Abstract
Objective:
Prior studies on the epidemiology of Whipple’s disease are limited by small sample size and case series design. We sought to characterize the epidemiology of Whipple’s disease in the United States utilizing a large population-based database.
Methods:
We queried a commercial database (Explorys Inc, Cleveland, OH), an aggregate of Electronic Health Record (EHR) data from 26 major integrated healthcare systems in the US. We identified a cohort of patients with a diagnosis of Whipple’s disease based on Systemized Nomenclature of Medical terminology (SNOMED-CT) codes. We calculated the overall and age, race, ethnicity, and gender based prevalence of Whipple’s disease and prevalence of associated diagnoses using univariate analysis.
Results:
A total of 35,838,070 individuals were active in the database between November 2012 and November 2017. Of these, 350 individuals had a SNOMED-CT diagnosis of Whipple’s disease, with an overall prevalence of 9.8 cases per 1 million. There was no difference in prevalence based on sex. However, prevalence of Whipple’s disease was higher in Caucasians, non-Hispanics, and individuals >65 years old. Individuals with a diagnosis of Whipple’s disease were more likely to have associated diagnoses/findings of arthritis, CNS disease, endocarditis, diabetes, malignancy, dementia, vitamin D deficiency, iron deficiency, chemotherapy, weight loss, abdominal pain, and lymphadenopathy.
Conclusions:
To our knowledge, this is the largest study to date examining the epidemiology of Whipple’s disease. In this large population-based study, the overall prevalence of Whipple’s disease in the US is 9.8 cases per 1 million people. It affects men and women at similar rates and is more common in Caucasians, non-Hispanics, and people >65 years old.
Keywords: Whipple’s Disease, Tropheryma whipplei, Epidemiology, GI tract infections
Introduction:
Whipple’s disease is a rare infection characterized by arthalgias, weight loss, diarrhea, and abdominal pain caused by the bacteria Tropheryma whipplei (1). T. whipplei, is ubiquitous in the environment. The bacterium has been detected in sewage and is more prevalent in the fecal samples of sewage workers than the general population (2–3). The pathogenesis of Whipple’s disease remains obscure and asymptomatic carriage is common (4–8). However, some hypothesize that underlying host immune deficiency and secondary immune downregulation induced by the bacterium play a role in the development of clinical disease (9–24).
It is often reported that the case prevalence of Whipple’s disease is 1 in 1,000,000 (25) and that the disease primarily affects middle-aged Caucasian men (26–27). However, these studies have been limited by small sample sizes, case series design, and referral bias. In contrast to US data, data from Germany actually shows there is a higher case prevalence among women (28). To date, there has been no large population-based epidemiologic study of Whipple’s disease. Given the limitations in prior studies, we sought to describe the epidemiology of Whipple’s disease in the United States over a 5- year period between 2012 and 2017 by using a population-based database. The aims of the study were to identify cases of Whipple’s disease, identify diagnoses and findings associated with the diagnosis of Whipple’s disease, and estimate overall prevalence of Whipple’s disease in the United States and among different age-, race- and gender-based subgroups. These data will help better define the epidemiology of Whipple’s disease.
Methods:
Database
We performed a retrospective analysis of a large population-based, commercial database (Explorys Inc, Cleveland, OH). This database contains an aggregate of Electronic Health Record data from 26 major integrated healthcare systems spread over 50 states in the United States from 1999 to 2017. Explorys contains de-identified patient data from participating institutions and uses a health data gateway server behind the firewall of each participating healthcare organization that collects de-identified data from various health information systems- Electronic Health Record using billing inquiries. Data are then standardized and normalized by Explorys. Diagnoses and findings are mapped into the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) hierarchy. Each participating healthcare institution has access to Explorys online (password protected), which provides from browsing of the data from all participating healthcare institutions. Explorys data are automatically updated at least once every 24 hours. Explorys is a Health Insurance Portability and Accountability Act- complaint platform and thus Institutional Review Board is not required.
Patient Selection
Using the Explorys search tool, we identified an aggregated patient cohort of eligible patients with Whipple’s disease between November 2012 and November 2017. Whipple’s disease patients were defined as those having a SNOMED CT diagnosis of “Whipple’s disease”.
Associated Medical Conditions of Interest
We identified multiple medical conditions and findings associated with Whipple’s disease as demonstrated by prior studies (1, 25, 29–39). Data on these conditions were extracted using SNOMED CT diagnostic terms for these disorders and findings. The associated diagnoses studied included arthritis, CNS disease, endocarditis, diabetes, malignancy, dementia, Vitamin D deficiency, seizures, iron deficiency, HIV, pericarditis, myocarditis, and Vitamin B12 deficiency. Associated findings studied included immunosuppression, chemotherapy, weight loss, abdominal pain, lymphadenopathy, HLA B27, skin hyperpigmentation, hypoalbuminemia, and ataxia. We restricted our analysis on associated diagnoses to a 3-year period surrounding the Whipple’s disease diagnosis to examine co-morbidities within a more restricted time frame.
Statistical Analysis
For patients with Whipple’s disease, demographics and associated diseases and findings were characterized by descriptive statistics. Univariate analysis was performed to assess the differences in the prevalence of associated medical conditions in patients with Whipple’s disease and control patients (patients in the database between November 2012 and November 2017 without diagnosis of Whipple’s disease) by calculating odds ratios (ORs) and 95% confidence intervals (CIs). For calculation of overall prevalence, we identified all patients in the database with Whipple’s disease from November 2012 to November 2017. We then divided this number by the total number of patients in the database (from November 2012 to November 2017). Similarly, age-, gender-, race-, and ethnicity-based prevalence rates were calculated. The OR and the 95% CI were calculated according to Altman (40) using the MedCalc Statistical Software (41) with a case-control design.
Results:
A total of 35,838,070 individuals in the database from November 2012 to November 2017 made up the source population. Of these, 350 patients had a SNOMED CT diagnosis of Whipple’s disease, with an overall prevalence of 9.8 cases per 1 million individuals. Forty individuals had a first ever diagnosis of Whipple’s disease in the last year of the study period, making the one-year period prevalence of first ever diagnosis of Whipple’s disease at 1.1 per 1 million people.
In sensitivity analyses, of the 350 cases of Whipple’s disease found in this study, 270 (77%) of cases had at least 2 counts of Whipple’s disease diagnosis while 80 (23%) cases had only one count of Whipple’s disease diagnosis. This is important since those with multiple coded encounters for Whipple’s disease likely have a lower risk of diagnosis miscoding.
Of the 350 individuals with Whipple’s disease, there was no significant difference in case prevalence with regards to sex. The prevalence in males was 10.6 cases per 1 million compared to females at 9.6 cases per 1 million (OR 1.1; 95% CI 0.9–1.4; p= 0.4). However, the prevalence differed according to race, ethnicity, and age. Prevalence in Caucasians was 13.9 cases per 1 million compared to 7.6 cases per 1 million in African Americans (OR 1.82; 95% CI 1.3–2.7; p= 0.002). With regards to ethnicity, data was available for Hispanic and non-Hispanic individuals. Prevalence in Hispanics was 7.8 cases per 1 million compared to 13.7 cases per 1 million in non-Hispanics (OR 0.6; 95% CI 0.4–0.9; p=0.02). Prevalence was 7.9 cases per 1 million in individuals ≤65 years old compared to 24.4 cases per 1 million in individuals >65 years old (OR 0.3; 95% CI 0.3 – 0.4; p< 0.0001) (Figure 1). Moreover, the prevalence increased with age until 85 and then decreased (Figure 2).
Figure 1.
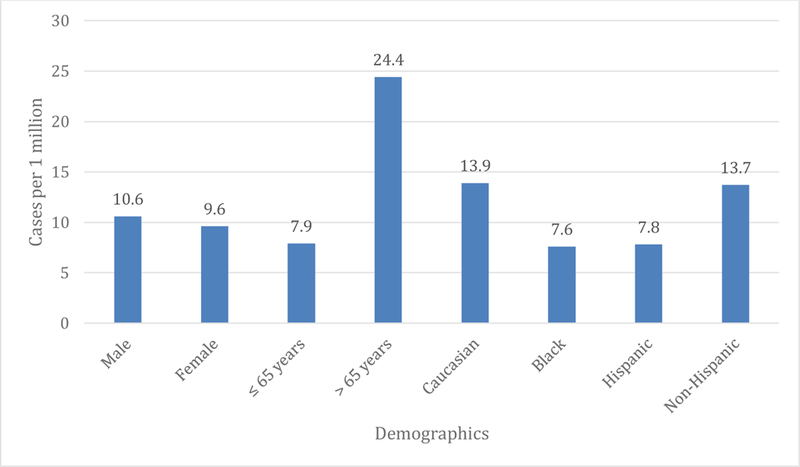
Demographics of Whipple’s cases.
Figure 2.
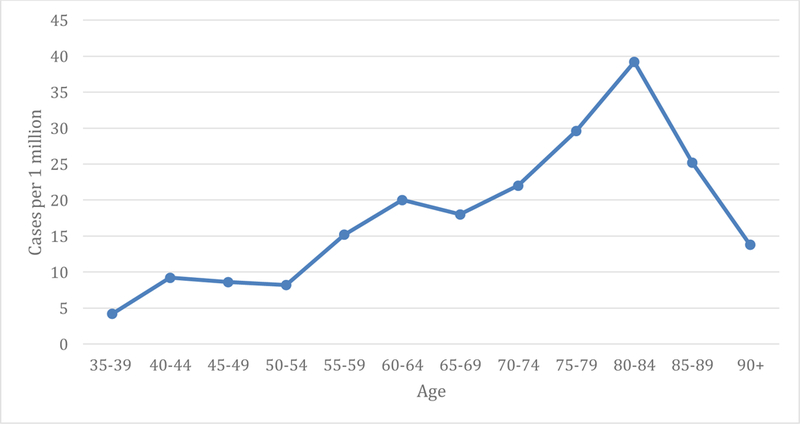
Number of Whipple’s Disease cases by age.
Within the cohort of patients with Whipple’s disease, 10 of the associated diagnoses and findings examined had a case prevalence of 0%. These diagnoses and findings with a 0% case prevalence included HIV, pericarditis, myocarditis, vitamin B12 deficiency, HLA B27, skin hyperpigmentation, hypoalbuminemia, ataxia, seizures, and immunosuppression. All other associated diagnoses and findings were significantly associated with the diagnosis of Whipple’s disease. Of patients with Whipple’s disease, arthritis was present in 37% (OR 4.0; 95% CI 3.3–5.0; p<0.0001), diarrhea was present in 23% (OR 11; 95% CI 9.2–15; p<0.0001), CNS disease was present in 66% (OR 17; 95% CI 14–22; p<0.0001), endocarditis was present in 5.7% (OR 22; 95% CI 14–34; p<0.0001), diabetes was present in 29% (OR 7.0; 95% CI 5.5–8.8; p<0.0001), malignancy was present in 43% (OR 8.5; 95% CI 6.9–11; p<0.0001), chemotherapy was present in 14% (OR 7.53; 95% CI 5.6–10.; p<0.0001), weight loss was present in 14% (OR 22; 95% CI 17–30.; p<0.0001), abdominal pain was present in 31% (OR 6.5; 95% CI 5.2–8.1; p<0.0001), lymphadenopathy was present in 5.7% (OR 8.3; 95% CI 5.3–13; p<0.0001), dementia was present in 5.7% (OR 6.6; 95% CI 4.2–10.; p<0.0001), vitamin D deficiency was present in 17% (OR 6.0; 95% CI 4.6–7.9; p<0.0001), and iron deficiency was present in 11% (OR 12; 95% CI 8.4–16; p<0.0001) (Table 1).
Table 1.
Associated diagnoses and findings. P<0.0001 for all odds ratios. See appendix for listing of SNOMED CT codes included for each association.
| Associations | n | % | Odds Ratio |
|---|---|---|---|
| Arthritis | 130 | 37% | 4.0 (3.3–5.0) |
| Diarrhea | 80 | 23% | 12 (9.2–15) |
| CNS Disease | 230 | 66% | 17 (14–22) |
| Immun osuppression | 0 | 0% | * |
| Endocarditis | 20 | 5.7% | 22 (14–34) |
| HLA B27 | 0 | 0% | * |
| HIV | 0 | 0% | * |
| Diabetes | 100 | 29% | 7.0 (5.5–8.8) |
| Malignancy | 150 | 43% | 8.5 (6.9–11) |
| Chemotherapy | 50 | 14% | 7.5 (5.6–10.) |
| Weight loss | 50 | 14% | 22 (17– 30.) |
| Abdominal Pain | 110 | 31% | 6.5 (5.2–8.1) |
| Lymphadenopathy | 20 | 6% | 8.3 (5.3–13.1) |
| Dementia | 20 | 6% | 6.6 (4.2–10.4) |
| Pericarditis | 0 | 0% | * |
| Myocarditis | 0 | 0% | * |
| Vitamin D Deficiency | 60 | 17% | 6.0 (4.2–10.) |
| Vitamin B12 Deficiency | 0 | 0% | * |
| Skin Hyperpigmentation | 0 | 0% | * |
| Hypoalbuminemia | 0 | 0% | * |
| Seizures | 0 | 0% | * |
| Ataxia | 0 | 0% | * |
| Iron Deficiency | 40 | 11% | 12 (8.4–16) |
In order ascertain whether the original pre-selected co-morbidities as noted above were actually associated with Whipple’s disease or were an artifact of a large sample size, we selected three diagnoses not known to be related to Whipple’s disease and that would be unlikely to be a confused with Whipple’s disease: Schizophrenia, Grave’s disease, and ventricular tachycardia. None of the individuals with Whipple’s disease had SNOMED-CT diagnoses for the above three medical conditions.
Discussion:
In this study, we evaluated the prevalence of Whipple’s disease over 5 years in the Explorys database between 2012 and 2017. To our knowledge, this is the largest study to date that estimates the prevalence of this condition in the United States at the national level. This is also the first large study to describe race-, age- and sex-based prevalence of Whipple’s disease in the United States from national-level data. Additionally, we examined prevalence of diagnoses and findings associated with Whipple’s disease.
Whipple’s disease is rare, though there has been no valid estimate of its actual prevalence prior to the current study (26). A case prevalence of 1/1,000,000 is often quoted (25). However, this estimate is an extrapolation based on an estimated 15002000 Whipple’s disease cases from the time of its discovery up until 1985 (42). While less precise, more reliable data from postmortem studies estimates the frequency of the disease is less than 0.1% (43). In this large population based national study, we estimated the overall prevalence of Whipple’s disease to be 9.8/1,000,000. Given that in our study, we had large numbers of Whipple’s disease cases and total number of individuals in the database, our estimate of prevalence may be more precise than prior estimates. However, a direct comparison with the estimate derived from Dobbins (42) would not be possible because of the difference in the time period over which the prevalence was estimated.
In the current study, we found that Whipple’s disease affects men and women at similar rates. This finding is in contrast to the widely held belief that Whipple’s disease mainly affects men (42, 44). Dobbins (27) performed a meta-analysis of a total of 696 cases of Whipple’s disease, 617 of which had been published in the literature up to that time.
Eighty-six percent of these cases were in men (27). Puechal (44) described 231 reported cases of Whipple’s disease and found that 85% of cases were in men. Both of these prior studies are limited by case series design and potential selection bias. Von Herbay et al. (28) examined data from 110 Whipple’s disease cases reported in Germany from 1965 – 1995, finding that throughout the time period of the study there was an increasing proportion of women diagnosed with Whipple’s from 4% at the onset of the study to 22% at its conclusion 30 years later. The current study found that prevalence of Whipple’s disease was similar between men and women which can be explained by the possibility that true prevalence has been increasing in females or that historically Whipple’s disease was underdiagnosed in women.
Whipple’s disease has been thought to be a disease affecting individuals in middle age (26, 44). Dobbins (27) found a mean age at diagnosis of 49 years. Similarly, Puechal (44) found a mean age at diagnosis of 50.4 years old. However, von Herbay (1997) found there was a significant increase in the mean age of patients throughout the time period of their study. The mean age of patients was 48.7 +/− 3.98 years from 1965–75 and mean age increased to 57.0 +/− 2.80 years at the conclusion of the study, from 1986–95. In the current study, we are unable to calculate a mean age of diagnosis because the Explorys database categorizes individuals by age group, rather than raw age. While this limitation exists in our data, prior studies are limited in their case series design and potential selection bias.
In the current study, older age was associated with an increased risk of Whipple’s disease. Prevalence was 7.9 cases per 1 million in individuals <65 years old compared to 24.4 cases per 1 million in individuals >65 years old. Case prevalence of Whipple’s disease peaked at 39.2 cases per 1 million in the 80–84 year old age group (Figure 2). It has been hypothesized that Whipple’s disease may be affecting individuals later in life due to our increased exposure to antibiotics in the modern era which may mask clinical Whipple’s disease for a period of time (28). The increase in age in Whipple’s disease cases in the current study could also reflect the shifting demographics of society in which a growing proportion of the population is >65 years old. It is also possible that elderly individuals are more likely to have the immune milieu necessary for Whipple’s disease to manifest.
While we did not look at region-wise burden of Whipple’s disease, it is safe to assume that regional differences in gender, age and race distribution cannot alone account for our findings of Caucasian, non-Hispanic and elderly (>65 years) predominance of Whipple’s disease. Furthermore, health institutions affiliated with Explorys cover all 50 states and span the East, Midwest, South, Central and West divisions of the US [45], thus providing a broad regional and climatic distribution of source population. Further genetic, environmental and behavioral studies are needed to understand the epidemiology of Whipple’s disease.
To our knowledge, there has only been one large case series to examine the association between race and Whipple’s disease. Dobbins (27) found that 97% of individuals in his large case series were Caucasian. In the current study, we found that the prevalence of Whipple’s disease is higher in Caucasians as compared to African Americans (13.9 versus 7.6 cases per 1 million respectively) and the prevalence is lower in Hispanics compared to non-Hispanics (7.8 versus 13.7 cases per 1 million respectively). As such, the current study provides further evidence that Whipple’s disease is more commonly diagnosed in Caucasians but is still not uncommon in African-Americans and Hispanics.
This is the first study to examine diagnoses and findings associated with Whipple’s disease. Within the cohort of patients with Whipple’s disease, ten of the associated diagnoses and findings examined had a case prevalence of 0% including HIV, pericarditis, myocarditis, vitamin B12 deficiency, HLA B27, skin hyperpigmentation, hypoalbuminemia, ataxia, seizures, and immunosuppression. The 0% prevalence of these previously associated diagnoses and findings can be attributed to several factors. Firstly, if an associated diagnosis or findings is rare (HIV for example) we may not have picked up an association in a sample of 350 Whipple’s disease cases. Secondly, some associations are rarely tested for (HLA B27 for example), so although there may have been individuals with HLA B27 within our study population, they may not have been identified. Thirdly, it is possible that some findings are less likely to have been coded with SNOMED CT codes. All other associated diagnoses and findings were significantly associated with the diagnosis of Whipple’s disease including arthritis, diarrhea, CNS disease, endocarditis, diabetes, malignancy, chemotherapy, weight loss, abdominal pain, lymphadenopathy, dementia, and iron deficiency. These associations can aid in identification of individuals who have a higher pre-test probability of Whipple’s disease, and thus may be more likely to benefit from further diagnostic testing.
This study has a few limitations that should be acknowledged. First, regarding the estimate of Whipple’s disease prevalence rates, we may have underestimated the true prevalence of disease because Whipple’s disease is difficult to diagnose and some true cases of Whipple’s disease may have been classified as other gastrointestinal or rheumatologic disorders. Validation of the SNOMED CT diagnostic code for Whipple’s disease was not possible because the patient information in the database is de-identified. However, although the ICD-9 and SNOMED CT are both medical terminology systems for recording medical diagnoses and concepts, SNOMED CT has many more concepts to be coded per clinical document than the ICD-9 (46) that makes it more accurate in terms of enlisting pertinent clinical information. Besides misclassification, another limitation of this study is the inability to capture information that is unavailable in the Explorys database. This unavailable information includes socioeconomic status and geographic data on patient population, including the regional distribution of Whipple’s disease, endoscopic abnormalities, and histology reports. Moreover, although Explorys uses a master patient identifier to match the same patient across different healthcare institutions and combine the data, (47) a few patients might have received care in multiple institutions within Explorys healthcare partners and thus could have been counted multiple times. However, this is countered by the fact that Explorys uses a robust patient- matching algorithm (47) and thus the effect of this error might be minimal and may affect the Whipple’s disease and control groups equally.
In summary, the analysis of the only population based national sample of Whipple’s disease cases thus far, from the large commercial database Explorys, estimates the overall prevalence of Whipple’s disease at 9.8/1,000,000. This estimate is larger than what has previously been extrapolated from case series. Consistent with prior case series data, we found that the prevalence of Whipple’s disease is higher in Caucasians as compared to African Americans. In contrast to prior data, though, we found that the prevalence of Whipple’s disease is similar between males and females and more common in individuals >65 years old.
Current Knowledge and What is New.
-
-
Whipple’s disease is a rare infection.
-
-
Previously no good estimate of its prevalence
-
-
Prior case series show Whipple’s disease is more common in white, middle-aged men.
-
-
Current study estimates overall prevalence of 9.8 cases per 1 million
-
-
Current study shows that Whipple’s disease is more common in Caucasians, but that it is still prevalent in African Americans and Hispanics
-
-
Current study shows that prevalence of Whipple’s disease is similar between men and women
-
-
Current study shows that prevalence of Whipple’s disease rises until age group 80–84, and is more common in people > 65 years old
-
-
Current study shows association between Whipple’s disease and arthritis, diarrhea, CNS disease, endocarditis, diabetes, malignancy, chemotherapy, weight loss, abdominal pain, lymphadenopathy, dementia, vitamin D deficiency, and iron deficiency
Abbreviations:
- SNOMED CT
systematized nomenclature of medicine clinical terms
- CNS
central nervous system
- HIV
human immunodeficiency virus
- HLA
human leukocyte antigen
- OR
odds ratio
- CI
confidence interval
- ICD-9
international classification of diseases−9
Appendix:
Abdominal pain included SNOMED CT codes for abdominal pain, generalized abdominal pain, chronic abdominal pain, acute abdominal pain. Malignancy included SNOMED CT codes for malignant neoplastic disease, neoplastic disease, primary malignant neoplasm. Seizure included SNOMED CT codes for seizure, seizure disorder, generalized seizure, partial seizure, epileptic seizure, and seizure related finding. Iron deficiency included SNOMED CT codes for iron deficiency and iron deficiency anemia. Ataxia included SNOMED CT codes for ataxia and cerebellar ataxia. Skin hyperpigmentation included SNOMED CT codes for disorder of skin pigmentation. Hypoalbuminemia included SNOMED CT codes for hypoalbuminemia. Diabetes included SNOMED CT codes for diabetes mellitus, diabetes mellitus without complication, type 2 diabetes mellitus, type 1 diabetes mellitus. Vitamin B 12 deficiency included SNOMED CT codes for Vitamin B12 deficiency (non-anemic) and Cobalamin deficiency. Vitamin D deficiency included SNOMED CT codes for Vitamin D deficiency. Myocarditis included SNOMED CT codes for myocarditis, acute myocarditis, viral myocarditis, bacterial myocarditis. Pericarditis included SNOMED CT codes for pericarditis. Dementia included SNOMED CT codes for dementia, presenile dementia, senile dementia, uncomplicated senile dementia, uncomplicated presenile dementia, senile dementia with delirium, and presenile dementia with delirium. Lymphadenopathy included SNOMED CT codes for lymphadenopathy, cervical lymphadenopathy, thoracic lymphadenopathy, pelvic lymphadenopathy, mediastinal lymphadenopathy, hilar lymphadenopathy, lymphadenopathy of head and/or neck. Chemotherapy included SNOMED CT codes for chemotherapy, antineoplastic agent, intravenous chemotherapy, intramuscular chemotherapy, subcutaneous chemotherapy. Weight loss included SNOMED CT codes for abnormal weight loss, weight loss finding, weight decreased. HIV included SNOMED CT codes for human immunodeficiency virus infection and HIV positive. Arthritis included SNOMED CT codes for arthritis, arthralagia of upper arm, arthropathy, arthralgia of the lower leg, arthralgia of the pelvic region and thigh, arthralgia of the ankle and/or foot. CNS disease included SNOMED CT codes for disorder of the nervous system. Immunosuppression included SNOMED CT codes for immunosuppressant, immunosuppressives, immunomodulator, immunotherapeutic agent. Diarrhea included SNOMED CT codes for diarrheal disorder, diarrhea, chronic diarrhea, diarrhea and vomiting, acute diarrhea, infectious diarrheal disease, functional diarrhea, nausea/vomiting/and diarrhea, irritable bowel syndrome with diarrhea. Endocarditis included SNOMED CT codes for endocarditis or endocardial disease. HLA-B27 included SNOMED CT codes for HLA-B27.
Footnotes
Conflicts of interest: There are no potential conflicts (financial, professional, or personal) to disclose by all the authors (Jamie Ann Elchert, Emad Mansoor, Mohannad Abou-Saleh and Gregory S. Cooper).
Writing Assistance: There is no writing assistance to disclose.
Contributor Information
Jamie Ann Elchert, Department of Internal Medicine, University Hospitals Cleveland Medical Center, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, Ohio 44106, Jamie.Elchert@UHhospitals.org.
Emad Mansoor, Department of Internal Medicine, University Hospitals Cleveland Medical Center, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, Ohio 44106.
Mohannad Abou-Saleh, Department of Internal Medicine, University Hospitals Cleveland Medical Center, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, Ohio 44106.
Gregory S. Cooper, Department of Internal Medicine, University Hospitals Cleveland Medical Center, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, Ohio 44106
References
- 1.Durand DV, Lecomte C, Cathebras P, Rousset H, & Godeau P (1997). Whipple disease. Clinical review of 52 cases. The SNFMI research group on whipple disease. Societe nationale francaise de medecine interne. Medicine, 76(3), 170–184. [DOI] [PubMed] [Google Scholar]
- 2.Schoniger-Hekele M, Petermann D, Weber B, & Muller C (2007). Tropheryma whipplei in the environment: Survey of sewage plant influxes and sewage plant workers. Applied and Environmental Microbiology, 73(6), 2033–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenollar F, Trani M, Davoust B, Salle B, Birg ML, Rolain JM, et al. (2008). Prevalence of asymptomatic tropheryma whipplei carriage among humans and nonhuman primates. The Journal of Infectious Diseases, 197(6), 880–887. [DOI] [PubMed] [Google Scholar]
- 4.Ehrbar HU, Bauerfeind P, Dutly F, Koelz HR, & Altwegg M (1999). PCR-positive tests for tropheryma whippelii in patients without whipple’s disease. Lancet (London, England), 353(9171), 2214–6736(99)01776–6. [DOI] [PubMed] [Google Scholar]
- 5.Street S, Donoghue HD, & Neild GH (1999). Tropheryma whippelii DNA in saliva of healthy people. Lancet (London, England), 354(9185), 1178–1179. [DOI] [PubMed] [Google Scholar]
- 6.Zinkernagel AS, Gmur R, Fenner L, Schaffner A, Schoedon G, & Schneemann M (2003). Marginal and subgingival plaque--a natural habitat of tropheryma whipplei? Infection, 31(2), 86–91. [DOI] [PubMed] [Google Scholar]
- 7.Dutly F, & Altwegg M (2001). Whipple’s disease and “tropheryma whippelii”. Clinical Microbiology Reviews, 14(3), 561–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amsler L, Bauernfeind P, Nigg C, Maibach RC, Steffen R, & Altwegg M (2003). Prevalence of tropheryma whipplei DNA in patients with various gastrointestinal diseases and in healthy controls. Infection, 31(2), 81–85. [DOI] [PubMed] [Google Scholar]
- 9.Marth T, Roux M, von Herbay A, Meuer SC, & Feurle GE (1994). Persistent reduction of complement receptor 3 alpha-chain expressing mononuclear blood cells and transient inhibitory serum factors in whipple’s disease. Clinical Immunology and Immunopathology, 72(2), 2 1 7–2 2 6. [DOI] [PubMed] [Google Scholar]
- 10.Ectors N, Geboes K, De Vos R, Heidbuchel H, Rutgeerts P, Desmet V, et al. (1992). Whipple’s disease: A histological, immunocytochemical and electronmicroscopic study of the immune response in the small intestinal mucosa. Histopathology, 21(1), 1–12. [DOI] [PubMed] [Google Scholar]
- 11.Marth T, Neurath M, Cuccherini BA, & Strober W (1997). Defects of monocyte interleukin 12 production and humoral immunity in whipple’s disease. Gastroenterology, 113(2), 442–448. [DOI] [PubMed] [Google Scholar]
- 12.Marth T, Kleen N, Stallmach A, Ring S, Aziz S, Schmidt C, et al. (2002). Dysregulated peripheral and mucosal Th1/Th2 response in whipple’s disease. Gastroenterology, 123(5), 1468–1477. [DOI] [PubMed] [Google Scholar]
- 13.Schneider T, Stallmach A, von Herbay A, Marth T, Strober W, & Zeitz M (1998). Treatment of refractory whipple disease with interferon-gamma. Annals of Internal Medicine, 129(11), 875–877. [DOI] [PubMed] [Google Scholar]
- 14.Ectors NL, Geboes KJ, De Vos RM, Heidbuchel HP, Rutgeerts PJ, Desmet VJ, et al. (1994). Whipple’s disease: A histological, immunocytochemical, and electron microscopic study of the small intestinal epithelium. The Journal of Pathology, 172(1), 73–79. [DOI] [PubMed] [Google Scholar]
- 15.Bai JC, Sen L, Diez R, Niveloni S, Maurino EC, Estevez ME, et al. (1996). Impaired monocyte function in patients successfully treated for whipple’s disease. Acta Gastroenterologica Latinoamericana, 26(2), 85–89. [PubMed] [Google Scholar]
- 16.Moos V, Schmidt C, Geelhaar A, Kunkel D, Allers K, Schinnerling K, et al. (2010). Impaired immune functions of monocytes and macrophages in whipple’s disease. Gastroenterology, 138(1), 210–220. [DOI] [PubMed] [Google Scholar]
- 17.Maiwald M, von Herbay A, Persing DH, Mitchell PP, Abdelmalek MF, Thorvilson JN, et al. (2001). Tropheryma whippelii DNA is rare in the intestinal mucosa of patients without other evidence of whipple disease. Annals of Internal Medicine, 134(2), 115–119. [DOI] [PubMed] [Google Scholar]
- 18.Schinnerling K, Moos V, Geelhaar A, Allers K, Loddenkemper C, Friebel J, et al. (2011). Regulatory T cells in patients with whipple’s disease. Journal of Immunology (Baltimore, Md.: 1950), 187(8), 4061–4067. [DOI] [PubMed] [Google Scholar]
- 19.Schinnerling K, Geelhaar-Karsch A, Allers K, Friebel J, Conrad K, Loddenkemper C, et al. (2015). Role of dendritic cells in the pathogenesis of whipple’s disease. Infection and Immunity, 83(2), 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desnues B, Lepidi H, Raoult D, & Mege JL (2005). Whipple disease: Intestinal infiltrating cells exhibit a transcriptional pattern of M2/alternatively activated macrophages. The Journal of Infectious Diseases, 192(9), 1642–1646. [DOI] [PubMed] [Google Scholar]
- 21.Desnues B, Ihrig M, Raoult D, & Mege JL (2006). Whipple’s disease: A macrophage disease. Clinical and Vaccine Immunology: CVI, 13(2), 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desnues B, Raoult D, & Mege JL (2005). IL-16 is critical for tropheryma whipplei replication in whipple’s disease. Journal of Immunology (Baltimore, Md.: 1950), 175(7), 4575–4582. [DOI] [PubMed] [Google Scholar]
- 23.Moos V, Kunkel D, Marth T, Feurle GE, LaScola B, Ignatius R, et al. (2006). Reduced peripheral and mucosal tropheryma whipplei-specific Th1 response in patients with whipple’s disease. Journal of Immunology (Baltimore, Md.: 1950), 177(3), 2015–2022. [DOI] [PubMed] [Google Scholar]
- 24.Trotta L, Weigt K, Schinnerling K, Geelhaar-Karsch A, Oelkers G, Biagi F, et al. (2017). Peripheral T-cell reactivity to heat shock protein 70 and its cofactor GrpE from tropheryma whipplei is reduced in patients with classical whipple’s disease. Infection and Immunity, 85(8), 10.1128/IAI.00363-17. Print 2017 August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider T, Moos V, Loddenkemper C, Marth T, Fenollar F, & Raoult D (2008). Whipple’s disease: New aspects of pathogenesis and treatment. The Lancet.InfectiousDiseases, 8(3), 179–190. [DOI] [PubMed] [Google Scholar]
- 26.Fenollar F, Puechal X, & Raoult D (2007). Whipple’s disease. The New England Journal of Medicine, 356(1), 55–66. [DOI] [PubMed] [Google Scholar]
- 27.Dobbins WO III. Whipple’s Disease, Charles C Thomas, Springfield 1987 [Google Scholar]
- 28.von Herbay A, Otto HF, Stolte M, Borchard F, Kirchner T, Ditton HJ, et al. (1997). Epidemiology of whipple’s disease in germany. analysis of 110 patients diagnosed in 1965–95. Scandinavian Journal of Gastroenterology, 32(1), 52–57. [DOI] [PubMed] [Google Scholar]
- 29.Marth T (2015). Systematic review: Whipple’s disease (tropheryma whipplei infection) and its unmasking by tumour necrosis factor inhibitors. Alimentary Pharmacology & Therapeutics, 41(8), 709–724. [DOI] [PubMed] [Google Scholar]
- 30.Lagier JC, Lepidi H, Raoult D, & Fenollar F (2010). Systemic tropheryma whipplei: Clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine, 89(5), 337–345. [DOI] [PubMed] [Google Scholar]
- 31.Moos V, & Schneider T (2011). Changing paradigms in whipple’s disease and infection with tropheryma whipplei. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology, 30(10), 1151–1158. [DOI] [PubMed] [Google Scholar]
- 32.Gunther U, Moos V, Offenmuller G, Oelkers G, Heise W, Moter A, et al. (2015). Gastrointestinal diagnosis of classical whipple disease: Clinical, endoscopic, and histopathologic features in 191 patients. Medicine, 94(15), e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori K, Ando I, & Kukita A (2001). Generalized hyperpigmentation of the skin due to vitamin B12 deficiency. The Journal of Dermatology, 28(5), 282–285. [DOI] [PubMed] [Google Scholar]
- 34.Louis ED, Lynch T, Kaufmann P, Fahn S, & Odel J (1996). Diagnostic guidelines in central nervous system whipple’s disease. Annals of Neurology, 40(4), 561–568. [DOI] [PubMed] [Google Scholar]
- 35.Matthews BR, Jones LK, Saad DA, Aksamit AJ, & Josephs KA (2005). Cerebellar ataxia and central nervous system whipple disease. Archives of Neurology, 62(4), 618–620. [DOI] [PubMed] [Google Scholar]
- 36.Elkins C, Shuman TA, & Pirolo JS (1999). Cardiac whipple’s disease without digestive symptoms. The Annals of Thoracic Surgery, 67(1), 250–251. [DOI] [PubMed] [Google Scholar]
- 37.Gubler JG, Kuster M, Dutly F, Bannwart F, Krause M, Vogelin HP, et al. (1999). Whipple endocarditis without overt gastrointestinal disease: Report of four cases. Annals of Internal Medicine, 131(2), 112–116. [DOI] [PubMed] [Google Scholar]
- 38.Fenollar F, Lepidi H, & Raoult D (2001). Whipple’s endocarditis: Review of the literature and comparisons with Q fever, bartonella infection, and blood culturepositive endocarditis. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 33(8), 1309–1316. [DOI] [PubMed] [Google Scholar]
- 39.Lepidi H, Fenollar F, Dumler JS, Gauduchon V, Chalabreysse L, Bammert A, et al. (2004). Cardiac valves in patients with whipple endocarditis: Microbiological, molecular, quantitative histologic, and immunohistochemical studies of 5 patients. The Journal of Infectious Diseases, 190(5), 935–945. [DOI] [PubMed] [Google Scholar]
- 40.Altman D (1991). Practical statistics for medical research. London, UK: Chapman and Hall. [Google Scholar]
- 41.MedCalc. (2016). Odds ratio calculator. Retrieved December/5, 2017, from https://www.medcalc.org/calc/odds_ratio.php
- 42.Dobbins WO III. Whipple’s disease: an historical perspective. Q J Med. 1985. September;56 (221):523–531. [PubMed] [Google Scholar]
- 43.Enzinger FM, & Helwig EB (1963). Whipple’s disease. [Whipple’s Disease] Virchows Arch. Path Anat, 336(238). [Google Scholar]
- 44.Puechal X (2001). Whipple disease and arthritis. Current Opinion in Rheumatology, 13(1), 74–79. [DOI] [PubMed] [Google Scholar]
- 45.Explorys team. “We unlock the power of BIG DATA to improve healthcare for everyone”. Explorys. 2015. Accessed March 15, 2018. <https://www.explorys.com/about-us.html>. [Google Scholar]
- 46.Nadkarni PM, & Darer JA (2010). Migrating existing clinical content from ICD-9 to SNOMED. Journal of the American Medical Informatics Association : JAMIA, 17(5), 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaelber DC, Foster W, Gilder J, Love TE, & Jain AK (2012). Patient characteristics associated with venous thromboembolic events: A cohort study using pooled electronic health record data. Journal of the American Medical Informatics Association: JAMIA, 19(6), 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]


