Abstract
Background
In the COAPT trial, transcatheter mitral valve repair (TMVr) with the edge-to-edge device led to reduced heart failure (HF) hospitalizations and improved survival in patients with symptomatic HF and 3-4+ secondary mitral regurgitation (MR) on maximally-tolerated medical therapy. Given the advanced age and comorbidities of these patients, improvement in health status is also an important treatment goal.
Objective
To understand the health status outcomes of patients with HF and 3-4+ secondary MR treated with TMVr versus standard care.
Methods
The COAPT trial randomized patients with HF and 3-4+ secondary MR to TMVr (n=302) or standard care (n=312). Health status was assessed at baseline and at 1, 6, 12, and 24 months with the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the SF-36 health status survey. The primary health status endpoint was the KCCQ overall summary score (KCCQ-OS; range 0-100; higher=better; minimum clinically important difference=5 points).
Results
At baseline, patients had substantially impaired health status (mean KCCQ-OS 52.4 ± 23.0). While health status was unchanged over time in the standard care arm, patients randomized to TMVr demonstrated substantial improvement in the KCCQ-OS at 1 month (mean between-group difference 15.9 points, 95% CI 12.3 to 19.5) with only slight attenuation of this benefit through 24 months (mean between-group difference 12.8 points, 95% CI 7.5 to 18.2). At 24 months, 36.4% of TMVr patients were alive with a moderately large (≥10-point) improvement vs. 16.6% of standard care patients (p<0.001), for a number needed to treat of 5.1 patients (95% CI 3.6 to 8.7). TMVr patients also reported better generic health status at each timepoint (24-month mean difference in SF-36 summary scores: physical 3.6 points, 95% CI 1.4-5.8; mental 3.6 points, 95% CI 0.8-6.4).
Conclusion
Among patients with symptomatic HF and 3-4+ secondary MR receiving maximally-tolerated medical therapy, TMVr with the edge-to-edge device resulted in substantial early and sustained health status improvement compared with medical therapy alone.
Keywords: mitral valve regurgitation, transcatheter valve, quality of life
CONDENSED ABSTRACT
In this prospective sub-study of the COAPT trial, we examined the disease-specific health status outcomes of patients with symptomatic HF and 3-4+ secondary mitral regurgitation randomized to transcatheter mitral valve repair (TMVr; n=302) versus standard care (n=312). Compared with standard care, patients randomized to TMVr demonstrated substantially better health status at 1 month with only slight attenuation of this benefit by 24 months. At 24 months, 36% of TMVr patients were alive with a moderately large improvement in health status vs. 17% of standard care patients (number needed to treat=5.1).
Introduction
Edge-to-edge transcatheter mitral valve repair (TMVr) with the edge-to-edge MitraClip device (Abbott, Santa Clara, CA) effectively reduces mitral regurgitation (MR) with low risk for periprocedural complications (1,2). Although the device was originally approved in the US exclusively for patients with degenerative (primary) MR at extreme surgical risk, the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial recently demonstrated that the benefit of TMVr extends to patients with heart failure (HF) and functional (secondary) MR as well (3). In this trial, TMVr reduced HF hospitalizations and all-cause mortality over 24 months compared with maximally-tolerated medical therapy alone.
Beyond prolonging survival and reducing hospitalizations, improving patients’ health status (i.e., symptoms, functional status, quality of life) is a key treatment goal of TMVr. In fact, among older patients with comorbidities and high symptom burden, health status improvement may be of greater importance to patients than improved survival (4,5). Prior uncontrolled studies among patients with symptomatic degenerative MR showed rapid and marked improvement in health status with TMVr.(6) However, the effect of TMVr on the health status of patients with HF and secondary MR is not known. To address this critical gap in knowledge and to better define the full clinical value of TMVr, we performed a prospective health status sub-study in the COAPT trial.
Methods
Study Design
The design(7) and primary results(3) of the COAPT trial (ClinicalTrials.gov: NCT01626079) have been published. Briefly, COAPT was a multicenter, randomized, open-label trial of TMVr with the MitraClip device in HF patients with left ventricular ejection fraction between 20 and 50% and 3-4+ secondary MR who remained symptomatic despite maximally-tolerated guideline-directed therapy (including use of cardiac resynchronization therapy when indicated). Patients were randomized 1:1 to TMVr or standard care and followed through a minimum of 1 year (and up to 2 years) for clinical events and health status. The study was approved by the institutional review board at each participating site, and all patients provided written informed consent.
Health Status Outcomes
Health status was evaluated at baseline and at 1, 6, 12, and 24 months from baseline with the Kansas City Cardiomyopathy Questionnaire (KCCQ)(8) and the Medical Outcomes Study Short-Form 36 (SF-36) Health Survey.(9) The KCCQ is a HF-specific health status measure that consists of 23 questions and 5 domains: physical limitation, symptoms, quality of life, social limitation, and self-efficacy. The first 4 domains are combined into an overall summary score (KCCQ-OS), which was the primary health status outcome of COAPT. Scores for domains and the summary score range 0 to 100 with higher scores indicating better health status. KCCQ-OS scores correlate roughly with New York Heart Association class as follows: class I ~KCCQ-OS 75-100; class II ~KCCQ-OS 60-74; class III ~KCCQ-OS 45-59; and class IV ~KCCQ-OS 0-44,(10) and changes in KCCQ-OS of 5, 10, and 20 points correspond with small, moderate, or large clinical changes, respectively.(11) The SF-36 is a generic health status measure that provides physical (SF-36 PCS) and mental (SF-36 MCS) summary scores, which are scaled to an overall population mean of 50 and standard deviation of 10; higher scores indicate better health status, and the minimal clinically important change is ~2.5 points (12).
Statistical Analysis
All analyses were performed from the time of randomization using an intention-to-treat approach. Within each treatment group, mean scores for each of the health status measures at each follow-up time point were compared with baseline using paired t-tests. For the primary analysis, between-group differences of health status scores of over time were estimated from piecewise linear regression models with a knot at 1 month. Models included time (linear and spline), treatment, and the interactions between treatment and time, age, sex, and severe lung disease. Subgroup analyses explored potential heterogeneity in health status differences at 1 year of follow-up by introducing interaction terms between treatment and patient factors: age (<74 vs. ≥74 years), sex, lung disease, etiology of cardiomyopathy (ischemic vs. non-ischemic), left ventricular end-diastolic volume index (<94 vs. ≥94 ml/m2), severity of mitral regurgitation (effective regurgitant orifice <0.4 vs. ≥0.4 cm2), gait speed on 5-meter walk test (<0.8 vs. ≥0.8 m/s), and dependency in activities of daily living.
To aid in clinical interpretability, we performed a series of categorical analyses among surviving patients and among all eligible patients (including those who died). At each time point, we calculated the proportion of patients in each treatment group who were alive with a moderately large health status improvement (change ≥10 points from baseline), alive with a large health status improvement (change ≥20 points from baseline), and “alive and well” as previously defined (KCCQ-OS ≥60 and no decline ≥10 points from baseline) (13). Proportions were compared between groups at each time point using chi-square tests, and absolute risk differences (with 95% CI) and numbers needed to treat were estimated. In addition, an ordinal analysis was performed that calculated the proportion of patients at each time point who were dead, had worse health status (change ≤−5), no change in health status (change >−5 to <5), or improved health status (change ≥5).
Finally, as a sensitivity analysis, survival and health status were jointly modeled using a Bayesian approach (14). An important limitation to health status analysis is that health status can only be assessed in surviving patients. Because patients with worse health status are more likely to die,(15–17) ignoring these deaths may bias the estimates of health status upward, as the sickest patients are systematically removed from the analyses due to death. In this novel approach, survival and health status were jointly modeled to allow the deaths to inform the health status estimates. A population level piece-wise linear model was fit with a knot at 1-month and patient- specific trajectories modeled by 3 random effects: baseline intercept, 1-month intercept, and post 1-month slope—the latter 2 parameters jointly modeled with the survival data. Joint modeling was done in a fully Bayesian framework, and priors were selected to be weakly informative, which stabilized the model while allowing the data to inform the posterior as much as possible.(18) Point estimates and credible intervals (CrI) were generated for the KCCQ-OS, SF- 36 PCS, and SF-36 MCS scores over time and the treatment effect of TMVr. The mean treatment effects from these joint analyses can be conceptualized as the expected benefit of TMVr if the patient survives to the time point of interest. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) and R (R foundation, Vienna Austria), and statistical significance was defined as a 2-sided p-value <0.05. The primary outcome was the between-group comparison of the KCCQ-OS over time with all other comparisons considered secondary; as such, there was no correction for multiple comparisons (19).
Results
Patient Population
Between December 2012 and June 2017, 614 patients were enrolled in COAPT at 78 centers in the United States and Canada; 302 were randomized to TMVr and 312 to standard care. Three patients in the standard care arm did not complete baseline health status measures and were excluded. Baseline characteristics were well balanced between treatment groups (Table 1). Mean age of the analytic cohort was 72±11 years, 64% were men, and mean left ventricular ejection fraction was 31.3±9.3%.
Table 1.
Baseline Characteristics
| TMVr (n=302) | Standard Care (n=309) | |
|---|---|---|
| Age—years | 71.7±11.8 | 72.7±10.6 |
| Male sex—no. (%) | 201 (66.6) | 191 (61.8) |
| Body surface area—m2 | 1.9±0.2 | 1.9±0.3 |
| Prior myocardial infarction—no. (%) | 156 (51.7) | 159 (51.5) |
| Peripheral vascular disease—no. (%) | 52 (17.2) | 57 (18.4) |
| Left ventricular ejection fraction—% | 31.3±9.1 | 31.2±9.6 |
| Prior stroke—no. (%) | 37 (12.3) | 35 (11.3) |
| Diabetes mellitus—no. (%) | 106 (35.1) | 122 (39.5) |
| Serum creatinine—mg/dL | 1.8±1.2 | 1.8±1.4 |
| Hemoglobin—g/dL | 16.4±22.1 | 15.9±20.6 |
| Atrial fibrillation—no. (%) | 168 (55.6) | 157 (50.8) |
| Chronic lung disease—no. (%) | 71 (23.5) | 71 (23.0) |
| Home oxygen—no. (%) | 10 (3.3) | 10 (3.2) |
| Prior pacemaker—no. (%) | 18 (6.0) | 25 (8.1) |
| Prior coronary artery bypass surgery—no. (%) | 121 (40.1) | 124 (40.1) |
TMVr, transcatheter mitral valve repair. 3 patients who were randomized to standard care did not complete the baseline health status surveys and were excluded from the health status study.
There were no significant differences between groups.
Baseline Health Status and Within-Group Comparisons
Compliance was high for health status measures over time, with ≥88% of eligible patients having KCCQ-OS data at each time point (Online Table 1). Mean KCCQ-OS score at baseline was 52.4±23.0, with the lowest domain score being quality of life at 44.9±25.7. Mean SF-36 PCS was 32.8±9.6, and mean SF-36 MCS was 46.0±12.9.
Among patients randomized to TMVr, KCCQ-OS increased by an average of 16.9 points by 1 month (95% confidence interval [CI] 14.2 to 19.6), with similar within-group changes at later time points (Table 2). All KCCQ domains improved significantly by 1 month, with the largest change in the quality of life domain (mean change 23.2 points, 95% CI 20.0 to 26.4). Scores on the SF-36 PCS and MCS both also increased significantly at 1 month, with changes of 6.0 points (95% CI 5.0 to 7.1) and 4.2 points (95% CI 2.8 to 5.6), respectively. Among patients randomized to standard care, the KCCQ-OS increased, on average, by 2.1 points in the first month (95% CI −0.1 to 4.3) with small but significant changes at later time points of 5-6 points and no significant changes in the SF-36 PCS or MCS scores.
Table 2.
Mean Scores and Within-Group Changes Compared With Baseline
| TMVr |
Standard Care |
|||||
|---|---|---|---|---|---|---|
| Mean ± SD | Paired Difference (95% CI) | P-Value | Mean ± SD | Paired Difference (95% CI) | P-Value | |
| KCCQ Overall Summary | ||||||
| Baseline | 53.2 ± 22.8 | 51.6 ± 23.3 | ||||
| 1 month | 70.9 ± 21.1 | 16.9 (14.2 to 19.6) | <0.001 | 54.6 ± 24.7 | 2.1 (−0.1 to 4.3) | 0.06 |
| 6 months | 73.0 ± 21.3 | 18.5 (15.4 to 21.6) | <0.001 | 59.0 ± 24.7 | 5.3 (2.4 to 8.3) | 0.001 |
| 12 months | 71.8 ± 22.2 | 17.0 (13.6 to 20.3) | <0.001 | 60.2 ± 24.5 | 5.1 (1.5 to 8.6) | 0.005 |
| 24 months | 70.9 ± 23.8 | 18.4 (13.7 to 23.2) | <0.001 | 61.2 ± 24.4 | 5.8 (0.2 to 11.4) | 0.04 |
| KCCQ Physical Limitations | ||||||
| Baseline | 58.3 ± 24.5 | 55.7 ± 26.0 | ||||
| 1 month | 69.9 ± 23.8 | 11.1 (8.3 to 13.9) | <0.001 | 55.8 ± 26.3 | −0.5 (−3.2 to 2.1) | 0.69 |
| 6 months | 72.2 ± 22.7 | 13.1 (9.9 to 16.3) | <0.001 | 59.0 ± 26.8 | 0.8 (−2.6 to 4.2) | 0.64 |
| 12 months | 69.7 ± 24.1 | 9.4 (5.9 to 12.9) | <0.001 | 57.7 ± 27.7 | −0.4 (−4.4 to 3.7) | 0.86 |
| 24 months | 67.6 ± 26.3 | 8.8 (3.7 to 14.0) | <0.001 | 59.5 ± 27.4 | 2.9 (−3.0 to 8.9) | 0.33 |
| KCCQ Total Symptoms | ||||||
| Baseline | 60.3 ± 24.9 | 58.9 ± 24.7 | ||||
| 1 month | 76.9 ± 20.9 | 15.5 (12.7 to 18.4) | <0.001 | 61.1 ± 24.9 | 1.3 (−1.2 to 3.8) | 0.31 |
| 6 months | 76.9 ± 22.4 | 14.9 (11.8 to 18.1) | <0.001 | 67.0 ± 24.0 | 5.5 (2.3 to 8.7) | 0.001 |
| 12 months | 76.2 ± 22.3 | 13.4 (9.9 to 16.8) | <0.001 | 68.4 ± 22.6 | 5.7 (1.8 to 9.6) | 0.004 |
| 24 months | 74.8 ± 23.5 | 14.8 (10.2 to 19.4) | <0.001 | 68.8 ± 22.4 | 7.4 (1.2 to 13.6) | 0.02 |
| KCCQ Quality of Life | ||||||
| Baseline | 45.2 ± 25.6 | 44.7 ± 25.8 | ||||
| 1 month | 68.9 ± 23.8 | 23.2 (20.0 to 26.4) | <0.001 | 49.8 ± 27.6 | 4.5 (1.8 to 7.1) | 0.001 |
| 6 months | 70.3 ± 24.4 | 24.6 (20.9 to 28.3) | <0.001 | 55.9 ± 28.1 | 9.0 (5.6 to 12.5) | <0.001 |
| 12 months | 71.4 ± 24.8 | 25.4 (21.3 to 29.4) | <0.001 | 57.4 ± 27.3 | 8.9 (4.9 to 12.9) | <0.001 |
| 24 months | 71.6 ± 26.0 | 28.3 (23.0 to 33.6) | <0.001 | 58.5 ± 28.1 | 7.5 (1.1 to 13.8) | 0.02 |
| KCCQ Social Limitation | ||||||
| Baseline | 49.5 ± 29.2 | 46.8 ± 30.4 | ||||
| 1 month | 67.9 ± 29.3 | 17.7 (13.9 to 21.5) | <0.001 | 50.8 ± 32.1 | 2.2 (−0.9 to 5.4) | 0.17 |
| 6 months | 72.2 ± 27.4 | 21.6 (17.2 to 25.9) | <0.001 | 54.0 ± 31.3 | 5.6 (1.8 to 9.3) | 0.004 |
| 12 months | 69.8 ± 28.6 | 19.0 (14.6 to 23.5) | <0.001 | 57.2 ± 31.9 | 6.6 (2.1 to 11.1) | 0.005 |
| 24 months | 68.5 ± 30.3 | 20.4 (13.8 to 27.1) | <0.001 | 58.0 ± 31.9 | 6.4 (−1.0 to 13.8) | 0.09 |
| SF-36 Physical Component Summary | ||||||
| Baseline | 33.0 ± 9.0 | 32.6 ± 9.9 | ||||
| 1 month | 39.2 ± 9.2 | 6.0 (5.0 to 7.1) | <0.001 | 33.6 ± 9.9 | 0.6 (−0.4 to 1.6) | 0.24 |
| 6 months | 38.9 ± 10.3 | 5.5 (4.2 to 6.8) | <0.001 | 34.2 ± 9.9 | 1.0 (−0.1 to 2.1) | 0.07 |
| 12 months | 38.7 ± 10.2 | 5.0 (3.6 to 6.4) | <0.001 | 34.6 ± 10.8 | 0.8 (−0.6 to 2.2) | 0.28 |
| 24 months | 38.1 ± 10.2 | 4.9 (3.1 to 6.8) | <0.001 | 34.1 ± 10.2 | 1.6 (−0.7 to 3.8) | 0.17 |
| SF-36 Mental Component Summary | ||||||
| Baseline | 46.7 ± 12.7 | 45.4 ± 13.0 | ||||
| 1 month | 51.4 ± 11.5 | 4.2 (2.8 to 5.6) | <0.001 | 46.1 ± 13.4 | 0.4 (−1.0 to 1.9) | 0.56 |
| 6 months | 52.0 ± 10.3 | 4.6 (3.1 to 6.2) | <0.001 | 47.0 ± 12.9 | 1.1 (−0.3 to 2.4) | 0.13 |
| 12 months | 51.3 ± 10.8 | 4.3 (2.5 to 6.1) | <0.001 | 48.2 ± 13.8 | 1.9 (−0.0 to 3.8) | 0.055 |
| 24 months | 50.1 ± 12.6 | 4.2 (1.6 to 6.8) | 0.002 | 48.9 ± 11.7 | −0.5 (−3.3 to 2.2) | 0.69 |
TMVr, transcatheter mitral valve repair; KCCQ, Kansas City Cardiomyopathy Questionnaire; SF, Medical Outcomes Study Short Form
Between-Group Comparisons and Subgroups
Among surviving patients at 1 month, the KCCQ-OS increased to a greater extent in the TMVr arm compared with standard care (mean difference 15.9 points, 95% CI 12.3 to 19.5, p<0.001; Central Illustration), with only slight attenuation of the treatment effect over time (mean difference among surviving patients of 14.5 points [95% CI 10.9 to 18.1] and 12.8 points [95% CI 7.5 to 18.2] at 12 months and 24 months, respectively; p<0.001 for both comparisons). Results were similar for each of the KCCQ domains (Online Table 2). A similar pattern was observed for both the SF-36 PCS and MCS, which improved significantly within 1 month in the TMVr arm compared with standard care, with mean between group differences of 5.3 (95% CI 3.8 to 6.8) and 5.2 (95% CI 3.3 to 7.1) points respectively, and only slight attenuation over time (Figure 1A and 1B). The health status benefit of TMVr compared with standard therapy was consistent across all subgroups (Table 3; all interaction p-values >0.2), excepting cause of cardiomyopathy; patients with an ischemic cardiomyopathy appeared to derive greater health status benefit from TMVr compared with those with a nonischemic cardiomyopathy (p-value for interaction=0.02). Notably, patients with chronic lung disease, slow gait speed, or dependency in an activity of daily living tended to have lower KCCQ-OS scores both at baseline and 1-year follow-up.
Figure 1. Generic Health Status Scores Over 24 Months.
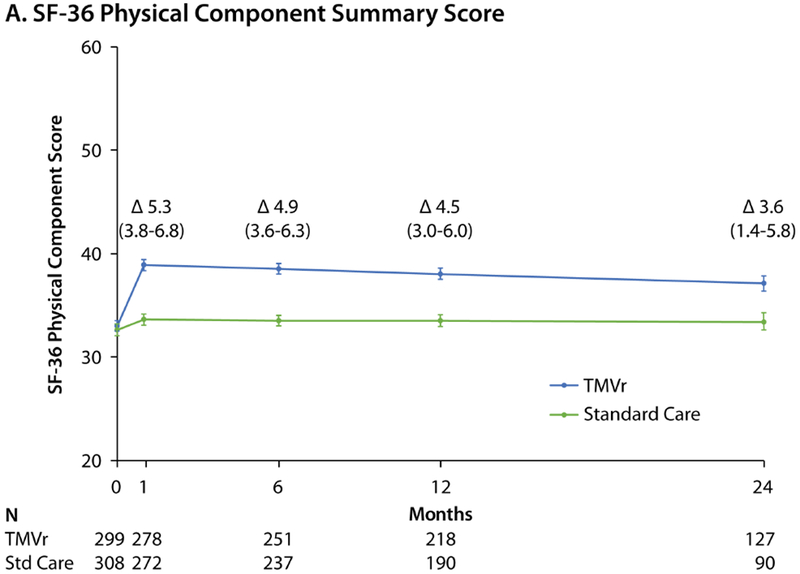
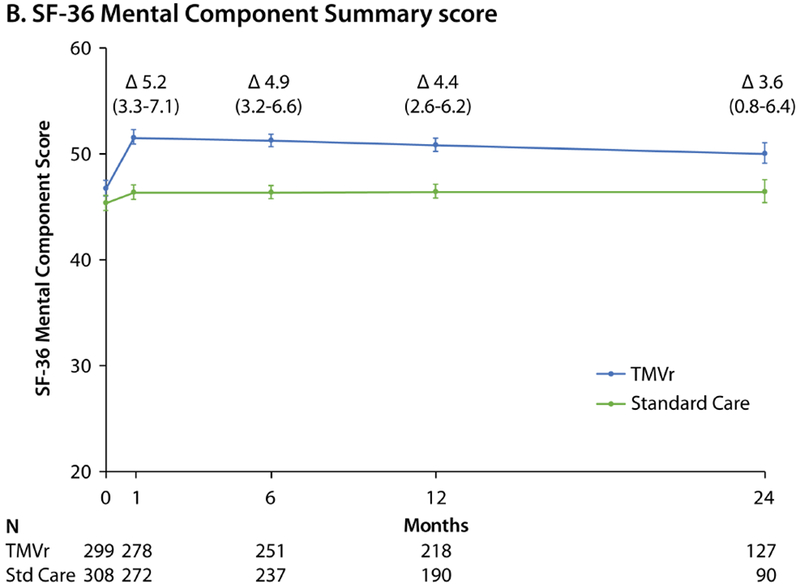
Predicted mean values with standard errors, as derived from piecewise linear regression models taking into account all available health status scores. Δ values represent mean between-group treatment difference and 95% CI. A. Short Form-36 Physical Component Summary score. B. Short-Form-36 Mental Component Summary score. Reductions in patient numbers in year 2 are due to a combination of death and administrative censoring.
Table 3.
Estimated Effect of TMVr on KCCQ-OS at 1-Year Among Key Patient Subgroups
| n | TMVr Mean | Standard Care Mean | Mean Difference (95% CI) | Interaction P-Value | |
|---|---|---|---|---|---|
| Age | 0.24 | ||||
| <74 years | 297 | 71.1 | 56.4 | 14.7 (9.6 to 19.8) | |
| ≥74 years | 317 | 68.3 | 54.3 | 14.1 (9.0 to 19.2) | |
| Sex | 0.67 | ||||
| Male | 393 | 70.9 | 55.5 | 15.3 (10.8 to 19.8) | |
| Female | 221 | 67.7 | 54.8 | 12.8 (6.8 to 18.8) | |
| Chronic lung disease | 0.33 | ||||
| Yes | 143 | 62.3 | 48.3 | 14.0 (6.3 to 21.8) | |
| No | 471 | 71.9 | 57.5 | 14.4 (10.3 to 18.5) | |
| Cause of cardiomyopathy | 0.02 | ||||
| Ischemic | 373 | 70.5 | 52.3 | 18.3 (13.6 to 22.9) | |
| Nonischemic | 241 | 68.3 | 60.6 | 8.4 (2.7 to 14.1) | |
| Left ventricular end diastolic volume index | 0.27 | ||||
| <94 mL/m2 | 269 | 66.2 | 51.9 | 14.3 (8.9 to 19.7) | |
| ≥94 mL/m2 | 298 | 72.3 | 57.0 | 15.3 (10.1 to 20.4) | |
| Severity of MR | 0.64 | ||||
| ERO <0.4 cm2 | 325 | 70.7 | 56.0 | 14.7 (9.8 to 19.6) | |
| ERO ≥0.4 cm2 | 266 | 68.7 | 53.3 | 15.3 (9.8 to 20.9) | |
| Walk speed | 0.90 | ||||
| <0.8 m/s | 268 | 65.2 | 50.3 | 14.9 (9.3 to 20.5) | |
| ≥0.8 m/s | 343 | 73.0 | 59.5 | 13.5 (8.9 to 18.1) | |
| ADL dependency | 0.80 | ||||
| Yes | 107 | 60.2 | 42.6 | 17.6 (8.6 to 26.6) | |
| No | 505 | 71.5 | 58.0 | 13.4 (9.6 to 17.3) |
TMVr, transcatheter mitral valve repair; KCCQ, Kansas City Cardiomyopathy Questionnaire; MR, mitral regurgitation; ERO, effective orifice area; ADL, activity of daily living.
Ordinal and Categorical Outcomes
Figure 2 shows the proportion of patients by treatment group at each time point who were dead, worse, unchanged, and improved from baseline according to the KCCQ-OS. At each time point, more patients who were randomized to TMVr were improved, fewer patients had significantly worsened from baseline, and fewer had died (with the exception of 1 month) compared with standard therapy. At 24 months, 39.3% of patients in the TMVr arm were alive and improved as compared with 20.8% in the standard care arm. Examining different thresholds of improvement at 24 months, 36.4% of TMVr patients were alive with a moderately large improvement in KCCQ-OS vs. 16.6% of standard care patients (number needed to treat [NNT] 5.1, 95% CI 3.6 to 8.7), and 29.1% of TMVr patients were alive with a large health status improvement vs. 11.7% of standard care patients (NNT 5.7, 95%CI 4.0 to 10.2; Table 4).
Figure 2. Ordinal Outcomes Integrating Survival and Health Status.
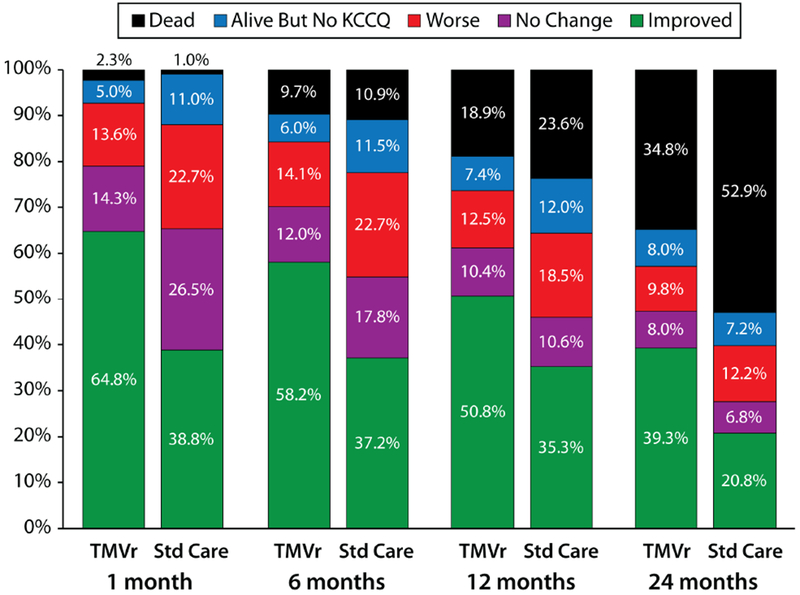
Worse=decrease in KCCQ-OS from baseline of ≥5 points; no change=change in KCCQ-OS of <5 points from baseline (increase or decrease); improved=increase in KCCQ-OS of ≥5 points from baseline. Patients who did not reach the time point of interest or withdrew from COAPT were excluded.
Table 4.
Categorical Outcomes
| TMVr | Standard Care | Risk Difference (95% CI) | P-Value | |
|---|---|---|---|---|
| Moderately improved | ||||
| 1 month | 163/279 (58.4%) | 71/272 (26.1%) | 32.3% (24.5, 40.1) | <0.001 |
| 6 months | 154/252 (61.1%) | 88/236 (37.3%) | 23.8% (15.2, 32.4) | <0.001 |
| 12 months | 125/219 (57.1%) | 86/188 (45.7%) | 11.3% (1.6, 21.0) | 0.022 |
| 24 months | 75/128 (58.6%) | 34/88 (38.6%) | 19.9% (6.7, 33.2) | 0.004 |
| Substantially improved | ||||
| 1 month | 106/279 (38.0%) | 34/272 (12.5%) | 25.5% (18.6, 32.4) | <0.001 |
| 6 months | 112/252 (44.4%) | 54/236 (22.9%) | 21.6% (13.4, 29.7) | <0.001 |
| 12 months | 91/219 (41.6%) | 41/188 (21.8%) | 19.7% (10.9, 28.5) | <0.001 |
| 24 months | 60/128 (46.9%) | 24/88 (27.3%) | 19.6% (6.9, 32.3) | 0.004 |
| Alive and moderately improved | ||||
| 1 month | 163/286 (57.0%) | 71/275 (25.8%) | 31.2% (23.4, 38.9) | <0.001 |
| 6 months | 154/281 (54.8%) | 88/269 (32.7%) | 22.1% (14.1, 30.2) | <0.001 |
| 12 months | 125/275 (45.5%) | 86/257 (33.5%) | 12.0% (3.7, 20.2) | 0.005 |
| 24 months | 75/206 (36.4%) | 34/205 (16.6%) | 19.8% (11.5, 28.1) | <0.001 |
| Alive and substantially improved | ||||
| 1 month | 106/286 (37.1%) | 34/275 (12.4%) | 24.7% (17.9, 31.5) | <0.001 |
| 6 months | 112/281 (39.9%) | 54/269 (20.1%) | 19.8% (12.3, 27.2) | <0.001 |
| 12 months | 91/275 (33.1%) | 41/257 (16.0%) | 17.1% (9.8, 25.0) | <0.001 |
| 24 months | 60/206 (29.1%) | 24/205 (11.7%) | 17.4% (9.8, 24.8) | <0.001 |
| Alive and well (KCCQ-OS ≥60 points and change of ≥−10 points from baseline) | ||||
| 1 month | 199/286 (69.6%) | 104/275 (37.8%) | 31.8% (23.9, 39.6) | <0.001 |
| 6 months | 176/281 (62.6%) | 108/269 (40.2%) | 22.5% (14.3, 30.6) | <0.001 |
| 12 months | 154/275 (56.0%) | 83/257 (32.3%) | 23.7% (15.5, 31.9) | <0.001 |
| 24 months | 87/206 (42.2%) | 44/205 (21.5%) | 20.8% (12.0, 29.5) | <0.001 |
TMVr, transcatheter mitral valve repair; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire Overall Summary Score.
Patients who had not yet reached the time point of interest, withdrew from COAPT, or were alive but did not complete the KCCQ-OS were excluded
Moderately improved: ≥10-point improvement in KCCQ-OS; substantially improved: ≥20-point improvement in KCCQ-OS
Joint Model Outcomes
Figure 3 shows the estimated health status trajectories for the KCCQ-OS, SF-36 PCS, and SF-36 MCS with TMVr vs. standard care as derived from piecewise linear regression (primary analysis) and the joint model that allowed the survival data to inform the health status estimates. Given the different mortality rates between the two groups, the treatment benefit of TMVr in the joint Bayesian model was greater than was estimated in the primary analysis at each time point. At 1 month, the mean treatment difference between TMVr and standard care in the KCCQ-OS was 18.5 points (95% CrI 14.3 to 22.7) using the joint approach, as compared with 15.9 points in the original model (Online Table 3). Furthermore, after accounting for the competing risk of mortality in the joint models, there was no attenuation in the treatment benefit of TMVr over time, with a mean difference in the KCCQ-OS of 18.7 points (95% CrI 14.1 to 23.3) at 12 months and 18.9 points (95% CrI 11.4 to 26.0) at 24 months. Similar patterns were observed for the SF-36 PCS and MCS.
Figure 3. Estimated Health Status Over 24 Months for TMVr and Standard Care According to the Piecewise Linear Regression (Primary Analysis) and Joint Bayesian Models.
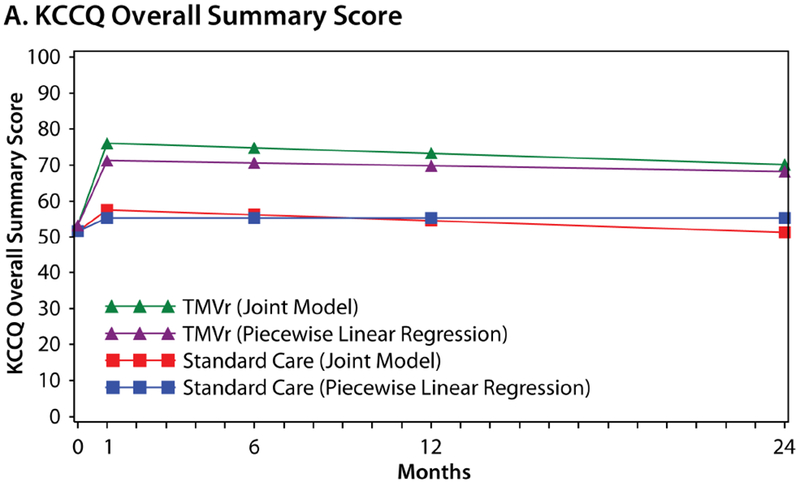
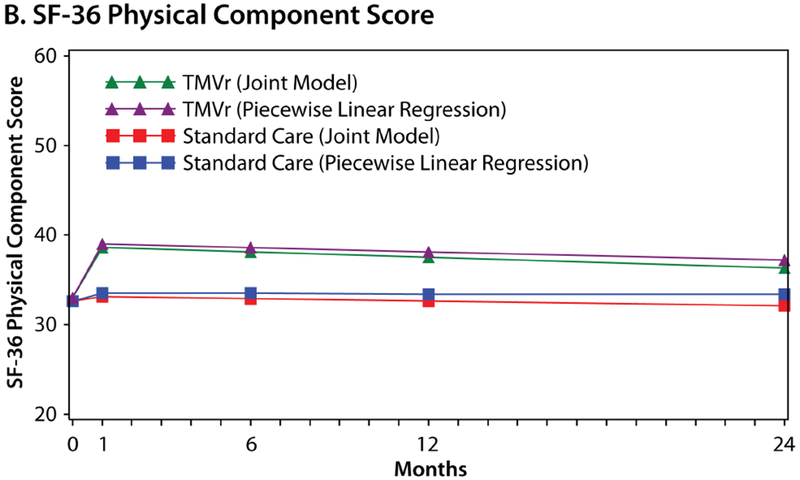
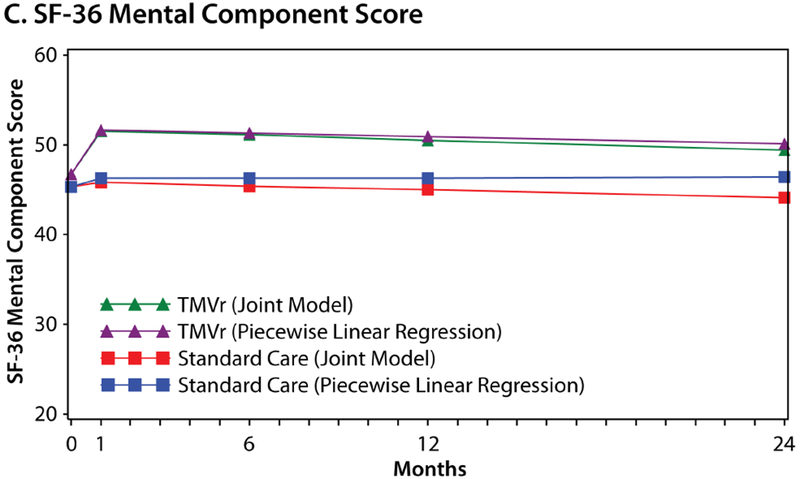
A. Kansas City Cardiomyopathy Questionnaire Overall Summary score. B. Short Form-36 Physical Component Summary score. C. Short-Form-36 Mental Component Summary score
Discussion
In the COAPT trial, TMVr led to a substantial reduction in mortality and HF hospitalizations over 24 months compared with standard care in patients with HF and 3-4+ secondary MR.(3) In this integrated sub-study, we found that TMVr also provided substantial benefits in terms of symptoms, functional status, and quality of life. The health status benefit of TMVr was moderately large and fully evident by 1 month. Among surviving patients, there was a slight attenuation of the health status effect over 24 months of follow-up; nonetheless, there was substantial sustained health status benefit at 12- and 24-month follow-up. In secondary analyses that accounted for bias due to exclusion of patients who died, the expected health status benefit with TMVr over standard therapy alone was stable over the full 24-month follow-up period. Although deaths were common in both treatment groups owing to advanced age, comorbidities, and underlying cardiomyopathy, a higher proportion of patients who were randomized to TMVr were alive with clinically meaningful improvement in health status at every follow-up time point. Indeed, our study suggests that only 5 patients would need to be treated with TMVr in order for 1 additional patient to be alive with a moderately large health status improvement at 24 months. Finally, the health status benefits of TMVr compared with standard care were consistent across all pre-specified subgroups except cause of cardiomyopathy, for which patients with ischemic cardiomyopathy appeared to derive greater health status benefit from TMVr compared with patients with nonischemic cardiomyopathy.
Since COAPT was the first randomized trial to collect detailed disease-specific and generic health status data for patients with secondary MR randomized to TMVr vs. standard care, there are limited data to serve as a basis for comparison. Two previous observational studies that included patients with both degenerative and functional MR (60-70% functional) found that TMVr was associated with mean reductions (improvements) of ~13 points in Minnesota Living with Heart Failure Questionnaire score compared with baseline(20,21)—roughly equivalent to a 14-point increase in the KCCQ-OS.(22) In a recent analysis from the ACC/STS Transcatheter Valve Therapy Registry, patients treated with TMVr (89% of whom had degenerative MR) had a mean change in KCCQ-OS of 25 points by 1 month, which remained stable among surviving patients through 1 year. Patients with degenerative MR appear to have greater improvements in health status after TMVr compared with those with functional MR, likely due to the greater relative contribution of the mitral regurgitation to the health status decrement, as opposed to any underlying cardiomyopathy. Importantly, in contrast to these prior studies, COAPT was restricted to patients with functional MR, provides longer-term health status follow-up, and is the only health status study to compare TMVr with a randomized standard care group.
In a study where mortality is not only high but differs between treatments, it is important to recognize the challenges of interpreting health status outcomes, which can only be observed in surviving patients. The 1-year quality of life analysis described in the main COAPT manuscript approached this challenge by imputing missing KCCQ-OS scores to patients who died from HF as the lowest KCCQ-OS value reported in that time period.(3) This resulted in a worst-case scenario but ignores the potential variability of health status scores of patients who died, had they survived. A second strategy (used in the current study) is to jointly model survival and health status, so that the informative missing data due to deaths can be integrated into the health status estimates. The resulting estimates thus denote the expected health status outcomes of the average COAPT patient had he or she survived. Finally, a third strategy (also employed in this study) is to examine combined outcomes (e.g., being alive with a clinically meaningful change in health status), which integrates the two most important considerations of patients and provides enhanced clinical interpretability.
Limitations
First, COAPT was a non-blinded trial which may introduce bias. While lack of blinding should have minimal impact on outcomes such as mortality, there is a possibility for a placebo effect when examining patient-reported measures. Given the magnitude of sustained health status benefit and the concordance of the results with other less subjective outcomes (including death and rehospitalization), it is unlikely that the health status benefit of TMVr is simply a placebo effect. Second, as discussed above, health status can only be measured in surviving patients. While we performed analyses that integrated mortality with health status and that used deaths to inform the health status outcomes, the true health status of patients who died, had they survived, is not knowable. Third, the durability of the health status benefit of TMVr beyond 24 months is not known, which is an important consideration in patients with underlying cardiomyopathy and comorbidities. Given the design of COAPT (which permits crossover to TMVr among surviving patients in the standard care group after 24 months), reliable longer term health status data will be limited to the TMVr group. Fourth, the interaction noted between cause of cardiomyopathy and health status should be considered exploratory as it may be a spurious finding, particularly given the number of secondary outcomes investigated.
Finally, the health status results observed in COAPT may not be generalizable to patients outside of the inclusion/exclusion criteria of the trial. Given the conflicting clinical results of the COAPT and MitraFR trials and the limited health status data collected in MitraFR, it will be important to investigate the health status outcomes of patients with HF and secondary MR treated with TMVr both in the real-world and among patients who would have been ineligible for COAPT.
In conclusion, in the COAPT trial, TMVr with the edge-to-edge device resulted in substantial health status improvement compared with standard care in symptomatic HF patients and 3-4+ secondary MR. This benefit emerged early, was consistent across key subgroups, and was sustained through 24 months follow-up. Considering the previously reported benefits of TMVr on survival and HF hospitalization, these health status findings further support the device as a valuable treatment option for HF patients with severe secondary MR who remain symptomatic despite maximally-tolerated guideline-directed medical therapy.
Supplementary Material
Central Illustration. Disease-Specific Health Status Scores Over 24 Months.
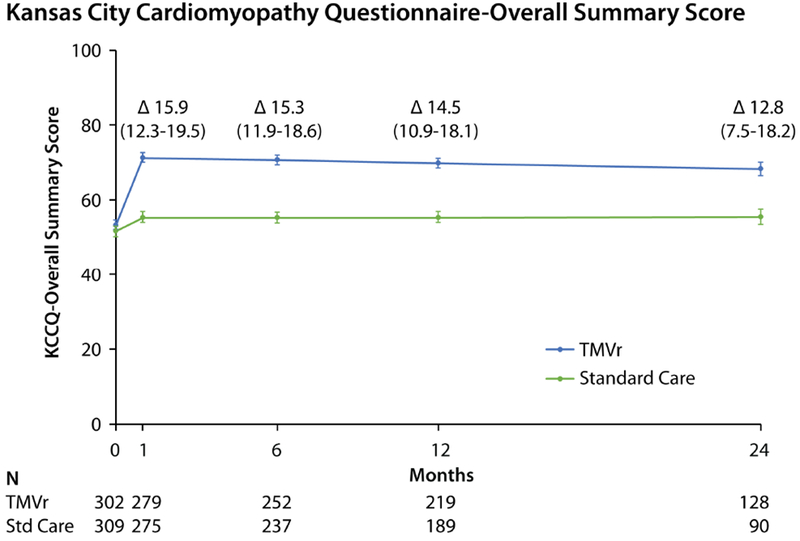
Predicted mean values with standard errors, as derived from piecewise linear regression models taking into account all available health status scores. Δ values represent mean between-group treatment difference and 95% CI. Reductions in patient numbers in year 2 are due to a combination of death and administrative censoring.
Perspectives.
Competency in Medical Knowledge
In patients with heart failure and secondary mitral regurgitation, TMVr using the edge-to-edge device results in rapid and sustained improvement in patients’ health status—their symptoms, functional status, and quality of life.
Competency in Patient Care
In order to maximize patients’ health and well-being, it is critical to understand the health status effects of any new therapy, so that treatments can be selected that positively impact patients’ quantity and quality of life.
Translational Outlook 1
Further research is needed to understand the generalizability of the health status results of COAPT—outside the strict inclusion/exclusion criteria of the trial and beyond experienced centers and operators—and also whether there is any heterogeneity of treatment benefit (beyond subgroup analyses).
Acknowledgments
Funding Source: The COAPT trial was sponsored by Abbott Vascular and designed collaboratively by the principal investigators and the sponsor. The present analysis was conducted by academic investigators at Saint Luke’s Mid America Heart Institute (Kansas City, MO). The authors had unrestricted access to the study data, drafted the manuscript, made the decision to submit for publication, and vouch for the veracity and completeness of its content. Dr. Arnold is supported by a Career Development Grant Award (K23 HL116799) from the National Heart, Lung, and Blood Institute.
Funding: Abbott; ClinicalTrials.gov number, NCT01626079.
Conflicts of Interest: JAS: owns the copyright for the KCCQ; equity interest in Health Outcomes Sciences; consulting income from Novartis, Bayer, AstraZeneca, V-wave, Corvia, Janssen; Advisory Board for United Healthcare; Board of Directors for Blue Cross Blue Shield of Kansas City. EAM: research grant support from Edwards Lifesciences, Medtronic, Boston Scientific, Abbott Vascular, and CSI. SJB: consulting income from Edwards LifeSciences and research grant support from Boston Scientific Inc. SK: research grant support from Abbott Vascular, Boston Scientific, Edwards Lifesciences, Mitralign; consulting income from Abbott Vascular and Boston Scientific. DSL: research grant support from Abbott Vascular; consulting income from Abbott Vascular. WTA: research grant support from Abbott Vascular; consulting income from Abbott Vascular. JAL: research grant support from AstraZeneca; consulting income from Abbott Vascular, Edwards Lifesciences, Boston Scientific, RESMED, Relypsa, Boehringer Ingelheim, V-Wave. GWS: consulting income from Claret, Medical Development Technologies, Backbeat, Sirtex, Matrizyme, Miracor, Neovasc, V-wave, Shockwave, Valfix, TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, and Ancora; speaker honoraria from Terumo and Amaranth; advisory board fees from QOOL Therapeutics and SpectraWAVE; equity/options in Ancora, Cagent, Qool Therapeutics, Aria, Caliber, MedFocus family of funds, Biostar family of funds, Applied Therapeutics and SpectraWAVE; director of SpectraWave; and his employer, Columbia University, receives royalties for sale of the MitraClip from Abbott. DJC: research grant support from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott Vascular; consulting fees from Medtronic and Edwards Lifesciences. The other authors report no conflicts.
ABBREVIATIONS
- CI
confidence interval
- CrI
credible intervals
- HF
heart failure
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- KCCQ-OS
KCCQ-overall summary score
- MR
mitral regurgitation
- NNT
number needed to treat
- SF-36
Medical Outcomes Study Short-Form 36 Health Survey
- SF-36 PCS
physical summary score
- SF-36 MCS
mental summary score
- TMVr
transcatheter mitral valve repair
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vakil K, Roukoz H, Sarraf M et al. Safety and efficacy of the MitraClip(R) system for severe mitral regurgitation: a systematic review. Catheter Cardiovasc Interv 2014;84:129–36. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl F, Schofer N, Lubos E, Blankenberg S, Schafer U. Critical evaluation of the MitraClip system in the management of mitral regurgitation. Vasc Health Risk Manag 2016;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone GW, Lindenfeld J, Abraham WT et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med 2018. [DOI] [PubMed] [Google Scholar]
- 4.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001;20:1016–24. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson LW, Hellkamp AS, Leier CV et al. Changing preferences for survival after hospitalization with advanced heart failure. Journal of the American College of Cardiology 2008;52:1702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold SV, Li Z, Vemulapalli S et al. Association of Transcatheter Mitral Valve Repair With Quality of Life Outcomes at 30 Days and 1 Year: Analysis of the Transcatheter Valve Therapy Registry. JAMA Cardiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack MJ, Abraham WT, Lindenfeld J et al. Cardiovascular Outcomes Assessment of the MitraClip in Patients with Heart Failure and Secondary Mitral Regurgitation: Design and rationale of the COAPT trial. Am Heart J 2018;205:1–11. [DOI] [PubMed] [Google Scholar]
- 8.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 9.Ware JE, Sherbourne CD The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 10.Heidenreich PA, Spertus JA, Jones PG et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol 2006;47:752–6. [DOI] [PubMed] [Google Scholar]
- 11.Spertus J, Peterson E, Conard MW et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–15. [DOI] [PubMed] [Google Scholar]
- 12.Ware J, Kosinski M, Bjorner JB, Turner-Bowkes DM, Gandek B, Maruish ME. Determining important differences in scores In: User’s Manual for the SF-36v2 Health Survey. Lincoln, RI: QualityMetric Incorporated, 2007. [Google Scholar]
- 13.Arnold SV, Spertus JA, Lei Y et al. How to Define a Poor Outcome After Transcatheter Aortic Valve Replacement: Conceptual Framework and Empirical Observations From the Placement of Aortic Transcatheter Valve (PARTNER) Trial. Circ Cardiovasc Qual Outcomes 2013;6:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spertus JV, Jones PG, Ho M et al. Integrating Quality of Life and Survival Outcomes in the PARTNER Trial (under review). [Google Scholar]
- 15.Heidenreich PA, Spertus JA, Jones PG et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol 2006;47:752–6. [DOI] [PubMed] [Google Scholar]
- 16.Kosiborod M, Soto GE, Jones PG et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation 2007;115:1975–81. [DOI] [PubMed] [Google Scholar]
- 17.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004;110:546–51. [DOI] [PubMed] [Google Scholar]
- 18.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis, Third Edition: CRC Press, Taylor & Francis Group, LLC, 2013. [Google Scholar]
- 19.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol 2001;54:343–9. [DOI] [PubMed] [Google Scholar]
- 20.Metze C, Matzik AS, Scherner M et al. Impact of Frailty on Outcomes in Patients Undergoing Percutaneous Mitral Valve Repair. JACC Cardiovasc Interv 2017;10:1920–1929. [DOI] [PubMed] [Google Scholar]
- 21.Maisano F, Franzen O, Baldus S et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052–61. [DOI] [PubMed] [Google Scholar]
- 22.Nassif ME, Tang Y, Cleland JG et al. Precision Medicine for Cardiac Resynchronization: Predicting Quality of Life Benefits for Individual Patients-An Analysis From 5 Clinical Trials. Circ Heart Fail 2017; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


