Abstract
We evaluated plasticity in speech supplemental motor area (SMA) tissue in two patients using functional magnetic resonance imaging (fMRI), following resection of tumors in or associated with the dominant hemisphere speech SMA. Patient A underwent resection of a anaplastic astrocytoma NOS associated with the left speech SMA, experienced SMA syndrome related mutism postoperatively, but experienced full recovery 14 months later. FMRI performed 32 months after surgery demonstrated a migration of speech SMA to homologous contralateral hemispheric regional tissue. Patient B underwent resection of a oligodendroglioma NOS in the left speech SMA, and postoperatively experienced speech hesitancy, latency and poor fluency, which gradually resolved over 18 months. FMRI performed at 64 months after surgery showed a reorganization of speech SMA to the contralateral hemisphere. These data support the hypothesis of dynamic, time based plasticity in speech SMA tissue, and may represent a noninvasive neural marker for SMA syndrome recovery.
Keywords: SMA, supplementary motor area, speech SMA, reorganization, contralateral
1. Introduction
Resection of tumors involving the dominant hemisphere speech supplementary motor area (SMA) often results in immediate postoperative speech deficits which can range from complete mutism to a less severe but global reduction in spontaneous speech (Damasio & Van Hoesen, 1980; Masdeu, Schoene & Funkenstein, 1978; Vassal, Charroud, & Deverdun, 2017; Ziegler, Kilian, & Deger, 1997). This is a form of a variably intense SMA syndrome directly related to the somatotopic organization of the SMA (Thulborn, Carpenter, & Just, 1999; Tombari, et al., 2004; Vassal, Charroud, & Deverdun, 2017). Speech SMA is represented by a cortical region anterior to the motor SMA, and contributes to the muscle groups supporting articulation and phonation (Bleasel, Comair, & Luders, 1996; Bogousslavsky & Regli, 1990; Pai, 1999; Riecker, Wildgruber, Grodd, & Ackermann, 2002; Rostomily, Berger, Ojemann, Lettich, Tsukerman, & Makuch, 1991; Rouiller, Babalian, Kazennikov, Moret, Yu, & Wiesendanger, 1994; Zentner, Hufnagel, Pechstein, Wolf, & Schramm, 1996). Recovery occurs spontaneously over weeks to months and is characterized by gradual return to fluent speech with little to no paraphasic errors and normal grammar (Damasio & Van Hoesen, 1980; Masdeu, Schoene, & Funkenstein, 1978; Ziegler, Kilian, & Deger, 1997). The mechanisms of the associated brain plasticity are incompletely understood, but likely involve cortical reorganization of the speech SMA.
Tumors grow, and induce modifications in local activity and connectivity, and therefore represent a model of brain plasticity (Thiel, et al., 2001; Thulborn, Carpenter, & Just, 1999; Tombari, et al., 2004; Vassal, Charroud, & Deverdun, 2017). While some infiltrated regions may retain their functionality, others migrate to the tumor periphery or there may be contralateral reorganization (Carey, Abbott, Egan, Bernhardt, & Donnan, 2005; Luft, et al., 2004; Tombari, et al., 2004; Vassal, Charroud, & Deverdun, 2017). Plasticity phenomena have indeed been observed before, during and after surgery, and a temporally trackable reorganization of sensorimotor networks following resection of tumors involving the motor SMA has been demonstrated (Fontaine, Capelle, & Duffau, 2002; Fox, et al., 1996; Fried I, et al., 1991; Indefrey & Levelt, 2004; Kim, et al., 2004; You, Jang, Kim, Kwon, Barrow, & Hallett, 2005). In contrast, only limited evidence exists to date regarding the functional reorganization in the speech SMA following dominant hemispheric lesions to this cortical region.
Imaging studies suggest that some eloquent dominant hemispheric cortical tissue (such as Wernicke’s area or Broca’s area) can functionally reorganize by recruiting homologous nondominant, contralateral tissue (Calvert, Brammer, Morris, Williams, King, & Matthews, 2000; Carpentier, et al., 2001; Karbe, Thiel, Weber-Luxenburger, Herholz, Kessler & Heiss, 1998). A functional magnetic resonance imaging (fMRI) study by Carpentier et al. found more bilateral activation in the speech SMA in patients with epileptogenic tissue in the dominant speech SMA when compared to control subjects (Carpentier, et al., 2001) In another fMRI study, Krainik et al. found increased language related activation in the nondominant speech SMA, contralateral to tumor locations, suggesting an atypical organization of the speech SMA in the presence of dominant hemispheric lesions (Krainik, et al., 2003). However, to date, the extent to which contralateral speech SMA representation of language activity reflects brain plasticity in functional recovery following dominant hemispheric lesions remains unknown.
The objective of this study was to directly identify the functional reorganization of the speech SMA, using fMRI, in patients with medial frontal lobe tumors who exhibited a postoperative SMA syndrome following surgical resection. We investigated this plasticity by assessing the language related speech SMA activity preoperatively and postoperatively, when full recovery of language abilities had been achieved. We also aimed to evaluate with fMRI whether the functional reorganization in speech SMA represented a dynamic shift over time following lesions of this region, by temporally tracking language related speech SMA activity.
2. Methods
2.1. Patients
Following Institutional Review Board (IRB) approval, we performed a retrospective chart and imaging review of two patients with lesions of the language dominant hemispheric frontal lobe. Patient A was a 39-year-old, right handed, Caucasian male with a 2.0 × 3.0 cm anaplastic astrocytoma NOS in the left frontal lobe. Patient B was a 35-year-old, right handed, Japanese-American male with a 3.5 × 2.5 cm oligodendroglioma NOS in the left parasagittal frontal lobe. English was the primary language for both subjects. Tumor resection was carried out without complication in both patients. All preoperative and postoperative neurological assessments were performed both by neurologists and neurosurgeons. Speech functions were assessed clinically with verbal comprehension, spontaneous speech, narrative tasks, verbal fluency, and repetition (Figure 1).
Figure 1.
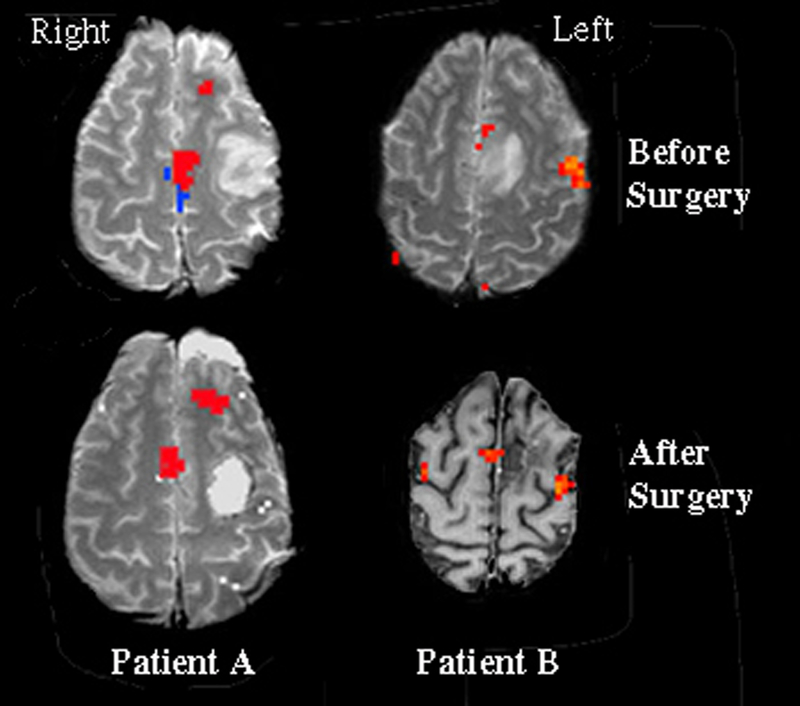
Statistical activation maps from language tasks were superimposed on T1 weighted anatomical images (Patient B, after surgery image is an inverted grey scale). Surgical resections were of tumorous tissue located in the left frontal lobes. Statistical analysis for Patient A was comprised of a conjunctive analysis, which demarcates only activated regions that were present in both language tasks. Statistical maps for Patient B reflect the activation pattern observed during the object naming task. For both patients, significant activation can be seen in the left speech SMA prior to surgery. However, following surgical resection of the tumorous tissue, the speech SMA was observed to relocate to the contralateral hemisphere. The region in blue represents tongue (or motor) SMA, in contrast with the region in red which represents speech SMA.
2.2. Imaging
Preoperative imaging was performed during the week prior to surgery. Postoperative imaging was acquired 32 months following neurosurgical tumor resection for Patient A and 64 months following surgery for Patient B. Magnetic resonance (MR) imaging was performed using a 3-Tesla magnet (General Electric Medical Systems [Milwaukee, WI] equipped with EPI from Advanced NMR [Wilmington, MA]). Prior to acquisition of functional images, a fast spin-echo sequence was used to obtain high-resolution structural maps (TR 5000 msec, TE 18 msec, flip angle 90 degrees, matrix size 256 × 256, field of view 24 × 18 cm, 36 3-mm-thick axial slices with no gap). For functional images, an EPI gradient-echo sequence (TR 2500 msec, TE 45 msec, matrix size 64 × 64; field of view 20 cm) was used to collect 84 functional images over 17 axial slices (4-mm-thick slices with a 1-mm gap). Each language task included three 30-second blocks with 30 seconds of rest between each activation block, starting and ending with a rest period. Language tasks employed were visual object naming and word list generation (tasks described below). Visual stimuli were presented through MR-compatible goggles, which were fitted over the patients’ eyes before the patients were placed in the imaging unit. Binaural auditory stimuli were delivered via MR-compatible headphones. Auditory and visual stimuli were controlled and presented using a Macintosh computer running Mac-Stim software (Apple Computers, Inc., Cupertino, CA).
2.3. fMRI preprocessing and analysis
Data preprocessing and analyses were performed with a single subject approach, as is necessary for precise functional localization for neurosurgical and clinical guidance. Task paradigm models used a categorical approach with boxcar functions consisting of fMRI activation during the speech tasks versus the respective rest periods. The boxcar functions were convolved with a model of the hemodynamic response by using software developed at our center (http://www.brainmapping.org) (Cohen, 1997). Following global normalization and smoothing, statistical analyses were performed for each task and each subject individually, using analytic technique previously described (Pouratian, Bookheimer, Rex, Martin, & Toga, 2002). Cortical extractions of each patient’s brain were used as a common space for comparing different mapping modalities. Fast spin-echo images of each patient’s brain were used to extract cortical surfaces for each patient. Images were corrected for radiofrequency nonuniformity (Pouratian, Bookheimer, & O’Farrell, 2000; Pouratian, Bookheimer, Rex, Martin, & Toga, 2002; Sled, Zijdenbos, & Evans, 1998). A three-dimensional active surface algorithm was used to generate an external cortical surface mesh for each patient. Functional MR imaging activations were aligned with the cortical extractions by aligning the coplanar high-resolution EPI images by rigid body transformations with the fast spin-echo images used to create the cortical extraction. This transformation was done based on the principal of maximizing mutual information. Electrocortical stimulation maps (ESM) were projected onto the cortical extraction by matching sulcal landmarks on the cortical extraction with the photograph of the exposed cortex covered by ESM tags and subsequently warping the image onto the surface. Most important in this process was that ESM sites directly overlapped with fMRI activations to be considered colocalized. Thus, statistical maps of activated regions were overlaid onto coplanar high-resolution anatomical images for each subject (Pouratian, Bookheimer, & O’Farrell, 2000; Pouratian, Bookheimer, Rex, Martin, & Toga, 2002). This utility of this technique to identify critical speech areas has previously been validated and its applicability demonstrated (Pouratian, Bookheimer, Rex, Martin, & Toga, 2002). Due to distortions introduced by mass effect related to tumor and the need to perform analyses in single subject space, standardized coordinates were not assessed as they are not likely to be generalizable.
2.4. Language tasks
Patients performed visual object naming and word generation language tasks while lying inside the MR magnet. Language tasks were performed covertly to minimize motion artifact.
2.5. Visual object naming
The patients were asked to silently name objects presented from the Boston Naming Test, which is a standardized test of object naming of line drawings (Kaplan, Goodglass, & Weintraub, 1983). Visual object naming is a robust activator of both essential and nonessential language cortices, especially of Broca’s area (Bookheimer, et al., 1997; Bookheimer, et al., 1995; Ojemann, 1993). Failure to complete this task is one of the most common symptoms of aphasia. Consequently, this is one of the most widely used tests for intraoperative mapping of eloquent cortices.
2.6. Word Generation.
Patients were asked to generate lists of words beginning with a certain letter or belonging to a certain category (for example, animals). Word generation has been demonstrated to consistently activate frontal language areas (Cannestra, et al., 2001; Cuenod, et al., 1995).
Both patients performed the visual object-naming task and only Patient A performed the word generation task. A conjunctive analysis was also performed for Patient A creating a statistical map that only displayed regions that were activated in both the visual object naming and word generation tasks.
3. Results
3.1. Patient A.
Patient A underwent fMRI five days prior to surgery. Analysis of the presurgical fMRI data in each of the language tasks revealed lateralized neural activation to the dominant (left) hemisphere, including speech SMA (Figure 1). This region of activation resided approximately 1.5–2.0 cm anterior to activation observed in the SMA-proper during separate tongue movement mapping (shown in blue in Figure 1). The patient underwent surgical resection of a 2.0 × 3.0 cm anaplastic astrocytoma NOS in the left frontal cortex. The surgery was performed with the patient awake and under local anesthesia using the asleep-awake-asleep approach (Hunke, Van De Wiele, Fried, & Rubinstein, 1998). Electrocorticography prior to resection identified Broca’s area consistent with the preoperative fMRI. The patients remained awake during the resection with continuous language assessment. The patient spoke normally throughout resection of the bulk of the tumor, until excision of the final, small piece of tumor located in white matter apparently connecting the speech SMA to other speech areas. The patient immediately stopped speaking in the OR. Once resection was completed he was placed back under general anesthesia. On the first day following surgery Patient A remained mute. He was able to generate a few simple words on days 2 – 4, improving rapidly until two weeks post-surgery when he spoke in complete sentences with occasional hesitations. At no time did he make paraphasic errors. He eventually returned to his work as a negotiator after full recovery of speech, which per clinical history and patient report occurred within the first two weeks postoperatively. At 14 months post-surgically, he underwent formal speech testing, which additionally documented complete functional recovery. Repeat fMRI language tissue mapping was performed 32 months following the surgical resection. Results demonstrated that the speech SMA had reorganized and was now located on the homologous contralateral hemispheric tissue (Figure 1). Thus, severing the dominant efferent and/or afferent pathways between the speech SMA and other language areas resulted in a migration of the speech SMA to the non-dominant hemisphere over time, which was accompanied by a full recovery of speech functioning.
3.2. Patient B.
Patient B received fMRI one day prior to surgery. Activation for visual object naming lateralized to the dominant (left) hemisphere, including speech SMA (Figure 1). This region of activation was approximately 1.0–2.0 cm anterior to activation observed in the SMA-proper during separate tongue movement mapping (shown in blue in Figure 1). The patient underwent surgical resection of a 3.5 × 2.5 cm oligodendroglioma NOS in the left parasagittal frontal lobe. The patient’s speech was normal preoperatively. Surgical resection involved a portion of the left speech SMA due to the tumor location. Clinical symptoms following surgery included hesitancy, slowness in speech, and poor fluency, without paraphasias. Repeat fMRI was performed 64 months following the surgical resection, by which time speech functioning had fully recovered. The fMRI results demonstrated that the speech SMA had reorganized and was now located on the homologous contralateral hemispheric tissue (Figure 1).
4. Discussion
The objectives of this study were to identify functional reorganization of the speech SMA using fMRI, specifically in association with the SMA syndrome, and to determine if this reorganization represented a dynamic shift over time. We observed in both patients a migration of speech SMA function from the dominant hemisphere to contralateral homologous region after full recovery from the SMA syndrome. Preoperative fMRI language maps lateralized to the left speech SMA in both patients, and surgery resulted in transient speech deficits postoperatively, consistent with reports of SMA syndromes, i.e., hesitance and/or mutism but without paraphasic errors or word finding difficulty more suggestive of aphasia. Notably, contralateral reorganization of the speech SMA was not only precipitated by direct lesioning of the dominant speech SMA (patient B), but also by severing of efferent and/or afferent white matter pathways between the speech SMA and other eloquent tissue (patient A).
Supplementary motor area syndromes can have diverse manifestations but most frequently present with impaired initiation of voluntary movement contralateral to the lesion. Although the SMA may be somatotopically organized, a pure language SMA syndrome is rare. (Kim, et al., 2010). Rather than isolated lesions of the SMA, disruption of associative white matter fiber tracts between Broca’s area and the ipsilateral SMA are more frequently implicated in pure language SMA syndromes (Anwander, Tittgemeyer, von Cramon, Friederici, & Knosche, 2007). The superior longitudinal fasciculus and the dorsal arcuate fasciculus are amongst those identified (Cona & Semenza, 2017; Hagoort, 2014). Against this backdrop, we note that the pure language SMA syndrome we observed in the two subjects reported herein may have different causative etiologies. The white matter fiber pathways mentioned were likely disrupted during surgical tumor resection in patient A. In contrast, the SMA itself was partially resected in patient B. The occurrence of a pure language SMA syndrome in both patients, even in the instance where connections to motoric speech areas (as in patient B) were not of themselves disrupted, is remarkable. Reorganization is likely made possible by the strong anatomic and functional connectivity between the two hemispheres by way of the corpus callosum and residual SMA function may or may not play a role in functional transfer to contralateral homologous brain (Acioly, Cunha, Parise, Rodrigues, & Tovar-Moll, 2015; Luppino, Matelli, Camarda, & Rizzolatti, 1993; Mitz & Wise, 1987; Pouratian, Bookheimer, & O’Farrell, 2000; Pouratian, Bookheimer, Rex, Martin, & Toga, 2002; Rouiller, Babalian, Kazennikov, Moret, Yu, & Wiesendanger, 1994).
The speech SMA is thought to reside within an area that has been distinguished from the SMA as the pre-SMA (Bradshaw, 1981; Cohen, 1997; Dapretto & Bookheimer, 1999, Picard & Strick, 2001; Sled, Zijdenbos, & Evans, 1998; Springer, et al., 1999). Anatomical and functional distinctions between pre-SMA and SMA-proper in motor control have been elaborated in monkeys and humans (Geschwind, 1969; Picard & Strick, 2001). The pre-SMA is associated more with complex and cognitive controls such as the acquisition of motor skills, motor selection, alternation of motor plans, and task switching (Kim, et al., 2010; Marsden et al., 1996; Barch et al., 2001; Gu et al., 2008). The pre-SMA is largely rostral to the genu of the arcuate sulcus in Brodmann’s area 6, whereas the SMA is located largely caudal to the genu of the arcuate sulcus in Brodmann’s area 6 (Picard & Strick, 1996; Picard & Strick, 2001). The pre-SMA is involved in higher level processes, while the SMA is more closely associated with motor activity; planning of a motor task may be managed by the pre-SMA, while the execution of the movement would be managed by the SMA (Luppino, Matelli, Camarda, & Rizzolatti, 1999; Kronfeld-Duenias, Amir, Erzati-Vinacour, Civier, & Ben-Shachar, 2016). More recently, this distinction has been extended to speech production (Alario, Chainay, Lehericy, & Cohen, 2006; Crosson, et al., 1999; Hertrich, Dietrich, & Ackermann, 2016; Indefrey & Levelt, 2004). The pre-SMA is involved with more complex linguistic operations such as word production, whereas the SMA is involved in overt articulation (Huang, Carr, & Cao, 2002; Palmer, Rosen, Ojemann, Buckner, Kelley, & Petersen, 2001; Picard & Strick, 1996). Anatomical studies have shown that the pre-SMA is coactivated with the prefrontal cortex and the caudate nucleus, while the SMA proper is mainly connected to the primary motor cortex and to the putamen (Kim, et al., 2010; Di Martino, et al., 2008). Moreoever, there is a high level of functional heterogeneity in the functional and anatomical segregation of the SMA proper, and difficult to determine precisely which subregions or subdivisions correspond to its various functionalities (Chung, Han, Jeong, & Jack, 2005; Marsden et al., 1996). The nature of speech necessarily comprises both elementary motor functions as well as higher order cognitive functions. Although it may involve the SMA-proper, in our study no activation was observed in the SMA-proper during language tasks. The activation all appeared to arise from the pre-SMA but in consideration of the difficulty in accurately localizing subregions and subdivisions of either the SMA-proper or the pre-SMA we chose to denote involved regions as the speech SMA area in the current study. Accordingly, then, the area referred to as the speech SMA in the current study is thought to represent a subdivision of the pre-SMA region because it resides anterior to the genu of the arcuate sulcus in medial area 6 and is also anterior to SMA activation generated during tongue movement mapping by approximately 1.0–2.0 cm. The present study therefore provides further evidence for the contribution of the pre-SMA, and more specifically the speech SMA, to higher level language processes as opposed to simple motor output programming.
The pattern of functional reorganization of the speech SMA observed here provides insight into the implications of lateralization of the “language apparatus.” The cortical reorganization in both patients was almost exclusively contralateral with little to no ipsilateral recruitment. It has been suggested that language lateralizes to avoid contralateral competition for control of midline vocal tract musculature.6 Of note, a study by Fox et al. demonstrated widespread bilateral over-activation of the motor system during a speech task in stuttering subjects (Fox, et al., 1996; Ojemann, 1993; Riecker, Wildgruber, Grodd, & Ackermann, 2002; Woods, Cherry, & Mazziotta, 1992; Lou, Peck, Brennan, Mallela, & Holodny, 2017)). The near complete contralateral relocation of the speech SMA that we observed in both patients may provide support for this notion that lateralization may prevent conflicting or mistimed motor signals that might create asynchronous motor input to muscles of articulation and phonation, resulting in deficient speech production.
Previous studies have demonstrated large scale reorganization of the SMA proper after surgical lesion resections involving the SMA, with recovery due to interhemispheric connectivity. This study demonstrates similar neural plasticity underlying the recovery of speech functioning following lesioning of the speech SMA or its associated afferent or efferent networks due to contralateral migration or recruitment of homologous tissue. In other words, our current findings suggest the ability of intact non-dominant hemisphere homologous tissue to compensate for damage to the dominant hemisphere speech SMA and/or its white matter pathways, accompanied by recovery of speech functioning (Riecker, Wildgruber, Grodd, & Ackermann, 2002).
5. Conclusion
Our study demonstrates an important aspect in functional recovery following resection of lesions involving or associated with the speech SMA: neural plasticity occurring through dynamic contralateral homologous functional recruitment. Although previous studies have demonstrated large scale reorganization of sensorimotor networks, this study adds to scant literature regarding such reorganization of tissue pertaining to speech. Findings from our current study and others that characterize functional reorganization of eloquent tissue provide useful noninvasive neural markers to assess plasticity, and guide speech and language therapy development. Identifying potential brain regions responsible for various clinical contexts, such as speech SMA syndrome, and understanding interhemispheric functional connectivity patterns may guide pharmacological, surgical or behavioral therapies in the future (Ojemann, 1983; Ojemann, 1993; Pai, 1999). Moreover, these results suggest intact interhemispheric connectivity and contralateral speech SMA are necessary for recovery from speech SMA syndromes after surgical resection of a lesion.
Table 1.
Patient clinical characteristics
| Patient A | Patient B | |
|---|---|---|
| Age (in years) | 39 | 35 |
| Sex | Male | Male |
| Handedness | Right | Right |
| Tumor type | Anaplastic astrocytoma NOS | Oligodendroglioma, NOS |
| Tumor size/location | 2.0 × 3.0 cm mass, left frontal lobe | 2.5 × 3.5 cm mass, left parasagittal frontal lobe |
| Midline shift | None | None |
| Preoperative speech | Normal | Mild word finding difficulties |
| Postoperative speech | Mute | Hesitance, slowness in speech, poor fluency |
| Documented speech recovery | Full recovery at 14 months | Full recovery at 18 months |
| Repeat fMRI mapping | 32 months after surgery | 64 months after surgery |
cm, centimeter; fMRI, functional magnetic resonance imaging
Acknowledgments
Sources of Support: The primary author (SC) is a current recipient of an NIH R25 award for mentored research. The research presented in this manuscript was not however, directly funded by public, commercial, or not-for-profit grants.
Footnotes
Financial Disclosure/Disclaimer: The authors have no vested financial interests in this study
Conflicts of Interest: None
REFERENCES
- Acioly MA, Cunha AM, Parise M, Rodrigues E, & Tovar-Moll F (2015). Recruitment of Contralateral Supplementary Motor Area in Functional Recovery Following Medial Frontal Lobe Surgery: An fMRI Case Study. Journal of Neurological Surgery Part A-Central European Neurosurgery, 76(6), 508–512. [DOI] [PubMed] [Google Scholar]
- Alario FX, Chainay H, Lehericy S, Cohen L (2006). The role of the supplementary motor area (SMA) in word production. Brain Research, 1076, 129–143. [DOI] [PubMed] [Google Scholar]
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, & Knosche TR (2007). Connectivity-Based Parcellation of Broca’s Area. Cerebral Cortex, 17(4), 816–825. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A (2001) Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex 11, 837–848. [DOI] [PubMed] [Google Scholar]
- Bleasel A, Comair Y, Luders HO (1996). Surgical ablation of the mesial frontal lobe in humans. Adv Neurol, 70, 217–235. [PubMed] [Google Scholar]
- Bogousslavsky J, Regli F (1990). Anterior cerebral artery territory infarction in the Lausanne Stroke Registry: Clinical and etiological patterns. Arch Neurol, 47, 144–150. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Malow BA, Gaillard WD, Sato S et al. (1997). A direct comparison of PET activation and electrocortical stimulation mapping for language localization. Neurology, 48, 1056–1065. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton TA, Gaillard W Theodore W (1995). Regional cerebral blood flow during object naming and word reading. Hum Brain Mapp, 3, 93–106. [Google Scholar]
- Bradshaw JL (1981). Hemispheric laterality and an evolutionary perspective. J Behav Brain Sci, 4, 21–22. [Google Scholar]
- Calvert GA, Brammer MJ, Morris RG, Williams SC, King N, Matthews PM (2000). Using fMRI to study recovery from acquired dysphasia. Brain Lang, 71, 391–399. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Pouratian N, Bookheimer SY, Martin NA, Beckerand DP, Toga AW (2001). Temporal spatial differences observed by functional MRI and human intraoperative optical imaging. Cereb Cortex, 11, 773–782. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Pugh KR, Westerveld M, Studholme C, Skrinjar O, Thompson JL, et al. (2001). Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia, 42, 1241–1254. [DOI] [PubMed] [Google Scholar]
- Carey LM, Abbott DF, Egan GF, Bernhardt J, Donnan GA (2005). Motor impairment and recovery in the upper limb after stroke. Stroke, 36, 625–629. [DOI] [PubMed] [Google Scholar]
- Chung GH, Han YM, Jeong SH, & Jack CR (2005). Functional heterogeneity of the supplementary motor area. AJNR Am J Neuroradiol, 26(7), 1819–1823. [PMC free article] [PubMed] [Google Scholar]
- Cohen MS (1997). Parametric analysis of fMRI data using linear systems methods. Neuroimage, 6, 93–103. [DOI] [PubMed] [Google Scholar]
- Cona G, & Semenza C (2017). Supplementary motor area as key structure for domain-general sequence processing: A unified account. Neuroscience and Biobehavioral Reviews, 72, 28–42. [DOI] [PubMed] [Google Scholar]
- Crosson B, Sadek JR, Bobholz JA, Gokcay D, Mohr CM, Leonard CM, et al. (1999). Activity in the paracingulate and cingulate sulci during word generation: an fMRI study of functional anatomy. Cereb Cortex, 9, 307–316. [DOI] [PubMed] [Google Scholar]
- Cuenod CA, Bookheimer SY, Hertz-Pannier L, Zeffiro TA, Theodore WH, Le Bihan D (1995). Functional MRI during word generation, using conventional equipment: a potential tool for language localization in the clinical environment. Neurology, 45, 1821–1827. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Van Hoesen GW (1985). Structure and function of the supplementary motor area. Neurology, 30, 359. [Google Scholar]
- Dapretto M, Bookheimer SY (1999). Form and content: dissociating syntax and semantics in sentence comprehension. Neuron, 24, 427–432. [DOI] [PubMed] [Google Scholar]
- Fontaine D, Capelle L, Duffau H (2002). Somatotopy of the supplementary motor area: evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery, 50, 297–305. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C (1996). A PET study of the neural systems of stuttering. Nature, 382: 158–162. [DOI] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, (1991). Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci, 11: 3656–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N (1969). Problems in the anatomical understanding of the aphasias In: Benton AL (Ed), Contributions to clinical neuropsychology (pp. 107–128). Chicago: Aldine [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, Choi CH, Lee JM, Kwon JS (2008). Neural correlates of cognitive inflexibility during task switching in obsessive compulsive disorder. Brain, 131, 155–164. [DOI] [PubMed] [Google Scholar]
- Hagoort P (2014). Nodes and networks in the neural architecture for language: Broca’s region and beyond. Current Opinion in Neurobiology, 28, 136–141. [DOI] [PubMed] [Google Scholar]
- Hertrich I, Dietrich S, & Ackermann H (2016). The role of the supplementary motor area for speech and language processing. Neuroscience & Biobehavioral Reviews, 68, 602–610. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y (2002). Comparing cortical activations for silent and overt speech using event-related fMRI. Hum Brain Mapp, 15, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunke K, Van de Wiele B, Fried I, Rubinstein EH (1998). The asleep-awake-asleep anesthetic technique for intraoperative language mapping. Neurosurgery, 42, 1312–1316. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM (2004). The spatial and temporal signatures of word production components. Cognition, 92, 101–144. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S (1983). Boston Naming Test. Philadelphia: Lee & Febiger [Google Scholar]
- Karbe H, Thiel A, Weber-Luxenburger G, Herholz K, Kessler J, Heiss WD Brain plasticity in poststroke aphasia: what is the contribution of the right hemisphere? Brain Lang, 64, 215–230. [DOI] [PubMed] [Google Scholar]
- Kim J-H, Lee J-M, Jo HJ, Kim SH, Lee JH, Kim ST, … Saad ZS (2010). Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. Neuroimage, 49(3), 2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Shin M, Lee K, Chu K, Woo SH, Kim YR, et al. (2004). Musical training-induced functional reorganization of the adult brain: Functional magnetic resonance imaging of transcranial magnetic stimulation study on amateur string players. Hum Brain Mapp, 23, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainik A, Lehericy S, Duffau H, Capelle L, Chainay H, Cornu P, et al. (2003). Postoperative speech disorder after medial frontal surgery: Role of the supplementary motor area. Neurology, 60, 587–594. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Civier O, & Ben-Shachar M (2016). The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Structure & Function, 221(1), 365–381. [DOI] [PubMed] [Google Scholar]
- Lou W, Peck KK, Brennan N, Mallela A, & Holodny A (2017). Left-lateralization of resting state functional connectivity between the presupplementary motor area and primary language areas. Neuroreport, 28(10), 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Waller S, Forrester L, Smith GV, Whitall J, Macko RF, et al. (2004). Lesion location alters brain activation in chronically impaired stroke survivors. Neuroimage, 21, 924–935. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda RM, Rizzolatti G (1993). Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol, 339, 114–140. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Deecke L, Freund HJ, Hallett M, Passingham RE, Shibasaki H, et al. (1996). The functions of the supplementary motor area. Summary of a workshop. Adv Neurol, 70, 477–487. [PubMed] [Google Scholar]
- Masdeu JC, Schoene WC, Funkenstein H (1978). Aphasia following infarction of the left supplementary motor area. Neurology, 28, 1220–1223. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Wise SP (1987). The somatotopic organization of the supplementary motor area: Intracortical microstimulation mapping. J Neurosci, 7, 1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ED, Rosen HJ, Ojemann JG, Buckner RL, Kelley WM, Petersen SE (2001). An event-related fMRI study of overt and covert word stem completion. Neuroimage, 14, 182–193. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996). Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex, 6, 342–354. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2001). Imaging the premotor areas. Curr Opin Neurobiol, 11, 663–672. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, O’Farrell AM (2000). Optical imaging of bilingual cortical representations. J Neurosurg, 93, 676–681. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW (2002). Utility of preoperative functionl magnetic resonance imaging for identifying language cortices in patients with vascular malformations. J Neurosurg, 97, 21–32. [DOI] [PubMed] [Google Scholar]
- Ojemann GA (1983). Brain organization for language from the perspective of electrical stimuation mapping. Behav Brain Sci, 35, 409–412. [Google Scholar]
- Ojemann GA (1993). Functional mapping of cortical language areas in adults. Intraoperative approaches. Adv Neurol, 63, 155–163. [PubMed] [Google Scholar]
- Pai MC (1999). Supplementary motor area aphasia: A case report. Clin Neurol Neurosurg, 101, 29–32. [DOI] [PubMed] [Google Scholar]
- Riecker A, Wildgruber D, Grodd W, Ackermann H (2002). Reorganziation of speech production at the motor cortex and cerebellum following capsular infarction: a follow-up functional magnetic resonance imaging study. Neurocase, 8, 417–423. [DOI] [PubMed] [Google Scholar]
- Rostomily RC, Berger MS, Ojemann GA, Lettich E, Tsukerman L, Makuch RL (1991). Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg, 75, 62–68. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M (1994). Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res, 102, 227–243. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging, 17, 87–97. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA,, Swanson SJ, Frost JA, Bellgowan PS, et al. (1999). Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain, 122, 2033–2046. [DOI] [PubMed] [Google Scholar]
- Thiel A, Herholz K, Koyuncu A, Ghaemi M, Kracht LW, Havedank B, et al. (2001). Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann Neurol, 50, 620–629. [DOI] [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA (1999). Plasticity of language-related brain function during recovery from stroke. Stroke, 30, 749–754. [DOI] [PubMed] [Google Scholar]
- Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher J, Tardy J, et al. (2004). A longitudinal fMRI study: in recovering and then in clinically stable subcortical stroke patients. Neuroimage, 23, 827–839. [DOI] [PubMed] [Google Scholar]
- Vassal M, Charroud C, Deverdun J (2017). Recovery of functional connectivity of the sensorimotor network after surgery for diffuse low-grade gliomas involving the supplementary motor area. J Neurosurg, 126, 1181–1190. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. (2005). Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke, 36, 1759–1763. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC (1992). Rapid automated algorithm for aligning and Reslicing PET images. J Comput Assist Tomogr, 16, 620–633. [DOI] [PubMed] [Google Scholar]
- You SH, Jang SH, Kim Y, Kwon Y, Barrow I, Hallett M (2005). Cortical reorganization induced by virtual reality therapy in a child with hemiparetic cerebral palsy. Dev Med Child Neurol, 47, 628–635. [PubMed] [Google Scholar]
- Ziegler W, Kilian B, Deger K (1997). The role of the left mesial frontal cortex in fluent speech: evidence from a case of left supplementary motor area hemorrhage. Neuropsychologia, 35, 1197–208. [DOI] [PubMed] [Google Scholar]
- Zentner J, Hufnagel A, Pechstein U, Wolf HK, Schramm J (1996). Functional results after resection procedures involving the supplementary motor area. J Neurosurg, 85, 542–549. [DOI] [PubMed] [Google Scholar]


