improve visual attention in infants during the first year, only a few positive long-term effects of prenatal DHA supplementation emerged from analyses of this follow-up. Increases in maternal blood DHA during pregnancy were related to verbal and full scale intelligence quotient (IQ) scores at 5 and 6 y, but these effects disappeared after controlling for SES. Maternal blood DHA concentrations at delivery were unrelated to outcomes, although maternal DHA at enrollment was related to productive vocabulary at 18 mo.
Conclusions
Although prenatal DHA supplementation substantially reduced early preterm birth and improved visual attention in infancy in this sample, no consistent long-term benefits were observed into childhood. Increases in maternal blood DHA concentration in pregnancy were related to higher IQs but this effect was confounded with SES and disappeared when SES was statistically controlled. This trial was registered at http://www.clinicaltrials.gov as NCT00266825 and NCT02487771.
Keywords: prenatal, docosahexaenoic acid, behavior, development, longitudinal, clinical trial
Introduction
DHA (22:6ω-3) is a long-chain PUFA, a member of the ω-3 fatty acid family. It is found in all cell membranes, but is particularly concentrated in the brain where it plays an important role in behavior and cognition (1). Diet is the predominant source for DHA; however, the average US adult consumes only ∼50 mg DHA/d. There is evidence for positive effects of DHA intake on sensory and cognitive function in both animals (2, 3) and humans (4–8), although the literature is mixed (9–17). The developmental effects of DHA have been most intensively studied in infancy, but recent work has focused on pregnancy and prenatal development because DHA accumulation in the brain is accelerated during the last intrauterine trimester (18–24). Given the hypothesis that improving DHA status during fetal development may promote postnatal behavioral and cognitive development, it is significant that very low baseline DHA status has been documented in several previous randomized controlled trials (RCTs) studying the effects of DHA during pregnancy in US women (25, 26).
The results on brain DHA deprivation in rodents show that even modest decreases in brain DHA during early development produce irreversible changes in neurotransmitter systems and cortical electrophysiology (27–29), thus raising the possibility that there could be similar critical windows for fetal programming in human fetal or neonatal life. The results of human prenatal DHA supplementation or status studies are also mixed between positive and null outcomes (30, 31). Observational studies of DHA status in mothers during pregnancy suggest that prenatal amounts of DHA may have long-term effects on their infants. Individual differences in maternal DHA status during pregnancy and lactation have been associated with individual differences in various measures of developmental outcome in infants and children (6, 32–41); more mature attention in infants and toddlers (33, 42, 43) and higher school performance at 7 y (44) have been reported in offspring of women with higher red blood cell phospholipids DHA at delivery.
Here, we report on the follow-up of the Kansas University DHA Outcomes Study (KUDOS), a randomized double-blind Phase III clinical trial (NCT02487771) to study the long-term effects of a prenatal dose of 600 mg of DHA on pregnancy and postnatal outcomes. Previous reports showed that a daily prenatal dose of 600 mg DHA compared with a placebo (0 mg) significantly increased birthweight and birth length, and also significantly reduced preterm births under 34 weeks of gestation (45); analogous effects have been seen with a daily dose of 800 mg of DHA compared with a placebo in pregnancy (46, 47). In addition, infants from mothers supplemented with 600 mg of DHA during pregnancy maintained higher levels of sustained attention measured during visual habituation at 4, 6, and 9 mo of age; infants whose mothers received the placebo declined in sustained attention over that same period (45).
Methods
Participants and design
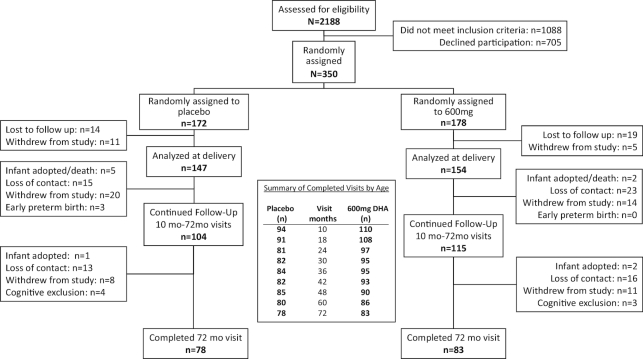
The Consolidated Standards of Reporting Trials (CONSORT) diagram for the follow-up phase of KUDOS is shown in Figure 1. The study was conducted following guidelines set forth by the Declaration of Helsinki; informed consent was obtained from all participants, and the study was reviewed and approved by the University of Kansas Medical Center Human Subjects Committee. Subjects were consented at enrollment (during pregnancy) for all measures through 18 mo; at the 18-mo appointment, subjects were invited to continue with follow-up visits from 24 through 72 mo, and those who accepted were consented into the second phase of the study. Details on the trial (enrollment, random assignment, blinding, data checking and integrity, inclusion and exclusion criteria) as well as information on compliance and demographics of the sample were reported in the 2 preceding articles from this RCT (45, 47).
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram for the follow-up study.
DHA was provided to the treatment group through 3 algae-oil capsules daily (200 mg DHA/capsule, DHASCO, from DSM Nutritional Products; formerly Martek Biosciences). Three capsules containing an equal proportion of soybean and corn oil were provided to the placebo group. Capsules were given beginning at enrollment (mean of 14.5 weeks of gestation) until birth. Both types of capsules were orange flavored. DSM Nutritional Products donated the capsules for the study but had no role in the study design, analysis, interpretation, or dissemination.
The first aim of the RCT was to determine the effect of prenatal DHA supplementation on pregnancy outcomes, and we have previously reported (47) increased gestation, birth weight, and birth length, and a reduction of early preterm birth (<34 weeks of gestation). The second primary aim of the trial was to determine the effects of prenatal DHA on the development of infants born to these mothers. We observed improvements in the quality of visual attention from 4 to 9 mo of age in a previous publication (45). The primary outcome for this aim was behavioral and cognitive development, which encompassed global developmental tests and assessments of language, executive function, and adaptive/regulative behavioral measures.
Longitudinal measures
The RCT featured a particularly rich and comprehensive longitudinal assessment schedule from 10 through 72 mo of age, all of which is reported on here. The measures are presented in groupings that reflect their underlying constructs and are summarized in Table 1.
TABLE 1.
Longitudinal schedule for the Kansas University DHA Outcomes Study follow-up1
| Age of administration (mo) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure type | Measure | 10 | 18 | 24 | 30 | 36 | 42 | 48 | 60 | 72 |
| Global standardized | BSID | — | × | — | — | — | — | — | — | — |
| WPPSI | — | — | — | — | × | — | × | — | × | |
| Literacy-language | MBCDI | — | × | — | — | — | — | — | — | — |
| TOPEL | — | — | — | — | — | × | — | — | — | |
| Sentence Repetition | — | — | — | — | × | × | × | — | — | |
| PPVT | — | — | — | — | — | — | — | × | — | |
| Executive function | Willatts Problem-Solving Task | × | — | — | — | — | — | — | — | — |
| Delayed Response | — | — | × | × | — | — | — | — | — | |
| DCCS | — | — | — | — | × | × | × | × | — | |
| Stroop tasks | — | — | — | — | × | × | × | × | — | |
| Tower of Hanoi | — | — | — | — | — | — | — | × | × | |
| Adaptive skills | BASC | — | — | — | — | × | — | × | × | × |
BASC, Behavioral Assessment Scale for Children; BSID, Bayley Scales of Infant Development; DCCS, Dimensional Change Card Sort; MBCDI, MacArthur-Bates Communicative Development Inventory; PPVT, Peabody Picture Vocabulary Test; TOPEL, Test of Preschool Early Literacy; WPPSI, Wechsler Preschool and Primary Scale of Intelligence.
Standardized tests
Bayley Scales of Infant Development II (18 mo)
The Bayley Scales of Infant Development II (BSID) is a commonly used instrument that assesses fundamental sensorimotor developmental milestones. The scale yields a Mental Development Index (MDI) and a Psychomotor Development Index (PDI), both expressed as standard scores on an intelligence quotient (IQ) scale.
Wechsler Preschool and Primary Scale of Intelligence—Third Edition (36, 48, and 72 mo)
The Wechsler Preschool and Primary Scale of Intelligence—Third Edition (WPPSI-III) (48) is among the most widely used standardized tests of early intelligence available. It is valid for ages 2.5 through 7.25 y and yields a number of subtests that in aggregate provide a Verbal IQ, a Performance IQ, a Processing Speed Quotient, and a Full Scale IQ score.
Emergent literacy/language tasks
MacArthur-Bates Communicative Development Inventory (18 mo)
The MacArthur-Bates Communicative Development Inventory (MBCDI) is a parent-report measure of early communicative competence that has been validated against actual child language diaries (49). At this age, the MBCDI yields numerous language-based variables, but we chose to focus on 3 continuous variables: the size of the child's productive (expressive) vocabulary, sentence length, and a measure of linguistic complexity.
The Test of Preschool Early Literacy (42 mo)
The Test of Preschool Early Literacy (TOPEL) (50) is a test for children aged 3–5 y that provides a reliable and valid means of monitoring children's progress toward literacy in vocabulary, phonological awareness, and print knowledge (51). The constructs measured by the TOPEL have been found to strongly predict school achievement (52).
Sentence Repetition tasks (36, 42, and 48 mo)
In Sentence Repetition (SR) tasks (53), children are presented with sentences and are instructed merely to repeat the sentences back verbatim. The test begins with simple sentences but these increase in difficulty as a function of length and syntactic complexity (54). These tasks are appropriate for preschoolers (55, 56), and performance is a strong predictor of later reading proficiency and fluency (57, 58). The score reflects the number of sentences the participant correctly repeats verbatim.
Peabody Picture Vocabulary Test, Third Edition (60 mo)
The Peabody Picture Vocabulary Test, Third Edition (PPVT-3) is a well-standardized and widely used measure of receptive vocabulary for individuals from 2.5 to 90 y of age (59). Form IIIB was used for all participants.
Laboratory-based tasks of executive function
Willatts problem-solving task (10 mo)
We employed a means-ends problem-solving test that measures complex cognitive abilities during the first year and which has been shown to be sensitive to DHA status and supplementation in earlier studies (4, 42, 60). Briefly, the child must retrieve a small toy under a cloth and behind a foam block obstacle. The task is successfully completed if the child can move the block out of the way, pull the cloth to bring the toy within reach, and uncover the toy. The entire 3-step problem is administered 3 times. Performance on the task is scored such that higher scores reflect more intentional levels of problem-solving ability.
Delayed Response spatial memory task (24 and 30 mo)
The Delayed Response (DR) task (61) is an assessment involving both attention and spatial memory. In full view of the child, a toy was buried 6 in (15.2 cm) to the left or right of the child's midline (counterbalanced across participants) in material in a 1 m × 0.5 m × 0.25 m box, and the child was required to locate it after a delay. Tests were conducted in 2 blocks comprised of 3 trials conducted with a 3-s delay at one location (i.e., right or left of midline), and 2 trials at the reversed location after a 10-s delay. For the second blockm the hiding locations were reversed relative to the first block. The accuracy of the child's searches was coded (in inches) from video recordings of the session.
Dimensional Change Card Sort task (36, 42, 48, and 60 mo)
This test, based on discrimination learning tasks (62, 63), is administered according to a standardized protocol (64). Children were presented with cards that could be sorted into boxes based on either object shape (rabbits or cars) or color (red or blue), were first asked to sort the cards on one dimension (e.g., shape), and were then asked to reverse (“switch”) the rule to the other dimension (e.g., to color). Thus, the task first provides data on rule learning, and the ability to inhibit use of the first rule after the switch. After 2 practice trials, 6 trials were conducted in which the child sorted cards. The child was then told that the sorting dimension was switched (i.e., if sorting on color, switch to sorting on object shape or vice versa) and another 6 trials were conducted. Responses were recorded for each card sorted and scored 1 for a correct sort and 0 for an incorrect sort in both the preswitch and postswitch phases. Task performance was quantified in each phase (pre- or postswitch) in terms of the total number of cards correctly sorted, as well as dichotomously as “pass/fail”; if the child sorted ≥5 cards correctly (out of 6 trials), the phase was scored as a “pass.”
Stroop tasks (36, 42, 48, and 60 mo)
In this task, children are asked to respond on the basis of a rule that conflicts with an intuitive response; this assesses both rule learning and inhibition. We used 2 variants of the task at each age. In the “Red/Yellow” variant (65), the child was presented with yellow and red squares on a computer monitor. The child was then asked to “trick” the experimenter by “pointing to the wrong color” when the experimenter named an object; for example, if the experimenter said, “Point to ketchup,” the child was asked to point to the yellow square and if the experimenter said “Point to mustard,” the child was asked to point to the red square. A pretest determined whether children understood their colors and the objects well enough to participate; and if they did not, the task was adjusted for terms that they did understand and which conformed appropriately to the task (e.g., mustard/yellow and ketchup/red, banana/yellow and apple/red, or Big Bird/yellow and Elmo/red). The second variant (the “Day/Night” form) is commonly administered to preschoolers (66): the child was presented with a yellow moon with stars on a black background, or a yellow sun on a white background. The child was then asked to say “day” when the moon was presented and “night” when the sun was presented. For both tasks, there are 4 practice trials and 16 test trials administered; lateral position, position of correct response, and order of correct response were counterbalanced across trials. In each case, the primary dependent measure was the number of correct responses.
Tower of Hanoi task (60 and 72 mo)
The Tower of Hanoi (TOH) task is a traditional problem-solving puzzle that has been used widely as a neuropsychological (67) and developmental (68, 69) assessment of rule-learning and maintenance, goal-directed behavior, planning, and error correction. To start, different-sized disks are stacked in ascending order of size on the first of 3 pegs arranged linearly on a board. The participant must move the disks from one end peg to the other by 1) moving only 1 disk at a time, 2) moving only the topmost disk from any stack, and 3) placing only smaller disks on top of larger ones. For assessing children, instructions are adapted to be age-appropriate, and the task is broken down into levels of increasing number of steps (from 2 to 7 steps), such that the child advances by solving each level in the optimal number of steps on 2 consecutive attempts with 6 attempts allowed on each level. If a child fails to complete 2 consecutive optimal solutions within the allotted attempts, this level is recorded as the maximum level attempted. The task is also scored based on a processing efficiency score which reflects how quickly a child achieved 2 consecutive optimal solutions at each level administered (70). The task has very good psychometric properties (71), with robust normative and developmental effects (69).
Parent-report measure of regulation and adaptive skills
Behavior Assessment System for Children-2 (36, 48, 60, and 72 mo)
The Behavior Assessment System for Children-2 (BASC) (72) is a widely used instrument for documenting self-regulation of mood and attention from ages 2 to 21 y. We used the Parent Rating Scales of the BASC-2, which was designed explicitly to be sensitive to developmental issues, and which measures both adaptive and problem behaviors in the community and home settings. The preschool (36, 48, and 60 mo) and child (72 mo) forms provide evaluation of the child on several subscales that measure adaptive and socioemotional function, many of which have been suggested as being potentially relevant to DHA effects. At the ages of assessment, the scale yields composite scores for Externalizing Problems, Internalizing Problems, Behavioral Symptoms, and Adaptive Skills.
Statistical analysis
The clinical trial was initially powered for pregnancy outcomes (47), but was designed to retain a total sample of n = 175 (n = ∼ 90 per group) throughout the follow-up period. Demographic characteristics of the entire sample and the arms of the trial have been provided in previous publications (45, 47). The follow-up sample size allowed for sufficient power (0.90) to detect, for example, a 5-point difference on the BSID and a 7-point difference on the WPPSI using an α level of P < 0.05. This report presents analyses comparing the DHA and placebo groups controlling for covariates that were observed to affect developmental outcomes, such as participants’ socioeconomic status (SES) and prepregnancy BMI, as well as the amount of additional DHA that mothers reported taking during pregnancy. SES was quantified by calculating a score derived from a principal component analysis of maternal age, maternal education, maternal performance on the PPVT, and household income derived from zip codes for families’ residence. This analysis yielded a single component (eigenvalue = 2.40) which accounted for 60.6% of shared variance among the variables entered.
We report here a series of mixed-models analyses, including subject as a random factor with a variance component covariance structure. DHA group (DHA compared with placebo) and sex (male compared with female) were factors in all analyses; visit (age) was included as a repeated-measure factor as appropriate. For the Dimensional Change Card Sort (DCCS) task “pass/fail” variable, we used a generalized estimating equations modeling process, which emulates mixed-models analyses for dichotomous outcomes. Additional within-session repeated factors were included for DR, DCCS, and Stroop tasks. SES, prepregnancy BMI, and additional DHA during pregnancy were covaried as main effects.
Results
For archival purposes, raw (i.e., unadjusted) means, SDs, and effect sizes for all variables are presented at all ages for global developmental tests (Table 2), language outcomes (Table 3), executive function measures (Table 4), and adaptive function/self-regulation constructs (Table 5). Statistical analyses of those data (including effects of covariates) are presented in the sections that follow.
TABLE 2.
Raw means, SDs, and effect sizes for global standardized tests at all ages for placebo and DHA-supplemented groups1
| Placebo | Supplemented | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessment | Subtest or variable | Age (mo) | Mean | SD | n | Mean | SD | n | Effect size2 (d) |
| BSID | MDI | 18 | 98.14 | 12.10 | 84 | 96.95 | 10.95 | 103 | 0.104 |
| PDI | 18 | 94.93 | 8.36 | 84 | 95.11 | 9.31 | 104 | 0.020 | |
| BRS total | 18 | 119.07 | 7.92 | 83 | 117.48 | 8.67 | 103 | 0.191 | |
| WPPSI | Verbal IQ | 36 | 100.99 | 14.22 | 77 | 101.10 | 16.51 | 91 | 0.007 |
| — | 48 | 99.30 | 15.31 | 67 | 100.13 | 16.13 | 80 | 0.052 | |
| — | 72 | 101.53 | 14.20 | 70 | 102.44 | 14.88 | 81 | 0.063 | |
| Performance IQ | 36 | 94.37 | 15.73 | 74 | 96.07 | 14.35 | 90 | 0.114 | |
| — | 48 | 96.87 | 14.84 | 76 | 98.74 | 15.52 | 88 | 0.123 | |
| — | 72 | 98.96 | 16.22 | 70 | 101.07 | 14.77 | 82 | 0.137 | |
| Processing Speed | 48 | 90.27 | 14.58 | 70 | 92.37 | 13.31 | 81 | 0.151 | |
| — | 72 | 99.09 | 12.79 | 70 | 101.38 | 12.84 | 82 | 0.179 | |
| Full Scale IQ | 36 | 97.78 | 15.43 | 74 | 98.75 | 15.75 | 88 | 0.062 | |
| — | 48 | 97.52 | 15.32 | 66 | 99.72 | 16.10 | 79 | 0.140 | |
| — | 72 | 100.67 | 14.70 | 69 | 102.21 | 14.02 | 81 | 0.108 | |
BRS, Behavior Rating Scale; BSID, Bayley Scales of Infant Development; IQ, intelligence quotient; MDI, Mental Development Index; PDI, Psychomotor Development Index; WPPSI, Wechsler Preschool and Primary Scale of Intelligence.
None of the effects reported here for placebo compared with supplemented attained statistical significance.
TABLE 3.
Raw means, SDs, and effect sizes for emergent language measures at all ages for placebo and DHA-supplemented groups1
| Placebo | Supplemented | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Subtest or variable | Age (mo) | Mean | SD | n | Mean | SD | n | Effect size2 (d) |
| MBCDI | Words Produced | 18 | 79.61 | 67.14 | 89 | 73.94 | 90.14 | 106 | 0.071 |
| Sentence Length | 18 | 1.84 | 0.85 | 88 | 1.79 | 1.14 | 105 | 0.050 | |
| Complexity | 18 | 1.96 | 3.84 | 88 | 1.64 | 4.30 | 105 | 0.078 | |
| Sentence Repetition | Score | 36 | 4.63 | 2.34 | 64 | 4.73 | 2.17 | 77 | 0.045 |
| — | 42 | 5.60 | 2.31 | 73 | 5.54 | 2.53 | 87 | 0.026 | |
| — | 48 | 6.96 | 1.97 | 72 | 6.96 | 2.11 | 79 | 0.002 | |
| TOPEL | Early Literacy Index | 42 | 92.99 | 18.76 | 71 | 95.08 | 18.80 | 87 | 0.111 |
| PPVT | Standard Score | 60 | 101.07 | 14.92 | 74 | 102.35 | 15.33 | 85 | 0.085 |
MBCDI, MacArthur-Bates Communicative Development Inventory; PPVT, Peabody Picture Vocabulary Test; TOPEL, Test of Preschool Early Literacy.
None of the effects reported here for placebo compared with supplemented attained statistical significance.
TABLE 4.
Raw means, SDs, and effect sizes for executive function measures at all ages for placebo and DHA-supplemented groups
| Placebo | Supplemented | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessment | Subtest or variable | Age (mo) | Mean | SD | n | Mean | SD | n | Effect size1 (d) |
| Willatts Task | Total score | 10 | 8.84 | 4.21 | 92 | 9.14 | 4.53 | 105 | 0.068 |
| Delayed Response | Initial trials | 24 | 4.00 | 2.82 | 74 | 3.81 | 2.93 | 81 | 0.063 |
| — | 30 | 3.98 | 3.20 | 73 | 4.91 | 3.52 | 81 | 0.278 | |
| Reversal trials | 24 | 5.38 | 3.58 | 75 | 4.95 | 3.14 | 81 | 0.128 | |
| — | 30 | 4.97 | 3.35 | 73 | 3.88 | 3.06 | 83 | 0.340 | |
| Dimensional Change Card Sort | Preswitch total | 36 | 4.75 | 1.68 | 79 | 5.16 | 1.61 | 93 | 0.252 |
| — | 42 | 5.82 | 0.89 | 76 | 5.62 | 1.08 | 91 | 0.202 | |
| — | 48 | 5.86 | 0.60 | 78 | 5.92 | 0.48 | 89 | 0.116 | |
| — | 60 | 5.87 | 0.78 | 74 | 6.00 | 0.00 | 84 | 0.369 | |
| Postswitch total | 36 | 2.13 | 2.11 | 79 | 1.97 | 2.43 | 93 | 0.070 | |
| — | 42 | 1.93 | 2.57 | 76 | 2.01 | 2.59 | 91 | 0.030 | |
| — | 48 | 3.17 | 2.81 | 78 | 3.27 | 2.88 | 89 | 0.036 | |
| — | 60 | 4.58 | 2.35 | 74 | 4.32 | 2.56 | 84 | 0.106 | |
| Percent passing | 36 | 0.39 | 0.49 | 79 | 0.52 | 0.50 | 93 | 0.250 | |
| — | 42 | 0.61 | 0.49 | 76 | 0.57 | 0.50 | 91 | 0.083 | |
| — | 48 | 0.74 | 0.44 | 78 | 0.75 | 0.43 | 89 | 0.021 | |
| — | 60 | 0.86 | 0.35 | 74 | 0.86 | 0.35 | 84 | 0.003 | |
| Stroop tasks | Total score | 36 | 7.46 | 5.32 | 56 | 8.32 | 4.73 | 67 | 0.171 |
| — | 42 | 8.05 | 4.69 | 67 | 8.77 | 4.56 | 81 | 0.157 | |
| — | 48 | 10.22 | 5.03 | 73 | 10.66 | 5.04 | 82 | 0.086 | |
| — | 60 | 12.45 | 4.63 | 74 | 13.13 | 4.07 | 83 | 0.156 | |
| Tower of Hanoi | Max steps | 60 | 5.28 | 1.45 | 71 | 5.11 | 1.54 | 79 | 0.113 |
| — | 72 | 6.15 | 1.18 | 66 | 6.17 | 0.87 | 79 | 0.013 | |
| Efficiency | 60 | 16.94 | 8.58 | 71 | 16.29 | 8.74 | 79 | 0.075 | |
| — | 72 | 24.56 | 8.34 | 66 | 24.15 | 6.79 | 79 | 0.055 | |
None of the effects reported here for placebo compared with supplemented attained statistical significance, except the main effect for Delayed Response (P < 0.05).
TABLE 5.
Raw means, SDs, and effect sizes for parent-report measures of adaptive behavior and self-regulation at all ages for placebo and DHA-supplemented groups
| Placebo | Supplemented | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessment | Subtest or variable | Age (mo) | Mean | SD | n | Mean | SD | n | Effect size2 (d) |
| BASC1 | Externalizing Problems | 36 | 48.17 | 6.86 | 71 | 49.11 | 7.85 | 82 | 0.127 |
| — | 48 | 48.87 | 8.22 | 74 | 50.08 | 8.22 | 85 | 0.148 | |
| — | 60 | 48.94 | 8.71 | 71 | 50.43 | 9.22 | 83 | 0.166 | |
| — | 72 | 50.10 | 9.68 | 67 | 49.37 | 6.76 | 75 | 0.090 | |
| Internalizing Problems | 36 | 49.31 | 9.67 | 71 | 49.10 | 8.05 | 82 | 0.024 | |
| — | 48 | 47.30 | 8.65 | 74 | 47.46 | 8.73 | 85 | 0.019 | |
| — | 60 | 49.99 | 7.96 | 72 | 49.21 | 8.75 | 83 | 0.093 | |
| — | 72 | 46.80 | 7.80 | 66 | 45.96 | 8.35 | 75 | 0.104 | |
| Behavioral Symptoms | 36 | 47.99 | 7.14 | 70 | 49.46 | 6.85 | 82 | 0.212 | |
| — | 48 | 49.42 | 8.62 | 74 | 50.59 | 7.20 | 85 | 0.149 | |
| — | 60 | 50.17 | 7.66 | 69 | 50.78 | 8.19 | 83 | 0.077 | |
| — | 72 | 48.99 | 8.09 | 66 | 49.17 | 6.72 | 75 | 0.026 | |
| Adaptive Skills | 36 | 54.00 | 7.49 | 71 | 52.56 | 7.24 | 82 | 0.196 | |
| — | 48 | 50.15 | 8.76 | 74 | 47.72 | 8.40 | 85 | 0.284 | |
| — | 60 | 52.09 | 8.18 | 71 | 50.96 | 8.14 | 83 | 0.137 | |
| — | 72 | 51.80 | 8.09 | 66 | 49.73 | 9.77 | 75 | 0.230 | |
BASC, Behavior Assessment System for Children.
None of the effects reported here for placebo compared with supplemented attained statistical significance.
Standardized tests
BSID
Analysis of the MDI and PDI from the BSID at 18 mo yielded no significant effects involving DHA group, or any other factor. Among the covariates, SES score was significantly and positively related to the MDI (P = 0.001) and prepregnancy BMI was significantly and negatively related to the PDI (P = 0.031).
WPPSI
Analyses of the WPPSI included Verbal, Performance, Processing Speed IQs, and the Full Scale IQ. Note that the Processing Speed is available only for 48 and 72 mo. In no analysis did DHA group emerge as a significant component, either as a main effect or in an interaction; the effects described below largely involve effects of visit and the covariates. Significant effects of visit were seen for each WPPSI outcome. For Verbal IQ (P = 0.015), scores were lower at 48 mo than at 36 or 72 mo, but for Performance IQ (P = 0.001), Processing Speed IQ (P < 0.001), and Full Scale IQ (P = 0.003) standard scores increased linearly across ages. For the Full Scale IQ, a significant sex × visit interaction emerged (P = 0.022); girls increased linearly from 36 to 72 mo but boys were essentially flat from 36 to 48 mo before increasing at 72 mo.
Covariates affected IQ outcomes in similar ways, with SES positively associated with Verbal, Performance, Processing Speed, and Full Scale IQs (all Ps < 0.001). Maternal prepregnancy BMI was negatively associated with Verbal IQ (P = 0.017), Performance IQ (P = 0.001), and Full Scale IQ (P = 0.003).
Emergent literacy/language tasks
MBCDI
The MBCDI as administered at 18 mo yields numerous outcomes, but we focused on continuous measures reflecting productive vocabulary (i.e., words produced), average sentence length, and linguistic complexity. No significant effects emerged from analyses for any of these outcomes, except for a significant sex effect for average sentence length (P = 0.014), where girls had longer sentence lengths than boys.
Prepregnancy BMI was negatively related to both productive vocabulary (P = 0.012) and average sentence length (P = 0.014); additional DHA intake was positively related to productive vocabulary (P = 0.035). SES was not associated with any of these outcomes.
SR
Analysis of the SR score yielded a significant main effect for visit (P < 0.001) as all children showed a linear improvement in scores from 36 to 48 mo. In addition, a significant visit × sex × DHA group interaction (P = 0.028) emerged. Univariate analyses conducted separately at each age to aid interpretation of this interaction were equivocal, as none yielded any significant terms involving DHA group or sex; the interaction may be attributable to a steeper developmental trajectory for girls in the placebo group (see Figure 2). SES was significantly and positively (P < 0.001) associated with performance on this task.
FIGURE 2.
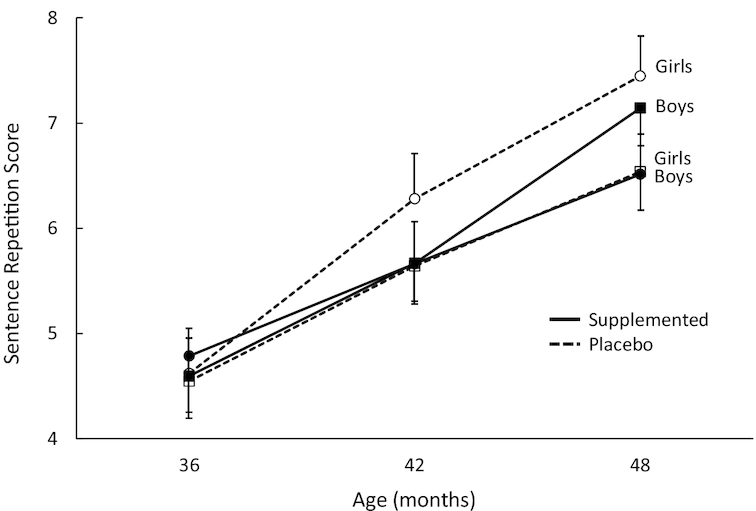
Plot of sex × visit × DHA group interaction for Sentence Repetition task scores. Overall sample sizes are 141 at 36 mo (n = 64 and 77 for placebo and DHA, respectively), 160 at 42 mo (n = 73 and 87, respectively), and 151 at 48 mo (n = 72 and 79, respectively). All means shown here are adjusted for covariates and the interaction represented here is statistically significant (P = 0.028). Error bars represent SEMs.
TOPEL
Analysis of the Early Literacy Index (the composite score from the TOPEL) at 42 mo yielded no significant effects. SES was significantly (P < 0.001) and positively related to performance on this measure.
PPVT
Analysis of the PPVT standard score at 60 mo yielded no significant effects. SES was significantly (P < 0.001) and positively related to receptive vocabulary.
Laboratory-based tasks of executive function
Willatts task
Analyses of scores on the Willatts task yielded only a main effect for sex (P = 0.023), with girls outperforming boys.
DR task
The variable analyzed from this task was distance from the target point (i.e., accuracy) for the spatial memory search; lower values represented better performance. Several significant effects emerged on this outcome involving the DHA group factor. Toddlers from supplemented mothers performed slightly better than toddlers from placebo mothers, producing a main effect for DHA group (P = 0.047); this was consistent across ages (see Figure 3). This main effect was qualified by 3 subsequent higher-order interactions: a DHA group × sex interaction (P = 0.005), a DHA group × visit × trial type × order interaction (P = 0.028), and a DHA group × visit × sex × trial type × order interaction (P = 0.026). To understand these interactions more clearly, we conducted separate analyses for each order. For order 1, the analysis yielded a significant DHA group × sex interaction (P = 0.045), as boys in the placebo group performed more poorly than girls in the same group; no significant terms involving DHA emerged from the separate analysis of order 2.
FIGURE 3.
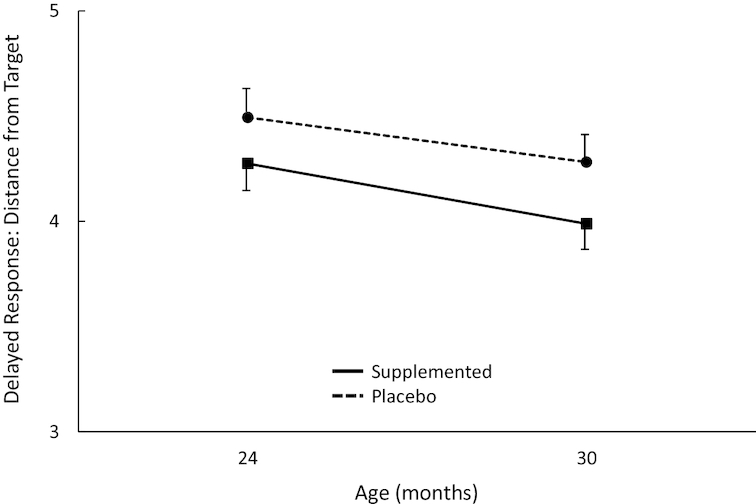
Plot of main effect for DHA group on Delayed Response accuracy across visits. Overall sample sizes are 161 at 24 mo (n = 77 and 84 for placebo and DHA, respectively) and 160 at 30 mo (n = 84 and 85, respectively). All means shown here are adjusted for covariates. The main effect for DHA group was statistically significant (P = 0.047) but was qualified by several significant higher-order interactions. Error bars represent SEMs.
Several expected significant main effects emerged: a trial type effect (P < 0.001) resulted from better search accuracy on initial trials than on trials on which spatial position was reversed and an order effect (P < 0.001) was attributable to performance being better on the first trial block than on the second as children's performance worsened over repeated reversal of hiding locations. Girls performed better than boys; the sex effect (P < 0.001) was significant. A visit × trial number interaction (P = 0.032) was attributable to 24-mo-olds performing more poorly than 30-mo-olds on the first trial, although both ages ultimately improved to attain equivalent accuracy on subsequent trials. Other significant interactions included visit × order × sex (P = 0.020); the general trend was for children's accuracy to decrease across orders over time, but 24-mo-old boys performed poorly across the board. A similar pattern was responsible for a significant sex × trial type × order (P = 0.019) interaction; boys showed a substantial decrease in accuracy across both trials and orders, but the effects of order and time on girls’ accuracy were more tempered.
Additional DHA consumed during pregnancy was modestly and positively related to accuracy on this task (P = 0.040).
DCCS task
Analysis of the DCCS total scores yielded no significant effects involving DHA group. There were main effects for visit (P < 0.001), as performance increased with age and, as expected, preschoolers performed significantly better on the preswitch trials than the postswitch trials, yielding a significant main effect for pre-post switch (P < 0.001). Both main effects were qualified by a significant visit × pre-post interaction (P < 0.001), as preswitch performance improved gradually from 36 to 60 mo, whereas performance on postswitch trials improved dramatically across the same ages. Maternal prepregnancy BMI was significantly and negatively related to performance on this task (P = 0.028).
The generalized estimating equations analysis of the DCCS pass/fail data yielded a significant DHA group × visit interaction (P = 0.048); children from DHA-supplemented mothers during pregnancy passed the task at a significantly higher rate at 36 mo than children from mothers assigned to the placebo group (see Figure 4), suggesting slightly accelerated executive function on this task as a result of prenatal DHA supplementation. Other significant main effects included visit (P < 0.001), with more children passing the DCCS criterion with age, and pre-post switch (P < 0.001), as more children passed the preswitch than the postswitch phases. Once again, a significant 2-way interaction between these factors (P < 0.001) was attributable to the fact that whereas the number of children passing the preswitch phase increased gradually across age, the number of children passing the postswitch phase increased more precipitously. All 3 covariates significantly affected pass rate: SES (P < 0.001) and additional DHA (P = 0.011) were positively related to pass rate, and maternal prepregnancy BMI (P = 0.015) was negatively related to pass rate.
FIGURE 4.
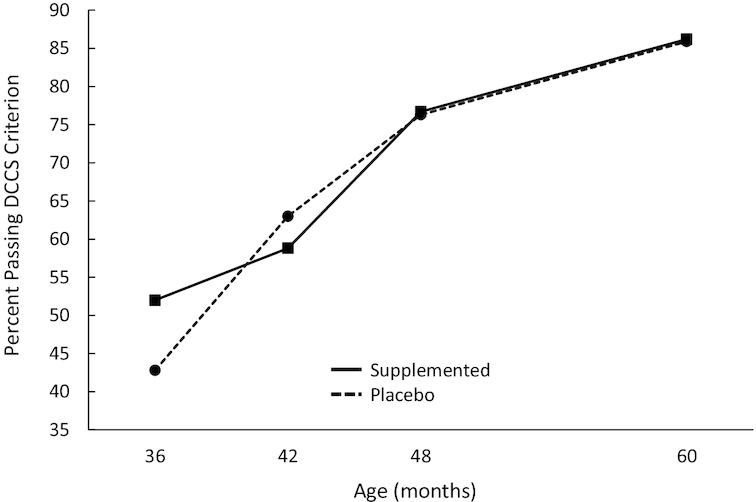
Percentage of children passing the DCCS criterion as a function of age and DHA group. Overall sample sizes are 172 at 36 mo (n = 79 and 93 for placebo and DHA, respectively), 167 at 42 mo (n = 76 and 91, respectively), 167 at 48 mo (n = 78 and 89, respectively), and 158 at 60 mo (n = 74 and 84, respectively). All means shown here are adjusted for covariates and the interaction represented here is statistically significant (P = 0.048). DCCS, Dimensional Change Card Sort.
Stroop tasks
Data from the 2 Stroop variants were combined and analyzed in a single mixed-model analysis, featuring factors of DHA group, sex, visit, and Stroop task. No significant terms emerged involving DHA group, although the main effect for DHA group showed a marginal effect (P = 0.087), with the children from supplemented mothers scoring consistently above children from placebo mothers. Other significant main effects emerged for Stroop task (P = 0.001), as the Red-Yellow task was easier than the Day-Night task, and for visit (P < 0.001), as performance improved linearly with age from 36 to 60 mo. These main effects were qualified by an interaction between the 2 terms (P < 0.001), as performance increased more precipitously for the Day-Night task than for the Red-Yellow task.
TOH task
The TOH task yields 2 variables: the maximum number of steps attempted to complete the task (a measure of an individual's performance ceiling, where higher values are better) and a processing efficiency score (a measure reflecting both how many steps they could complete overall and how many trials at each step were needed to achieve 2 consecutive optimal solutions, where higher values are better).
No effects or interactions involving DHA group emerged as significant in the analysis of the maximum steps attempted, although significant effects emerged for visit (P < 0.001), as 6-y-olds successfully attained a higher number of steps than 5-y-olds, and sex (P = 0.039), as girls successfully attained more steps than boys. No covariates were predictive of performance on this variable.
No significant effects involving DHA group were observed for the processing efficiency score, although significant main effects for visit (P < 0.001) and sex (P = 0.006) were qualified by a significant visit × sex interaction (P = 0.020). As expected, 6-y-olds were more efficient than 5-y-olds and girls were more efficient than boys. The interaction was attributable to girls improving more strongly from 5 to 6 y than boys. Once again, no covariates were correlated with this variable.
Parent-report measures of regulation and adaptive skills
The numerous individual items on the parent-report form of the BASC-2 yield a number of subtests that are aggregated into 2 overall composite scores: the Adaptive Skills index and the Behavioral Symptoms index (an overall composite of problematic behavioral symptoms); the latter index can be parsed into 2 sub-composite variables: Externalizing Problems (aggression, hyperactivity, and other conduct problems) and Internalizing Problems (anxiety, depression, and somatization).
Behavioral Symptoms index
No significant effects were observed that involved DHA group. Analysis of this composite yielded only a main effect for sex (P = 0.002), with boys scoring slightly but significantly higher than girls. None of the covariates significantly predicted this composite score. This aggregate scale can be broken down into 2 subcategories. The Externalizing Problems index analysis yielded only a main effect for sex (P = 0.010), with boys scoring slightly but significantly higher than girls. None of the covariates significantly predicted this composite score. The Internalizing Problems index analysis yielded only a significant main effect for visit (P < 0.001), with an unexpectedly nonlinear pattern of change across age (see Table 5) that was not readily interpretable. Once again, none of the covariates significantly affected this composite score.
Adaptive Skills index
Once again, no significant effects involving DHA group were observed. The analysis of this composite yielded significant main effects for sex (P = 0.005) and visit (P < 0.001). Girls scored significantly higher than boys and the developmental course mirrored the nonlinear and difficult-to-interpret pattern seen for the Internalizing Problems index. SES was positively related (P = 0.004) to this composite score.
Cluster analyses on DHA status change in pregnancy
Although participants were randomly assigned to placebo and supplementation groups, it was clear from previous work that there was considerable overlap in the 2 groups in terms of change in DHA concentrations during pregnancy. In order to objectively characterize patterns of change in DHA among mothers in our sample, we subjected maternal red blood cell DHA concentrations at enrollment and at delivery to hierarchical cluster analyses (Ward's method) to objectively generate groups of maternal participants. The cluster analyses generated a “best” solution (based on standard stopping rules involving changes in coefficients at each step) involving 2 groups: a “Flat” cluster (n = 166) composed of mothers whose DHA remained essentially unchanged from enrollment to delivery, and an “Increase” cluster (n = 97) composed of mothers whose DHA rose significantly from enrollment to delivery (see Figure 5).
FIGURE 5.
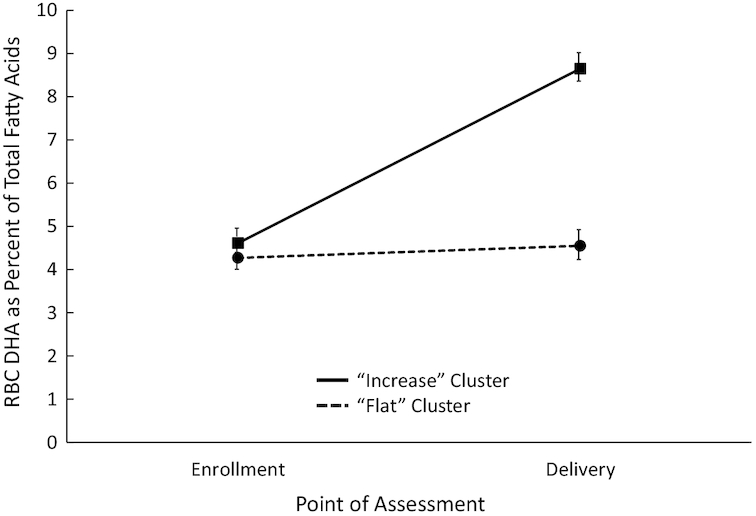
RBC DHA (percentage of total fatty acids) for “Flat” and “Increase” clusters across pregnancy. Overall sample size at both enrollment and delivery is 263 (n = 97 and 166 for Flat and Increase Clusters, respectively). Error bars represent SEMs. RBC, red blood cell.
Clusters and randomly assigned groups
There was significant (P < 0.001) although not perfect alignment between the 2 clusters and the randomly assigned groups, as 88 (89.8%) of the 98 mothers followed-up in the placebo group fell into the Flat Cluster, whereas 79 (66.9%) of the 118 mothers followed-up in the supplemented group fell into the Increase Cluster. Surprisingly, 39 mothers assigned to the supplemented group also had DHA change profiles classified as Flat. Given the importance of this biomarker, we believed that the clustering represented an indirect index of compliance with the RCT regimen. We also suspected that it could identify mothers who took additional DHA during pregnancy, especially in the placebo group; this point was strongly borne out by post hoc analyses (P < 0.001), as mothers receiving the placebo who were classified into the Increase Cluster took nearly 5 times the amount of DHA (M = 164.4 mg, SE = 28.96 mg) as mothers in the Flat Cluster (M = 35.7 mg, SE = 9.00 mg). It is worth nothing that although the Increase Cluster mothers in the supplemented group also took more additional DHA during pregnancy (M = 32.7 mg, SE = 9.37 mg) than Flat Cluster mothers in the supplemented group (M = 17.3 mg, SE = 9.86 mg), this difference was not statistically significant.
Correlates of clusters
We examined a number of birth and demographic characteristics of mothers in the 2 clusters, and some significant patterns emerged. Mothers in the Increase Cluster were older (P = 0.004), had higher education levels (P < 0.001), higher incomes (P = 0.002), higher overall SES scores (P < 0.001), and higher verbal IQ (PPVT scores; P = 0.009). In addition, infants of mothers in the Increase Cluster had significantly higher birthweights (P = 0.021) and head circumferences (P = 0.004). Overall, increases in DHA across pregnancy covaried with advantaged social status and better birth outcomes.
Clusters and child outcomes
The next set of analyses involved examination of selected child outcomes as a function of cluster membership. These analyses are represented in Table 6; for variables administered at a single point in the longitudinal schedule, univariate analyses were performed, whereas for repeated measures we used a mixed-models approach. The middle column of Table 6 shows P values for the main effect of Cluster membership. Note that, with standardized assessments administered at later ages (e.g., PPVT, WPPSI), there is a significant main effect for Cluster membership; in each case, the significant result favors the Increase cluster.
TABLE 6.
Effects of select analyses on DHA change status cluster1
| P values | |||||
|---|---|---|---|---|---|
| Measure | Ages (mo) | n | Variable | Cluster effect2 | Cluster effect with SES controlled |
| Willatts Task | 10 | 194 | Total score | 0.295 | 0.432 |
| BSID | 18 | 183 | MDI | 0.723 | 0.456 |
| — | 184 | PDI | 0.083 | 0.226 | |
| MBCDI | 18 | 191 | Words Produced | 0.641 | 0.573 |
| TOPEL | 42 | 154 | Early Literacy Index | 0.107 | 0.830 |
| PPVT | 60 | 156 | Standard score | 0.006 | 0.311 |
| Sentence Repetition | 36–48 | 137 | Total score | 0.407 | 0.357 |
| WPPSI | 36–72 | 143 | Verbal IQ | 0.010 | 0.788 |
| — | 148 | Processing Speed | 0.064 | 0.534 | |
| — | 149 | Performance IQ | 0.005 | 0.485 | |
| — | 141 | Full Scale IQ | 0.003 | 0.401 | |
| BASC | 36–72 | 141 | Externalizing Problems | 0.650 | 0.950 |
| — | 140 | Internalizing Problems | 0.294 | 0.382 | |
| — | 140 | Behavioral Symptoms Index | 0.217 | 0.236 | |
| — | 140 | Adaptive Skills | 0.090 | 0.055 | |
BASC, Behavioral Assessment Scale for Children; BSID, Bayley Scales of Infant Development; IQ, intelligence quotient; MBCDI, MacArthur-Bates Communicative Development Inventory; MDI, Mental Development Index; PDI, Psychomotor Development Index; PPVT, Peabody Picture Vocabulary Test; SES, socioeconomic status; TOPEL, Test of Preschool Early Literacy; WPPSI, Wechsler Preschool and Primary Scale of Intelligence.
Cluster effect refers to the P value of the statistical test between the 2 clusters. The first column shows the significance of the cluster effect alone; the far-right column shows the significance of the effect when SES is covaried. Note that all effects become nonsignificant in the latter column.
At face value, this would suggest that increases in DHA during pregnancy were associated with more positive long-range outcomes. However, given the pattern of correlates already described for cluster membership, we then reran analyses covarying SES. The P values for the results of those analyses are shown in the right-hand column of Table 6, which make it clear that the main effects of cluster membership disappear when SES is covaried. Thus, the more conservative and parsimonious conclusion here is that SES represents a classic third-variable in this analysis, mediating the perceived association between DHA increase during pregnancy and child outcomes.
Maternal and cord blood DHA concentrations and infant outcomes
We have so far examined the effects of assigned DHA group and direction of change in DHA during pregnancy on postnatal outcomes. The last set of analyses specifically regarding DHA to be presented here represent tests of maternal DHA concentrations during pregnancy and the effect on those same postnatal outcomes. Multiple regressions were run with maternal DHA concentrations at enrollment and at delivery as predictors of postnatal outcomes. For those variables measured repeatedly across time, we extracted a component score based on a principal components analysis performed on the repeated measures across ages. In each case where this was done, one (and only one) component emerged with an eigenvalue of ≥1.0, thus suggesting that this score was capturing the primary variance in the measure across time; the variance accounted for by the extracted components ranged from 67.4% to 85.3%.
Regressions were conducted simultaneously entering maternal DHA concentrations at enrollment and delivery to determine their effect on outcomes. We also collected cord blood DHA concentrations and entered that as a predictor of outcomes. Any significant effects observed were followed up with regressions in which SES was entered as a control. As shown in Table 7, cord blood was unrelated to any of the outcomes, although a number of significant effects emerged separately for maternal blood DHA concentrations during pregnancy. However, for all outcomes but 1, these effects did not remain statistically significant after controlling for SES. The 1 variable that did remain significant after covarying SES was Words Produced on the MBCDI; the coefficient there was +0.198 (P = 0.010), with higher DHA concentrations related to higher productive vocabulary. However, as this was the only effect to emerge from the conduct of so many tests, it is possible that this result was spurious.
TABLE 7.
Effects of maternal and cord blood DHA concentrations on selected outcomes1
| P values | ||||||
|---|---|---|---|---|---|---|
| Measure | Ages2 (mo) | n | Variable | Enrollment | Delivery | Cord blood |
| Willatts Task | 10 | 194 | Total score | 0.193 | 0.208 | 0.133 |
| BSID | 18 | 183 | MDI | 0.425 | 0.425 | 0.683 |
| — | 184 | PDI | 0.864 | 0.080 | 0.089 | |
| MCBDI | 18 | 191 | Words Produced | 0.0184 | 0.889 | 0.766 |
| TOPEL | 42 | 154 | Early Literacy Index | 0.452 | 0.0115 | 0.310 |
| PPVT | 60 | 156 | Standard score | 0.158 | 0.0045 | 0.405 |
| Sentence Repetition | 36–48 | 137 | Total score | 0.291 | 0.342 | 0.743 |
| WPPSI | 36–72 | 143 | Verbal IQ | 0.1135 | 0.0135 | 0.297 |
| — | 148 | Processing Speed3 | 0.0555 | 0.0135 | 0.574 | |
| — | 149 | Performance IQ | 0.0225 | 0.716 | 0.272 | |
| — | 141 | Full Scale IQ | 0.0045 | 0.0415 | 0.138 | |
| BASC | 36–72 | 141 | Externalizing Problems | 0.425 | 0.844 | 0.521 |
| — | 140 | Internalizing Problems | 0.991 | 0.367 | 0.564 | |
| — | 140 | Behavioral Symptoms Index | 0.308 | 0.272 | 0.510 | |
| — | 140 | Adaptive Skills | 0.0445 | 0.123 | 0.482 | |
BASC, Behavioral Assessment Scale for Children; BSID, Bayley Scales of Infant Development; IQ, intelligence quotient; MBCDI, MacArthur-Bates Communicative Development Inventory; MDI, Mental Development Index; PDI, Psychomotor Development Index; PPVT, Peabody Picture Vocabulary Test; SES, socioeconomic status; TOPEL, Test of Preschool Early Literacy; WPPSI, Wechsler Preschool and Primary Scale of Intelligence.
A principal component score derived across ages was the criterion variable.
This subscale is assessed only at 48 and 72 mo.
Effect of DHA remains significant after controlling for SES.
Effect of DHA does not remain significant after controlling for SES.
Discussion
The long-term results from this clinical trial on prenatal supplementation with a substantial dose of DHA show limited and relatively isolated benefits to children's cognitive and behavioral outcomes. Despite a dramatic reduction in early preterm birth and other adverse perinatal outcomes (47) and an advantage in the development of attention (45) during the first year of life that has been reported elsewhere as a function of this supplementation, we observed only a few scattered effects representing positive outcomes in any of the measures taken from 10 mo through 6 y of life on the behavioral outcomes collected. There was a positive effect of prenatal supplementation on attention and spatial memory (the DR task) at 24 and 36 mo that was somewhat qualified by interactions with sex and task variables. There was a significant improvement of high-level performance on rule learning and flexibility (the DCCS task) at 36 mo, but this effect dissipated at older ages. Finally, there was a trend for children from DHA-supplemented mothers to show better inhibitory control (Stroop tasks) across all ages, although the effect did not attain statistical significance. There were no effects on any standardized tests of intelligence or language, and no effects on any measures of adaptive or regulative function. This pattern of null findings is consistent with the results of 1 other large trial on prenatal supplementation (73–75). Given the rich and comprehensive longitudinal schedule administered here, we cannot contend that the findings reported here are attributable to the use of inappropriate measures, the omission of critical ages from the developmental design, or an overreliance on the use of global developmental outcomes (76).
In addition to these analyses, we also conducted tests on the effects of change of DHA status during pregnancy as well as overall DHA concentrations in mothers and infants on long-term outcomes. These additional analyses yielded some positive results favoring increases in DHA over pregnancy or increased concentrations during pregnancy on some meaningful outcomes in later childhood but because increases in, or higher concentrations of, DHA during pregnancy were generally confounded by SES, we took care to covary SES in subsequent follow-up analyses of these positive results. When SES was statistically controlled, virtually every positive outcome for DHA change or concentration receded below statistical significance, suggesting that SES was acting as a classic third-variable in these observational analyses. In addition, we analyzed the association between maternal DHA concentration at enrollment and delivery and various child outcomes, with the same fundamental finding: the few associations we observed showing DHA concentrations to be positively related to outcomes dissipated when SES was covaried. The single association to remain after SES was covaried was the relation between maternal DHA at enrollment and productive vocabulary at 18 mo.
These results showing limited and scattered benefit of prenatal DHA supplementation stand in stark contrast to our findings of broadly distributed long-term benefit in postnatal supplementation with long-chain PUFAs that we have previously reported (77, 78). The prenatal and postnatal trials we have conducted in the last decade vary in sample size, dose, and demographic characteristics. When the results of the 2 trials are compared, the pattern of outcomes suggests that early postnatal supplementation of infants themselves may have long-term cognitive benefit due to early programming, but supplementation of the mother during pregnancy may produce benefits only during pregnancy and to infants only briefly during the first year, with any positive influences fading shortly thereafter. It could also be the case that the universal postnatal consumption of DHA by infants in this trial from a combination of human milk and DHA-containing infant formulas allowed both placebo and DHA-supplemented groups to achieve sufficient DHA exposure for cognitive development.
ACKNOWLEDGEMENTS
We thank Lori Blanck, Aiden Bondurant, Caitlin Brez, Katlin Cedeño, Carol Cheatham, Danielle Christifano, Lori Curtindale, Tasha Doty Follmer, Amy Kepler, Ke Liao, and Sara Macone for assistance in data collection, coding, and entry.
The authors’ responsibilities were as follows—SEC: was the principal investigator on the original trial; JC and SEC: were principal investigators on the follow-up; KG and BJG: were co-investigators; DJS: oversaw data collection, data entry, and data integrity; EK and JMT: coordinated the clinical trial; JC: analyzed the data and wrote the manuscript; and all authors read and approved the final manuscript. JC and SEC have served as consultants to Nestlé, Wyeth, Fonterra Brands, and Mead Johnson Nutrition. None of the other authors reported a conflict of interest related to the study.
Notes
Supported by US NIH grants R01HD047315 (to SEC) and U54 HD090216 (to JC).
DSM Nutritional Products donated the capsules for the study but had no role in the study design, analysis, interpretation, or dissemination.
Abbreviations used: BASC, Behavioral Assessment Scale for Children; BSID, Bayley Scales of Infant Development; DCCS, Dimensional Change Card Sort; DR, Delayed Response; IQ, intelligence quotient; MBCDI, MacArthur-Bates Communicative Development Inventory; MDI, Mental Development Index; PDI, Psychomotor Development Index; PPVT, Peabody Picture Vocabulary Test; RCT, randomized controlled trial; SES, socioeconomic status; SR, Sentence Repetition; TOH, Tower of Hanoi; TOPEL, Test of Preschool Early Literacy; WPPSI, Wechsler Preschool and Primary Scale of Intelligence.
References
- 1. Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci U S A. 1986;83(11):4021–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.. Jensen CL, Heird WC, Anderson RE. Effect of maternal docosahexaenoic (DHA) supplementation on milk and infant plasma DHA. Pediatr Res. 1996;39(4):1857. [Google Scholar]
- 3. Wainwright PE, Huang YS, Coscina DV, Levesque S, McCutcheon D. Brain-effects and behavioral-effects of dietary n-3 deficiency in mice: a 3-generational study. Dev Psychobiol. 1994;27(7):467–87. [DOI] [PubMed] [Google Scholar]
- 4. Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet. 1998;352(9129):688–91. [DOI] [PubMed] [Google Scholar]
- 5. Forsyth JS, Willatts P, Ross P, Mires GJ. Relationship of prenatal LCPUFA status to infant visual and cognitive function. J Reprod Infant Psyc. 2003;21(3):253. [Google Scholar]
- 6. Willatts P, Forsyth JS, Agostoni C, Bissenden J, Casaear P, Boehm G. Long-chain polyunsaturated fatty acid supplementation in infancy and cognitive function in later childhood. J Reprod Infant Psyc. 2003;21(3):257–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gibson RA, Makrides M. Polyunsaturated fatty acids and infant visual development: a critical appraisal of randomized clinical trials. Lipids. 1999;34(2):179–84. [DOI] [PubMed] [Google Scholar]
- 8. SanGiovanni JP, Berkey CS, Dwyer JT, Colditz GA. Dietary essential fatty acids, long-chain polyunsaturated fatty acids, and visual resolution acuity in healthy fullterm infants: a systematic review. Early Hum Dev. 2000;57(3):165–88. [DOI] [PubMed] [Google Scholar]
- 9. Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr Res. 1998;44(2):201–9. [DOI] [PubMed] [Google Scholar]
- 10. Carlson SE, Ford AJ, Werkman SH, Peeples JM, Koo WWK. Visual acuity and fatty acid status of term infants fed human milk and formulas with and without docosahexaenoate and arachidonate from egg yolk lecithin. Pediatr Res. 1996;39(5):882–8. [DOI] [PubMed] [Google Scholar]
- 11. Carlson SE, Werkman SH, Rhodes PG, Tolley EA. Visual-acuity development in healthy preterm infants: effect of marine-oil supplementation. Am J Clin Nutr. 1993;58(1):35–42. [DOI] [PubMed] [Google Scholar]
- 12. Uauy RD, Birch DG, Birch EE, Tyson JE, Hoffman DR. Effect of dietary omega-3-fatty-acids on retinal function of very-low-birth-weight neonates. Pediatr Res. 1990;28(5):485–92. [DOI] [PubMed] [Google Scholar]
- 13. Auestad N, Halter R, Hall RT, Blatter M, Bogle ML, Burks W, Erickson JR, Fitzgerald KM, Dobson V, Innis SM et al.. Growth and development in term infants fed long-chain polyunsaturated fatty acids: a double-masked, randomized, parallel, prospective, multivariate study. Pediatrics. 2001;108(2):372–81. [DOI] [PubMed] [Google Scholar]
- 14. Auestad N, Montalto MB, Hall RT, Fitzgerald KM, Wheeler RE, Connor WE, Neuringer M, Connor SL, Taylor JA, Hartmann EE. Visual acuity, erythrocyte fatty acid composition, and growth in term infants fed formulas with long chain polyunsaturated fatty acids for one year. Pediatr Res. 1997;41(1):1–10. [DOI] [PubMed] [Google Scholar]
- 15. Bakker EC, Ghys AJA, Kester ADM, Vles JSH, Dubas JS, Blanco CE, Hornstra G. Long-chain polyunsaturated fatty acids at birth and cognitive function at 7y of age. Eur J Clin Nutr. 2003;57(1):89–95. [DOI] [PubMed] [Google Scholar]
- 16. Jensen CL, Prager TC, Fraley JK, Chen HM, Anderson RE, Heird WC. Effect of dietary linoleic/alpha-linolenic acid ratio on growth and visual function of term infants. J Pediatr. 1997;131(2):200–9. [DOI] [PubMed] [Google Scholar]
- 17. Innis SM, Akrabawi SS, Diersen-Schade DA, Dobson MV, Guy DG. Visual acuity and blood lipids in term infants fed human milk or formulae. Lipids. 1997;32(1):63–72. [DOI] [PubMed] [Google Scholar]
- 18. Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE. Essential fatty acids in visual and brain development. Lipids. 2001;36(9):885–95. [DOI] [PubMed] [Google Scholar]
- 19. Martinez M. Developmental profiles of polyunsaturated fatty acids in the brain of normal infants and patients with peroxisomal diseases: severe deficiency of docosahexaenoic acid in Zellweger's and pseudo-Zellweger's syndromes. World Rev Nutr Diet. 1991;66:87–102. [DOI] [PubMed] [Google Scholar]
- 20. Green P, Glozman S, Kamensky B, Yavin E. Developmental changes in rat brain membrane lipids and fatty acids: the preferential prenatal accumulation of docosahexaenoic acid. J Lipid Res. 1999;40(5):960–6. [PubMed] [Google Scholar]
- 21. Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extra-uterine fatty-acid accretion in infant brain: implications for fatty-acid requirements. Early Hum Dev. 1980;4(2):131–8. [DOI] [PubMed] [Google Scholar]
- 22. Carlson SE. Docosahexaenoic acid supplementation in pregnancy and lactation. Am J Clin Nutr. 2009;89(2):678S–84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muskiet FAJ, van Goor SA, Kuipers RS, Velzing-Aarts FV, Smit EN, Bouwstra H, Dijck-Brouwer DAJ, Boersma ER, Hadders-Algra M. Long-chain polyunsaturated fatty acids in maternal and infant nutrition. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):135–44. [DOI] [PubMed] [Google Scholar]
- 24. Hadders-Algra M, Bouwstra H, van Goor SA, Dijck-Brouwer J, Muskiet FAJ. Prenatal and early postnatal fatty acid status and neurodevelopmental outcome. J Perinat Med. 2007;35:S28–34. [DOI] [PubMed] [Google Scholar]
- 25. Smuts CM, Huang MZ, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol. 2003;101(3):469–79. [DOI] [PubMed] [Google Scholar]
- 26. Gustafson KM, Carlson SE, Colombo J, Yeh HW, Shaddy DJ, Li S, Kerling EH. Effects of docosahexaenoic acid supplementation during pregnancy on fetal heart rate and variability: a randomized clinical trial. Prostaglandins Leukot Essent Fatty Acids. 2013;88(5):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res. 2004;152(1):49–57. [DOI] [PubMed] [Google Scholar]
- 28. Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89(3):695–702. [DOI] [PubMed] [Google Scholar]
- 29. Anderson GJ, Neuringer M, Lin DS, Connor WE. Can prenatal n-3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatr Res. 2005;58(5):865–72. [DOI] [PubMed] [Google Scholar]
- 30. Gibson RA, Chen WX, Makrides M. Randomized trials with polyunsaturated fatty acid interventions in preterm and term infants: functional and clinical outcomes. Lipids. 2001;36(9):873–83. [DOI] [PubMed] [Google Scholar]
- 31. Gould JF, Smithers LG, Makrides M. The effect of maternal omega-3 (n−3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97(3):531–44. [DOI] [PubMed] [Google Scholar]
- 32. Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB, Lammi-Keefe CJ. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am J Clin Nutr. 2002;76(3):608–13. [DOI] [PubMed] [Google Scholar]
- 33. Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, Blaga OM, Carlson SE. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75(4):1254–67. [DOI] [PubMed] [Google Scholar]
- 34. Innis SM, Friesen RW. Essential n-3 fatty acids in pregnant women and early visual acuity maturation in term infants. Am J Clin Nutr. 2008;87(3):548–57. [DOI] [PubMed] [Google Scholar]
- 35. Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the Inuit of arctic Quebec. J Pediatr. 2008;152(3):356–64. [DOI] [PubMed] [Google Scholar]
- 36. Malcolm CA, McCulloch DL, Montgomery C, Shepherd A, Weaver LT. Maternal docosahexaenoic acid supplementation during pregnancy and visual evoked potential development in term infants: a double blind, prospective, randomised trial. Arch Dis Child. 2003;88(5):F383–F90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bouwstra H, Dijck-Brouwer J, Decsi T, Boehm G, Boersma ER, Muskiet FA, Hadders-Algra M. Neurologic condition of healthy term infants at 18 months: positive association with venous umbilical DHA status and negative association with umbilical trans-fatty acids. Pediatr Res. 2006;60(3):334–9. [DOI] [PubMed] [Google Scholar]
- 38. van Goor SA, Dijck-Brouwer DA, Doornbos B, Erwich JJ, Schaafsma A, Muskiet FA, Hadders-Algra M. Supplementation of DHA but not DHA with arachidonic acid during pregnancy and lactation influences general movement quality in 12-week-old term infants. Br J Nutr. 2010;103(2):235–42. [DOI] [PubMed] [Google Scholar]
- 39. Escolano-Margarit MV, Ramos R, Beyer J, Csábi G, Parrilla-Roure M, Cruz F, Perez-Garcia M, Hadders-Algra M, Gil A, Decsi T et al.. Prenatal DHA status and neurological outcome in children at age 5.5 years are positively associated. J Nutr. 2011;141(6):1216–23. [DOI] [PubMed] [Google Scholar]
- 40. Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am J Clin Nutr. 2011;93(5):1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Jong C, Kikkert HK, Seggers J, Boehm G, Decsi T, Hadders-Algra M. Neonatal fatty acid status and neurodevelopmental outcome at 9 years. Early Hum Dev. 2015;91(10):587–91. [DOI] [PubMed] [Google Scholar]
- 42. Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Influence of long-chain polyunsaturated fatty acids on infant cognitive function. Lipids. 1998;33(10):973–80. [DOI] [PubMed] [Google Scholar]
- 43. Kannass KN, Colombo J, Carlson SE. Maternal DHA levels and toddler free-play attention. Dev Neuropsychol. 2009;34(2):159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Wurff ISM, Bakker EC, Hornstra G, Kirschner PA, Gielen M, Godschalk RWL, Kremers S, Zeegers MP, de Groot RHM. Association between prenatal and current exposure to selected LCPUFAs and school performance at age 7. Prostaglandins Leukot Essent Fatty Acids. 2016;108:22–9. [DOI] [PubMed] [Google Scholar]
- 45. Colombo J, Gustafson KM, Gajewski BJ, Shaddy DJ, Kerling EH, Thodosoff JM, Doty T, Brez CC, Carlson SE. Prenatal DHA supplementation and infant attention. Pediatr Res. 2016;80(5):656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yelland LN, Gajewski BJ, Colombo J, Gibson RA, Makrides M, Carlson SE. Predicting the effect of maternal docosahexaenoic acid (DHA) supplementation to reduce early preterm birth in Australia and the United States using results of within country randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2016;112:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH, Shaddy DJ. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97(4):808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence—III. Upper Saddle River, NJ: Pearson; 2002. [Google Scholar]
- 49. Fenson L, Dale PS, Reznick JS, Bates E, Thal DJ, Pethick SJ. Variability in early communicative development. Monogr Soc Res Child Dev. 1994;59(5):1–173.; discussion 174–85. [PubMed] [Google Scholar]
- 50. Lonigan C, Wagner R, Torgesen J. The Test of Preschool Early Literacy. Greenville, SC: Super Duper Publications; 2007. [Google Scholar]
- 51. Lonigan C. Development, assessment, and promotion of preliteracy skills. Early Educ Dev. 2006;17:91–114. [Google Scholar]
- 52. Lemelin JP, Boivin M, Forget-Dubois N, Dionne G, Seguin JR, Brendgen M, Vitaro F, Tremblay RE, Perusse D. The genetic-environmental etiology of cognitive school readiness and later academic achievement in early childhood. Child Dev. 2007;78(6):1855–69. [DOI] [PubMed] [Google Scholar]
- 53. Spreen O, Benton A. Neurosensory Center comprehensive examination for aphasia. Victoria, Canada: University of Victoria;1969. [Google Scholar]
- 54. Montgomery MM, Stephens MI, Montgomery AA. Sentence repetition in preschoolers: effects of length, complexity, and word familiarity. J Psycholinguist Res. 1978;7(6):435–52. [DOI] [PubMed] [Google Scholar]
- 55. Devescovi A, Caselli MC. Sentence repetition as a measure of early grammatical development in Italian. Int J Lang Commun Disord. 2007;42(2):187–208. [DOI] [PubMed] [Google Scholar]
- 56. Chafetz J. The closed-class vocabulary as a closed set. Appl Psycholinguist. 1994;15(3):273–87. [Google Scholar]
- 57. Manis F, Lindsey K, Bailey C. Development of reading in grades K-2 in Spanish-speaking English-language learners. Learn Disabil Res Pract. 2004;19:214–24. [Google Scholar]
- 58. Shankweiler D, Smith ST, Mann VA. Repetition and comprehension of spoken sentences by reading-disabled children. Brain Lang. 1984;23(2):241–57. [DOI] [PubMed] [Google Scholar]
- 59. Dunn LM, Dunn DN. Peabody Picture Vocabulary Test. 4th ed San Antonio, TX: Pearson/PsyCorp; 2007. [Google Scholar]
- 60. Willatts P, Forsyth JS. The role of long-chain polyunsaturated fatty acids in infant cognitive development. Prostaglandins Leukot Essent Fatty Acids. 2000;63(1–2):95–100. [DOI] [PubMed] [Google Scholar]
- 61. Schutte AR, Spencer JP, Schoner G. Testing the dynamic field theory: working memory for locations becomes more spatially precise over development. Child Dev. 2003;74(5):1393–417. [DOI] [PubMed] [Google Scholar]
- 62. Kendler TS. Development of discrimination learning: a levels-of-functioning explanation. In: Reese H, editor Advances in child development and behavior. New York: Academic Press; 1979. p. 83–117. [PubMed] [Google Scholar]
- 63. Reese HW, Lipsitt LL. Experimental child psychology. New York: Wiley; 1970. [Google Scholar]
- 64. Zelazo PD. The Dimensional Change Card Sort (DCCS): a method of assessing executive function in children. Nat Protoc. 2006;1(1):297–301. [DOI] [PubMed] [Google Scholar]
- 65. Carlson SM, Moses LJ. Individual differences in inhibitory control and children's theory of mind. Child Dev. 2001;72(4):1032–53. [DOI] [PubMed] [Google Scholar]
- 66. Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: performance of children 3.5–7 years old on a Stroop-like day-night test. Cognition. 1994;53(2):129–53. [DOI] [PubMed] [Google Scholar]
- 67. Casey BJ, Vauss YC, Chused A, Swedo SE. Cognitive functioning in Sydenham's Chorea. 2. Executive functioning. Dev Neuropsychol. 1994;10(2):89–96. [Google Scholar]
- 68. Schoner G, Thelen E. Using dynamic field theory to rethink infant habituation. Psychol Rev. 2006;113(2):273–99. [DOI] [PubMed] [Google Scholar]
- 69. Liu T, Abrams J, Carrasco M. Voluntary attention enhances contrast appearance. Psychol Sci. 2009;20(3):354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gottlieb J, Balan P, Oristaglio J, Suzuki M. Parietal control of attentional guidance: the significance of sensory, motivational and motor factors. Neurobiol Learn Mem. 2009;91(2):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rawley JB, Constantinidis C. Neural correlates of learning and working memory in the primate posterior parietal cortex. Neurobiol Learn Mem. 2009;91(2):129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Reynolds C, Kamphaus R. Behavioral Assessment System for Children—2. Upper Saddle River, NJ: Pearson; 2006. [Google Scholar]
- 73. Smithers LG, Gibson RA, Makrides M. Maternal supplementation with docosahexaenoic acid during pregnancy does not affect early visual development in the infant: a randomized controlled trial. Am J Clin Nutr. 2011;93(6):1293–9. [DOI] [PubMed] [Google Scholar]
- 74. Gould JF, Makrides M, Colombo J, Smithers LG. Randomized controlled trial of maternal omega-3 long-chain PUFA supplementation during pregnancy and early childhood development of attention, working memory, and inhibitory control. Am J Clin Nutr. 2014;99(4):851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010;304(15):1675–83. [DOI] [PubMed] [Google Scholar]
- 76. Colombo J, Carlson SE. Is the measure the message: the BSID and nutritional interventions. Pediatrics. 2012;129(6):1166–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Colombo J, Carlson SE, Cheatham CL, Shaddy DJ, Kerling EH, Thodosoff JM, Gustafson KM, Brez C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr. 2013;98(2):403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T. Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr Res. 2011;70(4):406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]