Abstract
Negative symptoms, such as blunted facial affect, are core features of psychotic disorders that predict poor functional outcome. However, it is unknown whether these impairments occur prior to the onset of psychosis. Understanding this phenomenon in the psychosis risk period has significant relevance for elucidating pathogenic processes, as well as potential for informing a viable new behavioral marker for broader social dysfunction and clinical course. The current study sought to determine the nature of facial expression deficits among individuals at clinical high-risk (CHR) for developing psychosis using a comprehensive approach, incorporating clinical interview ratings and automated facial expression coding analysis. A total of 42 CHR and 42 control participants completed clinical interviews and digitally taped segments were submitted into an automated, computerized tool to assess for seven basic facial expressions (joy, anger, surprise, fear, contempt, disgust, sadness). Furthermore, relationships between facial expressions and social functioning and available scores on a psychosis conversion risk calculator from a total of 78 participants (39 CHR and 39 controls) were examined. Relationships between measures were also investigated (data was available for the Prodromal Inventory of Negative Symptoms among 33 CHR and 25 controls). Findings from clinical interview indicated that the CHR group exhibited elevated blunting. Furthermore, automated analyses showed that the CHR group displayed blunting in expressions of joy but surprisingly, increased anger facial expressions. Lastly, irregularities in facial expressions were related to decreased social functioning and increased psychosis conversion risk calculator scores, signaling heightened likelihood of conversion to psychosis. These findings suggest that alterations in facial expressivity occur early in the pathogenesis of psychosis and provide evidence for the efficacy of higher resolution measures of facial expressivity in psychosis research.
Keywords: clinical high-risk, schizophrenia, facial expressions, negative symptoms, functioning
Introduction
Negative symptoms are core features of psychotic disorders that predict a number of poor clinical outcomes and are resistant to currently available treatments (Fervaha, Foussias, Agid, & Remington, 2014; Foussias, Agid, Fervaha, & Remington, 2014; Kirkpatrick, Fenton, Carpenter, & Marder, 2006; Millan, Fone, Steckler, & Horan, 2014; Strauss, Harrow, Grossman, & Rosen, 2010). Blunted affect, which refers to a reduction of emotional expression in the face, voice, and body gestures (Andreasen, 1982; Kirkpatrick, et al., 2006) is a key negative symptom domain. In adults with psychotic disorders, there is consistent evidence for blunted facial expressions as measured via multiple methods (e.g., behavioral observation, electromyography, automated computer-based analyses) (Bobes, Arango, Garcia-Garcia, & Rejas, 2010; Evensen et al., 2012; Gur et al., 2006; Malla et al., 2002), and these deficits have a number of detrimental social consequences, such as interpersonal relationship quality and social adjustment (Bellack, Morrison, Wixted, & Mueser, 1990; Hooley, Richters, Weintraub, & Neale, 1987). Blunting occurs in response to both laboratory and naturalistic contexts, and although these deficits are pronounced, individuals with psychotic disorders do exhibit micro-expressions consistent with the valence of stimuli they are exposed to that are unobservable to the naked eye (e.g., showing zygomatic activity, muscular facial activity related to smiling, to positive stimuli) (Kring & Earnst, 1999; Kring, Kerr, & Earnst, 1999). Evidence for valence-related deficits in facial expressivity is inconsistent (Kring & Moran, 2008). Most studies show that both men and women with schizophrenia have less zygomatic activation than controls in response to positively valenced evocative stimuli; however, findings in relation to negative stimuli are more mixed, with several studies indicating increased corrugator activity in schizophrenia patients, and others indicating no difference between schizophrenia patients and controls (Kring, et al., 1999; Mote, Stuart, & Kring, 2014; Wolf, Mass, Kiefer, Wiedemann, & Naber, 2006). It has been indicated that reductions in emotion expression may be disconnected from subjective experiences of emotion, which appear to be intact in schizophrenia (Kring & Moran, 2008).
CHR individuals are considered at imminent risk for transitioning to a psychotic disorder in a short window (Cannon et al., 2008; Fusar-Poli et al., 2012; Fusar-Poli et al., 2013). This group experiences positive symptoms but in an attenuated fashion and additionally, exhibits functional and cognitive decline (Cannon, et al., 2008; Fusar-Poli, et al., 2012). Using scales such as the Structured Interview for Prodromal Syndromes (SIPS), negative symptoms have been found to be frequent (e.g., 82% of cases) (Piskulic et al., 2012) and typically precede the emergence of attenuated positive symptoms by several years. Furthermore, a prominent feature among this group are decreases in social functioning (Addington, Penn, Woods, Addington, & Perkins, 2008; Corcoran et al., 2011; Cornblatt et al., 2007; Lencz, Smith, Auther, Correll, & Cornblatt, 2003; Yung et al., 2005). Although there is clear evidence for blunted facial expressions in psychotic disorders that are not due to antipsychotic medication side effects (Kring & Earnst, 1999; Kring & Elis, 2013), it is unclear if these deficits emerge as a consequence of processes after illness onset, or if the phenomenon reflects pathogenic processes that occur prior to onset.
Similar to first-generation negative symptom scales used to evaluate schizophrenia, CHR scales used to make determinations about negative symptoms have a number of conceptual and methodological limitations that prevent a clear determination of which specific symptom domains are contributing to severity ratings. CHR youth display a multitude of negative symptoms (e.g., avolition, anhedonia), and these may be confused for deficits in other domains like blunted affect, when rating scales do not parse constructs according to modern conceptualizations. Additionally, CHR youth display a number of comorbid psychiatric conditions (e.g., depression, anxiety) that also frequently display blunted facial expressions and can sometimes be prescribed antipsychotic medications that induce blunting. Thus, while clinical rating scales provide vital information that there is an abnormality in CHR youth, their lack of precision make it impossible to isolate the nature of this deficit or trust that the deficit truly reflects emotional expressivity and not some other psychological process.
It is now possible to use automated computerized analyses of facial expressivity to remove the ambiguity and subjectivity associated with findings obtained from clinical rating scales. Historically, a common approach to assess for facial expressions involved video coding, such as the Facial Affective Coding System (FACS) developed by Paul Ekman and Wallace Friesen (Ekman & Friesen, 1978). The FACS coding technique uses Action Units (AUs) that correspond with muscle movements observed from images or videos; collections of action units formulate specific expressions such as joy or sadness. Together, FACS coding has been proven to be effective in assessing for blunting in facial expressions in adults with schizophrenia (Gaebel & Wölwer, 2004; Trémeau et al., 2005). One of the strengths of video coding techniques is that facial expressions can be captured in naturalistic settings. For example, Walker and colleagues (1993) coded video home-movies taken at birthdays and family/holiday gatherings of patients that later developed schizophrenia. This group found by coding these videos that girls who developed schizophrenia showed blunting in facial expressions particularly in the expression of joy as a child up until adolescence compared to siblings without a diagnosis. Although video coding shows promise, this technique requires extensive training time and can be subject to rater bias. Specialized software now makes it possible to automate the extensive features previously done manually by coding systems like FACS. Early approaches of automated facial expression analysis used static images to classify facial expressions into broad emotion categories such as joy, fear, and disgust (Pantic & Rothkrantz, 2004). One downfall of coding static images is the temporal content is lost. As such, more recent work has begun to utilize automated analysis of continuous video output which produces ratings of facial action units (Cohen, Morrison, & Callaway, 2013; Hamm, Kohler, Gur, & Verma, 2011). There is evidence for the efficacy of automated facial expression analysis in populations such as schizophrenia (Hamm, et al., 2011), individuals with schizotypal traits (Cohen, et al., 2013), autism (Owada et al., 2018), and Parkinson’s disease (Bandini et al., 2017).
The Present Study.
The present study sought to determine (1) if CHR youth exhibit higher blunting scores from a clinical interview compared to a matched healthy control (HC) group, (2) if CHR youth exhibit blunting in all facial expressions assessed through automated analysis (joy, anger, surprise, fear, contempt, disgust, sadness) compared to the HC group and (3) if blunted facial expressions were associated with social functioning, psychosis conversion risk scores, and depressive symptoms in CHR youth. Facial expressions were measured using a clinical interview and automated analysis, capitalizing on the strengths of each. First, blunted facial expressions were measured drawing from the Prodromal Inventory of Negative Symptoms (PINS) (Pelletier-Baldelli, Strauss, Visser, & Mittal, 2017) blunted affect item to determine the nature of facial expressivity from the perspective of clinical interview ratings and examine unique contributions and overlaps with automated analysis. Second, facial expressions during participant clinical interviews were measured using a FACS-based automated analysis software to provide an objective measure of facial expressivity, provide an index of a broader range of emotive dysfunction and determine specificity (analyzing specific expressions), and test the feasibility of a more easily disseminated assessment method of facial expressions alternative to clinical interviews.
Based on previous research indicating patients diagnosed with psychotic disorders exhibit blunting in facial expressions (Andreasen, 1982; Evensen, et al., 2012; Kelley, Haas, & van Kammen, 2008; Kirkpatrick, et al., 2006; Kring & Elis, 2013; Malla, et al., 2002), and at-risk populations experience emotive dysfunctions (Addington et al., 2012; Addington, Saeedi, & Addington, 2006; Amminger et al., 2011; Berenbaum, Snowhite, & Oltmanns, 1987; Corcoran et al., 2015; Kohler & Martin, 2006; van't Wout, Aleman, Kessels, Larøi, & Kahn, 2004), we predicted that CHR youth would exhibit blunting in facial expressions compared to control youth on the PINS interview and from the automated analysis. Further, based on findings indicating that negative symptoms predict decreased social functioning (Corcoran et al., 2011), we predicted that blunted facial expressions would be associated with lower social functioning scores among CHR youth. Additionally, in line with research that has found that negative symptoms predict conversion to psychosis (Cannon, et al., 2008; Corcoran, et al., 2011; Piskulic, et al., 2012), we predicted that blunting in facial expressions would be associated with higher scores on a psychosis conversion risk calculator indicating increased likelihood of transition to psychosis. We also examined relationships between facial expression measures and depression scores. Finally, exploratory analyses were employed to examine influences of (1) anhedonia and (2) biological sex on facial expression measures.
Method
Participants
A total of 84 participants (42 CHR and 42 control youth; note that 39 CHR and 39 controls had psychosis conversion risk score data and 33 CHR and 25 controls and negative symptom data), aged 12–21 (M = 18.90, SD = 1.91), were recruited through the Adolescent Development and Preventive Treatment (ADAPT) Program using Craigslist, e-mail announcements, newspaper advertisements, flyers, and community health referrals. Exclusion criteria consisted of head injury, the presence of a neurological disorder, and lifetime substance dependence. The presence of an Axis I psychotic disorder (e.g., schizophrenia, schizoaffective disorder, schizophreniform) was an exclusion criterion for CHR participants. The presence of any category of Axis I disorder or a psychotic disorder in a first-degree relative was an exclusion criterion for control participants. In order to improve generalizability of our sample, participants were included if they were on antipsychotic medications, however, this was only a small subsample of the total participants in the study (n = 3). The protocol and informed consent procedures were approved by the university Institutional Review Board (protocol #10–0398).
The SIPS (Miller et al., 2003) was administered to diagnose a prodromal syndrome. CHR participants in the present study met SIPS criteria for a prodromal syndrome, defined by moderate-to-severe but not psychotic levels of positive symptoms (rated from 3 to 5 on a 6-point scale) or a decline in global functioning accompanying the presence of schizotypal personality disorder or a family history of psychosis (Miller, et al., 2003). The SIPS gauges several distinct categories of prodromal symptom domains, including positive, negative, and disorganized dimensions. A mean score for each category is used as an indicator of the respective dimensions of symptomatology.
The Structured Clinical Interview for the DSM-IV Axis I Disorders (First, Spitzer, Gibbon, & Williams, 1995) was also administered to rule out a psychotic disorder diagnosis. This measure has been demonstrated to have excellent interrater reliability in adolescent populations (Martin, Pollock, Bukstein, & Lynch, 2000). Training of advanced doctoral student interviewers was conducted during a 2-month period for both clinical interviews, and interrater reliabilities exceeded the minimum study criterion (κ ≥ 80). All participants included in this study consented to being video-recorded during clinical interviews.
Facial Expression Measures
Prodromal Inventory of Negative Symptoms (PINS)
The PINS is a newly developed assessment that has shown promise in assessing negative symptoms among CHR youth and has a specific severity item relating to blunted facial expressions (PINS BA) (Pelletier-Baldelli, Strauss, Visser, & Mittal, 2017). The PINS entails 13-items and was modeled after the Clinical Assessment Interview for Negative Symptoms (CAINS) (Horan, Kring, Gur, Reise, & Blanchard, 2011) and the Brief Negative Symptom Scale (BNSS) (Kirkpatrick et al., 2011; Strauss et al., 2012). The PINS BA severity item includes information obtained from participant reports (e.g., “Do others tell you that you rarely show your emotions on your face?”) and clinical observation (e.g., “Are their facial features expressive?”). PINS BA severity scores range from 1 to 6, with 1 indicating questionably present and 6 representing extreme (e.g., no facial emotion expressions). The PINS consummatory anhedonia item was also used to examine the relationships between experience of emotion (how the individual feels when actually participating in social interactions) and expressivity. This item falls on a 1–6 scale, with 1 representing questionably present consummatory anhedonia and 6 indicating a complete inability to enjoy interactions.
Automated Analysis
Segments of the first five-minutes of video-recorded clinical interviews of the SIPS (content included obtaining information regarding demographics) were submitted into iMotions (2016) computerized software for facial expression analysis. The use of five-minute clips is supported by meta-analytic evidence indicating that “thin slices” of behavior (assessed in under 5 minutes) can yield considerable predictive accuracy with longer observations not resulting in greater accuracy (Ambady & Rosenthal, 1992). The software uses a commercial automated facial coding tool (Emotient FACET) (https://imotions.com) which grew out of The Computer Expression Recognition Toolbox (CERT) (Littlewort et al., 2011). The module is based on the well-established FACS approach (Ekman & Friesen, 1978) and evaluates 33 action units to derive indicators of basic emotions (anger, contempt, disgust, joy, sadness, fear, surprise). The iMotions (2016) Emotient module uses a predetermined algorithm and provides evidence scores based off of AUs detected. The evidence score indicates the likelihood that an expression fits the predetermined AU-based definition of “joy” for example. The variable of interest is the likelihood facial expressions are present, indicating the percent likelihood an expressions matches the predetermined algorithm. Then, using a threshold (allowing for detection of moderate facial expressions), iMotions (2016) provides the average percent of frames in the video segment detected. Over 90% of video frames were recognized by the iMotions (2016) software in this sample at a sampling rate of 30 Hz, suggesting the video recording conditions were appropriate for automated analysis of objective facial expressions and the software was able to detect the recordings. There were no significant differences between CHR and control youth in instances in which the automated analysis tool was able to register the video recording of the face, t(82) = .02, p = .98.
This software has been validated for use of assessing facial expressions (Littlewort, et al., 2011; Taggart, Dressler, Kumar, Khan, & Coppola, 2016) and has been employed successfully in research with clinical populations, including children with autism spectrum disorder populations (Trevisan, Bowering, & Birmingham, 2016). This approach holds potential for high-volume and low-cost screening and diagnosis of CHR youth based on facial expressions – including detection of emotional signals on the face that escape the human eye.
Social Functioning
The Global Functioning Scale: Social (GFS-S) (Auther, Smith, & Cornblatt, 2006; Cornblatt, et al., 2007) was used to assess for social functioning. A score is given on a 1–10 scale, with 1 indicating low levels of social functioning and 10 indicating very high levels of social functioning (e.g., number of friends, how often the individuals engages in social activity, any reports of arguments or falling outs).
Psychosis Conversion Risk Scores
Each CHR participant was assessed for the percent likelihood of conversion to psychosis in one year. We assessed risk to conversion using a freely available online calculator (http://riskcalc.org:3838/napls/) recently developed by the North American Prodrome Longitudinal Study (NAPLS) (Cannon et al., 2016). In the initial development, Cannon and colleagues (2016) showed in a well powered study that the calculator had good discriminant ability to classify CHRs who did or did not convert to psychosis. Carrión and colleagues (2016) also replicated these results in an independent sample using the same calculation procedure (Carrión et al., 2016). The measures used to create the risk calculator score included the same materials as described in initial development of the calculator (Dean, Walther, Bernard, & Mittal, 2018), collected during the clinical interviews and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) (Nuechterlein et al., 2008) cognitive battery to input information into the online calculator. Family history of psychosis in a first degree relative was collected during the clinical interview. The SIPS scores for unusual thought content and suspiciousness were readjusted and summed based on the described procedure in Cannon and colleagues (2016). Additional measures included age, Brief Assessment of Cognition in Schizophrenia symbol coding score (Keefe et al., 2004), the Hopkins Verbal Learning Test–Revised sum score for trials 1–3 (Brandt & Benedict, 2001), the total sum score of 31 negative life events aggregated from the Research Interview Life Events Scale (Dohrenwend, Askenasy, Krasnoff, & Dohrenwend, 1978),and the GFS-S (Auther, et al., 2006; Cornblatt, et al., 2007) were also used. The number of traumas was calculated based on information given in the SCID interview.
Statistical Approach
Independent t tests and chi-square tests were employed to examine differences between groups in continuous and categorical demographic variables, respectively. Analysis of Covariance (ANCOVA) was used to look at group differences in facial expression measures, controlling for depression scores. All correlational analyses were conducted within the CHR group. Specifically, bivariate correlations were used to investigate relationships between facial expression measures and 1) social functioning, 2) psychosis conversion risk calculator scores, and 3) depressive symptoms. Partial correlations (controlling for depressive symptoms) were used in analyses in which significant correlations were observed between facial expression measures and depressive symptoms. Relationships between facial expression measures and social functioning and psychosis conversion risk calculator scores were examined in facial expression variables that were significantly different between groups. To examine the impacts of the experience of emotion on expressivity, exploratory analyses were conducted to (1) determine group differences in the PINS consummatory anhedonia item controlling for depression and (2) employ correlations between this variable and the PINS BA item, and automated analysis variables. Additionally, exploratory analyses were conducted to determine the influence of sex on facial expression using two-way ANCOVA with sex (male/female) and group (CHR/control) also when controlling for depression. While the total sample size of participants in the current study was 84 (42 CHR and 42 controls), data was missing for the PINS interview and psychosis conversion risk calculator resulting in a sample size for PINS data of 33 CHR participants and 25 controls and a sample size of 39 CHR and 39 controls with psychosis conversion risk calculator data. Controlling for antipsychotic medications did not change the direction or magnitude of results. A false-discovery rate (FDR) correction was applied to analyses to account for multiple comparisons. A note has been made to indicate after each set of findings whether the significant results survived the FDR correction.
Results
Demographics
CHR youth did not differ from control youth in age t(82) = 1.58, p = .12 or parental education t(74) = -.44, p = .66, but did differ in biological sex χ2(1) = 5.93, p = .02. The influence of sex on facial emotion variables was evaluated in exploratory analyses noted below. As expected, the CHR group endorsed more positive, t(1,82) = 15, p ≤ .001, d = 3.28, 95% CI [9.73, 12.75], and negative symptoms t(1,82) = 8.58, p ≤ .001, d = 1.87, 95% CI [6.97, 11.26]. Furthermore, the CHR group had significantly higher Beck Depression Inventory (BDI) scores compared to controls, t(75 ) = 6.39, p ≤ .001. When assessing differences between the CHR group and controls in social functioning and psychosis conversion risk calculator scores, the CHR group exhibited significantly lower scores in social functioning, t(82) = -7.46, p ≤ .001 and higher psychosis conversion risk calculator scores, t(76) = 6.21, p ≤ .001 compared to controls. See Table 1 for demographic characteristics.
Table 1.
Demographic details.
| CHR | Control | Statistic | p | |
|---|---|---|---|---|
| Age mean (SD) | 18.90 (1.91) | 18.12 (2.61) | t(82) = 1.58 | .12 |
| Biological Sex (counts) | χ2(1) = 5.93 | .02 | ||
| Male | 23 | 12 | ||
| Female | 19 | 30 | ||
| Total | 42 | 42 | ||
| Parent Education (years) mean (SD) | 15.51 (2.10) | 15.76 (2.89) | t(74) = −.44 | .66 |
| Automated Analysis Frames Detected (%) | 90 | 90 | t(82) = .02 | .98 |
| Beck Depression Inventory mean (SD) | 18.37 (11.89) | 4.81 (5.40) | t(75 ) = 6.39 | ≤.001 |
| Social Functioning mean (SD) | 6.83 (1.41) | 8.60 (.59) | t(82) = −7.45 | ≤.001 |
| Psychosis Risk Scores mean (SD) (%) | 9.52 (5.65) | 3.81 (1.07) | t(76) = 6.21 | ≤.001 |
| Symptoms Domains mean (SD) | ||||
| Positive | 11.69 (4.70) | .45 (1.19) | t(1,82) = 15 | ≤.001 |
| Negative | 9.55 (6.84) | .43 (.83) | t(1,82) = 8.58 | ≤.001 |
Note. Parental education is the average of mother and father education. Automated analysis frames detected indicate the total percent of time in which the automated analysis tool was able to register the video recording of the face. The Beck Depression Inventory (BDI) represents a total sum score. Social functioning scores are given on a 1–10 scale, with 1 indicating low levels of social functioning and 10 very high levels. Psychosis risk scores represent psychosis risk conversion calculator scores (signaling heightened likelihood of conversion to psychosis in 1 year). Symptom domains are sum scores of items for positive and negative symptoms from the Structured Interview for Prodromal Syndromes (SIPS).
Group Differences in Facial Expressions
Clinical Interview-Based Assessment
There were higher levels of blunting in the CHR group (M = 1.29, SD = 1.53) from the PINS BA severity item compared to controls (M = .04, SD = .20), F(1,50) = 4.01, p = .05, ηp2 = .07, 95% CI [-.002, 1.58].
Automated Analysis
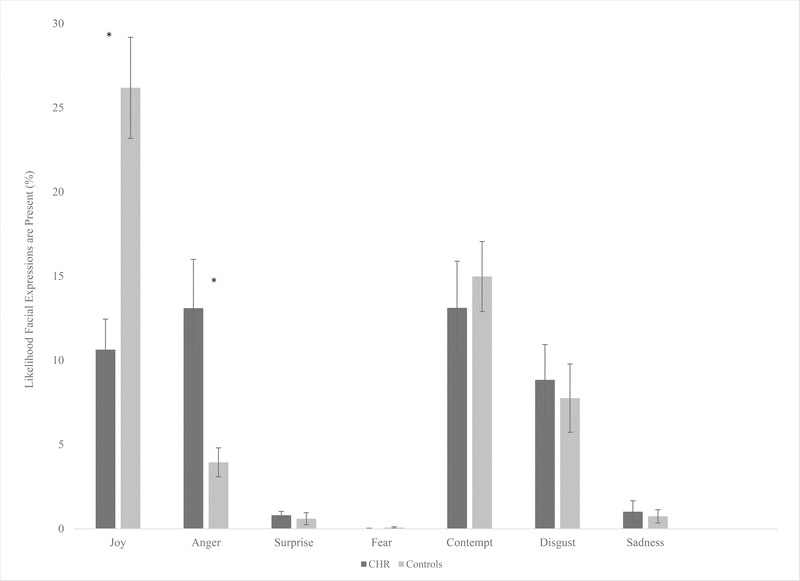
When examining group differences in the automated variables, the CHR group showed lower levels of joy expressions, F(1,74) = 9.72, p = .003, ηp2 = .12, 95% CI [-23.93, -5.27], and increased anger expressions, F(1,74) = 7.50, p = .008, ηp2 = .09, 95% CI [2.79, 17.69] compared to control youth. There were no differences between CHR and control youth in expressions of surprise, F(1,74) = .05, p = .83, fear F(1,74)= .72, p = .40, contempt F(1,74) = .002, p = .96, disgust F(1,74) = .21, p = .65, or sadness F(1,74) = 1.64, p = .21. All noted significant results remained significant after the FDR correction. (See Figure 1).
Figure 1.
Group differences in facial expressions measured by automated analysis. Scores are based off of the percent likelihood an expression is present. *p ≤ .05. Error bars represent standard error.
Relationships Between Facial Expression Measures
There was a moderate effect for the relationship between the PINS BA severity item and automated analysis; higher scores on the PINS BA severity item was significantly correlated with lower joy expressions. However, the PINS BA severity item was not related to anger expressions. The significant results survived a FDR correction. See Table 2.
Table 2.
Correlations between facial expression measures.
| iMot Joy | iMot Anger | PINS BA | |
|---|---|---|---|
| iMot Joy | - | ||
| iMot Anger | −0.33 | - | |
| PINS BA | −0.37* | 0.14 | - |
Note. The blunted affect item from the Prodromal Inventory of Negative Symptoms (PINS) is denoted by “PINS BA.” The automated analysis variables are abbreviated as “iMot Joy” (joy expressions) and “iMot Anger” (anger expressions). Numbers shown are r values.
p ≤ .05
Facial Expression Measures and Depressive Symptoms
Relationships between facial expression measures and depressive symptoms were examined. Findings indicate that the BDI was not significantly related with PINS BA severity, r = .29, p = .12, or automated analysis variables (joy expressions, r = -.30, p = .07 and anger expressions, r = -.09, p = .59).
Facial Expressions and Social Functioning
Overall, higher levels of blunted facial expressions were associated with lower levels of social functioning. Specifically, higher PINS BA severity scores were associated with lower levels of social functioning, r = -.40, p = .02. Similarly, lower levels of joy expressions were associated with lower levels of social functioning, r = .37, p = .02,. Anger expression was not associated with social functioning, r = .02, p = .90. Significant results survived the FDR correction.
Facial Expressions and Psychosis Conversion Risk Scores
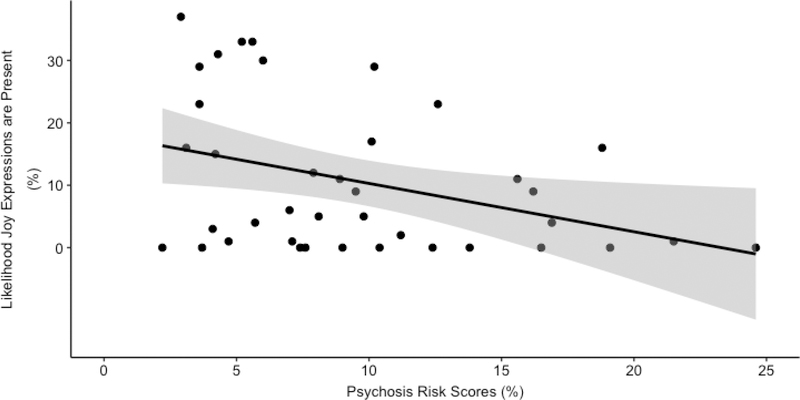
Overall, higher levels of blunted facial expressivity were associated with higher levels of psychosis conversion risk scores. Specifically, higher scores on the PINS BA severity item were associated with higher psychosis conversion risk scores, r = .39, p = .03. Similarly, lower levels of joy expressions were associated with higher conversion risk scores, r = -.37, p = .02. Anger expressions were not associated with psychosis conversion risk, r = .11, p = .51. Results passed the FDR correction. See Figure 2.
Figure 2.
Relationship between joy expressions from automated analysis and psychosis conversion risk calculator scores within the CHR group. Both variable are represented as percents. The shaded grey indicates the 95% confidence interval boundary.
Facial Expressions and Anhedonia
Results from exploratory analyses indicate that there were group differences in PINS item assessing consummatory anhedonia, F(1,53) = 9.61, p = .003, ηp2 = .15 in that the CHR group had higher severity scores (M = 1.06, SD = 1.24) compared to the control group (M = .04, SD = .20). Furthermore, correlational analyses revealed that the PINS consummatory anhedonia item was not significantly associated the PINS BA item, r = .18, p = .30. The PINS consummatory anhedonia item was also not significantly correlated with the automated analysis variables; joy expressions r = -.19, p = .28, or anger expressions r = .02, p = .90. Significant results survived the FDR correction.
Facial Expressions and Biological Sex
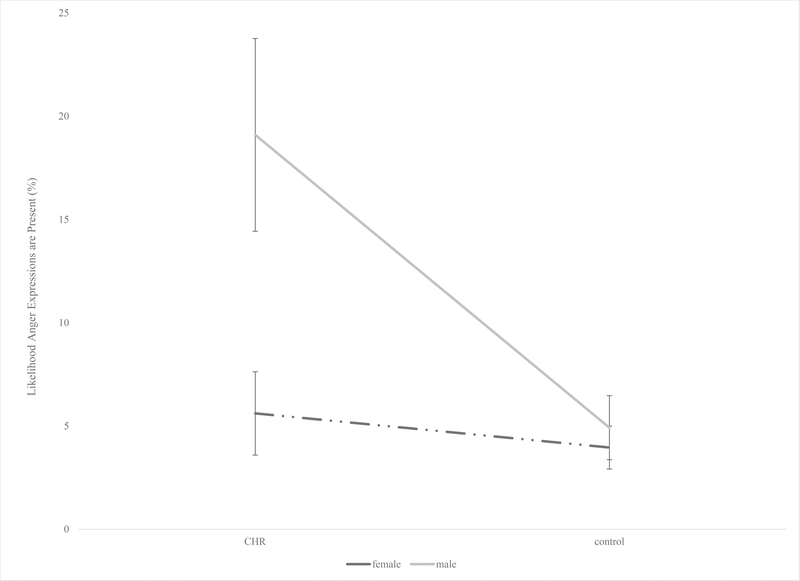
We looked at the influence of sex on facial expression measures in a series of exploratory analyses and while there were no findings for the majority of the measures used to assess facial expressions, we did detect a significant interaction for one expression. Specifically, there was a significant interaction between sex (male/female) and group (CHR/control) on anger facial expressions from the automated analysis, F(1,72) = 6.14, p = .02, ηp2 = .08. Post hoc analyses revealed that there were significant differences in anger expressions between CHR and control males in that the CHR males had greater anger expressions compared to the control males, F(1,29) = 8.80, p = .006, ηp2 = .23. There were no significant differences between CHR and control females, F(1,42) = .20, p = .66. See Figure 3. These findings survived the FDR correction.
Figure 3.
Interaction between sex (male/female) and group (CHR/control) in anger facial expressions from automated analysis. Error bars represent standard error.
Discussion
The current study investigated facial expressivity among CHR youth using multiple measures (clinical interviews and automated analysis of objective facial expressions) and furthermore, determined relationships with social functioning and psychosis conversion risk calculator scores. Findings revealed that CHR youth in this sample exhibited alterations in facial expressions (blunting in joy expressions, but increased anger expressions). Furthermore, these data indicate that blunting in facial expressions of joy is related to decreased social functioning and higher scores on a psychosis conversion risk calculator. Each of the measures used in the study offered different perspectives of assessing facial expressions. The use of the PINS interview allowed us to hone in on facial expressivity scores from clinical interviews. Including the use of automated analysis allowed us to extend information obtained from clinical interviews and derive finding regarding specific emotions and in an objective manner. Given the main two ways in which individuals receive information about the emotions from others are through vision (e.g., facial expressions) and auditory perception, these data have critical clinical implications. Taken together, results contribute to the current literature and may be informative for understanding the pathogenesis of psychotic disorders.
Findings suggesting CHR youth exhibited alterations in facial expressions contribute to the growing literature investigating emotional processing and are consistent with evidence among schizophrenia populations (Kring & Elis, 2013; Kring, Gur, Blanchard, Horan, & Reise, 2013). In our study, the PINS and automated analysis of objective facial expressions converged suggesting that CHR youth exhibit impairments in facial expressivity, including blunting. Evidence from exploratory analyses indicating that consummatory anhedonia was not significantly related to the PINS BA item and automated analysis variables assessing facial expressivity further support that alterations in facial expressions are not better explained by the experience of emotion in this sample. Alterations in facial expressions may be a marker in the pathophysiology of psychosis. Furthermore, these data provide additional evidence for the utility of clinical interviews and automated analysis as a means to assess facial expressions among CHR youth.
While we were able to detect blunting in facial expressions in both measures, automated analysis revealed additional data that would not have been available with clinical interviews alone. Specifically, we found that CHR youth displayed increased anger expressions compared to controls. These findings provide a nuanced perspective and shed light on the need to investigate specific facial expressions in order to understand facial expressions more broadly among this group. Furthermore, these data highlight the potential for automated analysis to detect increased frequency of emotion in some domains, in contrast to clinical interviews, which emphasize symptom deficits. These data are in line with a previously noted prospective study in which boys and girls that went on to develop schizophrenia expressed more negative (e.g., anger, sadness) emotion compared to siblings (Walker, Grimes, Davis, & Smith, 1993). Even so, results indicating increased anger among CHR youth are still difficult to interpret. Given work from Horan and colleagues (2006) suggesting that patients with schizophrenia have difficulties inhibiting the experience of negative emotion, it is possible that these interpretations may translate to the expression of negative emotion among CHR youth, representing a larger emotion regulation deficit. Alternatively, increased anger expressions could be more representative of difficulties in concentration or regulating cognitive demand given that the muscle movements of anger and concentration overlap on an AU level (Ekman & Friesen, 1978). Future work is warranted to understand facial expressions in both positive and negative emotion among this group.
Results suggested that the BDI was not correlated with the PINS BA item or automated analysis. Depressive symptoms have been reported in other samples of CHR youth (Corcoran, et al., 2011). These data contribute to the ongoing debate of the role of primary and secondary symptoms in at-risk and psychosis populations. Generally, these data are in line with Corcoran and colleagues (2011) in which the authors found in a sample of 56 CHR youth that negative symptoms uniquely explained lower social functioning scores above and beyond depressive symptoms.
When investigating correlations between facial expression measures, we found that the PINS BA severity item was significantly related to automated analysis joy expressions but not anger expressions. This finding may be related to the differences between the two approaches. The PINS BA severity score is deduced from a clinical interview and examines blunted affect broadly. In contrast, automated analysis is objective and provides information about specific expressions (e.g., joy, anger, etc). Nonetheless, more research is needed to examine convergence and divergence between different approaches (e.g., clinical interviews, automated coding).
Several studies have reported that negative symptoms are impactful on social functioning in both schizophrenia and CHR populations (Corcoran, et al., 2011; Evensen, et al., 2012; Gur, et al., 2006; Meyer et al., 2014; Schlosser et al., 2015) which are consistent with our current findings indicating blunting in facial expressivity was related to decreases in social functioning. Additionally, social impairments have been found to be uniquely predictive of transition to psychosis (Cannon, et al., 2008). These data support findings from Schlosser and colleagues (2015) in which negative symptoms were predictive of social functioning in a sample of CHR youth. However, the present study differs from the literature in that we found a specific negative symptom category to be associated with decreased social functioning. This approach offers further support in the utility of investigating individual negative symptom categories and functioning. Furthermore, it highlights the importance of facial expressions in the context of social interactions. Given that social interactions involve not just verbal but also nonverbal information such as facial expressivity, facial expressions can be helpful for building and maintaining social relationships.
It has been suggested that negative symptoms contribute to the transition to psychosis and these data add to the larger literature investigating vulnerability markers that contribute to the onset of psychosis (Cannon, et al., 2008; Fusar-Poli, et al., 2012). The present results showed that blunting in facial expressivity measured by the PINS BA severity item and automated analysis was correlated with higher scores on a psychosis conversion risk calculator. Additionally, the present findings are in line with results from a study in which blunted or inappropriate affect (measured from the Assessment of Prodromal and Schizotypal Symptoms) (McGorry, Copolov, & Singh, 1990), among other CHR symptom items, were highly predictive of transition to psychosis (Mason et al., 2004). However, longitudinal data is still needed to better understand the relationship between facial expressivity and transition to psychosis.
Lastly, when exploratory analyses were conducted to determine sex differences in facial expression measures, findings indicated that CHR males showed greater anger expressions compared to control males. This is in support of the sensitivity of automated analysis of facial expressions in picking up these differences that could have been missed with clinical interviews. Replication and additional studies are needed to provide information regarding the impacts of biological sex on negative symptoms.
While there are several strengths to the study such as the multi-method approach, which allowed us to examine (a) generalizability (across measures) and (b) specificity (in terms of specific emotions) for alterations in facial expressivity, there are also important limitations to consider. The use of clinical interviews is a limitation and may not induce a full range of facial emotions and the content of the interview may influence results. Additionally, it is possible that when using the first-five minutes of the clinical interviews, we were getting a baseline read of facial expressions influenced by social relatedness, rapport, and comfort with the interviewer. Studies using other paradigms that are able to better control for these issues, such as an evocative film clip viewing task, are warranted. Furthermore, it is possible that interviewer biases can inflate correlational analyses (this limitation highlights the potential value of automated analysis given the objective nature of this approach), which provides further support regarding the need for replication studies using experimental paradigms. Additional studies are required to better understand the validity of automated analysis. Similarly, we used a freely available online calculator to calculate psychosis conversion risk scores. However, it is important to note that the online calculator was based on some of the items from the inventory used to detect a prodromal syndrome (SIPS) and we did use the first five-minutes of clinical interviews for facial expression analysis. This provides further evidence for the potential utility of experimental tasks to examine the relationships between facial expressivity and psychosis conversion risk calculator scores. Future work should investigate differences in biological sex in facial expressivity using a more evenly distributed number of males and females and the relationships between trauma and facial expressions.
Even though the sample size is comparable to studies in schizophrenia populations (Kring & Elis, 2013), larger samples sizes could deem useful in future work. It is important to note that in our study, we used correlational analysis so causation cannot be inferred. Furthermore, while we used psychosis conversion risk calculator scores, these data do not provide information regarding conversion; longitudinal data could provide important insights regarding the impacts of facial expressivity and conversion to psychosis over time. While we did examine anhedonia, there is still more work needed to better understand the relationships between the experience and expression of emotion among CHR populations. It may be useful for future work to continue to disentangle the mechanisms underlying alterations in facial expressivity, particularly testing to see if these abnormalities are a result of difficulties identifying and discriminating between emotions, impairments in perspective-taking, or deficits in motor abnormalities. Additionally, questions still remain within the literature regarding facial expressions and depressive symptoms and additional research should continue to unravel the role of primary and secondary negative symptoms.
Acknowledgments
Funding for this study was provided by the National Institute of Mental Health Grants R01MH094650, R21MH110374 to V.A.M, R21MH115231 to C.M.H. and V.A.M and T32 NS047987 (Ken Paller). The aims of the study are original and new. No authors have any potential conflicts of interest.
This study suggests that individuals at clinical high-risk (CHR) for psychosis exhibit alterations in facial expressivity which are related to impairments in social functioning and increased psychosis conversion risk calculator scores.
References
- Addington J, Penn D, Woods SW, Addington D, & Perkins DO (2008). Social functioning in individuals at clinical high risk for psychosis. Schizophrenia Research, 99(1), 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Piskulic D, Perkins D, Woods S, Liu L, & Penn D (2012). Affect recognition in people at clinical high risk of psychosis. Schizophenia Research, 140(1–3), 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Saeedi H, & Addington D (2006). Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophrenia research, 85(1), 142–150. [DOI] [PubMed] [Google Scholar]
- Ambady N, Rosenthal R (1992). Thin slices of expressive behavior as predictors of interpersonal consequences: A meta-analysis. Schizophrenia Bulletin, 111(2), 256–274. [Google Scholar]
- Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Schlögelhofer M, Mossaheb N, . . . McGorry PD (2011). Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophrenia Bulletin, 38(5), 1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC (1982). Negative symptoms in schizophrenia. Archives of General Psychiatry, 39(784–788), 564. [DOI] [PubMed] [Google Scholar]
- Auther A, Smith C, & Cornblatt B (2006). Global Functioning: Social Scale (GF: Social). Glen Oaks, NY: Zucker-Hillside Hospital. [Google Scholar]
- Bandini A, Orlandi S, Escalante HJ, Giovannelli F, Cincotta M, Reyes-Garcia CA, . . . Manfredi C (2017). Analysis of facial expressions in parkinson's disease through video-based automatic methods. Journal of Neuroscience Methods, 281, 7–20. [DOI] [PubMed] [Google Scholar]
- Bellack AS, Morrison RL, Wixted JT, & Mueser KT (1990). An analysis of social competence in schizophrenia. The British Journal of Psychiatry, 156(6), 809–818. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Snowhite R, & Oltmanns TF (1987). Anhedonia and emotional responses to affect evoking stimuli. Psychological Medicine, 17(3), 677–684. [DOI] [PubMed] [Google Scholar]
- Bobes J, Arango C, Garcia-Garcia M, & Rejas J (2010). Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. Journal of Clinical Psychiatry, 71(3), 280. [DOI] [PubMed] [Google Scholar]
- Brandt J, & Benedict RH (2001). Hopkins verbal learning test--revised: professional manual: Psychological Assessment Resources. [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, . . . McGlashan T (2008). Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Archives of General Psychiatry, 65(1), 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, . . . McGlashan TH (2016). An individualized risk calculator for research in prodromal psychosis. American Journal of Psychiatry, 173(10), 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión RE, Cornblatt BA, Burton CZ, Tso IF, Auther AM, Adelsheim S, . . . Sale TG (2016). Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP project. American Journal of Psychiatry, 173(10), 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Morrison SC, & Callaway DA (2013). Computerized facial analysis for understanding constricted/blunted affect: Initial feasibility, reliability, and validity data. Schizophrenia Research, 148(1), 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Keilp J, Kayser J, Klim C, Butler P, Bruder G, . . . Javitt D (2015). Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychological Medicine, 45(14), 2959–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Kimhy D, Parrilla-Escobar M, Cressman V, Stanford A, Thompson J, . . . Moore H (2011). The relationship of social function to depressive and negative symptoms in individuals at clinical high risk for psychosis. Psychological Medicine, 41(2), 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, & Cannon TD (2007). Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin, 33(3), 688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Walther S, Bernard JA, & Mittal VA (2018). Motor Clusters Reveal Differences in Risk for Psychosis, Cognitive Functioning, and Thalamocortical Connectivity: Evidence for Vulnerability Subtypes. Clinical Psychological Science, 2167702618773759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BS, Askenasy AR, Krasnoff L, & Dohrenwend BP (1978). Exemplification of a method for scaling life events: The PERI Life Events Scale. Journal of health and social behavior, 205–229. [PubMed] [Google Scholar]
- Ekman P, & Friesen WV (1978). Manual for the facial action coding system: Consulting Psychologists Press. [Google Scholar]
- Evensen J, Røssberg JI, Barder H, Haahr U, ten Velden Hegelstad W, Joa I, . . . Opjordsmoen S (2012). Flat affect and social functioning: a 10 year follow-up study of first episode psychosis patients. Schizophrenia research, 139(1), 99–104. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, & Remington G (2014). Impact of primary negative symptoms on functional outcomes in schizophrenia. European Psychiatry, 29(7), 449–455. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (1995). Structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute. [Google Scholar]
- Foussias G, Agid O, Fervaha G, & Remington G (2014). Negative symptoms of schizophrenia: clinical features, relevance to real world functioning and specificity versus other CNS disorders. European Neuropsychopharmacology, 24(5), 693–709. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, . . . McGuire P (2012). Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry, 69(3), 220–229. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, . . . Seidman LJ (2013). The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry, 70(1), 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebel W, & Wölwer W (2004). Facial expressivity in the course of schizophrenia and depression. European Archives of Psychiatry and Clinical Neuroscience, 254(5), 335–342. [DOI] [PubMed] [Google Scholar]
- Gur RE, Kohler CG, Ragland JD, Siegel SJ, Lesko K, Bilker WB, & Gur RC (2006). Flat affect in schizophrenia: relation to emotion processing and neurocognitive measures. Schizophrenia Bulletin, 32(2), 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J, Kohler CG, Gur RC, & Verma R (2011). Automated facial action coding system for dynamic analysis of facial expressions in neuropsychiatric disorders. Journal of Neuroscience Methods, 200(2), 237–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM, Richters JE, Weintraub S, & Neale JM (1987). Psychopathology and marital distress: The positive side of positive symptoms. Journal of Abnormal Psychology, 96(1), 27. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Gur RE, Reise SP, & Blanchard JJ (2011). Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophrenia Research, 132(2), 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Green MF, Kring AM, Nuechterlein KH (2006). Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? Journal of Abnormal Psychology, 115(3), 496–508. [DOI] [PubMed] [Google Scholar]
- iMotions Biometric Research Platform 6.0, iMotions A/S, Copenhagen, Denmark, 2016. [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, & Coughenour L (2004). The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research, 68(2), 283–297. [DOI] [PubMed] [Google Scholar]
- Kelley ME, Haas GL, & van Kammen DP (2008). Longitudinal progression of negative symptoms in schizophrenia: a new look at an old problem. Schizophrenia Research, 105(1), 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, & Marder SR (2006). The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia bulletin, 32(2), 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, & Marder SR (2011). The brief negative symptom scale: psychometric properties. Schizophrenia Bulletin, 37(2), 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, & Martin EA (2006). Emotional processing in schizophrenia. Cognitive Neuropsychiatry, 11(3), 250–271. [DOI] [PubMed] [Google Scholar]
- Kring AM, & Earnst KS (1999). Stability of emotional responding in schizophrenia. Behavior Therapy, 30(3), 373–388. [Google Scholar]
- Kring AM, & Elis O (2013). Emotion deficits in people with schizophrenia. Annual review of clinical psychology, 9, 409–433. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, & Reise SP (2013). The clinical assessment interview for negative symptoms (CAINS): final development and validation. American Journal of Psychiatry, 170(2), 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Kerr SL, & Earnst KS (1999). Schizophrenic patients show facial reactions to emotional facial expressions. Psychophysiology, 36(2), 186–192. [PubMed] [Google Scholar]
- Kring AM, & Moran EK (2008). Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia Bulletin, 34(5), 819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Smith CW, Auther AM, Correll CU, & Cornblatt BA (2003). The assessment of" prodromal schizophrenia": unresolved issues and future directions. Schizophrenia Bulletin, 29(4), 717. [DOI] [PubMed] [Google Scholar]
- Littlewort G, Whitehill J, Wu T, Fasel I, Frank M, Movellan J, & Bartlett M (2011). The computer expression recognition toolbox (CERT). Paper presented at the Automatic Face & Gesture Recognition and Workshops (FG 2011), 2011 IEEE International Conference on. [Google Scholar]
- Malla AK, Takhar JJ, Norman RM, Manchanda R, Cortese L, Haricharan R, . . . Ahmed R (2002). Negative symptoms in first episode non‐affective psychosis. Acta Psychiatrica Scandinavica, 105(6), 431–439. [DOI] [PubMed] [Google Scholar]
- Martin CS, Pollock NK, Bukstein OG, & Lynch KG (2000). Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug & Alcohol Dependence, 59(2), 173–176. [DOI] [PubMed] [Google Scholar]
- Mason O, Startup M, Halpin S, Schall U, Conrad A, & Carr V (2004). Risk factors for transition to first episode psychosis among individuals with ‘at-risk mental states’. Schizophrenia Research, 71(2), 227–237. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Copolov DL, & Singh BS (1990). Royal Park Multidiagnostic Instrument for Psychosis: Part I. Rationale and Review. Schizophrenia Bulletin, 16(3), 501–515. [DOI] [PubMed] [Google Scholar]
- Meyer EC, Carrión RE, Cornblatt BA, Addington J, Cadenhead KS, Cannon TD, . . . Walker EF (2014). The relationship of neurocognition and negative symptoms to social and role functioning over time in individuals at clinical high risk in the first phase of the North American Prodrome Longitudinal Study. Schizophrenia Bulletin, 40(6), 1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Fone K, Steckler T, & Horan WP (2014). Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. European Neuropsychopharmacology, 24(5), 645–692. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, . . . Woods SW (2003). Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin, 29(4), 703. [DOI] [PubMed] [Google Scholar]
- Mote J, Stuart BK, & Kring AM (2014). Diminished emotion expressivity but not experience in men and women with schizophrenia. Journal of Abnormal Psychology, 123(4), 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, . . . Gold JM (2008). The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. American Journal of Psychiatry, 165(2), 203–213. [DOI] [PubMed] [Google Scholar]
- Owada K, Kojima M, Yassin W, Kuroda M, Kawakubo Y, Kuwabara H, . . . Yamasue H (2018). Computer-analyzed facial expression as a surrogate marker for autism spectrum social core symptoms. PloS One, 13(1), e0190442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantic M, & Rothkrantz LJ (2004). Facial action recognition for facial expression analysis from static face images. IEEE Transactions on Systems, Man, and Cybernetics, Part B (Cybernetics), 34(3), 1449–1461. [DOI] [PubMed] [Google Scholar]
- Pelletier-Baldelli A, Strauss GP, Visser KH, & Mittal VA (2017). Initial development and preliminary psychometric properties of the Prodromal Inventory of Negative Symptoms (PINS). Schizophrenia Research, 189, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskulic D, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, . . . Walker EF (2012). Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Research, 196(2), 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser DA, Campellone TR, Biagianti B, Delucchi KL, Gard DE, Fulford D, . . . Vinogradov S (2015). Modeling the role of negative symptoms in determining social functioning in individuals at clinical high risk of psychosis. Schizophrenia Research, 169(1), 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Harrow M, Grossman LS, & Rosen C (2010). Periods of recovery in deficit syndrome schizophrenia: a 20-year multi–follow-up longitudinal study. Schizophrenia Bulletin, 36(4), 788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, . . . Kirkpatrick B (2012). Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophrenia Research, 142(1), 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart RW, Dressler M, Kumar P, Khan S, & Coppola JF (2016). Determining emotions via facial expression analysis software. Proceedings of Student-Faculty Research Day, CSIS, Pace University. [Google Scholar]
- Trémeau F, Malaspina D, Duval F, Corrêa H, Hager-Budny M, Coin-Bariou L, . . . Gorman JM (2005). Facial expressiveness in patients with schizophrenia compared to depressed patients and nonpatient comparison subjects. American Journal of Psychiatry, 162(1), 92–101. [DOI] [PubMed] [Google Scholar]
- Trevisan DA, Bowering M, & Birmingham E (2016). Alexithymia, but not autism spectrum disorder, may be related to the production of emotional facial expressions. Molecular Autism, 7(1), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Wout M, Aleman A, Kessels RP, Larøi F, & Kahn RS (2004). Emotional processing in a non-clinical psychosis-prone sample. Schizophrenia Research, 68(2), 271–281. [DOI] [PubMed] [Google Scholar]
- Walker EF, Grimes KE, Davis DM, & Smith AJ (1993). Childhood precursors of schizophrenia: facial expressions of emotion. The American Journal of Psychiatry, 150(11), 1654. [DOI] [PubMed] [Google Scholar]
- Wolf K, Mass R, Kiefer F, Wiedemann K, & Naber D (2006). Characterization of the facial expression of emotions in schizophrenia patients: preliminary findings with a new electromyography method. The Canadian Journal of Psychiatry, 51(6), 335–341. [DOI] [PubMed] [Google Scholar]
- Yung AR, Yung AR, Pan Yuen H, Mcgorry PD, Phillips LJ, Kelly D, . . . Killackey E (2005). Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Australian & New Zealand Journal of Psychiatry, 39(11–12), 964–971. [DOI] [PubMed] [Google Scholar]