Abstract
Background.
Isovaleric Acid (IVA) is a 5-carbon branched chain fatty acid present in fermented foods and produced in the colon by bacterial fermentation of leucine. We previously reported that the shorter, straight chain fatty acids acetate, propionate and butyrate, differentially affect colonic motility; however, the effect of branched chain fatty acids on gut smooth muscle and motility is unknown.
Aims.
To determine the effect of IVA on contractility of colonic smooth muscle.
Methods.
Murine colonic segments were placed in a longitudinal orientation in organ baths in Krebs buffer and fastened to force transducers. Segments were contracted with acetylcholine (ACh) and the effects of IVA on ACh-induced contraction were measured in the absence and presence of tetrodotoxin (TTx) or inhibitors of nitric oxide synthase (L-N-nitroarginine (L-NNA)) or adenylate cyclase (SQ22536). The effect of IVA on ACh-induced contraction was also measured in isolated muscle cells in the presence or absence of SQ22536 or protein kinase A (PKA) inhibitor (H-89). Direct activation of PKA was measured in isolated muscle cells.
Results.
In colonic segments, ACh-induced contraction was inhibited by IVA in a concentration-dependent fashion; the IVA response was not affected by TTx or L-NNA but inhibited by SQ22536. Similarly, in isolated colonic muscle cells, AChinduced contraction was inhibited by IVA in a concentration-dependent fashion and the effect blocked by SQ22536 and H-89. IVA also increased PKA activity in isolated smooth muscle cells.
Conclusions.
The branched chain fatty acid IVA acts directly on colonic smooth muscle and causes muscle relaxation via the PKA pathway.
Keywords: Colon, Smooth Muscle, Isovaleric Acid, Branched-Chain Fatty Acid, Cyclic AMP, Adenylyl Cyclase, Protein Kinase A, SQ22536, H-89, Tetrodotoxin, Nitric oxide synthase, L-N-nitroarginine
Introduction
Fatty acids are carboxylic acids with an alkyl tail of varying carbon length. Short chain fatty acids (SCFA) have fatty acids with lengths of 2 to 6 carbons, medium chain fatty acids (MCFA) have lengths of 7 to 12 carbons and long chain fatty acids (LCFA) have lengths of 13 to 21 carbons. Branched chain fatty acids (BCFA) possess a fork in the carbon chain of at least a methyl-group or longer.
The primary source of dietary SCFA is fermentation by the resident bacteria of the colon of hydrolysis-resistant starches which pass unabsorbed through the small intestine [1,2]. Short chain fatty acids are present in significant concentrations in feces with actual concentrations ranging widely between individuals based on diet, length along the colon, pH, and microbiota status [1–6]. In general, total concentrations of fatty acids in feces are in the mM range whereas, concentrations in the blood are in the high μM to low mM level. The main SCFA in both feces and blood are the straight chain fatty acids acetate, butyrate, and propionate. Recent interest has been directed at these SCFA because of their potential role in gut function, glucose homeostasis, appetite, regulation of metabolism, inflammation and immune competence, as well as tumorigenesis and colon cancers. These have been the topic of many recent reviews [3,5,7–12].
In contrast to SCFA, BCFA are produced when proteins pass through the small intestine unabsorbed and the branched amino acids valine, isoleucine, and leucine are respectively fermented to isobutyric acid, 2-methylbutyric acid, and isovaleric acid (IVA) [5,6,13–16]. In addition, BCFA are derived from ingested foods especially dairy and beef products [17,18]. While SCFA have been studied in detail and considered beneficial, BCFA and the products of protein fermentation are not well characterized and are generally considered deleterious to the gut. Even though produced in the colon or via ingested foods, IVA is one of the most prominent fatty acids in human blood. Although much less is known about blood levels of BCFA and because concentrations vary based on the diet, methods, time period and sampling in portal, hepatic and peripheral blood, significant concentrations have been measured. In fasting patients in Denmark, Jakobsdottir et al. found that IVA concentration in venous blood from control subjects ranked second at 40 μM, following acetic acid (245 μM), and at higher levels than propionic acid and the other prevalent BCFA isobutyric acid (13 μM) [19]. Blood levels of IVA can rise dramatically in pathological conditions, such as isovaleric acidemia where a genetic defect in the gene for isovaleryl-CoA dehydrogenase produces either a reduced efficiency or ineffective enzyme leading to a buildup of IVA [20]. In one reported case study from Japan, stable patients had IVA levels of up to 78 μM as compared to 6 μM in controls and up to 7960 μM during crisis [21]. In cases of bacterial overgrowth in small intestine, total SCFA concentrations can range from 210 μM to 12 mM and the levels of IVA can range from 2 μM to nearly 700 μM. Consistent with their production in the colon by fermentation, IVA is present in feces in significant amounts. Studies in human feces indicate that while the generation of SCFA predominate in the proximal colon, the contributions of BCFA increase from 17% of the total in proximal colon to 38% in distal colon [3,5]. IVA alone represents approximately 3.5 to 5% of SCFA in feces [22–24]. Specifically, estimates range from 0.8 to 22.0 mmol/Kg feces (wet weight) in different studies and depending on diet [6,25–28]. The significant concentrations of IVA in colon and blood raise the possibility of its intraluminal and/or extraluminal effects.
The effect of SCFA on mucosal health and function has been studied more extensively than the effects on motility although several studies suggest that SCFA increase colonic motility and secretion via stimulation of neural and/or serotonin mediated pathways [29–35]. Direct contractile effects of SCFA on rat colonic smooth muscle have been reported, however, little is known of the effects of BCFA on colonic smooth muscle [36]. The present study examined the effects of IVA, the most prevalent BCFA, on colonic smooth muscle strips and isolated cells. The results show that IVA causes a concentration-dependent inhibition of ACh-evoked contraction (i.e.,relaxation) of smooth muscle from the longitudinal muscle layer, which is mediated by activation of PKA.
METHODS
Materials
Chemicals not specified were acquired from Sigma-Aldrich (St. Louis, MO). Tetrodotoxin, NG-nitro-L-arginine (L-NNA), SQ22536 and H-89 were obtained from Tocris Bio-Techne (Minneapolis, MN). Isovaleric acid was obtained from Sigma-Aldrich and dissolved in Krebs buffer (pH 7.2). Eagle’s essential amino acid mixture was obtained from ThermoFisher, (Waltham, MA). Adenosine 5’-triphosphate [γ−32P] 6000 Ci/mmol was acquired from PerkinElmer (Boston, MA). Kemptide was acquired from Cayman Chemical (Ann Arbor, MI).
Animals
Mus muscularis C57BL/6J were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the AAALAC-accredited Division of Animal Resources facility, Virginia Commonwealth University (VCU). Both male and female mice of 6–8 weeks age were used in this study. As no statistical significance in responses was detected between colons from the sexes, the data were combined. Mice were euthanized by CO2 asphyxiation under protocols approved by VCU Institutional Animal Care and Use Committee, and all procedures were in their accordance.
Measurement of contraction and relaxation in colonic segments
The colon was removed, the mesentery trimmed, and the lumen flushed with Krebs buffer warmed to 37°C. Krebs buffer was prepared as follows: 118 mM NaCl, 4.75 mM KCl, 1.19 mM KH2PO4, 1.2 mM MgSO4, 2.54 mM CaCl2 and 25 mM NaHCO3, 11 mM glucose. Krebs buffer was bubbled with 95% O2 and 5% CO2 and adjusted to pH 7.4 before use as a control solution or as the bathing solution.
Segments of approximately 4 mm in length were cut from the colon and tied at both ends with surgical silk suture without occluding the lumen. One end of suture was attached to a glass hook which was submerged in Krebs buffer in a 5 ml water jacketed organ bath maintained at 37°C (Radnoti, Monrovia, CA). The silk suture on the opposite end of the colonic segment was attached to a model FT03C Force Transducer (Grass Technologies, Quincy, MA) which was connected to a Powerlabs 8/35 with Octal Bridge Amp (ADInstruments, Colorado Springs, CO). Continuous data was collected and analyzed with LabChart 8 (ADInstruments) software.
The colon tension was adjusted to an initial tension of 1.0 g and the segment equilibrated for 60 minutes. The Krebs buffer was replaced at 15-minute intervals 3 times before the initial experiment. Isovaleric acid (IVA) was added in single concentrations and the bath was rinsed with at least 3 bath volumes of Krebs buffer at 15-minute intervals between each addition of IVA.
Following equilibration, the viability of the strips was tested by contraction with acetylcholine (ACh) (10 μM). The effects of IVA at different concentrations was tested by precontracting the strip with ACh. When the contraction to ACh was stable (about 10 minutes after addition), IVA was added and the response measured. Vehicle controls for Krebs buffer, citrate buffer, DMSO and water had not significant effect. In some segments, the effects of 50 mM IVA were compared to equal concentrations of acetic acid and propionic acid measured in the same colonic preparation. To test the site and mechanism of action of IVA, some colon segments were pretreated with the neural toxin tetrodotoxin (TTx; 10 μM in citrate buffer), the nitric oxide synthase inhibitor L-NNA (100 μM in water), or the adenylate cyclase inhibitor SQ22536 (10 and 500 μM in DMSO). All inhibitors were added before ACh and were in contact with the segment during measurement of the effect of IVA.
The response to IVA was calculated as the decrease in tension from the stable ACh-induced contraction. The mean tension for a 60 second period in the presence of ACh alone immediately prior to addition of IVA was compared to the mean tension for a 30 second period measured at peak decrease in tone after addition of IVA. The decrease in tone was measured in grams and the percent relaxation induced by IVA was calculated as the percent decrease from the maximum ACh level of tone.
Measurement of contraction and relaxation in dispersed colonic muscle cells
Smooth muscle cells were isolated from colon after dissection by gently scraping off the mucosa and placing strips of colon in HEPES buffer medium containing 25 mM HEPES, 120 mM NaCl, 4 mM KCl, 2.6 mM KH2PO4, 0.6 mM MgCl2, 14 mM glucose and 2.1% Eagle’s essential amino acid mixture [37–39]. After washing the strips with medium, tissue was incubated for 30 minutes at 31 °C in 10 mL 0.1% collagenase II and 0.1% soybean trypsin inhibitor. The tissue was washed with collagenase-free medium and resuspended in collagenase-free medium for an additional 30 minute to allow spontaneous dispersion of cells. Dispersed muscle cells were filtered through 500 μM Nitex mesh.
A cell suspension containing 104 muscle cells/mL was treated with 1 μM ACh for 1 minute to induce contraction. Separate suspensions of cells were incubated with IVA (1–100 μM) for 1 and 5 minutes before addition of ACh and then cell lengths were measured. The percent decrease in cell length in response to ACh was calculated in the presence or absence of IVA (1–100 μM). In some experiments cells were incubated for 10 minutes with SQ22536 (1 and 10 μM) or cAMP-dependent protein kinase (PKA) inhibitor H-89 (10 μM) prior to the addition of ACh and IVA. After treatments, cells were fixed with 1% acrolein and cell lengths were measured by scanning micrometry (Lasico Los Angeles, CA). A decrease in cell length in response to ACh was considered as contraction and inhibition of contraction by IVA was considered as relaxation. A minimum of 50 cells were counted in each treatment group and separate animals were used in each experiment.
Measurement of PKA activation in dispersed colonic muscle cells
Colonic smooth muscle cells were isolated as previously described [41]. The cell suspensions were then treated with 1 μM vasoactive intestinal peptide (VIP) or 10 or 100 μM IVA for 5 minutes. VIP was used as a control peptide well known to activate the cAMP/PKA signaling pathway in gut smooth muscle [41]. The reaction was stopped by quick centrifugation and the pellet was suspended in a lysis buffer consisting of 1% NP40, 0.5% deoxycholic acid, 0.1% SDS, 10 mM sodium pyrophosphate, 150 mM NaCl, 50 mM Tris hydrochloride pH 7.4, and 2% 50X BD BaculoGold protease inhibitor cocktail (Sigma, St Louis, MO). The lysate was then centrifuged at 10,600 g and the supernatant saved and frozen at −80 °C overnight.
To measure PKA activity, 40 μL of cell lysate was mixed with 40 μL of PKA reaction buffer including 50 mM Tris Hydrochloride pH 7.4, 12.5 mM MgCl2, 25 mM KCl, 25 μM DTT, 150 μM Kemptide, and 250 μM ATP and [32P]ATP (6000 Ci/mmol). Reactions were performed in the presence (total activity) or absence (stimulated activity) of 5 μM cAMP. Non-specific activity was measured in the presence of 10 μM PKA inhibitor (myritoylated PKI) and was subtracted from total and stimulated activities. The samples were incubated at 37 °C for 15 minutes, and then 45 μL of sample absorbed onto Whatman Grade P81 filter paper. The filter paper was rinsed 5 times at 20-minute intervals with 75 mM H3PO4 and dried overnight. The samples were then placed in a scintillation vial in 5 mL of ScintiSafe (Fisher Scientific, Waltham, MA) and γ32P measured with a PerkinElmer Tri-Carb 2810 TR Liquid Scintilation Analyzer. PKA activity was calculated and represented as a ratio of counts per minute (CPM) in the absence or presence of cAMP [41].
Immunofluorescence Staining and Imaging
Colonic segments were fixed in 4% paraformaldehyde in PBS for 2 hours.. For antigen retrieval, they were submerged in a solution of 10 mM sodium citrate and 0.05% Tween 20 at pH 6.0 overnight at 4 °C, boiled for 3 minute with gentle stirring, followed by submersion in 30% sucrose in PBS overnight at 4 °C. Tissue was embedded in OCT compound (Tissue-Tek, VWR, Radnor, PA) and frozen at −80 °C, and 10 μM sections cutwith a cryo-microtome. Sections were dried for 1.5 hours at room temperature and kept at 4 °C. Sections were washed 4 times with PBS-Tween 0.5%, twice with PBS, then incubated 30 minute with 5% normal goat serum (Jackson ImmunoResearch, West Grove, PA) in PBS. Sections were then incubated overnight with 1:200 Thermo PA535298 OR51E1 antibody in 5% normal goat serum or in the absence of primary antibody as a control at 4 °C. Slides were washed again 4 times with PBS-Tween 0.5%, twice with PBS, then incubated 1 hour at room temperature with goat anti-rabbit Alexafluor 594 1:100 in 2.5% normal goat serum in PBS, washed 4 times with PBSTween 0.5% and twice with PBS before covering with Fluoroshield DAPI (Abcam, Cambridge, MA).
Slides were imaged with a Zeiss Imager Z1 controlled with ZEN software and using an EXFO X-Cite Series 120 fluorescence illumination source. Slides were imaged with 400 ms exposure for rhodamine filter, 200 ms for DAPI filter. Channels were combined using ImageJ164 (NIH) with FIJI [37], and contrast and brightness kept constant between images.
Statistical Analysis
In colon segment experiments, IVA-induced relaxations were calculated as decrease in tone from maximum ACh-induced increases in tone. Effects of inhibitors on IVA-induced relaxation were compared to responses in the absence of inhibitors. In isolated muscle cell experiments, the decrease in cell length from control response to ACh was compared to the response in the presence of ACh plus IVA alone (representing relaxation) and in the presence of inhibitors. Values are reported as mean ± standard error (segments) or ±σ to indicate standard deviation (isolated cells). Statistically significant difference was determined by one-way ANOVA, N-way ANOVA, or repeated measures ANOVA as applicable followed by Bonferroni test post-hoc when multiple groups compared, paired t-test when only 2 treatments within same segment were compared, and unpaired t-test when two different groups of segments compared. Results were considered significant at P<0.05. Statistical tests were carried out using MATLAB R2018a with the Statistics and Machine Learning Toolbox version 11.3.
RESULTS
Effect of IVA on ACh-induced contraction in colonic segments
As noted earlier, no statistically significant differences in response to ACh or IVA were noted between colons from male and female mice so data from mice of both sexes were combined. Similarly, when responses from proximal, middle, and distal section of the colon were compared, no statistically significant differences between responses of the regions were noted. Thus, data from colonic segments of males and females and from all regions were combined and hereafter referred to as colonic segments or colonic responses.
In colonic segments, ACh (10 μM) induced contraction, which peaked rapidly but then maintained a stable plateau phase for a prolonged period lasting over 10 minutes. The response to IVA was tested by addition of single concentrations of IVA at 10 minutes after addition of ACh. The mean increase in tension in response to ACh at this point was 1.02 ± 0.05 g above basal tone. Addition of IVA at the sustained plateau contraction consistently caused relaxation (i.e., inhibited ACh-induced contraction). The relaxation phase began almost immediately after the addition of IVA, and peak relaxation usually occurred within a minute (Figure 1). The decrease in tension partially recovered over a 20 minute period and was reversed to basal levels by repeated washing with fresh Krebs buffer.
Figure 1:
Effect of IVA on acetylcholine-induced contraction. Representative tracings illustrating contraction in response to acetylcholine (10 μM) and the inhibition of contraction upon application of IVA (50 mM).
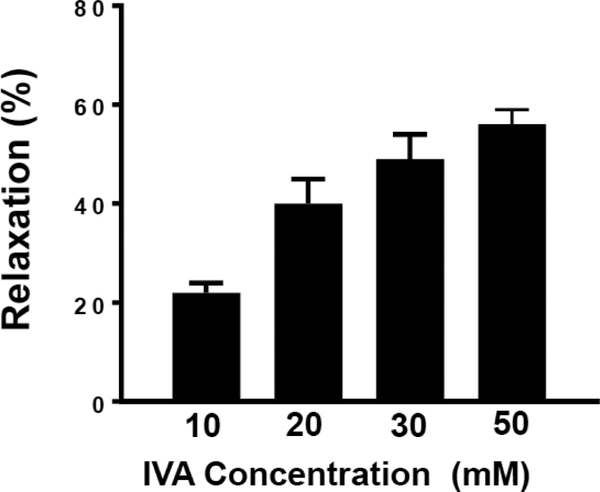
The relaxation in response to IVA was concentration-dependent over the range tested (Figure 2). IVA caused a 22 ± 1% relaxation at 10 mM, a 40 ± 4% relaxation at 20 mM, a 49 ± 4% relaxation at 30 mM, and a relaxation of 52 ± 3% at 50 mM.
Figure 2: Concentration-dependent relaxation by IVA in colonic segments.
Colonic segments were equilibrated in an organ bath. The segments were allowed to equilibrate at 1 g tension for 1 h before the addition of acetylcholine (10 μM). During the sustained phase of ACh-induced contraction, segments were treated with different concentrations of IVA (10–50 mM). IVA inhibited ACh-induced contraction in a concentration-dependent fashion. Results are expressed as percent of inhibition of ACh-induced contraction by IVA. Values are means±SEM of 35–50 experiments.
Straight chain fatty acids compared to IVA on ACh-induced contraction in colonic segments.
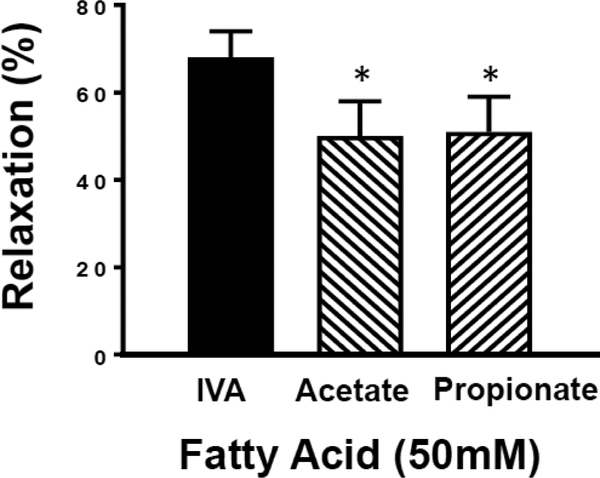
To determine whether the relaxation mediated by the branched chain fatty acid IVA differs from that of the shorter unbranched fatty acids common in stool and blood, we tested 50 mM propionic or acetic acid on colon segments and compared them to the effect of 50 mM IVA in the same colonic segment (Figure 3). IVA-induced relaxation of ACh-precontracted segments was 68 ± 6%, and the same segments relaxed 50 ± 8% and 51 ± 8% in response to acetic acid and propionic acid, respectively. Thus, IVA caused a greater relaxation than either of the straight chain fatty acids (n=6–13 p<0.05 for each straight chain FA vs IVA). This trend is the same when comparing decrease in tone measured in grams force. For ACh-contracted segments, the relaxation was 0.9 ± 0.1 g for IVA treatment, 0.5 ± 0.1 g for acetic acid treatment, and 0.6 ± 0.1 g, for propionic acid (P<0.05 for each versus IVA).
Figure 3: Comparison of relaxation of colon segments induced by 50 mM IVA, acetic acid and propionic acid.
Colonic segments were equilibrated in an organ bath. The segments were allowed to equilibrate at 1 g tension for 1 h before the addition of acetylcholine (10 μM). Initial IVA-induced relaxation of ACh-contracted segments was 68 ± 6% (n=13). After washout of IVA, ACh-contracted colon segments relaxed 50 ± 8% or 51 ± 8% in response to acetic acid (n=7) or propionic acid (n=6), respectively. Values are means±SEM; * indicates P<0.05
Effect of TTx, L-NNA and SQ22536 on IVA-induced relaxation in colonic segments.
To determine the effect of neuronal activity as potentially mediating the effects of IVA, colon segments were pretreated with the neuronal conduction blocker 10 μM TTx for 30 minutes prior to the addition of 10 μM ACh and 50 mM IVA. Neither TTx nor its citric acid vehicle control had a significant effect on the IVA-induced relaxation. In the same strips, the relaxation response to IVA was 41 ± 6% in the absence of TTx and 57 ± 7% in the presence of TTx (P>0.05; n=5). The decrease in tone in response to IVA expressed as grams force was also similar in the presence and absence of TTx (0.37 ± 0.06 g relaxation and 0.34 ± 0.07 g relaxation in the absence and presence of TTx, respectively, P>0.05). This suggests that the effect of IVA is direct on smooth muscle rather than mediated through neuronal mechanisms.
To determine the involvement of nitric oxide on the relaxant effects of IVA, colon segments were pretreated with the NOS inhibitor 100 μM L-NNA for at least 25 minutes prior to the addition of IVA and ACh. L-NNA did not significantly affect relaxation in response to IVA. In the absence to L-NNA, IVA caused a 53 ± 5% relaxation of ACh induced contraction and a 70 ± 9% relaxation of ACh induced contraction in the presence of L-NNA (P>0.05; n=6). The decrease in tone in response to IVA expressed as grams force was also similar in the presence and absence of L-NNA (0.41 ± 0.07 g relaxation and 0.48 ± 0.13 g relaxation in the presence and absence of L-NNA, respectively, P<0.05). Consistent with the effect of TTx, the lack of a statistically significant effect of L-NNA suggest that NO did not mediate the inhibitory effect of IVA on colonic muscle.
To examine the involvement of GαS-coupled pathways in IVA mediated relaxation, colonic segments were pre-treated with a selective adenylyl cyclase inhibitor SQ22536 (500 μM). Treatment with SQ22536 caused an inhibition in IVA-induced relaxation from that obtained in the absence of SQ22536, however, the difference in relaxation was not statistically significant (48 ± 4% relaxation of ACh-induced contraction in the absence of SQ22536 vs 37 ± 3% relaxation in the presence of SQ22536 n=4, P<0.05). In these strips, the vehicle, DMSO, caused a slight increase in the relaxation in response to IVA. When this is taken into account, the inhibition by SQ22536 was significant (53± 3% in the presence of the DMSO vehicle control vs 37 ± 3% in the presence of SQ22536, n=4; P<0.05) This suggested that the response to IVA might be mediated by activation of adenylyl cyclase and activation of the cAMP/PKA signaling pathway. This notion was tested further in isolated smooth muscle cells.
Effect of IVA on ACh-induced contraction in colonic smooth muscle cells.
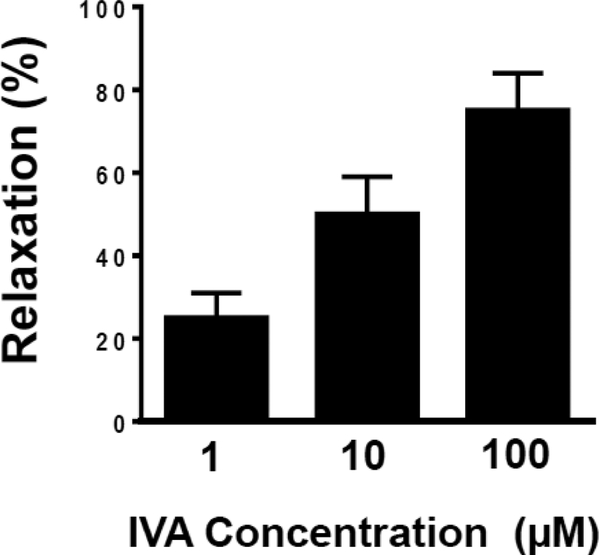
The lack of effect of L-NNA or TTx on IVA-induced relaxation in colonic segments suggests a direct effect of IVA on smooth muscle cells. To test this notion, the effect of IVA on ACh-induced contraction was examined in isolated colonic muscle cells. Muscle cells were treated with different concentrations of IVA (1 μM 100 μM) for 1 or 5 minutes before the addition for ACh (1 μM). Addition of ACh caused a significant 37% ±σ 6% decrease in cell length (control cell length 61 ±σ 4 μm, in the presence of ACh 38 ±σ 4 μm). Pretreatment of muscle cells with IVA for either 1 or 5 minutes inhibited AChinduced contraction in a concentration-dependent fashion (Figure 4). Inhibition of contraction by IVA was referred to as relaxation and represented as percent inhibition of ACh contraction. With 1 minute of pre-treatment with IVA, ACh-induced decrease in cell length was 30 ±σ 8% (or 25% relaxation) at 1 μm IVA, 20 ±σ 6% (or 50% relaxation) at 10 μM IVA and 10 ±σ 6% (or 75% relaxation) at 100 μM IVA(P<0.01). The relaxation of isolated muscle cells after pretreatment with IVA for 5 minute before addition of ACh was similar to the results obtained with 1 minute pretreatment (data not shown).
Figure 4: Concentration-dependent relaxation by IVA in isolated colonic muscle cells.
Relaxation of muscle cells was measured as decrease in maximal cell contraction induced by ACh. Muscle cells were treated with ACh (1 μM) in the absence or presence of pre-treatment with different concentrations (1–100 μM) of IVA. IVA inhibited (i.e. caused relaxation) ACh-induced contraction in a concentration-dependent fashion. Relaxation was expressed percent inhibition of contraction. Values are means±SD.
Effect of SQ22536 and H-89 on IVA-induced relaxation in colonic smooth muscle cells.
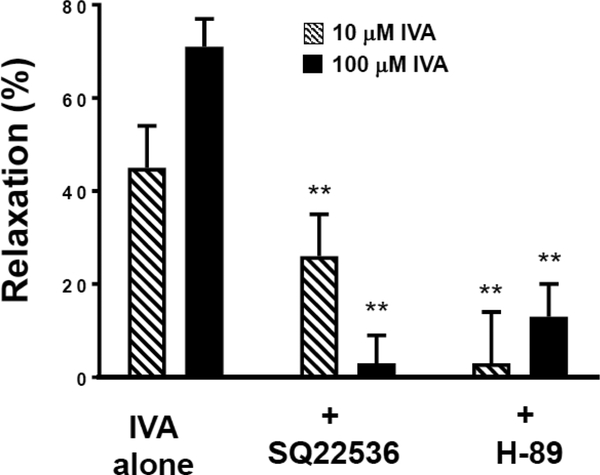
Studies in colonic segments suggested that the response to IVA was mediated by activation of adenylyl cyclase and production of cAMP/PKA. To further examine the involvement of GαS-coupled cAMP/PKA pathway in IVA mediated relaxation, isolated muscle cells were incubated with SQ22536 (1 and 10 μM) or PKA inhibitor H-89 (10 μM) prior to the addition of IVA (10 and 100 μM) and ACh (1 μM) (Figure 5). The decrease in cell length in response to ACh and ACh plus IVA was measured and the percent relaxation calculated. Experiments were repeated in the presence and absence of SQ22536 and H-89. SQ22536 inhibited relaxation in response to both 10 and 100 μM IVA. Relaxation was 45 ±σ 9% and 71 ±σ 6% at 10 and 100 μM IVA, respectively, whereas in the presence of SQ22536 (10 μM) relaxation was 26 ±σ 9% and 3 ±σ 6% at 10 and 100 μM IVA, respectively (Figure 5). The inhibition of relaxation by SQ22536 was dose-dependent as inhibition of relaxation was decreased with 1 μM SQ22536. The 100 μM IVA relaxation was 71 ±σ 6% in the absence of SQ22536, and in the presence of 1 μM SQ22536 was 23 ±σ 7% and 3 ±σ 6 % in the presence of 10 μM SQ22536 (P<0.01). H-89 also inhibited relaxation in response to both 10 and 100 μM IVA. In the presence of H-89 (10 μM) relaxation was 3 ±σ 11% and 13 ±σ 7% at 10 and 100 μM of IVA, respectively (P<0.01). Consistent with data obtained in the colonic segments, the results in isolated muscle cells suggest that IVA-induced relaxation was mediated by Gαs/cAMP/PKA pathway.
Figure 5: Effect of SQ22536 and H-89 on IVA-induced relaxation in colonic muscle cells.
Relaxation of muscle cells was measured as decrease in maximal cell contraction induced by ACh. Muscle cells were treated with ACh (1 μM) alone or in the presence of IVA (10 and 100 μM). In some experiments cells were incubated with SQ22536 (10 μM) or H-89 (10 μM) prior to the addition of IVA. IVA-inhibited contraction (i.e., caused relaxation) in response to ACh. Relaxation was expressed percent inhibition of contraction. IVA-induced relaxation was suppressed by both SQ22536 and H-89. Values are means±SD of 4 experiments. ** indicates P<0.01.
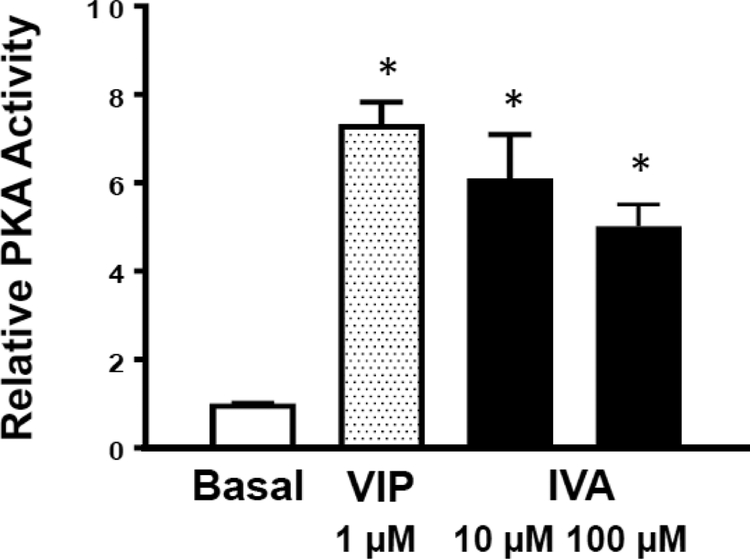
Effect of IVA on PKA activation.
The effect of the PKA-inhibitor H-89, and the adenylate cyclase inhibitor SQ22536, on cell length implies an involvement of the cAMP/PKA-mediated pathway in mediating relaxation by IVA. This notion was examined by direct measurements of PKA activity in response to IVA and vasoactive intestinal peptide (VIP), a ligand for Gαs-coupled VPAC2 receptors in gastrointestinal smooth muscle [41]. Both 10 and 100 μM IVA caused a significant increase in PKA activity in colonic smooth muscle cells. Cells treated with 10 μM showed 6.5±σ 1.0 more PKA activity and 100 μM IVA showed 5.0 ±σ 0.5 times more PKA activity than the untreated cells (1.0 ±σ 0.01), compared to 1 μM VIP which showed 7.3 ±σ 0.4 times more PKA activity than the untreated cells (Figure 6). These results suggests that activation of PKA by IVA is involved in mediating relaxation response and is consistent with the inhibition of IVA-induced relaxation by the PKA inhibitor H-89.
Figure 6: IVA-induced PKA activation in colonic muscle cells.
PKA activity in smooth muscle cells was measured by an in vitro kinase assay by the phosphorylation of PKA substrate kemptide using [32P]ATP. PKA activity was measured as described in the Methods and represented as relative PKA activity (ratio of counts per minute in the absence of added cAMP (stimulated activity) or in the presence of added cAMP (total activity)). Values are means±SD; * indicates P<0.05.
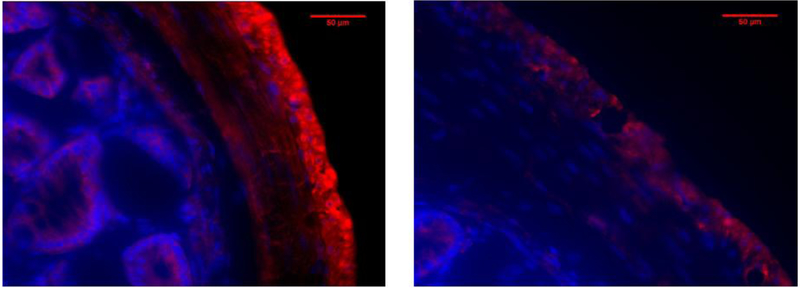
IVA-sensitive receptor Olfr558 (OR51E1 homolog) on colon smooth muscle.
Relaxation of smooth muscle with IVA via a cAMP/PKA pathway would indicate the presence of a G protein coupled receptor sensitive to IVA which activates adenylate cyclase in a Gαs-like manner. Murine receptor Olfr558, homologous to human receptor OR51E1, is Gαolf-linked receptor, similar to Gαs in that it activates adenylate cyclase (specifically, ACIII). IVA is an activating ligand for OR51E1 while shorter unbranched fatty acids such as propionic and acetic are not [38, 39]. Immunofluorescent staining of mouse colon for Olfr558 shows staining for the receptor on the longitudinal smooth muscle on the outer layer of the colon, as well as some staining in the circular layer of smooth muscle (Figure 7). Control stains without the primary antibody did not show specific staining of the secondary antibody in the smooth muscle layers (Figure 7).
Figure 7: Fluorescent staining of mouse colon for Olfr558 (OR51E1).
Crossections (10 μm thick) of mouse colon demonstrate staining for Olfr558 (Red) in the longitudinal layers of smooth muscle, with some noticeable staining in the circular muscle and epithelium (A). Sections without primary antibody (B) did not show any noticeable fluorescence for the secondary antibody. Blue indicates nuclear DAPI staining. Magnification is 400X.
DISCUSSION
In the present study, we have demonstrated that the branched chain fatty acid isovaleric acid causes relaxation of colonic smooth muscle. We have also identified that relaxation was mediated via cAMP/PKA pathway raising the possibility that IVA interacts with receptors coupled to Gαs-like protein. The evidence for the notion that IVA acts directly on smooth muscle cells was based on the following results. 1) IVA causes relaxation in a concentration-dependent fashion by inhibiting ACh-induced muscle contraction in colonic segments; 2) IVA-induced relaxation in colonic segments was not affected by L-NNA that blocks neuronal nitric oxide synthase activity and generation of nitric oxide, a key inhibitory neurotransmitter in the colon; 3) IVA-induced relaxation in colonic segments was also not affected by TTx that blocks neural activation to release neurotransmitters. A direct effect of IVA on smooth muscle was confirmed by demonstrating that IVA causes relaxation in a concentration-dependent fashion by inhibiting ACh-induced decrease in length in isolated muscle cells. This preparation is devoid of enteroendocrine cells, neurons and interstitial cell of Cajal (ICC) allowing the examination of the direct effect on smooth muscle cells and avoiding the confounding effects of other cell types (40). Activation of PKA and/or protein kinase G (PKG) plays a key role in smooth muscle relaxation. Lack of effect of L-NNA suggests a PKGindependent pathway probably involving PKA. The involvement of PKA pathway in relaxation was tested by H-89, which blocks protein kinase (PKA) by binding to the ATP-binding site in the PKA catalytic site, and by direct measurement of PKA activity in response to IVA in smooth muscle cells. IVA caused a significant increase in PKA activity and the stimulatory effect is similar to that of VIP, a known activator of Gαscoupled VPAC2 receptors in gastrointestinal smooth muscle [41]. In addition, IVAinduced relaxation was blocked by H-89 suggesting activation of PKA by IVA causes muscle relaxation. The similarity of action to VIP which is known to induce relaxation via the Gαs/cAMP/PKA pathway and the ability of the adenylate cyclase inhibitor SQ22536 to strongly inhibit IVA induced relaxation support the notion that the Gαs/cAMP/PKA mediates the response to IVA in the present study. However, it should be noted that other non-canonical mechanism may also activate PKA and cannot be ruled out as alternative or additive pathways activated by IVA in colonic smooth muscle [44–49].
An essential step in smooth muscle contraction in response to contractile agonists such as ACh is phosphorylation of the 20-kDa regulatory light chain of myosin II (MLC20) by Ca2+/calmodulin-dependent myosin light-chain kinase (MLCK) [40]. The increase in Ca2+ required for activation of MLCK is derived either by release of Ca2+ from the sarcoplasmic reticulum or influx of Ca2+ from the extracellular medium via Ca2+ channels. MLC20 phosphorylation is sustained even after the termination of initial raise in Ca2+ by inhibition of MLC phosphatase (MLCP) which normally terminates contractions by dephosphorylation of MLC20. Each of these steps plays a role in initiating or sustaining contractions [reviewed in [40]. The activation of PKA produced by IVA as evidenced by the effects of H-89 on relaxation and measured directly by PKA substrate phosphorylation in the present study could cause relaxation of ACh-induced contraction by several mechanisms at the level of the smooth muscle cell. PKA could reduce the increase intracellular Ca2+ levels by (a) inhibiting IP3 formation and/or IP3dependent Ca2+ release, (b) stimulating Ca2+ uptake into the stores and/or stimulating Ca2+ efflux, and (c) inhibiting Ca2+ influx. In addition, PKA could inhibit muscle contraction by stimulating MLCP activity thereby reducing phosphorylation of MLC20. Although the exact mechanism by which activation of PKA pathway in response to IVA mediates muscle relaxation has not been determined in the present study, mechanisms have been demonstrated for other relaxant agents such as VIP, which activates Gαscoupled cAMP/PKA pathway [reviewed in [40][41–43].
The relaxation of colonic smooth muscle by IVA could be mediated additionally through activation of K+ channels. Previous studies have shown the BCFA valproic acid activated TREK-1, a TWIK-related K+ channel. Activation of K+ channels causes hyperpolarization leading to inhibition of Ca2+ influx. Expression of TREK-1 in the smooth muscle and inhibition of contraction by valproic in rat and guinea pig stomach and tenia coli have been reported [50–52]. IVA has also been shown to activate KCNQ24 K+ channels, which are present on murine gastrointestinal smooth muscle [54–56]. The potential role of these K+ channels in additionally mediating the relaxation effects of IVA in smooth muscle of the mouse colon remains to be determined.
Recently, several members of a different class of receptors, the olfactory receptors (Olfr), have been suggested as a possible mediator of SCFA and BCFA responses in a variety of tissues [38,39,57–59]. The Olfr78 has been demonstrated in renal juxtaglomerular apparatus and smooth muscle cells of the small resistance vessels of the kidney where they have been postulated to regulate renin secretion and vasodilatation in response to SCFA produced by colonic microbiota [59]. The olfactory receptor Olfr558 has been shown to be sensitive to a number of medium and short chain fatty acids. Recent studies demonstrated the presence of Olfr558 in enterochromaffin cells where it induces serotonin release [38]. Notably, Olfr558 was shown to be most responsive to IVA (EC50: 8.9 μM) and to a much lesser extent isobutyrate and not responsive to the SCFAs acetate, butyrate or propionate. The effect is mediated by activation of Gαolf and activation of adenylate cyclase III, activating the cAMP/PKA signaling pathway. We demonstrate the presence of the Olfr588 on colonic muscle cells (Figure 7). However, the putative antagonist of this receptor [39], the BCFA 2-Ethylhexanoic acid, caused relaxation itself of colonic smooth muscle in our study (data not shown). Thus, although the olfactory receptors are a potential mediators of the response to IVA, we were unable to determine if they mediated the relaxation of colonic smooth muscle by IVA.
While little is known of the mechanisms of response to BCFA in the gut, the responses to SCFAs (mainly acetate, propionate, and butyrate) are mediated through the fatty acid receptor 2 (FFA2) and/or 3 (FFA3) formerly known as GPR43 and GPR41, respectively. These receptors are expressed in a variety of cells in the gut including enteroendocrine cells, adipocytes, pancreatic cells, immune cells, and enteric neurons; however, their expression in smooth muscle has not been studied [4,5,7–10,30–35]. Similarly, their role in mediating the response to BCFA has not been studied in any of these cell types in any detail. It is unlikely that either of these FFA receptors mediate the relaxant effects of IVA in mouse colonic smooth muscle in this study because of their well-known signaling mechanisms. FFA2 is coupled to Gαi/o and/or Gαq/11 subunits of the G protein and FFA3 is coupled to Gαi. Thus, activation of FFA2 or 3 would lead to an increase in Ca2+ and/or decrease in cAMP generation and result in contraction of smooth muscle cells rather than the increase in PKA activation in smooth muscle and the relaxation we have observed. In addition, recent studies in proximal colon demonstrate that FFA3 receptors are located on enteric neurons and that FFA3-specific agonists inhibit circular muscle contraction by inhibiting neuronal ACh release [35]. This infers that FFA3 mediated effects on circular smooth muscle are neuronal, not directly on smooth muscle cells.
As with other microbial products, the site of action of SCFA is likely to be multidimensional. SCFA have been shown to act intraluminally to release hormones and paracrine agents from enteroendocrine cells which then activate enteric neurons as well as enter the circulation. It is increasingly being recognized that microbial agents can also have effects after uptake through mucosa into vasculature. For example, recent studies suggest that GLP-1 secretion from the proximal intestine in response to FFA1 agonists is the result of vascular rather than luminal receptor activation [60]. A role of IVA from stool has also been correlated with depression in human suggesting that vascular mediate effects may be more widespread than expected [61]. Considering that BCFA are also produced intraluminally and ingested in the diet, it is also likely that they have both intraluminal effects as evidenced in their ability to release serotonin from enterochromaffin cells and extraluminal vascular effects. The use of nonperfused segmental colonic preparations and isolated smooth muscle cells in the in the current study, strongly indicate a direct smooth muscle effect. This does not exclude an indirect effect mediated by release of endocrine or paracrine agents. The effects of released endocrine and paracrine agents may be more evident in preparations of intact colon designed to investigate colonic motility patterns rather than direct contraction and relaxation of colonic smooth muscle.
In summary, the present study demonstrates a direct effect of the branched chain fatty acid, isovaleric acid, on smooth muscle cells of the longitudinal layer of the mouse colon. The effect is the result of activation of a PKA signaling cascade, which is well known to mediate the relaxation of gut smooth muscle by a variety of other neurotransmitters and hormones that are coupled to similar signaling cascade.
Acknowledgements:
This work was supported by grants DK 34153 (JR Grider) and DK28300 and DK15564 (KS Murthy) from the National Institute of Diabetes and Kidney Diseases
References
- 1.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981; 22: 763–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006; 40: 235–243. [DOI] [PubMed] [Google Scholar]
- 3.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987; 28: 1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016; 165: 1332–1345. [DOI] [PubMed] [Google Scholar]
- 5.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012; 95: 50–60. [DOI] [PubMed] [Google Scholar]
- 6.David LA, Maurice CF, Carmody RN et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014; 505: 559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016; 7: 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolognini D, Tobin AB, Milligan G, Moss CE. The Pharmacology and Function of Receptors for Short-Chain Fatty Acids. Mol Pharmacol. 2016; 89: 388–98. [DOI] [PubMed] [Google Scholar]
- 9.Soldavini J, Kaunitz JD. Pathobiology and potential therapeutic value of intestinal short-chain fatty acids in gut inflammation and obesity. Dig Dis Sci. 2013; 58: 2756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaji I, Kaunitz JD. Luminal chemosensing in the gastroduodenal mucosa. Curr Opin Gastroenterol. 2017; 33: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Keefe SJD. Diet, microorganisms and their metabolites and colon cancer. Nat Rev Gastroenterol Hepatol. 2016; 13: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder HJ. Role of Colonic Short-Chain Fatty Acid Transport in Diarrhea. Annu Rev Physiol. 2010; 72: 297–313. [DOI] [PubMed] [Google Scholar]
- 13.Andriamihaja M, Davila A-M, Eklou-Lawson M et al. Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet. Am J Physiol Liver Physiol. 2010; 299: G1030–G1037. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl. 1996; 216: 132–48. [DOI] [PubMed] [Google Scholar]
- 15.Russell WR, Gratz SW, Duncan SH et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011; 93: 1062–1072. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Rose DJ. Long-term dietary pattern of fecal donor correlates with butyrate production and markers of protein fermentation during in vitro fecal fermentation. Nutr Res. 2014; 34: 749–759. [DOI] [PubMed] [Google Scholar]
- 17.Ran-Ressler RR, Glahn RP, Bae S, Brenna JT. Branched-chain fatty acids in the neonatal gut and estimated dietary intake in infancy and adulthood. Nestle Nutr Inst Workshop Ser. 2013; 77: 133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ran-Ressler RR, Bae S, Lawrence P, Wang DH, Brenna JT. Branched-chain fatty acid content of foods and estimated intake in the USA. Br J Nutr. 2014; 112: 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakobsdottir G, Bjerregaard JH, Skovbjerg H, Nyman M. Fasting serum concentration of short-chain fatty acids in subjects with microscopic colitis and celiac disease: No difference compared with controls, but between genders. Scand J Gastroenterol. 2013; 48: 696–701. [DOI] [PubMed] [Google Scholar]
- 20.Vockley J, Ensenauer R. Isovaleric acidemia: new aspects of genetic and phenotypic heterogeneity. Am J Med Genet C Semin Med Genet. 2006; 142C: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shigematsu Y, Sudo M, Momoi T et al. Changing plasma and urinary organic acid levels in a patient with isovaleric acidemia during an attack. Pediatr Res. 1982; 16: 771–5. [DOI] [PubMed] [Google Scholar]
- 22.Weir TL, Manter DK, Sheflin AM et al. Stool Microbiome and Metabolome Differences between Colorectal Cancer Patients and Healthy Adults. PLoS One. 2013; 8: e70803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farup PG, Rudi K, Hestad K. Faecal short-chain fatty acids - a diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol. 2016; 16: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Høverstad T, Bjørneklett A, Fausa O, Midtvedt T. Short-chain fatty acids in the small-bowel bacterial overgrowth syndrome. Scand J Gastroenterol. 1985; 20: 492–9. [DOI] [PubMed] [Google Scholar]
- 25.Høverstad T, Fausa O, Bjørneklett A, Bøhmer T. Short-chain fatty acids in the normal human feces. Scand J Gastroenterol. 1984; 19: 375–381. [PubMed] [Google Scholar]
- 26.François IEJA, Lescroart O, Veraverbeke WS et al. Effects of Wheat Bran Extract Containing Arabinoxylan Oligosaccharides on Gastrointestinal Parameters in Healthy Preadolescent Children. J Ped Nutr. 2014; 58:647–653. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson C, Ahrné S, Molin G et al. Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: A randomized controlled trial. Atherosclerosis. 2010; 208: 228–233. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson U, Johansson M, Nilsson Å, Björck I, Nyman M. Dietary supplementation with β-glucan enriched oat bran increases faecal concentration of carboxylic acids in healthy subjects. Eur J Clin Nutr. 2008; 62: 978–984. [DOI] [PubMed] [Google Scholar]
- 29.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2006; 292: G429–37. [DOI] [PubMed] [Google Scholar]
- 30.Reigstad CS, Salmonson CE, Rainey JF et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015; 29: 1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiba Y, Inoue T, Kaji I et al. Short-chain fatty acid sensing in rat duodenum. J Physiol. 2015; 593: 585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaji I, Akiba Y, Konno K et al. Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon. J Physiol. 2016; 594: 3339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukumoto S, Tatewaki M, Yamada T et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003; 284: R1269–76. [DOI] [PubMed] [Google Scholar]
- 34.Bhattarai Y, Schmidt BA, Linden DR et al. Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT3 receptor expression via acetate production. Am J Physiol Gastrointest Liver Physiol. 2017; 313: G80–G87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaji I, Akiba Y, Furuyama T et al. Free fatty acid receptor 3 activation suppresses neurogenic motility in rat proximal colon. Neurogastroenterol Motil. 2018; 30: e13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cherbut C, Aubé AC, Blottière HM et al. In vitro contractile effects of short chain fatty acids in the rat terminal ileum. Gut. 1996; 38: 53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindelin J, Arganda-Carreras I, Frise E et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012; 9: 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellono NW, Bayrer JR, Leitch DB et al. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways Article Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017; 170: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jovancevic N, Dendorfer A, Matzkies M et al. Medium-chain fatty acids modulate myocardial function via a cardiac odorant receptor. Basic Res Cardiol. 2017; 112: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006; 68: 345–374. [DOI] [PubMed] [Google Scholar]
- 41.Murthy KS, Jin JG, Grider JR, Makhlouf GM. Characterization of PACAP receptors and signaling pathways in rabbit gastric muscle cells. Am J Physiol. 1997; 272: G1391–9. [DOI] [PubMed] [Google Scholar]
- 42.Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Liver Physiol. 2003; 284: G1006–G1016. [DOI] [PubMed] [Google Scholar]
- 43.Murthy KS, Makhlouf GM. Regulation of Adenylyl Cyclase Type V/VI in Smooth Muscle: Interplay of Inhibitory G Protein and Ca 2 Influx. Mol Pharm.1998;54: 122–128. [DOI] [PubMed] [Google Scholar]
- 44.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The Transcriptional Activity of NF-κB Is Regulated by the IκB-Associated PKAc Subunit through a Cyclic AMP–Independent Mechanism. Cell. 1997; 89: 413–424. [DOI] [PubMed] [Google Scholar]
- 45.Sriwai W, Mahavadi S, Al-Shboul O, Grider JR, Murthy KS. Distinctive G ProteinDependent Signaling by Protease-Activated Receptor 2 (PAR2) in Smooth Muscle: Feedback Inhibition of RhoA by cAMP-Independent PKA. PLoS One. 2013; 8: e66743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sriwai W, Zhou H, Murthy KS. G(q)-dependent signalling by the lysophosphatidic acid receptor LPA(3) in gastric smooth muscle: reciprocal regulation of MYPT1 phosphorylation by Rho kinase and cAMP-independent PKA. Biochem J. 2008; 411: 543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elrick MM, Samson WK, Corbett JA et al. Neuronostatin acts via GPR107 to increase cAMP-independent PKA phosphorylation and proglucagon mRNA accumulation in pancreatic α-cells. Am J Physiol Regul Integr Comp Physiol. 2016; 310: R143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su S-F, Yang A-M, Yang S-B et al. Intracerebroventricular administration of neuronostatin delays gastric emptying and gastrointestinal transit in mice. Peptides. 2012; 35: 31–5. [DOI] [PubMed] [Google Scholar]
- 49.Amato A, Baldassano S, Caldara G, Mulè F. Neuronostatin: peripheral site of action in mouse stomach. Peptides. 2015; 64: 8–13. [DOI] [PubMed] [Google Scholar]
- 50.Kristev A, Peichev L, Zaprianov G, Lukanov J. Effect of sodium valproate on the spontaneous contractile and bioelectric activity of smooth muscle fibres isolated from experimental animals. Folia Med (Plovdiv). 1994; 36: 11–9. [PubMed] [Google Scholar]
- 51.Hwang SJ, O’Kane N, Singer C et al. Block of inhibitory junction potentials and TREK-1 channels in murine colon by Ca2+ store-active drugs. J Physiol. 2008; 586: 1169–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alcaino C, Farrugia G, Beyder A. Mechanosensitive Piezo Channels in the Gastrointestinal Tract. Curr Top Membr. 2017; 79: 219–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manville RW, Abbott GW. Ancient and modern anticonvulsants act synergistically in a KCNQ potassium channel binding pocket. Nat Commun. 2018; 9: 3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jepps TA, Greenwood IA, Moffatt JD, Sanders KM, Ohya S. Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2009; 297: G107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salzer I, Erdem FA, Chen W-Q et al. Phosphorylation regulates the sensitivity of voltage-gated Kv7.2 channels towards phosphatidylinositol-4,5-bisphosphate. J Physiol. 2017; 595: 759–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mani BK, Robakowski C, Brueggemann LI et al. Kv7.5 Potassium Channel Subunits Are the Primary Targets for PKA-Dependent Enhancement of Vascular Smooth Muscle Kv7 Currents. Mol Pharmacol. 2016; 89: 323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalbe B, Schlimm M, Wojcik S et al. Olfactory signaling components and olfactory receptors are expressed in tubule cells of the human kidney. Arch Biochem Biophys. 2016; 610: 8–15. [DOI] [PubMed] [Google Scholar]
- 58.Priori D, Colombo M, Clavenzani P et al. The olfactory receptor OR51E1 is present along the gastrointestinal tract of pigs, co-localizes with enteroendocrine cells and is modulated by intestinal microbiota. PLoS One. 2015; 10: e0129501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pluznick JL, Protzko RJ, Gevorgyan H et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013; 110: 4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christensen LW, Kuhre RE, Janus C, Svendsen B, Holst JJ. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol Rep. 2015; 3. doi: 10.14814/phy2.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szczesniak O, Hestad KA, Hanssen JF, Rudi K. Isovaleric acid in stool correlates with human depression. Nutr Neurosci. 2016; 18: 1–12. [DOI] [PubMed] [Google Scholar]