Abstract
Background:
Kabuki (Niikawa-Kuroki) syndrome (KS) is caused by disease causing variants in either of two components (KMT2D, KDM6A) of the histone methylation machinery. Nearly all individuals with KS have cognitive difficulties and most have Intellectual Disability (ID). Recent studies on a mouse model of KS suggest disruption of normal adult neurogenesis in the granule cell layer of the dentate gyrus of the hippocampus. These mutant mice also demonstrate hippocampal memory defects compared to littermates, but this phenotype is rescued postnatally with agents that target the epigenetic machinery. If these findings are relevant to humans with KS, we would expect significant and disproportionate disruption of visuospatial functioning in these individuals.
Methods:
To test this hypothesis, we have compiled a battery to robustly explore visuospatial function. We prospectively recruited 22 patients with molecularly confirmed KS and 22 IQ-matched patients with ID.
Results:
We observed significant deficiencies in visual motor, visual perception and visual motor memory in the KS group compared to the IQ-match group on several measures. In contrast, language function appeared to be marginally better in the KS group compared to the IQ matched group on a sentence comprehension task.
Conclusions:
Together, our data suggest specific disruption of visuospatial function, likely linked to the dentate gyrus, in individuals with KS and provides the groundwork for a novel and specific outcome measure for a clinical trial in a KS population.
Keywords: KMT2D, epigenetics, dentate gyrus, intellectual disability, neurocognitive testing, histone machinery
INTRODUCTION:
Kabuki (Niikawa-Kuroki) syndrome (KS; MIM#147920) is a genetic disorder occurring in about 1 in every 30,000 births (Adam, Hudgins, 2005) characterised by five cardinal features; dysmorphic facial features, skeletal anomalies, persistence of fetal fingertip pads, postnatal growth deficiency, and intellectual disability (ID) (Niikawa et al., 1988). The gene most likely to carry disease causing variants in patients with KS is KMT2D – a histone methyltransferase that adds mono- and trimethylation to the fourth lysine (K4) of histone 3, modifications found at enhancers and actively transcribed promoters, respectively (Ng et al., 2010). Disease causing variants in KDM6A – a histone demethylase that removes H3K27me3, a modification seen in closed chromatin – represent another known, but less common cause of KS (MIM#300867) (Lederer et al., 2012). Together, KMT2D and KDM6A account for 24–56% of clinically defined KS (Paderova et al., 2016), indicating locus heterogeneity. KS is also an example of a Mendelian disorder of the epigenetic machinery, a rapidly emerging cause of ID (Fahrner, Bjornsson, 2014).
Previously, a mouse model of KS (Kmt2d+/βGeo mice) has been shown to have many features of individuals with the syndrome (growth retardation, skeletal and craniofacial abnormalities) (Bjornsson et al., 2014). This model has also demonstrated abnormalities in visuospatial hippocampal memory as seen as decreased performance in novel object recognition and in platform crossing in a Morris water maze (Bjornsson et al., 2014) accompanied with deficits in adult neurogenesis in the dentate gyrus of the hippocampus and a thinner granule cell layer (Bjornsson et al., 2014). Importantly, these cognitive and histopathologic deficits were corrected with postnatal administration of a histone deacetylase inhibitor (Bjornsson et al., 2014). Although in vivo human studies of adult neurogenesis are currently impossible, the dentate gyrus – and postnatal neurogenesis in this region – has been linked to spatial pattern separation and thus tests that examine visuospatial reasoning and memory at least partially localise to this brain region and would be expected to be abnormal in individuals with KS if these mouse studies are predictive of the human pathophysiology (Brickman, Stern & Small, 2011, Kesner, 2013, Kesner et al., 2014, Kesner, Rolls, 2015, Morris et al., 2012, Epp, Haack & Galea, 2011).
To date, there have been a limited number of studies exploring the cognitive profile of humans with KS and none that link this cognitive profile with the basic science insights regarding the molecular and neuroanatomical underpinnings of the disease. The original report by Niikawa et al. in 1981 suggested that individuals with KS had moderate to severe ID (DQ/IQs between 22 and 77) (Niikawa et al., 1981). Several more recent reports including a 1988 review of 62 cases suggested that most patients with KS have mild to moderate ID with about 10% of patients falling in the range of normal IQ and some scattering into severe and profound (Niikawa et al., 1988, Philip et al., 1992, Schrander-Stumpel et al., 1994). Mervis et al. performed a set of cognitive and adaptive measures in 11 participants with KS (Mervis et al., 2005). They saw clear weaknesses in visuospatial construction with relative strengths in verbal reasoning (Mervis et al., 2005), however none of these patients were molecularly confirmed. A longitudinal case study of one patient with KS who had clinical neuropsychological assessment at different ages also demonstrated strengths in verbal reasoning with weaknesses in visuospatial construction and this difference became more pronounced over time even as the overall IQ improved (Sanz et al., 2010). Lehman et al. studied 31 patients with documented KMT2D disease causing variants. They found that those with nonsense variants had lower full scale IQs than those with other disease causing variants (Lehman et al., 2017). In addition, they found that many patients had much higher verbal comprehension and working memory scores than perceptual reasoning and processing speed scores (Lehman et al., 2017). More recently, a study by Caciolo et al. compared molecularly confirmed KS vs. clinically defined KS and again found visuospatial weaknesses and no significant differences between the two groups (Caciolo et al., 2018). A brief retrospective review done in conjunction with the Kmt2d+/βGeo mouse work did suggest that the deficit pattern in KS could map to the dentate gyrus (Bjornsson et al., 2014). To date, no prospective studies have aimed to neuroanatomically localise the cognitive deficits in KS, to compare their profiles to IQ matched controls, nor to create a repeatable cognitive outcome measure that could be used in a clinical trial.
Based on the mouse and human data, we hypothesised that patients with KS have deficits in dentate gyrus neurogenesis which would be expected to lead to measurable impairment in visuospatial memory and reasoning tasks. Here we describe a novel testing battery designed to test this hypothesis by detailed characterisation of cognitive functions strongly linked to the dentate gyrus. Here, we demonstrate that individuals with KS have disproportionate visuospatial abnormalities that differentiate them from typically developing children but also from age and IQ-matched controls. These data suggest that visuospatial measurements could act as a potential therapeutic outcome measures in clinical trials targeting the neurocognitive phenotype in KS.
METHODS:
Subjects
We recruited 23 children and adults with molecularly confirmed KS ages 7 and older with likely pathogenic variant in KMT2D or KDM6A from our Epigenetics and Chromatin clinic (https://www.hopkinsmedicine.org/institute-genetic-medicine/patient-care/genetics-clinic/about/epigenetics-chromatin-clinic/index.html) or from patient organised KS conferences. Exclusion criteria included presence of another known genetic syndrome, inability to participate in testing due to significant visual or hearing impairment or profound ID although the latter is rare for KS type 1. One child was excluded after investigation determined that his KMT2D variant was unlikely to be pathogenic leaving 22 participants. All participants had clinical neuropsychological assessments in the past which included a measurement of full scale IQ. The study was approved by the Johns Hopkins Medical Institutions Institutional Review Board and all participants underwent a written informed consent process.
Controls
Controls were recruited from other clinics at Kennedy Krieger Institute, primarily clinics that specialise in evaluating learning, development and cognition and not genetic clinics. 22 controls were recruited to match participants in the KS group based on chronologic age (+/− 1.5 years for <18 years old, +/− 3.5 years for 18 or older) and full scale IQ (+/− 5 points). Gender matching was not performed. Exclusion criteria included presence of a known genetic syndrome or inability to participate in testing due to significant visual or hearing impairment. Controls also signed a written informed consent.
Cognitive Assessment Battery
Subtests were selected from clinically established and validated neuropsychological measures. Subtests selected were areas of particular weakness in patients with KS on review of clinical neuropsychological testing of our previous patients and testing reported in the literature (Sanz et al., 2010, Bjornsson et al., 2014, Mervis et al., 2005, Lehman et al., 2017). Subtests were also selected if they specifically targeted functions linked to the dentate gyrus (Brickman, Stern & Small, 2011, Kesner, 2013, Kesner et al., 2014, Kesner, Rolls, 2015, Morris et al., 2012, Epp, Haack & Galea, 2011). One verbal comprehension and one reading measure were also added for comparison. Subtests were selected so that administration of the entire battery would take under 2 hours and be applicable to a range of ages. The testing battery consisted of: Raven Progressive Matrices, Benton Judgement of Line Orientation, Rey Complex Figure Test, Wide Range Achievement Test-3 - Word Reading and Sentence Comprehension, Beery Developmental Tests of Visual Motor Integration and Visual Perception, Delis-Kaplan Executive Functioning System - Trail Making and Color Word Interference Tests, Wechsler Intelligence Scale for Children-IV - Coding, Cancellation, and Block Design as appropriate for age. (Supplementary Table 2) (Delis, Kaplan & Kramer, 2001, Meyers, Meyers, 1996, Wilkinson, Robertson, 2006, Beery, Buktenica, 2004, Wechsler, 2003,).
Statistical Analysis
All statistical analyses were done in R version 3.5.1. For subtests for which age-based standard scores were available for all ages tested, those standard scores were used. If the tests were not designed for all ages, raw scores were used. In addition, because all participants – KS and controls – scored so poorly on the Benton Judgement of Line Orientation test, raw scores were used there as well. Scaled scores were used for Wide Range Achievement Test Word Reading and Sentence Comprehension, Beery Visual Motor Integration and Visual Perception, Delis-Kaplan Executive Functioning System Trail Making and Color Word Tests, and Wechsler Block Design. T-scores were used for Rey Complex Figure Test. Raw scores were used for Raven Progressive Matrices, Benton Judgement of Line Orientation, and WISC-IV Coding and Cancellation. A P value of <0.05 was considered statistically significant. All result distributions were evaluated to determine whether they were parametric and then Student’s t-tests or Wilcoxon rank-sum tests were used to compare the performance on each of the cognitive measures between patients with KS and controls as appropriate. The Benjamini-Hochberg adjustment was performed to account for multiple comparisons (Benjamini, Hochberg, 1995).
RESULTS:
Twenty of the 22 subjects had disease causing variants in KMT2D and two in KDM6A. Of the 20 KMT2D disease causing variants, 17 were truncating variants – occurring throughout the protein and likely leading to nonsense mediated decay - and 3 were missense (Figure 1). There were not enough subjects with KDM6A disease causing variants nor with missense KMT2D disease causing variants to make accurate comparisons between groups although both patients with KDM6A disease causing variants were on the lower end of the cohort for their full scale IQ (Supplementary Table 1). Controls were well-matched to cases in that there was no significant difference in FSIQ between the groups (Table 1); however, there were significantly more females than males in the KS cohort and the controls were equally divided between genders (Supplementary Table 1, Table 1). Because there were only 3 males in the KS cohort we also cannot make accurate comparisons between genders in the KS group.
Figure 1: KMT2D disease causing variants in our prospective cohort.
Our schematic of the gene demonstrates major domains found in KMT2D. Domain position is based on InterPro. Frameshift or nonsense variants are shown above the schematic of the gene but these are expected to lead to nonsense mediated decay or in some cases dominant negative effects. Missense variants are shown below the schematic of the gene.
Table 1:
Cognitive Assessment of KS vs. IQ Matched Controls
| Mean KS | Mean Control |
p-adjusted | Effect size |
||
|---|---|---|---|---|---|
|
Baseline Characteristics |
Gender | F=19/22 | F=11/22 | 0.0096 | |
| Age | 15.68 | 14.95 | 0.758 | ||
| Full Scale IQ | 67.82 | 69.36 | 0.8709 | ||
|
Cognition/Visual Perception |
Raven Progressive Matrices | 18.14 | 20.36 | 0.6102 | |
| Executive Functioning | D-KEFS TMT Condition 2 | 2.59 | 2.55 | 0.8744 | |
| D-KEFS TMT Condition 3 | 3.18 | 2.55 | 0.9494 | ||
| D-KEFS TMT Condition 4 | 3.00 | 2.00 | 0.4023 | ||
| D-KEFS CW Condition 1 | 4.45 | 4.45 | 0.9494 | ||
| D-KEFS CW Condition 2 | 4.59 | 3.00 | 0.4023 | ||
| D-KEFS CW Condition 3 | 2.59 | 3.00 | 0.9494 | ||
| D-KEFS CW Condition 4 | 3.09 | 2.73 | 0.9362 | ||
|
Executive Function/ Visual Perception |
D-KEFS TMT Condition 1 | 3.00 | 2.95 | 0.6102 | |
| WISC-IV Coding* | 27.18 | 33.32 | 0.4602 | ||
| WISC-IV Cancellation* | 41.41 | 50.45 | 0.3887 | ||
| Language | WRAT Word Reading | 78.36 | 79.23 | 0.8771 | |
| WRAT Sentence Comprehension | 82.95 | 72.18 | 0.0658 | ||
| Visual Motor | Beery Visual Motor Integration | 50.23 | 69.09 | 0.0014 | 1.4055 |
| RCFT Copy* | 8.93 | 22.93 | 0.0003 | 1.5746 | |
| Visual Perception | Wechsler Block Design | 2.45 | 4.64 | 0.0035 | 1.1645 |
| Beery Visual Perception | 56.55 | 72.23 | 0.0035 | 1.0642 | |
| Benton Judgement of Line Orientation |
5.82 | 11.00 | 0.142 | ||
| RCFT Recognition | 23.14 | 26.50 | 0.7161 | ||
| Visuospatial Memory | RCFT Immediate Recall | 20.68 | 31.50 | 0.0003 | 2.0326 |
| RCFT Delayed Recall | 20.36 | 27.59 | 0.0003 | 1.457 | |
| Motor | D-KEFS TMT Condition 5 | 4.14 | 4.59 | 0.8197 |
=Raw scores used
= reaches statistical significance
F= females, D-KEFS = Delis-Kaplan Executive Functioning System, TMT = Trail Making Test, CW = Color Word, WRAT = Wide Ranging Achievement Test, WISC-IV = Wechsler Intelligence Scale for Children, RCFT = Rey Complex Figure Test
Table 1 shows the means for the KS cohort and matched controls for each subtest administered as well as the adjusted p-value for the difference between these means. There was no difference between the groups in measures of general cognition or executive functioning. In contrast, we observed significant differences between the groups on all measures of visual motor (Beery VMI, RCFT copy) and visual motor memory performance (RCFT immediate and delayed) and some of the measures of visuospatial perception (Beery VP and Block Design). All of these significant results are depicted in Figure 2. There was no significant difference between the groups on the motor speed task. The KS group had a higher mean score on the sentence comprehension task although the difference between these means just above our cutoff of statistical significance. As expected, both the cohort of KS patients and their matched controls had lower mean scores than the general population test mean for every subtest (Figures 2 and 3).
Figure 2: Performance of KS subjects, IQ-matched controls and a normal population (test norms).
A: Full scale IQ – no significant difference; B: Block Design scaled scores: significant difference between KS and controls; C: Wide Range Achievement Test Sentence Comprehension scaled scores: KS patients performed better than control but difference did not reach statistical significance; D: Beery Visual Motor Integration scaled scores: significant difference between KS and controls; E: Beery Visual Perception scaled scores: significant difference between KS and controls; F: Rey Complex Figure Test Immediate Recall T-scores: significant difference between KS and controls; G: F: Rey Complex Figure Test Delayed Recall T-scores: significant difference between KS and controls.
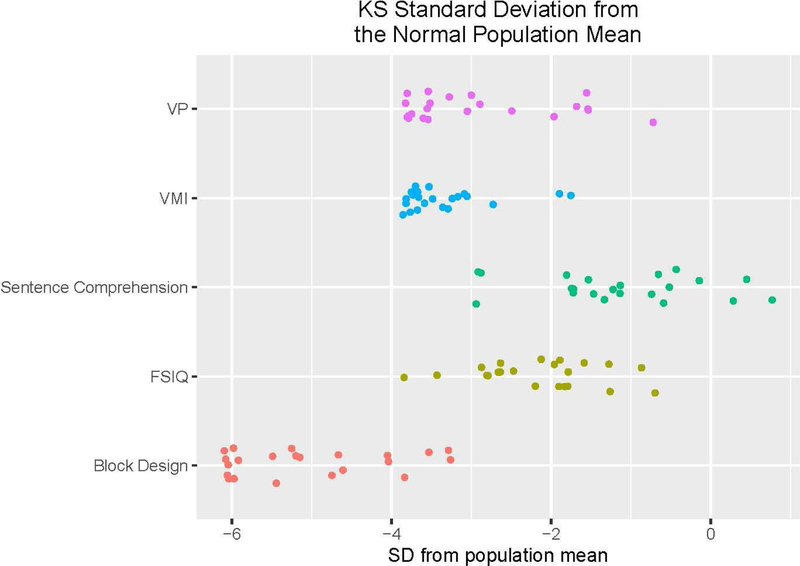
Figure 3: Deficiencies compared to the normal population of KS subjects for different subtests.
Standard deviations below normal test mean for KS subjects’ performance on subtests. Visuospatial tests are many SDs below the mean and lower than FSIQ or WRAT Sentence Comprehension. For instance, many individuals with KS are 6 SD below the norm of this test for block design.
DISCUSSION:
There has been rapid discovery of molecular causes of ID in the last decade (Vissers, Gilissen & Veltman, 2016) and the scientific community now has knowledge of the majority of causes of ID. This is a unique opportunity to consider unifying features and/or molecular pathways among patients with ID but also an opportunity for scientists and providers to seek subtle differences among individual groups within this patient population. Although, traditionally ID has been considered to lead to global intellectual dysfunction of multiple aspects of cognition, it is now becoming clear that among patients with ID there are disproportionate strengths and weaknesses of intellectual functioning and these differences may be clues into the pathogenesis of this disease entity (Waite et al., 2014). For instance, patients with Williams syndrome have disproportionate visuospatial defects but relative sparing of language abilities (Landau, Ferrara, 2013, Mervis et al., 2000). In contrast, the 7q11.23 duplication of the same region (7q11.23 duplication syndrome) leads to speech apraxia, and is therefore a molecular and phenotypic opposite to Williams syndrome (Velleman, Mervis, 2011). Similarly, there have been other recent descriptions of relatively isolated speech apraxia (Pilarowski et al., 2018). Thus, there are disorders that lead to disproportional problems with individual aspects of cognitive function.
Here we add KS to the list of such disorders by showing that molecularly confirmed patients with KS show a disproportionate deficiency of visuospatial abilities as seen by more extensive abnormalities in visual motor, visual memory, and visual perception subtests compared to measures of language comprehension and general cognition; this observation is strengthened by the fact that it holds even when KS patients are compared to IQ matched controls. In addition, there was not a significant difference between the groups in a pure motor task suggesting that this is a deficit in visuospatial perception and memory and not just a motor skills issue. In contrast, although not statistically significant, the KS subjects performed better on the language measure – particularly the one dealing with comprehension and not the one dealing purely with reading which may be affected by visuospatial issues. However, we are aware that since the controls are matched based on IQ and the KS patients clearly have relative weaknesses in visuospatial perception and memory that the non-significant difference seen in the language measure may be secondary to this matching strategy. In all, these data strongly support the hypothesis that the hippocampus and specifically the dentate gyrus (Kesner, 2013, Kesner et al., 2014, Kesner, Rolls, 2015, Brickman, Stern & Small, 2011, Epp, Haack & Galea, 2011, Morris et al., 2012) plays a role in the pathophysiology of the ID in KS. This hypothesis can be further explored in future neuropsychological studies and also through imaging studies.
Studies such as ours may help cluster individual causes of ID into groups with shared pathophysiology. It has not escaped our attention that Kabuki and Williams syndromes have many shared neurocognitive and neurobehavioural features in addition to the disproportionate visuospatial defects, including a relative strength in social interaction and communicative skills and anxiety (Mervis et al., 2005, Lehman et al., 2017, Caciolo et al., 2018), this may indicate that the pathophysiology of these two disorders is linked in some unknown manner. One potential hypothesis would be that the transcription factors thought to be causative for the ID in Williams syndrome (GTF2IRD1, GTF2I) may use co-activators such as KMT2D to exercise their developmental programming but this remains to be tested in future studies.
Our current study has several limitations. First, we mainly have representation for patients with KS type 1, since this is the majority of patients with molecularly confirmed KS that are available and able to participate in such studies. Since KS2 is rare the affected boys tend to be much more severely affected and therefore unable to complete the neuropsychological assessment. Second, although patients were collected in a prospective manner for this study, we observed a significant sex-bias with more females than males with KS recruited to our study. We have no good explanation for this observation, although it may indicate an ascertainment bias. Because our matched controls do not have the same gender distribution, it is possible this is acting as a confounder to the results although there is no reason to assume this to be the case. Third, we have very few patients with missense changes and are therefore unable to comment on similarities or differences dependent on mutational type although our sense is that perhaps patients with missense changes have a milder disease phenotype as has been previously published (Hannibal et al., 2011, Lehman et al., 2017). Fourth, we have not performed comprehensive assessment of all aspects of the cognitive phenotype due to practical restrictions. Nevertheless, we would argue that this study also has some unique strengths. We believe that by focusing on visuospatial functions (memory, perception, construction, and visuomotor) which have previously been described as weaker in KS that we confirm here that this area of cognition is disproportionately affected in this syndrome and we can make a stronger case that the dentate gyrus is indeed crucial in the neurological profile of KS. Further strengthening this conclusion is a study performed by van Dongen et al. and published simultaneously with this study which looks at an entirely different cohort of KS patients and compares them to a different control group and again finds specific weaknesses in visuoconstruction (van Dongen et al., in press). In addition, with this focused battery we begin to lay the groundwork for outcome measures in a potential clinical trial with mechanism-based treatments (such as but not limited to an HDAC inhibitor) and our battery may act as a starting point for other investigators. Furthermore, this is the first study to compare patients with KS to IQ matched controls and through this, we can confirm that this cognitive profile is specific to KS and not just secondary to the general ID itself.
Thus in summary, we find disproportionate defects of visuospatial functioning in a prospective study of patients with molecularly confirmed KS.
Supplementary Material
ACKNOWLEDGEMENTS:
We would like to thank the patients and the families that participated in this study. H.T.B. is funded through an Early Independence Award from the National Institute of Health (DP5OD017877) and a grant from the Louma G. Foundation. The Intellectual and Developmental Disabilities Center at Kennedy Krieger/Johns Hopkins University (U54 HD079123) also provided support. J.H., E.M.M. and H.T.B are consultants for Millennium Pharmaceuticals.
REFERENCES:
- Adam MP & Hudgins L 2005, “KS: a review”, Clinical genetics, vol. 67, no. 3, pp. 209–219. [DOI] [PubMed] [Google Scholar]
- Beery KE & Buktenica NA 2004, Beery-Buktenica Developmental Test of Visual-Motor Integration, 5th edn, NCS Pearson Inc, Minneapolis, MN. [Google Scholar]
- Benjamini Y & Hochberg Y 1995, “Controlling the False Discovery Rate - a new and powerful approach to multiple testing”, Journal of the Royal Statistical Society B, vol. 57, pp. 289–300. [Google Scholar]
- Benton AL, Varney NR & Hamsher K 1978, “Visuospatial Judgment”, Archives of Neurology, vol. 35, pp. 364–367. [DOI] [PubMed] [Google Scholar]
- Bjornsson HT, Benjamin JS, Zhang L, Weissman J, Gerber EE, Chen YC, Vaurio RG, Potter MC, Hansen KD & Dietz HC 2014, “Histone deacetylase inhibition rescues structural and functional brain deficits in a mouse model of KS”, Science translational medicine, vol. 6, no. 256, pp. 256ra135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Stern Y & Small SA 2011, “Hippocampal subregions differentially associate with standardised memory tests”, Hippocampus, vol. 21, no. 9, pp. 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caciolo C, Alfieri P, Piccini G, Digilio MC, Lepri F, Tartaglia M, Menghini D & Vicari S 2018, “Neurobehavioural features in individuals with KS”, Molecular Genetics & Genomic Medicine, vol. 6, no. 3, pp. 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E & Kramer JH 2001, Delis-Kaplan executive function system, The Psychological Corporation, San Antonio. [Google Scholar]
- Epp JR, Haack AK & Galea LAM 2011, “Activation and survival of immature neurons in the dentate gyrus with spatial memory is dependent on time of exposure to spatial learning and age of cells at examination”, Neurobiology of Learning and Memory, vol. 95, no. 3, pp. 316–325. [DOI] [PubMed] [Google Scholar]
- Fahrner JA & Bjornsson HT 2014, “Mendelian Disorders of the Epigenetic Machinery: Tipping the Balance of Chromatin States”, Annual Review of Genomics and Human Genetics, vol. 15, no. 1, pp. 269–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal MC, Buckingham KJ, Ng SB, Ming JE, Beck AE, McMillin MJ, Gildersleeve HI, Bigham AW, Tabor HK, Mefford HC, Cook J, Yoshiura K, Matsumoto T, Matsumoto N, Miyake N, Tonoki H, Naritomi K, Kaname T, Nagai T, Ohashi H, Kurosawa K, Hou JW, Ohta T, Liang D, Sudo A, Morris CA, Banka S, Black GC, Clayton-Smith J, Nickerson DA, Zackai EH, Shaikh TH, Donnai D, Niikawa N, Shendure J & Bamshad MJ 2011, “Spectrum of MLL2 (ALR) mutations in 110 cases of KS”, American journal of medical genetics.Part A, vol. 155A, no. 7, pp. 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP 2013, “An analysis of the dentate gyrus function”, Behavioural brain research, vol. 254, pp. 1–7. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hui X, Sommer T, Wright C, Barrera VR & Fanselow MS 2014, “The role of postnatal neurogenesis in supporting remote memory and spatial metric processing”, Hippocampus, vol. 24, no. 12, pp. 1663–1671. [DOI] [PubMed] [Google Scholar]
- Kesner RP & Rolls ET 2015, “A computational theory of hippocampal function, and tests of the theory: new developments”, Neuroscience and biobehavioural reviews, vol. 48, pp. 92–147. [DOI] [PubMed] [Google Scholar]
- Landau B & Ferrara K 2013, “Space and language in Williams syndrome: insights from typical development”, Wiley interdisciplinary reviews.Cognitive science, vol. 4, no. 6, pp. 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer D, Grisart B, Digilio MC, Benoit V, Crespin M, Ghariani SC, Maystadt I, Dallapiccola B & Verellen-Dumoulin C 2012, “Deletion of KDM6A, a histone demethylase interacting with MLL2, in three patients with KS”, American Journal of Human Genetics, vol. 90, no. 1, pp. 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman N, Mazery AC, Visier A, Baumann C, Lachesnais D, Capri Y, Toutain A, Odent S, Mikaty M, Goizet C, Taupiac E, Jacquemont ML, Sanchez E, Schaefer E, Gatinois V, Faivre L, Minot D, Kayirangwa H, Sang K‐LQ, Boddaert N, Bayard S, Lacombe D, Moutton S, Touitou I, Rio M, Amiel J, Lyonnet S, Sanlaville D, Picot MC & Geneviève D 2017, “Molecular, clinical and neuropsychological study in 31 patients with KS and KMT2D mutations”, Clinical Genetics, vol. 92, no. 3, pp. 298–305. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Becerra AM, Rowe ML, Hersh JH & Morris CA 2005, “Intellectual abilities and adaptive behaviour of children and adolescents with KS: a preliminary study”, American journal of medical genetics.Part A, vol. 132A, no. 3, pp. 248–255. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Bertrand J, Morris CA, Klein-Tasman BP & Armstrong SC 2000, “The Williams syndrome cognitive profile”, Brain and cognition, vol. 44, no. 3, pp. 604–628. [DOI] [PubMed] [Google Scholar]
- Meyers JE & Meyers KR 1996, Rey Complex Figure and Recognition Trial, Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Morris AM, Churchwell JC, Kesner RP & Gilbert PE 2012, “Selective lesions of the dentate gyrus produce disruptions in place learning for adjacent spatial locations”, Neurobiology of Learning and Memory, vol. 97, no. 3, pp. 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SB, Bigham AW, Buckingham KJ, Hannibal MC, McMillin MJ, Gildersleeve HI, Beck AE, Tabor HK, Cooper GM, Mefford HC, Lee C, Turner EH, Smith JD, Rieder MJ, Yoshiura K, Matsumoto N, Ohta T, Niikawa N, Nickerson DA, Bamshad MJ & Shendure J 2010, “Exome sequencing identifies MLL2 mutations as a cause of KS”, Nature genetics, vol. 42, no. 9, pp. 790–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikawa N, Kuroki Y, Kajii T, Matsuura N, Ishikiriyama S, Tonoki H, Ishikawa N, Yamada Y, Fujita M & Umemoto H 1988, “Kabuki make-up (Niikawa-Kuroki) syndrome: a study of 62 patients”, American Journal of Medical Genetics, vol. 31, no. 3, pp. 565–589. [DOI] [PubMed] [Google Scholar]
- Niikawa N, Matsuura N, Fukushima Y, Ohsawa T & Kajii T 1981, “Kabuki make-up syndrome: a syndrome of mental retardation, unusual facies, large and protruding ears, and postnatal growth deficiency”, The Journal of pediatrics, vol. 99, no. 4, pp. 565–569. [DOI] [PubMed] [Google Scholar]
- Paderova J, Holubova A, Simandlova M, Puchmajerova A, Vlckova M, Malikova M, Pourova R, Vejvalkova S, Havlovicova M, Senkerikova M, Ptakova N, Drabova J, Geryk J, Maver A, Krepelova A & Macek M Jr 2016, “Molecular genetic analysis in 14 czech KS patients is confirming the utility of phenotypic scoring”, Clinical genetics [DOI] [PubMed]
- Philip N, Meinecke P, David A, Dean J, Ayme S, Clark R, Gross-Kieselstein E, Hosenfeld D, Moncla A & Muller D 1992, “Kabuki make-up (Niikawa-Kuroki) syndrome: a study of 16 non-Japanese cases”, Clinical dysmorphology, vol. 1, no. 2, pp. 63–77. [PubMed] [Google Scholar]
- Pilarowski GO, Vernon HJ, Applegate CD, Boukas L, Cho MT, Gurnett CA, Benke PJ, Beaver E, Heeley JM, Medne L, Krantz ID, Azage M, Niyazov D, Henderson LB, Wentzensen IM, Baskin B, Sacoto MJG & Bowman GD 2018, “Missense variants in the chromatin remodeler CHD1 are associated with neurodevelopmental disability”, Journal of Medical Genetics, vol. 55, no. 8, pp. 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz JH, Lipkin P, Rosenbaum K & Mahone EM 2010, “Developmental profile and trajectory of neuropsychological skills in a child with KS: implications for assessment of syndromes associated with intellectual disability”, The Clinical neuropsychologist, vol. 24, no. 7, pp. 1181–1192. [DOI] [PubMed] [Google Scholar]
- Schrander-Stumpel C, Meinecke P, Wilson G, Gillessen-Kaesbach G, Tinschert S, Konig R, Philip N, Rizzo R, Schrander J & Pfeiffer L 1994, “The Kabuki (Niikawa-Kuroki) syndrome: further delineation of the phenotype in 29 non-Japanese patients”, European journal of pediatrics, vol. 153, no. 6, pp. 438–445. [DOI] [PubMed] [Google Scholar]
- van Dongen LCM, Wingbermuhle E, van der Veld WM, Stumpel C, Kleefstra T & Egger JIM 2019, “Exploring the cognitive phenotype of Kabuki (Niikawa-Kuroki) syndrome”, Journal of Intellectual Disability, in press. [DOI] [PMC free article] [PubMed]
- Velleman SL & Mervis CB 2011, “Children with 7q11.23 Duplication Syndrome: Speech, Language, Cognitive, and Behavioural Characteristics and their Implications for Intervention”, Perspectives on language learning and education, vol. 18, no. 3, pp. 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers LE, Gilissen CF & Veltman JA 2016, “Genetic studies in intellectual disability and related disorders”, Nature Reviews Genetics, vol. 17, no. 1, pp. 9–18. [DOI] [PubMed] [Google Scholar]
- Waite J, Heald M, Wilde L, Woodcock K, Welham A, Adams D, Oliver C 2014, “The importance of understanding the behavioural phenotypes of genetic syndromes associated with intellectual disability”, Paediatrics and Child Health, vol. 24, no. 10, pp. 468–472. [Google Scholar]
- Wechsler D 2003, Wechsler Intelligence Scale for Children - Fourth Edition, Harcourt Assessment, Inc, San Antonio, TX. [Google Scholar]
- Wilkinson GS & Robertson GJ 2006, Wide Range Achievement Test 4 Professional Manual, Psychological Assessment Resources, Lutz, FL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.