Abstract
Introduction: Maturity-onset diabetes mellitus affecting the elderly population is marked by insulin resistance and decreased insulin production. The relationship between periodontitis and diabetes is bidirectional. Type 2 diabetic patients are more prone to chronic periodontitis (CP) and severe periodontitis affects the glycemic control in such patients. Recently, dental diode laser has become an effective tool in controlling CP. To date, very few studies have been conducted to check the efficacy of diode laser in control of periodontal destruction in type 2 diabetes mellitus (DM2) patients. Hence, the need of the study was to evaluate whether diode laser helps improvement of periodontal outcome and reduction in anaerobic bacteria in elderly diabetic patients with CP.
Methods: Forty DM2 patients with CP were randomized into group A (control): scaling and root planing (SRP) only and group B (test): SRP followed by soft tissue dental diode laser (808 nm) application. Four patients (2 in each group) were lost during follow up. Clinical parameters, plaque samples and glycated hemoglobin levels were evaluated at both baseline and 90 days post-treatment.
Results: Improvement in clinical, microbiological and glycemic parameters were noted in the group that received SRP as well as SRP + LANAP (laser-assisted new attachment procedure). The reductions in clinical parameters were statistically significant after 3 months (P<0.001). The microbial analysis of plaque samples for Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg) decreased significantly after 3 months in group B than in group A. Glycated hemoglobin level (HbA1c) decreased significantly after 90 days in both the groups (P<0.001) with more reduction in the SRP+LANAP group (6.49%) in comparison to SRP alone (16.25% vs. 9.76%). However, on the intergroup comparison, the difference in HbA1c reduction was nonsignificant.
Conclusion: Laser as an adjunct to SRP is an effective procedure for improving clinical and microbiological parameters in maturity onset diabetes mellitus patients with CP. Also, there was a better improvement in glycemic control in the test group compared to control group after 3 months. Hence, medically compromised patients like DM2 with CP with delayed wound healing can effectively be treated by laser as an adjunct to nonsurgical periodontal therapy for better results
Keywords: Chronic periodontitis, Diode laser, Diabetes mellitus, HbA1c, LANAP, Microbiology, Nonsurgical periodontal therapy
Introduction
Chronic periodontitis (CP) is a multifactorial disease caused by various periodontopathogenic bacteria that results in the destruction of the tooth-supporting tissues i.e. periodontal tissue.1 Diabetes mellitus is a chronic metabolic disorder of the pancreas that results in marked hyperglycemia due to insulin resistance and decreased production of insulin by the pancreas.2,3 Non-insulin dependent diabetes mellitus (NIDDM) also known as type 2 diabetes mellitus (DM2) is more common in the elderly population. Periodontitis is considered a sixth complication in diabetic patients. The relationship between Periodontitis and NIDDM is bidirectional. NIDDM predisposes an individual to the risk of developing CP, and severe periodontitis affects glycemic control in diabetic patients.4 Few meta-analyses studies have confirmed that HbA1c reduction of around 0.4% can be anticipated following effective periodontal therapy.5
The primary etiological factor for the existence of periodontitis is pathogenic microorganisms within the subgingival biofilm. Specific periodontopathogenic bacterial species and their virulence factors have been recognized as etiological agents and they affect the rate of progression of the disease. Hence, they act as indicators for progression of periodontal breakdown.6 Today about a dozen oral microorganisms are classified as periodontal pathogens. Foremost among these are gram-negative organisms including Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg).7
The elimination of subgingival microorganism and regeneration of lost tissue is considered as the most important goal of periodontal therapy. Successful periodontal therapy is determined by control of destructive periodontal pathogens along with a shift in the microbial flora towards one that is typically present in health.8-10 This elimination of the pathogenic subgingival microbiota can be achieved by nonsurgical periodontal therapy which is considered as the gold standard for a plaque as well as microbial elimination.10-12 However, previous studies have shown that nonsurgical periodontal therapy alone may fail to completely eliminate the pathogenic subgingival microbiota because of limited access to the instruments, as a result, this may lead to persistent periodontopathogens.10,13-15 These limitations of mechanical therapy have led to a shift to other adjunctive measures such as the use of antimicrobial therapy.
The spread of localized inflammation which is closely associated with the destruction of the supporting periodontal tissue is promoted by the microbial colonization, especially the gram-negative anaerobic bacteria in deep inaccessible areas such as furcation areas and root concavities. This justifies the need for the bacterial elimination from periodontal pockets.16 Mechanical methods such as scaling and root planing (SRP) are not sufficient for the complete bacterial elimination.17,18 Systemic antibiotics have been employed in cases which are nonresponsive to conventional periodontal treatments in spite of the known systemic side effects of antibiotics and the development of antibiotic resistance.1,19 Dental diode laser system has become an effective tool when used along with conventional periodontal therapy resulting in significant bacterial reduction which makes the adjunctive use of antibiotics almost unnecessary.20
Lasers have been used in periodontal therapy since long for subgingival curettage, gingivectomy, frenectomy, removal of granulation tissue, during flap surgery, osseous recontouring, implant maintenance and management of peri-implantitis.
The antimicrobial efficacy of diode laser has been proven in a number of studies.7 Due to its exceptional ease of use and affordability as well as action on various pigmented periodontal pathogens the diode laser has become an integral tool in the dental armamentarium.21 It is well absorbed by various chromophores present in diseased periodontal tissues such as melanin and hemoglobin.22 These evidence in the dental literature states diode laser is a useful adjunct to nonsurgical periodontal therapy in producing improved results.23
Although several studies have shown the efficacy of soft tissue diode laser therapy over SRP alone in the treatment of CP, very few studies were done in NIDDM patients who are frequently affected by CP due to bidirectional relationship and to assess whether laser-assisted new attachment procedure (LANAP) has additional systemic beneficial effect on glycemic control, improvement in overall clinical outcome and the reduction in periodontal microbiology.
Methods
A total of 40 DM2 patients with CP were selected from the Department of Periodontology, JSS Dental College, and Hospital, Mysuru between April 2016 and September 2017.
Data Collection
Inclusion Criteria
Patients between 30-60 years of age with confirmed diagnosis of DM2 and chronic generalized periodontitis with ≥20 teeth remaining.
The periodontal pocket depth of 4-7 mm with clinical attachment level (CAL) of 2 mm or greater and each quadrant having at least 3 teeth.
Consenting patients who were cooperative and able to come for regular follow up.
Exclusion Criteria
Pregnant/lactating women.
Patients who were on antibiotic therapy in the previous six months.
Patients who had undertaken any periodontal therapy in past 6 months.
Smoking.
Alcoholism.
Sample Size
By purposive sampling, 40 patients were selected who fulfilled the inclusion criteria. Four patients (2 in test and 2 in control) were lost during follow-up. After baseline examination, 36 patients were assigned randomly to test and control group of 18 patients each (Figure 1).
Figure 1.
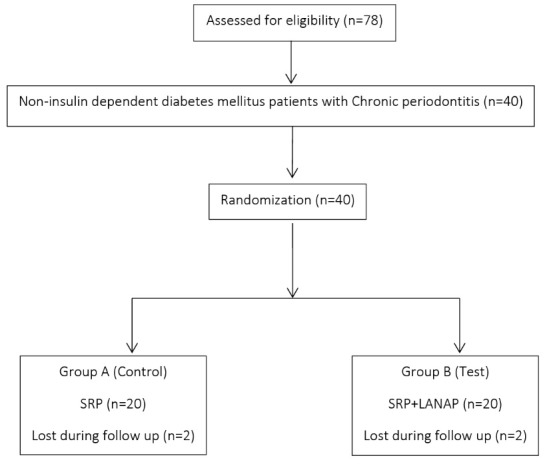
Consort Diagram of the Study.
Study Design
This was a parallel design, randomized, single-blind, and controlled clinical trial of 3 months duration. The study was conducted to compare and evaluate Diode Laser therapy as an adjunct to SRP in NIDDM patients with CP.
Group A (Control group): SRP followed by irrigation with normal saline.
Group B (Test group): SRP followed by diode laser application (laser assisted new attachment procedure, LANAP) and irrigation with normal saline.
Randomization
The randomization of 36 patients was done as follows. 36 type 2 diabetes and CP reported to the investigator of the study. The investigator who was blind to the study protocol then randomly assigned 36 patients into test and control group by sequentially numbered sealed opaque envelopes. The operator then according to the treatment assigned in the sealed envelope treated the patients either by SRP alone or SRP+LANAP and then recalled the patients after 3 months for follow-up.
All the patients who had participated in the study fulfilling the inclusion criteria had to fill an informed consent prior to the study.
Study Protocol
In the first visit oral hygiene instructions were given.
Patients were then recalled after 7 days for baseline blood investigation (HbA1c), a collection of subgingival plaque samples and measurements of clinical parameters followed by nonsurgical periodontal therapy in control group and SRP followed by LANAP in the test group.
Subgingival plaque samples were sent for microbiological analysis.
Laser application was done with the help of an active diode laser (Mikro® Sunny 808 nm diode laser). A wavelength 808 nm and a power setting of 1.5 W-1.8 W were used in continuous, contact mode with a thin flexible fiber-optic cable (320 nm). The fiber optic cable was introduced into the periodontal pocket with the laser beam directed towards the soft tissue of the pocket wall and moved in an apico-coronal direction, with a sweeping motion. The depth of the periodontal pocket in millimeters corresponds to the exposure time in seconds
Patients were again recalled at 3 months for assessment of clinical, microbiological and HbA1c level posttreatment.
Patients were monitored periodically for oral hygiene maintenance and recalled for further periodontal therapy as needed.
Clinical Parameters Assessed
Baseline and 3 months assessment of the following full mouth clinical parameters:
Plaque index (PI) (Silness and Loe,1964)24
Gingival index (GI) (Loe and Silness,1967)24
CAL - using a UNC-15 probe from a fixed reference point on the crown to the base of the pocket.
Probing depth (PD) - using a UNC-15 probe taken from the margin of the gingiva to the base of the pocket.
Microbiological Analysis
The collection of plaque sample was done in the Department of Periodontology, JSS Dental College, and Hospital, Mysuru. Collection of subgingival plaque samples was done by the same operator to standardize the sampling procedure. Before sampling, the adjacent teeth were isolated with cotton rolls. To standardize site selection and adequate sample volume, plaque sample was collected from all the quadrants and pooled in reduced transport fluid (RTF) medium for microbiological analysis. Plaque samples contaminated with blood and saliva were discarded. The samples were then immediately transferred for microbiological culturing of Aa and Pg which were then separately vortexed and inoculated in anaerobic jar according to the requirement for culturing and quantification of anaerobic bacteria.
Statistical Analysis
The values obtained from the clinical, microbiological and hematological data were subjected to following statistical analysis:
Descriptive statistics – mean and standard deviation.
Paired samples “t-test” for within group comparison.
Independent-samples t test for comparison between 2 groups.
The data obtained were processed using statistical software (SPSS 22 for Windows).
Results
The study outline is explained in Figure 1. The demographic data of the patients at baseline are illustrated in Table 1.
Table 1. Demographic Data of the Test (SRP+LANAP) and Control (SRP) Group at Baseline .
| SRP (n = 18) | SRP+LANAP (n = 18) | |
| Age | 50.6 (7.25) | 48.05 (6.05) |
| Gender | ||
| Male | 9 | 9 |
| Female | 9 | 9 |
| BMI | 23.9±0.6 | 23.7±0.7 |
| HbA1c mean (%) | 7.994±1.2744 | 8.122±1.2744 |
| PI | 2.3194±0.26352 | 2.45±0.25 |
| GI | 2.38667±0.369610 | 2.19±0.2234 |
| PD (mm) | 3.5911±0.33582 | 3.6406±0.3902 |
| CAL (mm) | 8.7361±0.7682 | 8.6739±0.7046 |
Abbreviations: PI, plaque index; GI: gingival index: PD: probing depth; CAL: clinical attachment level; SRP: scaling and root planning; LANAP: Laser-assisted new attachment procedure.
Clinical Parameters
There was a statistically significant improvement in clinical parameters from baseline to 3 months postoperatively (P < 0.001) (Table 2). The results are displayed in boxplots diagrams in Figure 2.
Table 2. Clinical Parameters Assessment at Day Zero and 90 Days in the Test and Control Groupsa .
| Clinical Parameter | Evaluation Point | Group A (SRP) | Group B (SRP+LANAP) | P Value |
| PI | Day 0 | 2.3194±0.26352 | 2.45±0.25 | 0.003b |
| 90 days | 1.56917±0.323484 | 1.26±0.22 | ||
| P value | 0.000b | 0.000b | ||
| GI | Day 0 | 2.38667±0.369610 | 2.19±0.2234 | 0.000b |
| 90 days | 1.5556±0.32333 | 1.04±0.2275 | ||
| P value | 0.000b | 0.000b | ||
| PD (mm) | Day 0 | 3.5911±0.33582 | 3.6406±0.3902 | 0.000b |
| 90 days | 2.6339±0.39313 | 1.8050±0.3278 | ||
| P value | 0.000b | 0.000b | ||
| CAL(mm) | Day 0 | 8.7361±0.7682 | 8.6739±0.7046 | 0.000b |
| 90 days | 7.5011±0.5242 | 6.6511±0.5315 | ||
| P value | 0.000b | 0.000b |
Abbreviations: PI, plaque index; GI, gingival index; PD, probing depth; CAL, clinical attachment level; SRP, scaling and root planning; LANAP, Laser-assisted new attachment procedure.
aData are shown as Mean ± SD.
bStatistically significant.
Figure 2.
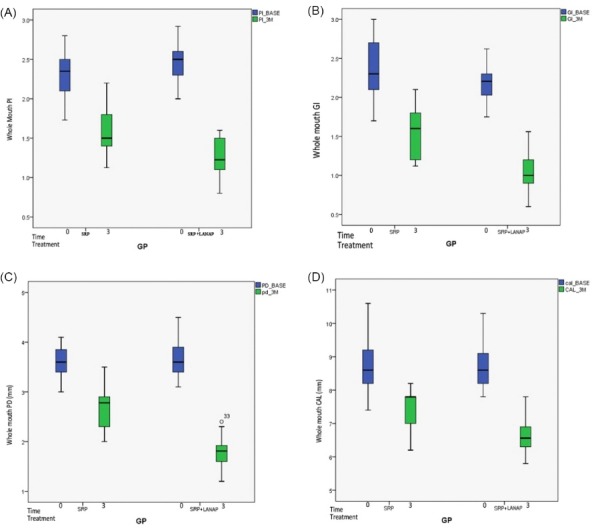
A) Plaque Index B) Gingival Index C) Probing Depth D) Clinical Attachment Level Assessed at Day Zero and 90 Days Posttreatment.
HbA1c(%)
The results of HbA1c values are depicted in Figure 3. The values of HbA1c in the test site decreased from 8.122± 1.26 at baseline to 6.833 ± 0.651 at 3 months and in the control, site decreased from 7.994 ± 1.27 at baseline to 7.217± 0.793 at 3 months. There was 6.49% more reduction in HbA1c levels in the SRP+LANAP group compared to SRP alone (16.25% vs. 9.76%) but on intergroup comparison the difference in HbA1c reduction was nonsignificant. The changes in HbA1c(%) are shown in Table 3.
Figure 3.
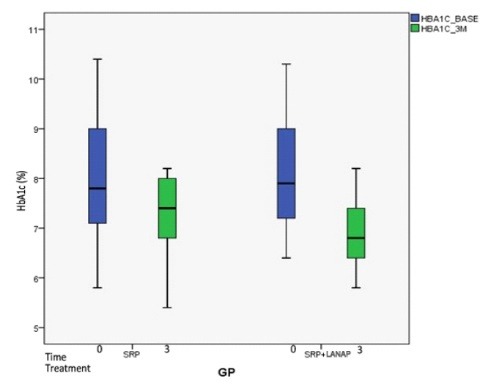
Mean HbA1c(%) Level at Day 0 and 90 Days in SRP Group and SRP+LANAP Group.
Table 3. Glycemic Parameters in Group A and Group Ba .
| Clinical Parameter | Evaluation Point | Group A (SRP) | Group B (SRP+LANAP) | P Value |
| HbA1c (%) | Day 0 | 7.994±1.2744 | 8.122±1.2744 | 0.121 |
| 90 days | 7.2178±0.79353 | 6.8333±.65169 | ||
| P value | 0.000b | 0.000b |
Abbreviation: HbA1c: Glycated hemoglobin level.
aData are shown as Mean ± SD.
bStatistically significant.
Microbiological Analysis
The collection of subgingival plaque samples for the culturing of anaerobic bacteria was done at baseline and 3 months postoperatively. The results in CFU/mL of Aa and Pg are shown in (Figure 4).
Figure 4.
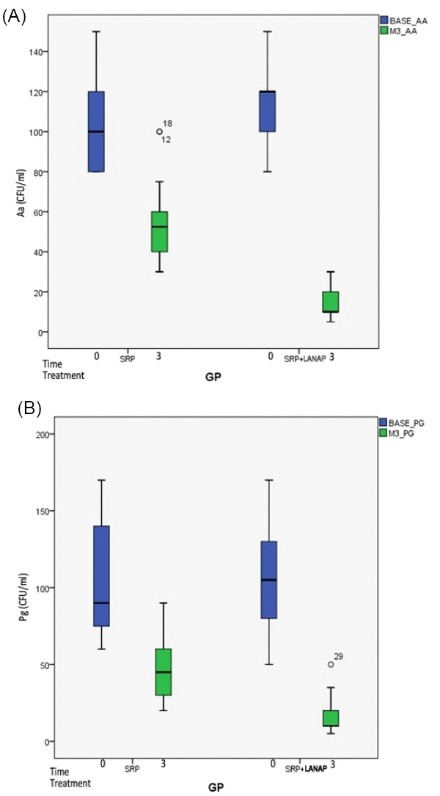
Mean Reduction in CFU/mL of Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg) From Baseline to 3 Months.
Colony Count of Aa (CFU/mL)
The colony count of Aa in control group decreased from 104.44 ± 20.643 to 56.11 ± 19.363 and from 112.78 ± 19.33 to 15.00 ± 8.22 in the test group. The comparison between the treatment groups revealed 46.27% reduction of Aa in control group and 86.69% reduction of Aa in test showing that test group had 40.4% more significant reduction in Aa than control. Thus, on intergroup comparison statistical significant reduction was observed in colony count of Aa in test group than in control after 3 months (Table 4, Figure 4)
Table 4. Microbiological Parameters in CFU/mL at Day Zero and 90 Days in the Test and Control Groupsa .
| Clinical Parameter | Evaluation Point | Group A (SRP) | Group B (SRP+LANAP) | P Value |
| Aa (CFU/mL) | Day 0 | 104.44±20.643 | 112.78±19.34 | 0.000b |
| 90 days | 56.11±19.369 | 15.00±8.225 | ||
| P value | 0.000b | 0.000b | ||
| Pg (CFU/mL) | Day 0 | 101.39±33.642 | 102.50±33.618 | 0.000b |
| 90 days | 50.00±21.35 | 14.72±12.18 | ||
| P value | 0.000b | 0.000b |
Abbreviations: Aa, Aggregatibacter actinomycetemcomitans; Pg, Porphyromonas gingivalis.
aData are shown as Mean ± SD.
bStatistically significant.
Colony Count of Pg (CFU/mL)
The colony count of Pg in CFU/ml decreased from 102.50 ± 33.61 at the baseline to 14.72 ± 12.18 at 3 months in the test group and from 101.39 ± 33.64 at the baseline to 50.00 ± 21.351 at three months in control. The comparison between the treatment groups revealed 85.15% reduction of Pg in test group and 50.64% reduction of Pg in control group showing that the test group had 35% more significant reduction of Pg than in control.
Thus, on intergroup comparison statistical significant reduction was observed in colony count of Pg in test group than in control after 3 months (Table 4, Figure 4).
Discussion
The current study shows a better reduction of HbA1c levels, in laser treated group (16.25%) than in SRP treated group (9.76%) after 3 months but this reduction was not statistically significant on the intergroup comparison from baseline to 3 months. The values of HbA1c at 3 months postoperatively decreased from 8.12% to 6.8% in the test group (SRP+LANAP) and 7.9% to 7.2% in control group (SRP). In the present study, the lowering of HbA1c levels after nonsurgical periodontal therapy was in accordance with previously reported studies.25-29
Previous studies had linked obesity and smoking as confounding factors to diabetes mellitus and HbA1c control in patients with CP.27 In the present study patients had confirmed diagnosis of DM2, with a mean BMI <24 kg/m2, and were nonsmokers, which positively helped in assessing the bidirectional relationship of DM2 and CP.
The outcome of the current study was similar with those of two recent investigations that examined the effect of PDT alone and in combination with SRP in patients receiving initial and supportive periodontal therapy. Because PDT had no reported systemic effect, there was no reason to assume that it would improve HbA1c levels.30,31
This is in contradiction to a recent study by Koçak and Sağlam which showed SRP+ Diode laser group showed statistically significant improvement in glycemic control (HbA1c) than in SRP group in patients with DM2 and CP.32
The present study showed improvement in all clinical parameters (PI, GI, PD, CAL) from baseline to 3 months in SRP and SRP+LANAP group which was statistically significant.
The value of plaque index reduced from 2.31 at baseline to 1.56 at 3 months in control and from 2.45 to 1.26 in the test group. This observation is in accordance with a study done by Berakdar et al.33
The value of GI score reduced from 2.23 at baseline to 1.55 at 3 months after therapy in control and from 2.19 to 1.04 in the test group. This is in accordance with a study by Fallah where after 6 weeks there is compelling evidence of improvement in gingival.34
The mean reduction of PD and CAL from baseline to 3 months in the present study were similar with those obtained by other studies.7,35-38 A study was done by Obradovic et al where laser as an adjunct to SRP showed significant improvement in gingival inflammation compared to SRP alone in NIDDM patients.39 Another histologic study by Obradovic et al, showed a faster healing response when SRP was combined with DL in NIDDM patients.40
In a study by Yukna et al laser as an adjunct to SRP resulted in significant reduction of PD with a gain in CAL.41 This reduction in PD can be explained due to better pocket epithelial lining removal by soft tissue diode laser leading to connective tissue attachment directly to root surface favoring regeneration as reported by Kreisler et al.36 A similar study conducted on animals by Romanos et al.42 showed better removal of pocket epithelial lining in DL+SRP group compared to conventional hand instruments. Hence, the abovementioned studies suggest that a better response in clinical parameters can be achieved when diode laser is used as an adjunct to conventional SRP.
The present study showed a significant reduction in colony count (CFU/mL) of Aa and Pg from base line to 3 months follow-up in both the groups. The colony count of Aa in control group decreased from 104.44 ± 20.643 to 56.11 ± 19.363 and from 112.78 ± 19.33 to 15.00 ± 8.22 in the test group. The microbial count of Aa revealed 46.27% reduction in control group and 86.69% reduction in test showing that test group had 40.4% more reduction in Aa than control. Also, the colony count of Pg (CFU/mL) decreased from 102.50 ± 33.618 at the baseline to 14.72 ± 12.184 at 3 months in the test group and from 101.39 ± 33.642 at the baseline to 50.00 ± 21.351 at 3 months in control. The mean percentage decrease of Pg was 50.64% in control group and 85.15% in test group showing that the test group had 35% more reduction of Pg than in control. This is because of the fact that diode laser is effective against many putative periodontal pathogens including Aa and Pg and its ability to reach inaccessible periodontal sites which had been proved in previous studies.43
Thus, the present study shows that diode laser application is effective in reducing inflammation due to the antimicrobial property of laser. A classic study by Pick and Colvard et al in 1998 on the treatment of periodontal pockets using 810 nm diode lasers showed immediate structural damage of gram-negative bacteria and a marked reduction of periodontopathogens namely Aa and Pa.7,44 Diode laser is effective not only in the elimination of bacteria from the periodontal pocket but also the complete removal of bacterial toxins from root cementum.45 Another study by Lin et al showed marked reduction of Aa and prevent further recolonization for up to 28 days using diode lasers.46
Conclusion
The present study showed statistically significant improvement in clinical and microbiological parameters in the test than in control group from baseline to 3 months. Thus we can conclude that the use of diode laser therapy after SRP is more effective than SRP alone in the overall improvement of clinical and microbiological parameters. Also, the adjunctive use of soft tissue diode laser resulted in better improvement in HbA1c in NIDDM patients than SRP alone after 3 months. The percentage decrease in HbA1c was (16.25%) in test versus (9.76 %) in control but the difference between the groups was not statistically significant. This suggests the need for further long-term randomized controlled clinical trials with larger sample size before generalizing that diode laser therapy results in improved glycemic control in maturity onset diabetes mellitus patients with CP.
Ethical Considerations
The study was conducted after obtaining ethical clearance form ethical review committee JSS Dental College and Hospital, Mysuru. The study was registered in clinical trials registry-India with (identifier: CTRI/2017/11/010348).
Conflict of Interests
The authors declare no conflict of interest.
Funding
The study was funded by JSS university research grant with registration number REG/DIR(R)/URG/ 54/2011-12/10888/1.
Acknowledgments
We sincerely acknowledge JSS Academy of Higher Education and Research for research grants support and respected Head of the department Dr. Sheela Kumar for her constant support and invaluable insights during this dissertation. Also, I would like to thank Dr. Lancy D Souza statistician and Mr. Patel who helped me in my dissertation work.
Please cite this article as follows: Chandra S, Shashikumar P. Diode laser - a novel therapeutic approach in the treatment of chronic periodontitis in type 2 diabetes mellitus patients: a prospective randomized controlled clinical trial. J Lasers Med Sci. 2019;10(1):56-63. doi:10.15171/jlms.2019.09.
References
- 1.Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. J Periodontol. 20001997;14:12–32. doi: 10.1111/j.1600-0757.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 2.Barr EL, Zimmet PZ, Welborn TA. et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation. 2007;116:151–1517. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 3.Löe H. Löe HPeriodontal diseaseThe sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. [PubMed] [Google Scholar]
- 4.Preshaw PM, Alba AL, Herrera D. et al. Periodontitis and diabetes: A two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teeuw WJ, Gerdes VEA, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33:421–427. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolf HF, Hassel TM. Colour Atlas of Dental Hygiene: Periodontology. Theme; 2006.
- 7.Moritz A, Schoop U, Goharkhay K, Schauer P. Treatment of periodontal pockets with a diode laser. Lasers Surg Med. 1998;22:302–311. doi: 10.1002/(sici)1096-9101(1998)22:5<302::aid-lsm7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Mousquès T, Listgarten MA, Phillips RW. Effect of scaling and root planing on the composition of the human subgingival microbial flora. J Periodontal Res. 1980;15:144–51. doi: 10.1111/j.1600-0765.1980.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 9.Hinrichs JE, Wolff LF, Pihlstrom B, Schaffer EM, Liljemark WF, Bandt CL. Effects of scaling and root planing on subgingival microbial proportions standardized in terms of their naturally occurring distribution. J Periodontol. 1985;56:187–94. doi: 10.1902/jop.1985.56.4.187. [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz Yilmaz. Effect of gallium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med. 2002;30(1):60–6. doi: 10.1002/lsm.10010. [DOI] [PubMed] [Google Scholar]
- 11.Garrett JS. Effects of non-surgical therapy on periodontitis in humans: a review. J Clin Periodontol. 1983;10:515–23. doi: 10.1111/j.1600-051x.1983.tb02181.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaldahl WB, Kalkwarf KL, Patil KD. A review of longitudinal studies that compared periodontal therapies. J Periodontol. 1993;64:243–253. doi: 10.1902/jop.1993.64.4.243. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan SA, Robertson PB. Calculus removal by scaling/root planing with and without surgical access. J Periodontol. 1987;58(3):159–163. doi: 10.1902/jop.1987.58.3.159. [DOI] [PubMed] [Google Scholar]
- 14.Matia JI, Bissada NF, Maybury JE, Richette P. Efficiency of scaling of molar furcation area with and without surgical access. Int J Periodontics Restorative Dent. 1986;6:25–35. [PubMed] [Google Scholar]
- 15.Adriaens PA, Edwards CA, De Boever JA, Loesche WJ. Ultrastructural observation on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol. 1988;59:493–503. doi: 10.1902/jop.1988.59.8.493. [DOI] [PubMed] [Google Scholar]
- 16.Loesche WJ. The antimicrobial treatment of periodontal disease: Changing the treatment paradigm. Crit Rev Oral Biol Med. 1999;10(3):245–275. doi: 10.1177/10454411990100030101. [DOI] [PubMed] [Google Scholar]
- 17.Herrera D, Sanz M, Jepsen S, Needleman I, Roldan S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002;29(Suppl 3):136–59. doi: 10.1034/j.1600-051x.29.s3.8.x. [DOI] [PubMed] [Google Scholar]
- 18.Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL, Socransky SS. Clinical and microbiological features of subjects with adult periodontitis who responded poorly to scaling and root planing. J Clin Periodontol. 1997;24(10):767–76. doi: 10.1111/j.1600-051x.1997.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 19.Lopez NJ, Gamonal JA, Martinez B. Repeated metronidazole and amoxicillin treatment of periodontitis A follow-up study. J Periodontol. 2000;71(1):79–89. doi: 10.1902/jop.2000.71.1.79. [DOI] [PubMed] [Google Scholar]
- 20.Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 21.Dortbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res. 2001;12(2):104–108. doi: 10.1034/j.1600-0501.2001.012002104.x. [DOI] [PubMed] [Google Scholar]
- 22.Coleton S. Lasers in surgical procedures and oral medicine. Dent Clin North Am. 2004;48:937–62. doi: 10.1016/j.cden.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Lang NP, Brägger U. Periodontal diagnosis in the 1990s. J Clin Periodontol. 1991;18:370–379. doi: 10.1111/j.1600-051x.1991.tb02303.x. [DOI] [PubMed] [Google Scholar]
- 24. Wolf HF, Rateitschak K. Periodontology. Vol 1. Thieme: 2005.
- 25.Iwamoto Y, Nishimura F, Nakagawa M. et al. The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes. J Periodontol. 2001;72:774–778. doi: 10.1902/jop.2001.72.6.774. [DOI] [PubMed] [Google Scholar]
- 26.Kiran M, Arpak N, Unsal E, Erdogan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–272. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 27.Koromantzos PA, Makrilakis K, Dereka X. et al. A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes Part I effect on periodontal status and glycaemic control. J Clin Periodontol. 2011;38:142–147. doi: 10.1111/j.1600-051X.2010.01652.x. [DOI] [PubMed] [Google Scholar]
- 28.Navarro-Sanchez AB, Faria-Almeida R, Bascones-Martinez Bascones-Martinez. Effect of non-surgical periodontal therapy on clinical and immunological response and glycaemic control in type 2 diabetic patients with moderate periodontitis. J Clin Periodontol. 2007;34:835–843. doi: 10.1111/j.1600-051X.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- 29.Borgnakke WS, Chapple IL, Genco RJ. et al. The multi-center randomized controlled trial (RCT) published by the journal of the American Medical Association (JAMA) on the effect of periodontal therapy on glycated hemoglobin (HbA1c) has fundamental problems. J Evid Based Dent Pract. 2014;14:127–132. doi: 10.1016/j.jebdp.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christodoulides N, Nikolidakis D, Chondros P. et al. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: A randomized, controlled clinical trial. J Periodontol. 2008;79:1638–1644. doi: 10.1902/jop.2008.070652. [DOI] [PubMed] [Google Scholar]
- 31.Chondros P, Nikolidakis D, Christodoulides N, Rössler R, Gutknecht N, Sculean A. Photodynamic therapy as an adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: A randomized controlled clinical trial. Lasers Med Sci. 2009;24:681–688. doi: 10.1007/s10103-008-0565-z. [DOI] [PubMed] [Google Scholar]
- 32.Koçak1 E, Sağlam M. Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics with periodontitis: a randomized clinical trial. Lasers Med Sci. 2016;31(2):343–53. doi: 10.1007/s10103-016-1868-0. [DOI] [PubMed] [Google Scholar]
- 33.Berakdar M, Callaway A, Eddin MF, Ross A, Willershausen B. Comparison between scaling-root-planing (SRP) and SRP/photodynamic therapy: a six-month study. Head Face Med. 2012;8:12. doi: 10.1186/1746-160X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fallah A. Effects of 980 diode laser treatments combined with scaling and root planing on periodontal pockets in chronic periodontitis patients. Lasers Dent. 2010;14:1–11. [Google Scholar]
- 35.Dukic W, Bago I, Aurer A, Roguljic M. Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: a randomized clinical study. J Periodontol. 2013;84:1111–1117. doi: 10.1902/jop.2012.110708. [DOI] [PubMed] [Google Scholar]
- 36.Kreisler M, Al Haj H, d’Hoedt B. Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med. 2005;37:350–355. doi: 10.1002/lsm.20252. [DOI] [PubMed] [Google Scholar]
- 37.Qadri T, Miranda L, Tuner J, Gustafsson A. The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. J Clin Periodontol. 2005;32:714–719. doi: 10.1111/j.1600-051X.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 38.Sağlam M, Kantarcı A, Dündar N, Hakkı SS. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci. 2014;29:37–46. doi: 10.1007/s10103-012-1230-0. [DOI] [PubMed] [Google Scholar]
- 39.Obradovic R, Kesic L, Mihailovic D. et al. Low-level lasers as an adjunct in periodontal therapy in patients with diabetes mellitus. Diabetes Technol Ther. 2012;14:799–803. doi: 10.1089/dia.2012.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obradovic R, Kesic L, Mihailovic D, Antic S. et al. A histological evaluation of a low-level laser therapy as an adjunct to periodontal therapy in patients with diabetes mellitus. Lasers Med Sci. 2013;28:19–24. doi: 10.1007/s10103-012-1058-7. [DOI] [PubMed] [Google Scholar]
- 41.Yukna RA, Carr RL, Evans GH. Histologic evaluation of a Nd: YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restorative Dent. 2007;27:577–587. [PubMed] [Google Scholar]
- 42.Romanos GE, Henze M, Banihashemi S. et al. Removal of epithelium in periodontal pockets following diode (980 nm) laser application in the animal model: an in vitro study. Photomed Laser Surg. 2004;22:177–183. doi: 10.1089/1549541041438597. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz F, Sculean A, Berakdar M, Georg T, Reich E, Becker J. Periodontal treatment with an Er: YAG laser or scaling and root planing A 2-year follow-up split-mouth study. J Periodontol. 2003;74(5):590–596. doi: 10.1902/jop.2003.74.5.590. [DOI] [PubMed] [Google Scholar]
- 44.Moritz A, Gutknecht N, Doertbudak O. et al. Bacterial reduction in periodontal pockets through irradiation with a diode laser: A pilot study. J Clin Laser Med Surg. 1997;15:33–7. doi: 10.1089/clm.1997.15.33. [DOI] [PubMed] [Google Scholar]
- 45.Pick RM, Colvard MD. Current status of lasers in soft tissue dental surgery. J Periodontol. 1993 Jul;64(7):589–602. doi: 10.1902/jop.1993.64.7.589. [DOI] [PubMed] [Google Scholar]
- 46.Lin PP, Rosen S, Beck FM, Matsue M, Horton JE. A comparative effect of the Nd: YAG Laser with root planing on subgingival anaerobes in periodontal pockets. J Dent Res. 1992;71:299. doi: 10.1902/jop.1999.70.11.1276. [DOI] [Google Scholar]


