Abstract
The K-Cl cotransporter KCC2 is essential in the development of the “GABA switch” that produces a change in neuronal responses to GABA signaling from excitatory to inhibitory early in brain development, and alterations in this progression have previously been hypothesized to play a causal role in autism spectrum disorder (ASD). We investigated the KCC2b (Slc12a5) heterozygous knockout mouse using a battery of rodent behavioral tests relevant to core and co-morbid ASD symptoms. Compared to wild-type littermates, KCC2+/− mice were normal in standard measures of locomotor activity, grooming and digging behaviors, and social, vocalization and anxiety-like behaviors. However, KCC2+/− mice exhibited increased social dominance behaviors, and increased amplitude of spontaneous postsynaptic currents in the medial prefrontal cortex (PFC) that were previously implicated in governing social hierarchy and dominance behaviors. Treatment of wild-type mouse brain slices with the KCC2 inhibitor VU0240511 increased the amplitude and frequency of excitatory postsynaptic currents, partially recapitulating the phenotype of KCC2+/− mice. These findings indicate that the activity of KCC2 plays a role in social dominance, in parallel with effects on PFC signaling, further suggesting that KCC2 function has some relevance to social behavior but without the breadth of impact on autism-like behavior suggested by previous studies. Further testing could assess whether KCC2 alters other circuits, and whether additional factors such as environmental insults may precipitate autism-related behavioral phenotypes.
Keywords: Autism, social, GABA, excitatory, inhibitory, dominance
Lay Summary
A mouse model of altered chloride transporter expression was used to look for a role in behaviors and brain function relevant to autism. There was an imbalance in signaling in the prefrontal cortex, and increased social dominance behavior, though other autism-related behaviors were not changed. These findings indicate that altered chloride transporter function affects prefrontal cortex function and social dominance without a broader impact on autism-like behaviors.
Introduction
The K-Cl cotransporter KCC2 is a neuronal ion transporter essential for the modulation of inhibitory synaptic transmission. Early in mammalian brain development, KCC2 expression relative to NKCC1 is low, resulting in high intra-neuronal chloride levels and excitatory responses to GABAA ionotropic receptor activation (Ben-Ari, Cherubini, Corradetti, & Gaiarsa, 1989; Dzhala et al., 2005). Neuronal KCC2 expression increases in early brain development (Clayton, Owens, Wolff, & Smith, 1998; Lu, Karadsheh, & Delpire, 1999; Rivera et al., 1999) so that the electrochemical Cl- potential equilibrium becomes hyperpolarized with respect to the membrane potential, thus enabling inhibitory responses to GABAA receptor activation. This so-called “GABA switch” from excitatory to inhibitory function is essential to establishing proper circuit function in the mature brain (Ben-Ari, 2002; Blaesse, Airaksinen, Rivera, & Kaila, 2009).
The critical influence of KCC2 on the primary inhibitory system in the brain has clear implications for normal development, and changes in KCC2 function are implicated in neurodevelopmental disorders such as epilepsy (Huberfeld et al., 2007; Kahle et al., 2014; Puskarjov et al., 2014), autism spectrum disorder, and schizophrenia (Arion & Lewis, 2011; Hyde et al., 2011; Merner et al., 2015). Consistent with clinical findings, mice with Slc12a5/KCC2 loss of function mutations exhibit respiratory failure and early death in complete nulls (Hubner et al., 2001), seizures and early death in KCC2b isoform nulls (Woo et al., 2002), and increased seizure susceptibility precipitated by administration of propofol and pentylenetetrazole (PTZ) in heterozygous null animals (Tornberg, Voikar, Savilahti, Rauvala, & Airaksinen, 2005; Woo et al., 2002). In addition, heterozygous neurons demonstrate hyper-excitability in vitro when compared to neurons isolated from wild-type (WT) mice (Woo et al., 2002; Zhu, Lovinger, & Delpire, 2005; Zhu, Polley, Mathews, & Delpire, 2008).
Ben-Ari and colleagues have hypothesized that defects in the GABA switch may cause or contribute to ASD symptoms, and this hypothesis aligns with the broader hypothesis that ASD results from an imbalance of normal ratio of excitatory to inhibitory neurotransmission (Ben-Ari, 2014; Tyzio et al., 2014). In two rodent models of ASD or relevant symptoms, rats exposed in utero to valproic acid (VPA) and in mice lacking the fragile X gene Fmr1, the GABA switch was delayed or impaired according to electrophysiological measures (Eftekhari et al., 2014; Tyzio et al., 2014). Chloride channel activity has emerged as a promising target for treatment of the excitatory-inhibitory neurotransmission imbalance, and related neurological disorders (Gagnon et al., 2013). Importantly, GABA switch defects were rescued by maternal treatment with the NKCC1 antagonist bumetanide, indicating that tipping the transporter balance in favor of KCC2 and lower intra-neuronal chloride concentrations could restore normal development of GABAergic inhibition (Tyzio et al., 2014). Further, the dysregulated behaviors relevant to ASD, including ultrasonic vocalization deficits, were rescued by maternal treatment with bumetanide (Eftekhari et al., 2014; Tyzio et al., 2014). These findings align with clinical study results from humans with ASD treated with bumetanide (Lemonnier et al., 2012; Lemonnier et al., 2017).
Based upon these reports and the overarching hypothesis of an altered chloride gradient driving diminished inhibitory effects of GABA in ASD, we hypothesized that mice with decreased KCC2 function should show deficits that relate to ASD symptoms and parallel findings from other rodent models of ASD symptoms such as VPA-exposed rats and Fmr1 null mice. To test this hypothesis, we assessed heterozygous mice expressing one WT gene for KCC2 and one mutant loss of function gene for KCC2b (+/−). The mutant allele allows expression of KCC2a, an isoform that starts at an upstream promoter but contributes to only 5–10% of KCC2 expression. These hypomorphic animals mimic the shift in NKCC1 to KCC2 balance hypothesized by Ben-Ari and colleagues (Woo et al., 2002). We hypothesized that KCC2+/− mice would exhibit alterations in rodent behavioral assays that are standardly used to asses ASD-relevant behaviors such as social interactions, vocalizations, repetitive and anxiety-like behaviors, as well as electrophysiological measures related to neuronal features that could impact some of these behaviors.
Methods
Behavior Testing
Behavior testing was approved by the Vanderbilt University Institutional Animal Care and Use Committee, and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Scoring of all behavior tests was performed by an experimenter blind to the genotype of the subjects.
KCC2 Mice
Mice with an exon 1b disruption in the Slc12a5 gene encoding the KCC2 protein (Woo et al., 2002) were bred from matings of heterozygous sires with a C57BL/6J background and WT dams at Vanderbilt University. Offspring were genotyped by standard protocol (Woo et al., 2002) and heterozygous (+/−) and WT (+/+) male mice were housed together with three to five mice per cage, with food and water available ad libitum. A total of 33 mice were used for all behavior tests, including 17 WT and 16 Slc12a5/KCC2+/− littermates. Heterozygous KCC2 mice have increased susceptibility to epileptic seizures (Woo et al., 2002). Of the animals tested, three KCC2+/− mice were observed to have motor movements consistent with seizures, and one died prior to social testing.
Testing began at 8 weeks of age and behavioral tests were conducted in the order laid out below, with at least two days in between tests. All tests were conducted in isolation, unless otherwise noted, with the subjects returning to group housing after each test. Male subjects were then allowed to mate with WT females, and offspring were tested for ultrasonic vocalizations. Behavior testing occurred during the light phase of the circadian cycle, in a separate behavior testing room, where animals were acclimated for 1 hour prior to testing.
Elevated Zero Maze
Anxiety-like behavior was measured in the elevated zero maze (EZM) for 5 minutes, as previously described (Zike et al., 2017). ANY-maze software (Stoelting, Wood Dale, IL) was used to analyze movement in the maze, including time spent in each arm of the maze, and total distance traveled and entries into arms as measures of total activity. The ratio of time spent in the open arms compared to the closed arms of the maze was calculated and used as the primary outcome variable, which was compared between genotypes by t-test, as were the activity measures (GraphPad Prism 6, La Jolla, CA). Welch’s correction was used when there were unequal variances.
Locomotor Activity
Spontaneous locomotor activity was measured in the open field test as previously described (Carter et al., 2011), with test time of 90 minutes. Distance traveled was assessed after 5 minutes, as a response to a novel environment, and after 90 minutes, as a measure of general activity level. Genotypes were compared using a t-test (GraphPad Prism 6), with Welch’s correction when there were unequal variances, and one subject (KCC2+/−) was removed as an outlier in the 90 minute analysis (>3 standard deviations from the mean).
Home cage Activity
HomeCageScan (Clever Sys, Reston, VA) was used as previously described (Veenstra-VanderWeele et al., 2012; Zike et al., 2017) to assess time spent in various activities over a 24-hour period in the home cage, including sleeping, consuming food or water, rearing, grooming, hanging, remaining low, sniffing, stretching, twitching and walking. A two-way repeated measures ANOVA was used to analyze the array of behaviors (GraphPad Prism 6).
Repetitive Behavior Tests
Spontaneous grooming was assessed as previously described (Zike et al., 2017), for 10 minutes in an empty cage.
To assess digging, each mouse was placed in a novel cage, with bedding, and allowed to habituate to the novel environment for 10 minutes. Then time spent digging was recorded with a stopwatch over a 10-minute period. Total digging time was analyzed by t-test for the two genotypes (GraphPad Prism 6).
Tube Test for Dominance
Dominance behavior was assessed in the tube test as described previously (Veenstra-VanderWeele et al., 2012). Each mouse was paired with a stranger mouse of the opposite genotype twice, starting once on each side of the tube, at least 2 hours apart from the previous test. Pairings continued “round robin” style until each mouse had completed 6 matches. The total number of wins achieved by mice of each genotype was compared with McNemar’s test (GraphPad.com). A t-test compared the number of wins each mouse achieved between the two genotypes (GraphPad Prism 6).
Three-Chamber Test for Sociability
The three-chamber test for sociability was performed as described previously (Veenstra-VanderWeele et al., 2012), adapted from Moy et al. (2009). Time spent in the chamber with a social stimulus, and time spent near the pencil cup containing the social stimulus (<1cm) were measured in a 10-minute period. Data were analyzed by two-way, repeated-measures ANOVA with the stimulus as a within-subjects variable (GraphPad Prism 6).
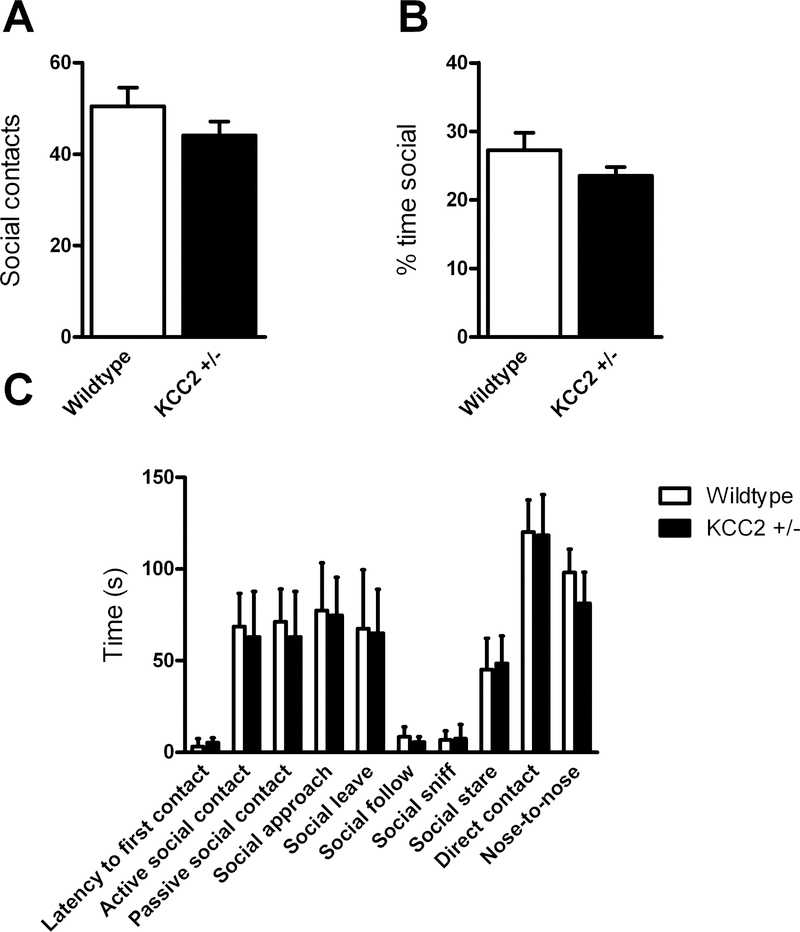
Reciprocal Social Behavior Test
Stranger age- and sex-matched mice of the same genotype were paired and placed in a clean cage. Behaviors, including latency to first contact, time spent in social contact, active and passive social contact, direct contact (touching or <0.5mm apart), nose-to-nose time, social sniffing time, time spent staring, approaching, following, or leaving the other mouse, and number of social contacts, were recorded for 10 minutes and assessed by SocialScan software (CleverSys, Inc, Reston, VA). Independent t-tests, with a False Discovery Rate (Q) value set to 1% to address multiple tests, were used to analyze the number of social contacts and the percent time engaged in social behaviors, while a two-way repeated-measures ANOVA was used to analyze the time spent engaged in specific social behaviors across genotypes (GraphPad Prism 6).
Ultrasonic Vocalizations
Ultrasonic vocalizations (USVs) were recorded as described previously (Veenstra-VanderWeele et al., 2012), with the modification of the age at test. Pups were removed from their cage and recorded at postnatal days 4, 8, and 12, to parallel the report in VPA-exposed rats and Fmr1 null mice (Tyzio et al., 2014). Two-way repeated-measures ANOVA was used to assess the effects of genotype and postnatal day on the number and duration of USVs emitted, after collapsing both sexes when no differences were detected and genotypes of males and females were each found to be in Hardy-Weinberg equilibrium. Tukey’s multiple comparisons test was used to determine the postnatal day effect (GraphPad Prism 6).
Electrophysiological Recording
Animal handling was approved by the Tulane University Institutional Animal Care and Use Committee, and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Acute Brain Slice Preparation
KCC2+/− and WT littermate mice aged P35–45 were anesthetized with isoflurane and rapidly decapitated. Brains were quickly removed and placed into ice-cold ACSF solution (composition in mM: 124 NaCl, 4 KCl, 26 NaHCO3, 1.26 NaH2PO4, 6 MgSO4, 1 CaCl2). A vibratome (Leica VT1200) was used to generate 300um thick medial prefrontal cortex (mPFC) coronal sections containing the anterior cingulate cortex (ACC) and prelimbic cortex (PrL). Slices were transferred to an oxygenated (95% oxygen, 5% CO2) holding chamber containing bicarbonate buffered ACSF at room temperature (composition in mM: 124 NaCl, 4 KCl, 26 NaHCO3, 1.26 NaH2PO4, 3 MgSO4, 2 CaCl2).
For the KCC2 inhibitor (VU0240511, Delpire et al., 2009) experiments, brains were removed from WT mice as described above, but then placed into ice-cold, modified NMDG-containing ACSF solution (composition in mM: 110 NMDG, 110 HCl, 3 KCl, 10 MgCl2 6H20, 1.1 NaH2PO4 H20, 0.5 CaCl2 dihydrate, 25 glucose, 3 pyruvic acid, 10 ascorbic acid, 25 NaHCO3). A vibratome (Leica VT1200S) was used to generate 300um thick mPFC coronal sections containing the ACC and PrL. Slices were allowed to recover in oxygenated (95% oxygen, 5% CO2) NMDG-based ACSF for 30 minutes at 34°C. Slices were then transferred to an oxygenated holding chamber at room temperature containing bicarbonate buffered ACSF as per above.
Electrophysiology Recordings
Following at least 1h of recovery, acute brain slices were placed in a recording chamber mounted to an upright microscope (Olympus BX61WI) with infrared-differential interference contrast optics at a magnification of 10 or 40× and perfused with room temperature bicarbonate buffered ACSF at a rate of 1–2 mL/min. All recordings were taken from layer V neurons of ACC and PrL regions in mPFC. All recordings were taken within 6 hours after slicing. The number of cells and animals from which recordings were done are noted in the Figure 3 legends.
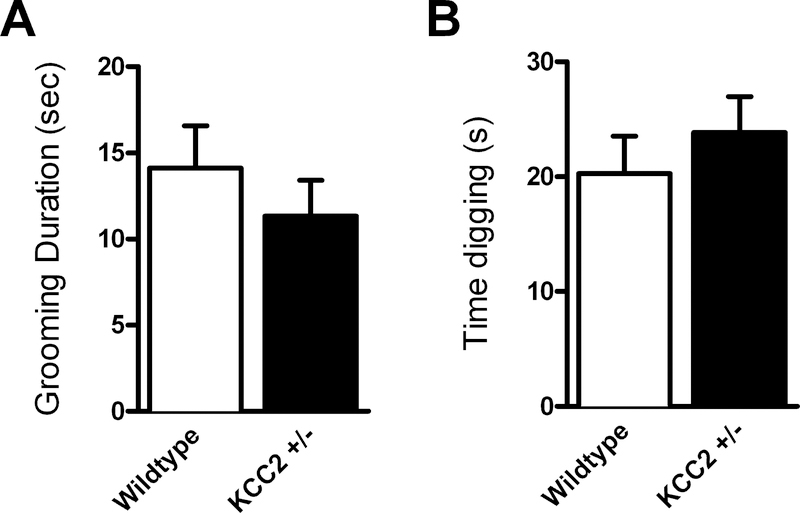
Figure 3.
No effect of KCC2 deficiency on repetitive behaviors grooming or digging in a novel cage. (KCC2+/− mice n = 16, WT mice n = 17)
Whole cell patch clamp recordings were acquired using a patch clamp amplifier (Warner Instruments, PC-505B) and Power Lab Digitizer (AD Instruments, 16/35). Current recordings were acquired for 90s, filtered at 2 kHz at a sampling rate of 10 kHz using Lab Chart software (AD Instruments). Pipette resistances ranged from 4–8 MΩ while series access resistance ranged from 7 to 20 MΩ and was monitored for consistency. Recordings were discarded if access resistance was greater than 20 MΩ or leak current rose above 200 pA.
For voltage-clamp recordings, a cesium methylsulfonate internal solution was used containing (in mM): 120 CsMeSO3, 10 HEPES, 1 MgSO4, 0.1 CaCl2, 1 EGTA, 2 KCl, 5 phosphocreatine disodium salt hydrate, 2 QX-314, 2 ATP disodium salt hydrate, 0.3 GTP sodium salt) (Hall et al. 2007). Excitatory and inhibitory currents were recorded from neurons held at −60 mV or 0 mV, respectively. TTX 1 uM, D-APV 100 uM, and picrotoxin 100 uM were added to the ACSF for mEPSC recordings, while mIPSC ACSF contained TTX 1 uM, NBQX 5 uM, and APV 100 uM. For experiments involving the KCC2 inhibitor, 3 uM VU0240511 was applied to the holding chamber as well as perfused onto slices for the duration of the study.
An additional electrophysiology rig was used that followed the aforementioned protocol, however, a MultiClamp 700B (Axon Instruments) amplifier, Digidata 1550 digitizer (Axon Instruments), upright microscope (Zeiss Axio Examiner D1), and pCLAMP10 software (Axon Instruments) were used for acquisition.
Electrophysiology Analysis and Statistics
Synaptic currents were analyzed using MiniAnalysis (Synaptosoft) and custom written macros in IgorPRO (Wavemetrics) software. Example traces were filtered for presentation. Data are shown as means + SEM. Student’s t-test was used to determine significance.
Results
Behavior Testing
KCC2+/− mice were compared to WT littermates in an extensive battery of behavioral tests that are standardly used to characterize rodent models of ASD. The only notable phenotype observed in KCC2+/− mice was in the tube test where mutant mice exhibited a significant increase of “wins” indicative of enhanced social dominance behaviors. There were no other significant genotype-dependent behavioral differences.
Elevated Zero Maze
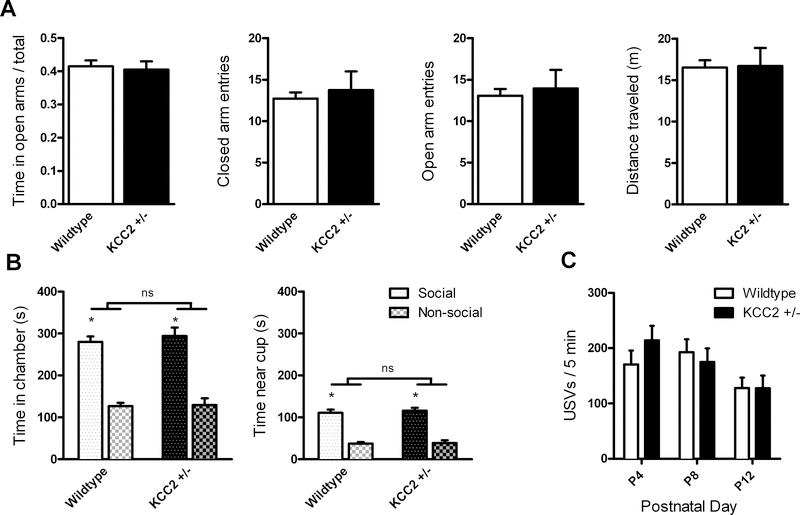
KCC2+/− mice did not significantly differ from WT on any measure of anxiety-like behavior or activity in the EZM (Fig. 1A), including the proportion of time spent in the open arms (t(31) = 0.334, p = 0.740), entries into closed (Welch’s t(18) = 0.442, p = 0.664) or open arms (Welch’s t(19) = 0.370, p = 0.715), or distance traveled (Welch’s t(19) = 0.079, p = 0.938).
Figure 1.
No effect of KCC2 deficiency on anxiety-like behavior, sociability, or ultrasonic vocalizations.
(A) KCC2+/− mice (n = 16) did not exhibit any difference compared to WT mice (n = 17) in anxiety-like behavior in the elevated zero maze, as measured by proportion of time spent in open arms (left), differences in number of closed or open arm entries (center left and right), and did not alter general activity in the maze (right). (B) KCC2+/− mice (n = 15) did not exhibit any difference compared to WT mice (n = 17) in sociability in the three-chamber test, measured by time spent in the chamber (left) or near the cup (right) containing the social stimulus, which was significantly higher than time spent in the non-social chamber or near the non-social cup for both KCC2+/− and WT mice. (C) USVs emitted in response to maternal separation decreased by postnatal day 12, but there was no interaction with genotype (KCC2+/− mice n = 33, WT mice n = 37). * significant effect of social stimulus, p<0.0001; ns non-significant effect of genotype
Locomotor Activity
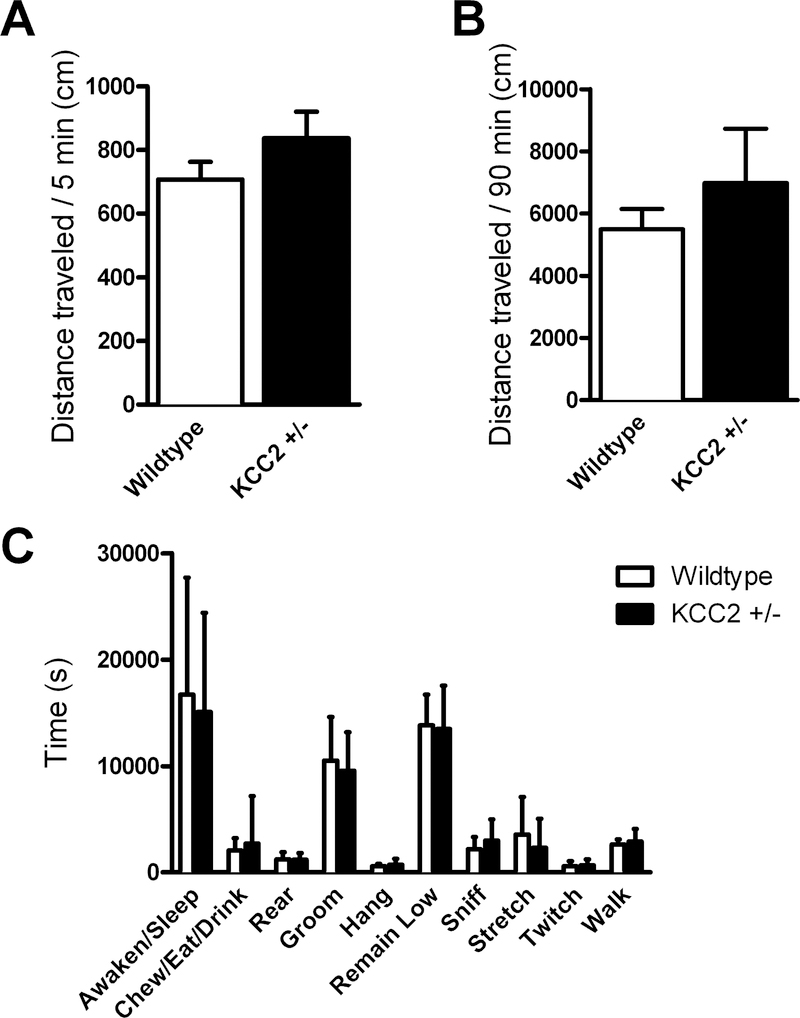
There was no significant difference between genotypes in activity in the open field in either the first 5 minutes (t(31) = 1.302, p = 0.203) or the entire 90 minutes of the test (Welch’s t(17) = 0.796, p = 0.437; Fig. 2A-B).
Figure 2.
No effect of KCC2 deficiency on activity or other home cage behaviors.
Activity level was not different between KCC2+/− (n = 16) and WT (n = 17) mice in the first 5 (A) or full 90 (B) minutes of the test in the open field. There was no interaction between activity and genotype (KCC2+/− mice n = 16, WT mice n = 15) in home cage behaviors (C).
Home cage Activity
There were no significant differences between genotypes for measures of behavior in the home cage activity scan (no interaction between genotype and behavior F(9,261) = 0.312, p = 0.971; no effect of genotype F(1,29) = 0.463, p = 0.502; effect of behavior F(9,261) = 61.44, p<0.0001; Fig. 2C).
Repetitive Behavior Tests
There were no significant differences between genotypes in time spent in grooming (t(31) = 0.869, p = 0.392) or digging (t(31) = 0.787, p = 0.437; Fig. 3) in a novel cage.
Three-Chamber Test for Sociability
There was no significant difference between genotypes in sociability (Fig. 1B), assessed either by time in each chamber (no interaction between genotype and stimulus: F(1,30) = 0.0951, p = 0.760) or time near the stimulus pencil cup (no interaction between genotype and stimulus: F(1,30) = 0.0592, p = 0.809). There was a main effect of social versus non-social stimulus in both chamber time (F(1,30) = 71.3, p < 0.0001) and time near the cup (F(1,30) = 108.9, p < 0.0001). There was no main effect of genotype in either chamber time (F(1,30) = 0.900, p = 0.350) or time near the pencil cup (F(1,30) = 0.378, p = 0.543).
Tube Test for Dominance
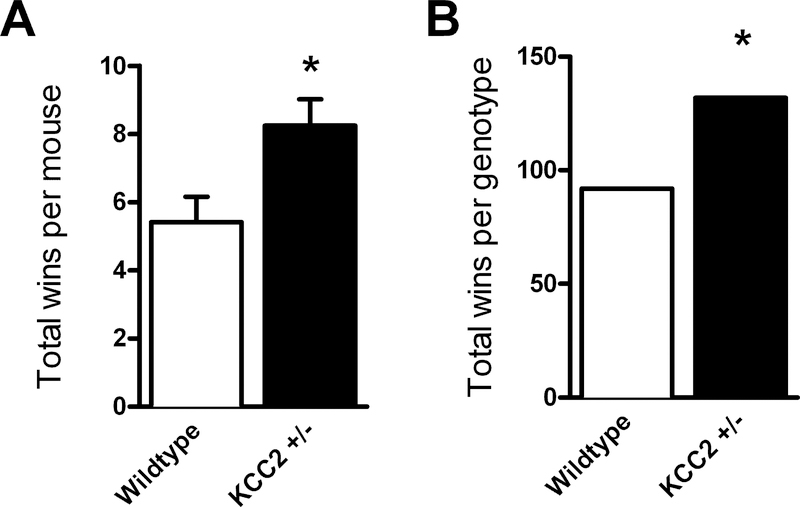
KCC2+/− mice won significantly more tube test bouts than WT mice (Fig. 4), when analyzed by the number of wins by each mouse (t(31) = 2.641, p = 0.013), and the same pattern was reflected in the total number of wins for each genotype (McNemar’s test χ2 = 6.79, df = 1, p = 0.0092).
Figure 4.
KCC2+/− mice exhibited higher levels of dominance behavior in the tube test.
Each mouse completed 12 matches with 6 subjects of the opposite genotype. KCC2+/− mice (n = 16) achieved an average of 8.25 wins, a significantly greater number than WT mice (n = 17) that averaged 5.41 wins (A). The total number of wins achieved by each genotype across all matches was also significantly higher in KCC2+/− mice than WT (B). *p<0.05
Reciprocal Social Behavior Test
There was no significant difference in social behavior in any measure of the reciprocal social behavior test (no interaction between genotype and behavior F(9,117) = 0.427, p = 0.918; no effect of genotype F(1,13) = 0.741, p = 0.405; effect of behavior F(9,117) = 75.39, p<0.0001; Fig. 5): In addition, there was no overt fighting, only one occasion of mounting behavior, from a WT mouse, and minimal latency to first contact overall.
Figure 5.
No effect of KCC2 deficiency on social interaction behavior in the social reciprocity test with another mouse of the same genotype. (KCC2+/− pairs n = 7, WT pairs n = 7)
Ultrasonic Vocalizations
There was no significant interaction between genotype and postnatal day (F(2,136) = 1.09, p = 0.339), and no main effect of genotype (F(1,68) = 0.143, p = 0.706) on the number of USVs emitted. There was a significant effect of postnatal day (F(2,136) = 5.51, p = 0.005), with the number of USVs decreasing significantly by P12 compared to both P4 and P8 (Fig. 1C).
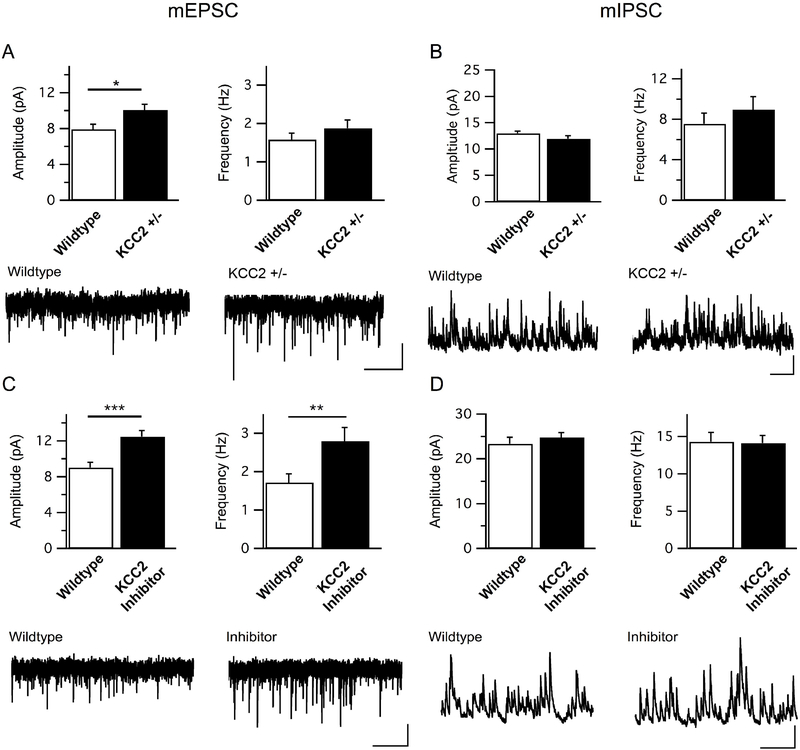
Electrophysiology Recordings
There was a significant effect of KCC2+/− genotype on the amplitude, but not frequency, of mEPSCs (amplitude: t(35) = 2.45, p = 0.02; frequency: t(35) = 1.10, p = 0.28; Fig. 6A), while there was no significant effect on mIPSCs (amplitude: t(22) = 1.48, p = 0.15; frequency: t(22) = 0.80, p = 0.43; Fig. 6B). When WT neurons were treated with the KCC2 inhibitor VU0240551, there was a significant increase in both amplitude and frequency of mEPSCs (amplitude: t(25) = 3.79, p = 0.001; frequency: t(25) = 2.52, p = 0.02; Fig. 6C), and no significant effect on mIPSCs (amplitude: t(22) = 0.80, p = 0.43; frequency: t(22) = 0.09, p = 0.93; Fig. 6D).
Figure 6.
Excitatory synaptic tone onto L5 mPFC neurons is increased by genetic reduction and pharmacological inhibition of KCC2.
(A) Quantitation and sample traces of mEPSC amplitudes and frequencies in WT and KCC2+/− mice show a significant increase in the amplitude of events in heterozygous neurons. (WT n = 18 cells/4 mice, KCC2+/− n = 19/4). *p = 0.02. Scale bar = 5 pA/2s (B) Quantitation and sample traces of mIPSC amplitudes and frequencies in WT and KCC2+/− mice revealed no significant changes in amplitude or frequency. (WT n = 11 cells/2 mice, KCC2+/− n = 13 cells/2 mice). Scale bar = 5 pA/500 ms (C) Quantitation and sample traces of mEPSC amplitudes and frequencies in WT cells incubated with 3 uM KCC2 inhibitor VU 0240551. KCC2 inhibition caused a significant increase in both the amplitude and frequency of mEPSCs in these neurons (WT n = 14/4, inhibitor n = 13/3). ***p = 0.001, **p = 0.02. Scale bar = 5 pA/2s (D) Quantitation and sample traces of mIPSC amplitudes and frequencies in WT cells incubated with 3 uM KCC2 inhibitor VU 0240551. KCC2 inhibition did not cause a significant change in mIPSC amplitude nor frequency (WT n = 12/2, inhibitor n = 12/3). Scale bar = 20 pA/500ms
Discussion
The increase of KCC2 expression in maturing neurons is critical for establishing GABAergic inhibition in the developing brain, and alterations of KCC2 function are implicated in neurodevelopmental disorders including epilepsy, ASD, and schizophrenia (Hyde et al., 2011; Merner et al., 2015). In this study, we investigated KCC2+/− mice as a model of ASD using a battery of tests relevant to ASD core and co-morbid symptoms. Previous findings implicate a shift in the NKCC1:KCC2 ratio in altered social communication and repetitive behaviors in VPA-exposed rats and Fmr1 null mice (Tyzio et al., 2014). However, we observed no overt phenotypes on parallel behavioral tests in mice with 50% reduction in KCC2 expression (Woo et al., 2002).
We observed a significant difference in KCC2+/− mice in only one behavioral assay, the tube test for dominance, in which KCC2+/− mice exhibited a significant increase of social dominance behaviors. Specifically, we found that KCC2+/− mice won significantly more matches than their WT counterparts in the tube test, demonstrating increased dominance behavior. Behavior in the tube test is altered in several genetic mouse models relevant to ASD and related syndromes (Greco et al., 2013; Spencer, Alekseyenko, Serysheva, Yuva-Paylor, & Paylor, 2005; Veenstra-VanderWeele et al., 2012). The test requires a social confrontation and forces a binary outcome, which is distinct from other measures of social function. It has been described as relevant for the aggression occasionally observed in ASD (Crawley, 2007), but the tube test appears to more meaningfully probe social judgment and social dominance, rather than aggression, which very rarely occurs during the test (Greco et al., 2013). Whereas abnormalities in the tube test have been reported in almost every genetic mouse model related to ASD that has been assessed, not all mouse models of ASD show effects in the same direction, with a similar number with increased versus decreased dominance (Bernardet & Crusio, 2006; de Esch et al., 2015; Greco et al., 2013; Hill et al., 2015; Irie, Badie-Mahdavi, & Yamaguchi, 2012; Jiang-Xie et al., 2014; Lijam et al., 1997; Shahbazian et al., 2002; Spencer et al., 2005; Veenstra-VanderWeele et al., 2012). The lack of significant effects of genotype on anxiety-like behavior, locomotor activity, and other individual behaviors in the homecage scan suggests that the effect seen in the tube test was not due to other behavioral changes that could impact the test. While this test does not directly correspond to social deficits commonly observed in ASD patients, our results suggest that KCC2 impacts the function of circuits that regulate social behavior circuitry.
Through the tube test and other tests of social behaviors in rodents, connections between the PFC and amygdala have been identified as regulating social behavior, as have thalamocortical connections (Felix-Ortiz, Burgos-Robles, Bhagat, Leppla, & Tye, 2016; Ferguson & Gao, 2018). This circuitry is dysregulated in mouse models of ASD (Ellegood et al., 2018; Muller et al., 2017), and these general findings correspond with evidence of dysregulated activity and connectivity of ASD patients (Green, Hernandez, Bookheimer, & Dapretto, 2017; and see meta-analysis by Patriquin, DeRamus, Libero, Laird, & Kana, 2016). Dominance behavior and social hierarchy are governed by multiple neuronal circuits, but manipulations of the prefrontal cortex have demonstrated causal effects on dominance behavior. Neurons of the dorsal medial prefrontal cortex (dmPFC) were active when mice exhibited dominant behaviors such as pushes to win a tube test, and activation (via optogenetics) or inactivation (via DREADDs) of these neurons led to increased wins or losses, respectively (Zhou et al., 2017).
While many studies have implicated GABAergic dysregulation in the expression of aggressive behavior (reviewed in Takahashi & Miczek, 2014), further investigations into the specific components of the GABA system have demonstrated that more aggressive BALB/cJ mice have lower levels of GABA and higher levels of Slc12a5/KCC2 gene expression compared to the closely related BABL/cByJ strain of mice (Jager et al., 2017). Specifically in the ventral ACC, within the PFC, Slc12a5 expression was upregulated in the more aggressive BALB/cJ mice. These results at first seem to contradict those of the present study, where lower Slc12a5 expression is associated with higher dominance. However, Jager et al. hypothesize that this alteration in Slc12a5 levels is a compensatory mechanism in response to the lower GABA levels, and so the overall lower GABAergic tone may be the critical factor in the behavioral effects. Another study showed that lower levels of GABA production due to decreased expression of GAD67 in NPY neurons, particularly evident in the PFC, led to significantly greater numbers of wins in the tube test for social dominance, in line with the present findings (Corder et al., 2018). Moreover, the excitatory/excitatory imbalance may be the common factor leading to the association with increased dominance or aggression. In support of this, a recent report showed that an excitatory/inhibitory imbalance in either direction was sufficient to decrease sociability in the three-chamber test (Ferguson & Gao, 2018). In this study, DREADDs were used to inhibit projections from the mediodorsal thalamus to the mPFC, decreasing GABAergic output in the mPFC, and/or used to excite parvalbumin interneurons in the PFC; either manipulation alone decreased social approach behavior relative to control conditions, while the simultaneous effect in both cell populations restored excitatory/inhibitory balance and rescued the social behavior. While the mechanism of KCC2 effects on social behavior dysregulation has yet to be elucidated, the present studies support a role for this protein in altering excitatory/inhibitory balance, acting specifically within the PFC.
Moreover, previous work has shown that synaptic strength in the layer 5 pyramidal neurons of the dmPFC is linked with social hierarchy status, and alterations in synaptic strength via manipulation of AMPAR expression can change an individual’s status (Wang et al., 2011). We examined KCC2+/− neurons and identified a greater amplitude, but not frequency, of mEPSCs, consistent with the results seen in dominant relative to subordinate mice in the tube test for dominance, supporting the role of mPFC synaptic strength in the relationship to dominance behavior (Wang et al., 2011). In extracellular hippocampal slice recordings from KCC2+/− mice, spontaneous events displayed both increased amplitude and frequency, highlighting a potential difference in the effects of lower KCC2 expression between brain regions (Zhu et al., 2008). However, we did not observe any change in mIPSC amplitude in the mPFC, despite the known action of KCC2 on chloride channels and expected effects on GABA signaling, and this effect is consistent with another study that recorded currents in the hippocampus, in which KCC2 protein expression was decreased by about 50%, though this was due to the knockout of amyloid precursor protein (Chen et al., 2017). This could suggest that the observed effects on mEPSCs reflect the summed impact of KCC2 loss across circuits, rather than simply reflecting effects within the dorsal medial PFC pyramidal neurons themselves.
To evaluate whether the change in synaptic signaling in KCC2+/− mice was mediated by KCC2 acutely, we used the KCC2 inhibitor VU0240511, which enhanced the frequency as well as amplitude of mEPSCs. Unfortunately, this tool compound with poor drug metabolism and pharmacokinetic profiling (Delpire et al., 2012) is unlikely to reach the brain after peripheral administration and is therefore not suitable for behavioral testing. The inconsistency between the KCC2 inhibitor and the KCC2+/− neurons is worth noting. The drug increased both amplitude and frequency of events; whereas the heterozygous expression of the channel only altered amplitude. One possible explanation is that the inhibitor effectively blocks all transporters, while the heterozygous animals merely show a decrease in the number of KCC2 transporters. It is also possible that developmental compensation or another mechanism, including strengthening synaptic signaling amplitude through development of dominance behavior in KCC2+/− mice prior to tissue harvest, accounts for this difference, though we are not aware of any evidence demonstrating an effect of dominance behaviors on changes in synaptic signaling.
While the present experiments did not address a mechanism of the effect of KCC2 reduction or inhibition on mEPSCs, previous research has demonstrated mechanisms by which alterations in KCC2 are related to differences in excitatory and inhibitory signaling, including differences in expression level and location of GABA receptors (Li, Zhou, Peng, & Zhao, 2017), or AMPA receptors (Chevy et al., 2015; Gauvain et al., 2011).
Given the essential role of KCC2 in maintaining the chloride gradient for GABAergic signaling in general and for the GABA switch in particular, it is possible that the change we observed in a single social behavior is relevant to ASD, even without observing the changes in the range of ASD-relevant symptoms including ultrasonic vocalizations, sociability, and repetitive behavior described in the previous studies of VPA-exposed rats and Fmr1 null mice (Eftekhari et al., 2014; Tyzio et al., 2014). Increases in measures of EPSCs were observed in the VPA-treated rats and Fmr1 null mice, in addition to the KCC2+/− mice in the present study, supporting the functional role of the decreased KCC2 level common across these models. The notable difference in behavioral effects between studies emphasizes that KCC2 alone is not responsible for the emergence of particular behavioral phenotypes, and must affect broader circuits through development and interaction with other neurobiological factors dysregulated in each model or individual.
Future work could examine whether heterozygous KCC2+/− mice would show autism-related changes using other paradigms. Our results indicating a lack of genotype effect on anxiety-like behavior in the EZM are in contrast with findings of increased anxiety-related behaviors in the elevated plus maze and light-dark box emergence test in mice with more dramatically decreased KCC2 levels: about 17% of WT (Tornberg et al., 2005), relative to about 50% in the mice used in this study (Woo et al., 2002). It is possible that either the difference in KCC2 levels reached a threshold for greater behavior effects in the studies by Tornberg et al., or that the procedures used are differentially sensitive to anxiety-related behaviors in these subjects. In studies of the KCC2+/− animals using measures related to pain and seizures, aberrant findings were only seen when the system was perturbed by administration of propofol or PTZ to precipitate effects (Woo et al., 2002; Zhu et al., 2005; Zhu et al., 2008). This may indicate that the threshold for reactivity is lower in the KCC2+/− mice relative to WT, even when they do not exhibit innate differences. It is possible, for example, that an early environmental insult such as maternal immune activation would precipitate a developmental cascade leading to abnormal behavior in the KCC2+/− mice. Further, the demonstrated effects of KCC2 alterations on seizure activity are relevant for ASD, where there is a high incidence of comorbidity with epilepsy (reviewed in Lee, Smith, & Paciorkowski, 2015), and will be important to study further to understand the underlying mechanisms.
The age of animals must be carefully considered as well. KCC2 expression in the PFC of mice is low and restricted to cell bodies at birth, but increases and moves to somatodendritic zones thereafter, reaching adult distribution levels by the third week of life (Amadeo et al., 2018; Markkanen et al., 2014). All of our tests (with the exception of USVs) were therefore conducted after the establishment of the presumed GABA switch and consequent development. Our behavior tests were conducted starting at 8 weeks of age, or young adulthood. This has the advantage of being near the P35–45 age of the subjects used for electrophysiology while also being during the stage at which the conducted tests are typically done, for comparison with other studies (Crawley, 2007). Future studies could assess autism-related behaviors across the range of developmental stages, which could reveal relevant alterations not observed in the present studies.
KCC2 heterozygous null mice demonstrated greater levels of dominance behavior in the tube test, relative to WT littermates. They also showed higher amplitude of mEPSCs in layer 5 neurons of the mPFC, where previous work links synaptic strength with social status. The mice did not display differences in any other social or repetitive behaviors tested, including those that were abnormal and rescued by bumetanide in two published rodent models of symptoms relevant to ASD. This may indicate that the role of KCC2 in development is likely to act in concert with other factors to impact adult physiology and behavior. Animals with reduced KCC2 expression do not appear to model all aspects of ASD, but KCC2 remains an intriguing target for modulation of social behavior.
Acknowledgements:
Krista Paffenroth (Vanderbilt University) contributed to the execution of some of the behavioral studies. The creation of the KCC2+/− mouse and the KCC2 inhibitor were supported by NIH grants R01NS36758 and R21NS53651 to ED, respectively. This work was supported by NIH grant 5P50MH096972 and by a contract from F. Hoffmann-La Roche. Dr. Veenstra-VanderWeele has consulted or served on an advisory board for Roche Pharmaceuticals, Novartis, and SynapDx, has received research funding from Roche Pharmaceuticals, Novartis, SynapDx, Seaside Therapeutics, and Forest, and has received an editorial stipend from Springer and Wiley. At the time of these experiments, Dr. Saxe was an employee of F. Hoffmann-La Roche and is now an employee of Novartis. At the time of these experiments Dr. Santarelli was an employee of F. Hoffmann-La Roche and is now an employee of Boehringer Ingelheim.
References
- Amadeo A, Coatti A, Aracri P, Ascagni M, Iannantuoni D, Modena D, … Becchetti A (2018). Postnatal Changes in K(+)/Cl(−) Cotransporter-2 Expression in the Forebrain of Mice Bearing a Mutant Nicotinic Subunit Linked to Sleep-Related Epilepsy. Neuroscience, 386, 91–107. doi: 10.1016/j.neuroscience.2018.06.030 [DOI] [PubMed] [Google Scholar]
- Arion D, & Lewis DA (2011). Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen Psychiatry, 68(1), 21–31. doi: 10.1001/archgenpsychiatry.2010.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y (2002). Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci, 3(9), 728–739. doi: 10.1038/nrn920 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y (2014). The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience, 279, 187–219. doi: 10.1016/j.neuroscience.2014.08.001 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, & Gaiarsa JL (1989). Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol, 416, 303–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardet M, & Crusio WE (2006). Fmr1 KO mice as a possible model of autistic features. ScientificWorldJournal, 6, 1164–1176. doi: 10.1100/tsw.2006.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, & Kaila K (2009). Cation-chloride cotransporters and neuronal function. Neuron, 61(6), 820–838. doi: 10.1016/j.neuron.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Carter MD, Shah CR, Muller CL, Crawley JN, Carneiro AM, & Veenstra-VanderWeele J (2011). Absence of preference for social novelty and increased grooming in integrin beta3 knockout mice: initial studies and future directions. Autism Res, 4(1), 57–67. doi: 10.1002/aur.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wang J, Jiang J, Zheng X, Justice NJ, Wang K, … Yang L (2017). APP modulates KCC2 expression and function in hippocampal GABAergic inhibition. Elife, 6. doi: 10.7554/eLife.20142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevy Q, Heubl M, Goutierre M, Backer S, Moutkine I, Eugene E, … Poncer JC (2015). KCC2 Gates Activity-Driven AMPA Receptor Traffic through Cofilin Phosphorylation. J Neurosci, 35(48), 15772–15786. doi: 10.1523/JNEUROSCI.1735-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton GH, Owens GC, Wolff JS, & Smith RL (1998). Ontogeny of cation-Cl- cotransporter expression in rat neocortex. Brain Res Dev Brain Res, 109(2), 281–292. [DOI] [PubMed] [Google Scholar]
- Corder KM, Cortes MA, Bartley AF, Lear SA, Lubin FD, & Dobrunz LE (2018). Prefrontal cortex-dependent innate behaviors are altered by selective knockdown of Gad1 in neuropeptide Y interneurons. PLoS One, 13(7), e0200809. doi: 10.1371/journal.pone.0200809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN (2007). Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol, 17(4), 448–459. doi: 10.1111/j.1750-3639.2007.00096.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Esch CE, van den Berg WE, Buijsen RA, Jaafar IA, Nieuwenhuizen-Bakker IM, Gasparini F, … Willemsen R (2015). Fragile X mice have robust mGluR5-dependent alterations of social behaviour in the Automated Tube Test. Neurobiol Dis, 75, 31–39. doi: 10.1016/j.nbd.2014.12.021 [DOI] [PubMed] [Google Scholar]
- Delpire E, Baranczak A, Waterson AG, Kim K, Kett N, Morrison RD, … Lindsley CW (2012). Further optimization of the K-Cl cotransporter KCC2 antagonist ML077: development of a highly selective and more potent in vitro probe. Bioorg Med Chem Lett, 22(14), 4532–4535. doi: 10.1016/j.bmcl.2012.05.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpire E, Days E, Lewis LM, Mi D, Kim K, Lindsley CW, & Weaver CD (2009). Small-molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A, 106(13), 5383–5388. doi: 10.1073/pnas.0812756106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, … Staley KJ (2005). NKCC1 transporter facilitates seizures in the developing brain. Nat Med, 11(11), 1205–1213. doi: 10.1038/nm1301 [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Shahrokhi A, Tsintsadze V, Nardou R, Brouchoud C, Conesa M, … Ben-Ari Y (2014). Response to Comment on “Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring”. Science, 346(6206), 176. doi: 10.1126/science.1256009 [DOI] [PubMed] [Google Scholar]
- Ellegood J, Yee Y, Kerr TM, Muller CL, Blakely RD, Henkelman RM, … Lerch JP (2018). Analysis of neuroanatomical differences in mice with genetically modified serotonin transporters assessed by structural magnetic resonance imaging. Mol Autism, 9, 24. doi: 10.1186/s13229-018-0210-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, & Tye KM (2016). Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience, 321, 197–209. doi: 10.1016/j.neuroscience.2015.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BR, & Gao WJ (2018). Thalamic Control of Cognition and Social Behavior Via Regulation of Gamma-Aminobutyric Acidergic Signaling and Excitation/Inhibition Balance in the Medial Prefrontal Cortex. Biol Psychiatry, 83(8), 657–669. doi: 10.1016/j.biopsych.2017.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, … De Koninck Y (2013). Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med, 19(11), 1524–1528. doi: 10.1038/nm.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvain G, Chamma I, Chevy Q, Cabezas C, Irinopoulou T, Bodrug N, … Poncer JC (2011). The neuronal K-Cl cotransporter KCC2 influences postsynaptic AMPA receptor content and lateral diffusion in dendritic spines. Proc Natl Acad Sci U S A, 108(37), 15474–15479. doi: 10.1073/pnas.1107893108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Manago F, Tucci V, Kao HT, Valtorta F, & Benfenati F (2013). Autism-related behavioral abnormalities in synapsin knockout mice. Behav Brain Res, 251, 65–74. doi: 10.1016/j.bbr.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, & Dapretto M (2017). Reduced modulation of thalamocortical connectivity during exposure to sensory stimuli in ASD. Autism Res, 10(5), 801–809. doi: 10.1002/aur.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DS, Cabrera R, Wallis Schultz D, Zhu H, Lu W, Finnell RH, & Wlodarczyk BJ (2015). Autism-Like Behavior and Epigenetic Changes Associated with Autism as Consequences of In Utero Exposure to Environmental Pollutants in a Mouse Model. Behav Neurol, 2015, 426263. doi: 10.1155/2015/426263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, & Rivera C (2007). Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci, 27(37), 9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, & Jentsch TJ (2001). Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron, 30(2), 515–524. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, … Kleinman JE (2011). Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci, 31(30), 11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie F, Badie-Mahdavi H, & Yamaguchi Y (2012). Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proc Natl Acad Sci U S A, 109(13), 5052–5056. doi: 10.1073/pnas.1117881109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager A, Amiri H, Bielczyk N, van Heukelum S, Heerschap A, Aschrafi A, … Glennon JC (2017). Cortical control of aggression: GABA signalling in the anterior cingulate cortex. Eur Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Jiang-Xie LF, Liao HM, Chen CH, Chen YT, Ho SY, Lu DH, … Gau SS (2014). Autism-associated gene Dlgap2 mutant mice demonstrate exacerbated aggressive behaviors and orbitofrontal cortex deficits. Mol Autism, 5, 32. doi: 10.1186/2040-2392-5-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Merner ND, Friedel P, Silayeva L, Liang B, Khanna A, … Rouleau GA (2014). Genetically encoded impairment of neuronal KCC2 cotransporter function in human idiopathic generalized epilepsy. EMBO Rep, 15(7), 766–774. doi: 10.15252/embr.201438840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Smith T, & Paciorkowski AR (2015). Autism spectrum disorder and epilepsy: Disorders with a shared biology. Epilepsy Behav, 47, 191–201. doi: 10.1016/j.yebeh.2015.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonnier E, Degrez C, Phelep M, Tyzio R, Josse F, Grandgeorge M, … Ben-Ari Y (2012). A randomised controlled trial of bumetanide in the treatment of autism in children. Transl Psychiatry, 2, e202. doi: 10.1038/tp.2012.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonnier E, Villeneuve N, Sonie S, Serret S, Rosier A, Roue M, … Ben-Ari Y (2017). Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Transl Psychiatry, 7(3), e1056. doi: 10.1038/tp.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhou Y, Peng L, & Zhao Y (2017). Reduced protein expressions of cytomembrane GABAARbeta3 at different postnatal developmental stages of rats exposed prenatally to valproic acid. Brain Res, 1671, 33–42. doi: 10.1016/j.brainres.2017.06.018 [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, … Wynshaw-Boris A (1997). Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell, 90(5), 895–905. [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, & Delpire E (1999). Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol, 39(4), 558–568. [PubMed] [Google Scholar]
- Markkanen M, Karhunen T, Llano O, Ludwig A, Rivera C, Uvarov P, & Airaksinen MS (2014). Distribution of neuronal KCC2a and KCC2b isoforms in mouse CNS. J Comp Neurol, 522(8), 1897–1914. doi: 10.1002/cne.23510 [DOI] [PubMed] [Google Scholar]
- Merner ND, Chandler MR, Bourassa C, Liang B, Khanna AR, Dion P, … Kahle KT (2015). Regulatory domain or CpG site variation in SLC12A5, encoding the chloride transporter KCC2, in human autism and schizophrenia. Front Cell Neurosci, 9, 386. doi: 10.3389/fncel.2015.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, … Lauder JM (2009). Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav, 8(2), 129–142. doi: 10.1111/j.1601-183X.2008.00452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CL, Anacker AM, Rogers TD, Goeden N, Keller EH, Forsberg CG, … Veenstra-VanderWeele J (2017). Impact of Maternal Serotonin Transporter Genotype on Placental Serotonin, Fetal Forebrain Serotonin, and Neurodevelopment. Neuropsychopharmacology, 42(2), 427–436. doi: 10.1038/npp.2016.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, DeRamus T, Libero LE, Laird A, & Kana RK (2016). Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum Brain Mapp, 37(11), 3957–3978. doi: 10.1002/hbm.23288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskarjov M, Seja P, Heron SE, Williams TC, Ahmad F, Iona X, … Kaila K (2014). A variant of KCC2 from patients with febrile seizures impairs neuronal Cl- extrusion and dendritic spine formation. EMBO Rep, 15(6), 723–729. doi: 10.1002/embr.201438749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, … Kaila K (1999). The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature, 397(6716), 251–255. doi: 10.1038/16697 [DOI] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, … Zoghbi H (2002). Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron, 35(2), 243–254. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, & Paylor R (2005). Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav, 4(7), 420–430. doi: 10.1111/j.1601-183X.2005.00123.x [DOI] [PubMed] [Google Scholar]
- Takahashi A, & Miczek KA (2014). Neurogenetics of aggressive behavior: studies in rodents. Curr Top Behav Neurosci, 17, 3–44. doi: 10.1007/7854_2013_263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornberg J, Voikar V, Savilahti H, Rauvala H, & Airaksinen MS (2005). Behavioural phenotypes of hypomorphic KCC2-deficient mice. Eur J Neurosci, 21(5), 1327–1337. doi: 10.1111/j.1460-9568.2005.03959.x [DOI] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, … Ben-Ari Y (2014). Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science, 343(6171), 675–679. doi: 10.1126/science.1247190 [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, … Blakely RD (2012). Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A, 109(14), 5469–5474. doi: 10.1073/pnas.1112345109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, & Hu H (2011). Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science, 334(6056), 693–697. doi: 10.1126/science.1209951 [DOI] [PubMed] [Google Scholar]
- Woo NS, Lu J, England R, McClellan R, Dufour S, Mount DB, … Delpire E (2002). Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus, 12(2), 258–268. doi: 10.1002/hipo.10014 [DOI] [PubMed] [Google Scholar]
- Zhou T, Zhu H, Fan Z, Wang F, Chen Y, Liang H, … Hu H (2017). History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science, 357(6347), 162–168. doi: 10.1126/science.aak9726 [DOI] [PubMed] [Google Scholar]
- Zhu L, Lovinger D, & Delpire E (2005). Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J Neurophysiol, 93(3), 1557–1568. doi: 10.1152/jn.00616.2004 [DOI] [PubMed] [Google Scholar]
- Zhu L, Polley N, Mathews GC, & Delpire E (2008). NKCC1 and KCC2 prevent hyperexcitability in the mouse hippocampus. Epilepsy Res, 79(2–3), 201–212. doi: 10.1016/j.eplepsyres.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zike ID, Chohan MO, Kopelman JM, Krasnow EN, Flicker D, Nautiyal KM, … Veenstra-VanderWeele J (2017). OCD candidate gene SLC1A1/EAAT3 impacts basal ganglia-mediated activity and stereotypic behavior. Proc Natl Acad Sci U S A, 114(22), 5719–5724. doi: 10.1073/pnas.1701736114 [DOI] [PMC free article] [PubMed] [Google Scholar]