Abstract
Background:
Baseline rostral anterior cingulate cortex (rACC) activity is a well-replicated, non-specific predictor of depression improvement. The rACC is a key hub of the default mode network (DMN), which prior studies indicate is hyperactive in major depressive disorder (MDD). As DMN downregulation is reliant on input from the salience network (SN) and frontoparietal network (FPN), an important question is whether rACC connectivity with these systems contributes to depression improvement.
Method:
Our study evaluated this hypothesis in outpatients (N=238; 151 females) enrolled in the EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care) 8-week randomized clinical trial of sertraline versus placebo for MDD. Depression severity was measured using the Hamilton Rating Scale for Depression, and electroencephalography was recorded at baseline and week 1. Exact Low Resolution Electromagnetic Tomography (eLORETA) was used to compute activity from the rACC, and key regions within the DMN (posterior cingulate cortex), FPN (left dorsolateral prefrontal cortex) and SN (right anterior insula; rAI). Connectivity in the theta (4.5-7 Hz) and beta (12.5-21 Hz) bands was computed using lagged phase synchronization.
Results:
Stronger baseline theta-band rACC-rAI (SN hub) connectivity predicted greater depression improvement across 8 weeks of treatment for both treatment arms (B=−0.57, 95% CI=−1.07, −0.08, p=0.03). Early increases in theta-band rACC-rAI connectivity predicted a greater likelihood of achieving remission at week 8 (odds ratio=2.90, p=0.03).
Conclusion:
Among patients undergoing treatment, theta-band rACC-rAI connectivity is a prognostic, albeit treatment non-specific indicator of depression improvement, and early connectivity changes may predict clinically meaningful outcomes.
Keywords: Depression, sertraline, rostral ACC, functional connectivity, salience network, EEG
Introduction
Although a variety of interventions exist for major depressive disorder (MDD), fewer than 50% of individuals respond to first-line treatment (1). Consequently, there is an urgent need to better understand which factors predict depression recovery. Abnormal rostral anterior cingulate cortex (rACC) activity is critically implicated in MDD pathophysiology and has emerged as a prognostic (i.e., treatment non-specific) predictor of depression improvement (2). First observed by Mayberg et al. (3), heightened pre-treatment rACC activity/metabolism predicts greater response to a range of antidepressants, including paroxetine (4), nortriptyline (5), citalopram (6), and fluoxetine (7), but also to placebo (8). Highlighting the robustness of this finding, a meta-analysis showed that depression improvement was linked to higher pre-treatment rACC activity in 19 separate studies (2), although a number of non-replications emerged (9–12). This finding was recently replicated a 20th time (13) in the EMBARC study (Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care), an 8-week clinical trial of sertraline for MDD (14). Importantly, pre-treatment rACC theta current density (associated with heightened rACC metabolism; 15) displayed incremental predictive validity in relation to treatment outcome (across both sertraline and placebo conditions), over and above a range of clinical and demographic factors previously associated with better MDD prognosis.
The rACC may influence treatment responsiveness by facilitating adaptive communication among large-scale functional networks (2). It is the main node within the anterior portion of the default mode network (DMN), and shows coordinated activity under task-free conditions with other regions in this network, including the posterior cingulate cortex (PCC; the main node within the posterior portion of the DMN), angular gyrus, middle and superior frontal gyri and middle temporal gyrus. The DMN is thought to support self-referential processing and exhibits greater activity under task-free conditions relative to conditions requiring external focus (16). Resting-state functional connectivity studies have revealed hyperconnectivity within the DMN in MDD, which might support persistent negative self-referential thinking (17).
Given its location within the DMN and structural connections with other areas of the prefrontal cortex, the rACC also communicates with the frontoparietal network (FPN) to support emotion regulation and goal-oriented responding (18) – two processes that require a downregulation of DMN activity. The FPN and DMN are typically anticorrelated (19), but meta-analyses indicate that individuals with MDD exhibit weaker anticorrelations between these networks (17), potentially leading to DMN interference in conditions requiring external focus. Similarly, a recent electroencephalography (EEG) source localization study showed that elevated connectivity between the DMN and FPN in the beta frequency band was linked to a more recurrent illness course (20), indicating that aberrant communication between these networks may be associated with MDD trajectory.
Finally, the rACC also has anatomical connections to regions in the salience network (SN), particularly the right anterior insula (rAI) (21, 22), which is thought to play a critical role in emotional processing (23). This network supports the detection of emotionally salient stimuli, and the rAI in particular is thought to coordinate anticorrelated activity between the DMN and FPN (24, 25). The SN is typically anticorrelated with the DMN (26), however, there is debate as to whether more or less anticorrelated rACC-SN activity may facilitate depression improvement. Weaker anticorrelated rACC and SN (particularly AI) activity has been observed in severely depressed individuals (21). Furthermore, greater baseline rACC-SN connectivity has been found to predict depression improvement following one week of placebo and 10 weeks of antidepressant treatment (22). It has been suggested that enhanced rACC-SN connectivity may confer a greater capacity for adaptively responding to emotionally salient stimuli, highlighting a potential link between rACC-SN connectivity and the responsiveness of the depressed state to intervention.
Together, these findings suggest that rACC activity may influence depression improvement via connections with other regions within the DMN, and also by facilitating DMN connectivity with other networks, such as the FPN and SN. Building on recent findings in the EMBARC study showing that baseline rACC theta activity prognostically predicted treatment outcome (13), this study examined whether theta-band synchronization between the rACC and other regions of the DMN, as well as the FPN and SN, predicts depression improvement. Since an independent study showed that elevated beta-band DMN-FPN connectivity was associated with a more recurrent depressive illness course (20), we also evaluated connectivity within the beta frequency.
In line with prior work (22), we hypothesized that greater depressive symptom reduction would be predicted by increased pre-treatment rACC-SN connectivity. In contrast, given prior work linking heightened within-DMN connectivity (17) and DMN-FPN connectivity (20) to greater depression severity, we hypothesized that greater depressive symptom reduction would be predicted by decreased rACC-DMN and rACC-FPN connectivity. Additionally, given that the local activity/baseline metabolism of a region has been found to determine that same region’s resting-state functional connectivity (27), we also examined whether rACC connectivity moderated or mediated the link between rACC activity and depression improvement. Finally, recent evidence (also based on data from the EMBARC trial) indicates that early changes in rACC cortical thickness following the first week of treatment with sertraline - potentially reflecting increases in cortical 5-HT1A receptor concentrations - predicted greater reduction in depressive symptoms over the course of treatment (28). Since sertraline may also have acute effects on functional connectivity of the rACC with other regions, we also examined whether early changes in rACC connectivity in the first week of treatment were associated with the likelihood of achieving remission.
Methods and Materials
The EMBARC study design, recruitment, randomization methods, power calculation and assessment measures, can be found elsewhere (14), and in the Supplementary Information. Methods pertinent to this study are outlined below.
Study design
Using a double-blind design, participants were randomly assigned to 8 weeks of sertraline or placebo. The primary outcome was depression severity on the 17-item clinician-rated Hamilton Rating Scale for Depression (HRSD-17; 29), administered at baseline, weeks 1, 2, 3, 4, 6, and 8. EEG was recorded at baseline and week 1.
Sample
Outpatients aged 18-65 meeting criteria for MDD based on the Structured Clinical Interview for DSM-IV (30) were recruited at Columbia University College of Physicians & Surgeons, Massachusetts General Hospital, the University of Michigan, and the University of Texas Southwestern Medical Center. A Quick Inventory of Depressive Symptomatology (QIDS-SR; 31) score of ≥14 (moderate depression) was required at screening and randomization visits. Study procedures were approved by the Institutional Review Boards of all sites. Participants provided written informed consent after receiving a complete study description.
From July 2011 to December 2015, 634 individuals were screened and 296 were randomized to sertraline or placebo. Nine dropped out before taking medication, 266 (92.3%) had EEG data collected, and 248 were included in the final model reported by Pizzagalli, Webb and colleagues (13). Ten subjects were excluded from the current study for having < 40 seconds of artifact-free segments available for connectivity analysis (the recommended amount), leaving a final sample of 238 subjects. The study flow diagram is shown in Fig. S1, with dropout reasons listed in Table S1.
EEG acquisition and preprocessing
EEG data were recorded in four 2-minute eyes open and eyes closed trials. Different EEG acquisition systems were used across sites, therefore a manual was developed to standardize recording techniques (see Supplementary Methods). Briefly, EEG data from each site were interpolated to a common, 72-channel montage and resampled at 256 Hz. Then, a standardized preprocessing pipeline was used to extract 2-second non-overlapping, artifact-free epochs for connectivity analyses (32). In line with prior work (20, 33), the first 40 seconds of artifact-free data were analyzed.
ROI selection
To probe FPN connectivity, a left DLPFC seed was defined using coordinates from Dosenbach et al. (34). For DMN connectivity analyses, a midline PCC seed was defined using coordinates from Yeo et al. (35). For SN analyses, a rAI seed was defined using coordinates from Seeley et al. (36), as this right hemisphere region is thought to modulate DMN and FPN activity (24). Finally, a rACC seed was defined using prior work examining predictors of treatment response (5, 13). Seed coordinates are shown in Table S2. Seeds were used to create regions of interest (ROIs; Fig. 1) consisting of gray matter voxels within a 10-mm radius of the seed. Intracortical current source density at each ROI was then computed using the linear inverse solution, eLORETA (33).
Figure 1.
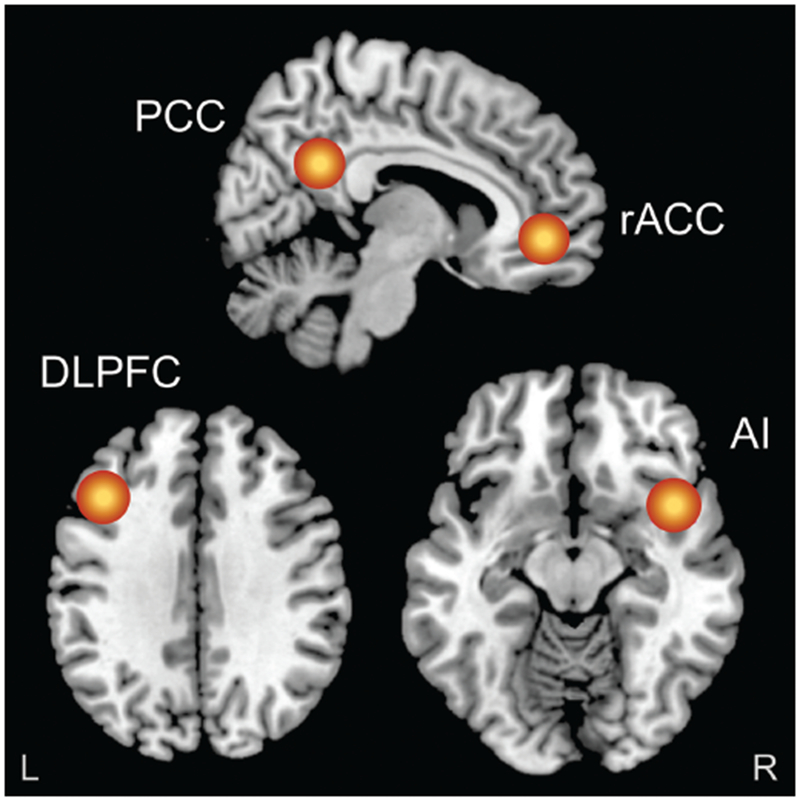
Figure shows the regions of interest (10 mm radius) that were created in the rostral anterior cingulate cortex (rACC), posterior cingulate cortex (PCC), left dorsolateral prefrontal cortex (DLPFC) and right anterior insula (rAI). Resting-state functional connectivity was then computed (by means of lagged phase synchronization) between the rACC, and the PCC (default mode network), left DLPFC (frontoparietal network) and rAI (salience network), in both the theta (4.5-7 Hz) and beta (12.5-21 Hz) frequency bands. For the purposes of visualization, regions of interest shown here are displayed on a 2 × 2 × 2 Montreal Neurological Institute template brain (5 mm resolution is used for analyses in eLORETA).
Source-based functional connectivity
Connectivity between sources was computed using lagged phase synchronization, which quantifies the nonlinear, non-instantaneous relationship between two signals (33). Instantaneous EEG-based connectivity measures have limited utility since they are susceptible to volume conduction, which leads to artificially correlated activity at different regions because the electrical signal spreads out laterally when it reaches the skull. However, non-instantaneous or “lagged” connectivity measures correct for this by computing the connectivity between two regions after any instantaneous contribution has been removed. Lagged phase synchronization was computed in the theta (4.5-7 Hz) and beta (12.5-21 Hz) frequencies (see Supplementary Methods for details).
Statistical analyses
Linear mixed effect models (implemented in STATA 13.1) evaluated whether rACC connectivity predicted HRSD score reductions across 8 weeks. Participants were treated as random effects, with subject-specific estimates for both intercept (estimated week 8 HRSD scores) and slope (weekly change in HRSD scores). Analyses were conducted in two stages. First, we entered demographic/clinical covariates linked to treatment response in MDD (Table S3), as well as the baseline rACC theta activity terms that were included in the final model reported in Table 2 of the earlier study published by Pizzagalli, Webb and colleagues (13). Second, Connectivity and Connectivity × Time (weeks 0, 1, 2, 3, 4, 6, 8, centered at week 8) terms were added to the model. We applied a conservative criterion (13) whereby connectivity terms had to be associated with both the intercept (Connectivity effect) and slope (Connectivity × Time interaction) at p<0.05 to be considered significant. For models containing significant connectivity terms, we used a likelihood ratio test to evaluate the goodness of fit of this extended model relative to the model containing only the covariates and baseline rACC theta activity terms.
Table 2.
Linear mixed model showing theta-band rACC-rAI connectivity as a predictor of HDRS score improvement across 8 weeks
| Model Term | Coef. | SE | Z | P |
|---|---|---|---|---|
| Time | −3.19 | 0.94 | −3.41 | <0.001 |
| Treatment | 5.86 | 2.68 | 2.19 | 0.03 |
| Time × Treatment | −0.19 | 0.25 | −0.74 | 0.46 |
| Site | 1.52 | 0.37 | 4.15 | <0.001 |
| Time × Site | 0.17 | 0.07 | 2.50 | 0.01 |
| Treatment × Site | −0.17 | 0.53 | −0.33 | 0.74 |
| Time × Treatment × Site | −0.02 | 0.10 | −0.25 | 0.80 |
| Depression Severity | 0.48 | 0.09 | 5.55 | <0.001 |
| Time × Depression Severity | −0.07 | 0.01 | −5.39 | <0.001 |
| Treatment × Depression Severity | −0.27 | 0.11 | −2.54 | 0.01 |
| Anxiety Severity | 0.10 | 0.05 | 2.21 | 0.03 |
| Age | 0.14 | 0.03 | 4.67 | <0.001 |
| Time × Age | 0.01 | 0.00 | 1.41 | 0.16 |
| Treatment × Age | −0.08 | 0.04 | −2.14 | 0.03 |
| Gender | −0.53 | 0.51 | −1.03 | 0.31 |
| Race | 0.33 | 0.34 | 0.99 | 0.32 |
| Time × Race | 0.06 | 0.06 | 0.99 | 0.32 |
| Marital Status | −0.96 | 0.31 | −3.10 | <0.001 |
| Employment Status | −0.08 | 0.35 | −0.23 | 0.82 |
| Treatment × Employment Status | 0.49 | 0.53 | 0.93 | 0.35 |
| rACC Theta | −3.82 | 1.37 | −2.80 | 0.01 |
| Time × rACC Theta | −0.57 | 0.25 | −2.28 | 0.02 |
| Theta-band rACC-rAI Connectivity | −3.01 | 1.35 | −2.23 | 0.03 |
| Time × Theta-band rACC-rAI Connectivity | −0.59 | 0.25 | −2.37 | 0.02 |
Note. rACC=rostral anterior cingulate cortex; rAI=right anterior insula; Anxiety Severity=Anxious Arousal subscale of the Mood and Anxiety Symptom Questionnaire; Depression Severity=Baseline Hamilton Depression Rating Scale total score.
For connectivity terms associated with both the intercept and slope, and which yielded a significantly improved model fit, we tested whether rACC connectivity moderated the relationship between baseline rACC theta activity and depression improvement by adding a Connectivity × rACC theta term, and a Connectivity × rACC theta × Time term. A significant interaction term was taken as evidence of moderation.
For mediation analyses, we evaluated a model wherein baseline rACC connectivity mediated the relationship between baseline rACC theta activity and HRSD score improvement (baseline-week 8). Since prior work has shown that rACC connectivity changes after one week of placebo are correlated with depressive symptom improvement (22), we tested a second mediation model wherein early change (baseline-week 1) in rACC connectivity was the mediator.
Finally, we examined whether connectivity was associated with clinically meaningful outcomes: (a) treatment response, defined as >50% reduction in HRSD scores by week 8, and (b) depression remission, defined as a HRSD score ≤7 at week 8.
Results
Sample characteristics of the 238 subjects included in this analysis are shown in Table 1, with further details show in Table S4.
Table 1.
Demographic and clinical characteristics of the analyzed sample (N=238)
| Whole sample (N=238) | CU site (n=75) | MG site (n=36) | TX site (n=83) | UM site (n=44) | P Value | |
|---|---|---|---|---|---|---|
| Age in years, M (SD) | 36.9 (13.2) | 33.5 (11.0)a | 33.2 (13.1)a | 43.5 (12.4)b | 33.4 (14.0)a | <0.001 |
| Female, No. (%) | 151 (63.4) | 49 (65.3) | 18 (50.0) | 52 (62.7) | 32 (72.7) | 0.21 |
| Years of education, M (SD) | 15.1 (2.4) | 15.6 (2.1) | 15.0 (2.5) | 14.6 (2.7) | 15.1 (2.3) | 0.09 |
| Caucasian, No. (%) | 163 (68.5) | 45 (60.0) | 26 (72.2) | 57 (68.7) | 5 (11.4) | 0.16 |
| Hispanic, No. (%) | 42 (17.6) | 19 (25.3)a | 2 (5.6)b | 18 (21.7)a | 3 (6.8)2 | 0.01 |
| Married, No. (%) | 49 (20.6) | 9 (12.0) | 7 (19.4) | 22 (26.5) | 11 (25.0) | 0.13 |
| Employed, No. (%) | 135 (56.7) | 41 (54.7) | 26 (72.2) | 40 (48.2) | 28 (63.6) | 0.07 |
| Age of MDD onset, M (SD) | 16.3 (5.7) | 17.1 (5.9)a | 16.2 (4.3)a,b | 16.8 (6.4)a,b | 14.2 (4.5)b | 0.04 |
| Current MDE length (months), median | 15.5 | 20.0 | 8.5 | 30.0 | 6.0 | 0.09 |
| Number of prior MDEs, median | 4 | 3 | 5 | 5 | 6 | 0.19 |
| QIDS, M (SD) | 18.2 (2.8) | 18.8 (2.8)a | 17.5 (2.8)a,b | 17.5 (2.5)b | 18.7 (3.1)a,b | 0.01 |
| HRSD 17-item, M (SD) | 18.5 (4.5) | 17.9 (4.4) | 19.9 (4.0) | 18.6 (4.5) | 18.0 (4.8) | 0.11 |
Note. MDD=Major Depressive Disorder; MDE=Major Depressive Episode; QIDS=Quick Inventory of Depressive Symptoms; HRSD=Hamilton Rating Scale for Depression; P values indicate the significance value associated with the main effect of Site. Where the main effect of Site was significant at p<0.05, superscript letters are used to denote the results of Bonferroni-adjusted pairwise comparisons between sites. Sites with the same superscript letter did not differ significantly from each other.
Effects of baseline rACC connectivity on depression improvement
A main effect of Connectivity (B=−3.01, 95% CI=−5.65, −0.37, p=0.03) and a Connectivity × Time interaction (B=−0.59, 95% CI=−1.07, −0.10, p=0.02) emerged for rACC-rAI (SN hub) connectivity in the theta band. Specifically, across the entire sample (placebo and sertraline groups), elevated theta-band rACC-rAI connectivity predicted lower week 8 HRSD scores and greater symptom improvement over 8 weeks, controlling for demographic/clinical covariates and baseline rACC theta activity. A likelihood ratio test showed that a model containing these two connectivity terms (Table 2) provided improved fit relative to a covariates + rACC theta activity only model (LR=6.69, p=0.04). Notably, when connectivity terms were entered into the model, both rACC theta activity terms remained significant predictors of symptom improvement (rACC theta term: B=−3.82, 95% CI=−6.50, −1.15, p=0.01; rACC theta × Time term: B=−0.57, 95% CI=−1.07, −0.08, p=0.02). Furthermore, baseline theta-band rACC-rAI connectivity was uncorrelated with rACC theta activity (r=0.06, p=0.39), indicating that these two metrics were independent predictors of depression improvement. Aligning with rACC theta activity findings reported by Pizzagalli, Webb and colleagues (13), connectivity terms did not interact with treatment condition in predicting symptom change (both ps>0.05), suggesting that they are treatment non-specific (i.e., prognostic) predictors of symptom improvement.
In contrast, neither theta-band rACC-PCC (the key posterior DMN region) connectivity, nor theta-band rACC-left dorsolateral prefrontal cortex (DLPFC; the key FPN region) connectivity emerged as predictors of depression improvement (all ps>0.05). Furthermore, when considering beta-band connectivity, no models showed both a significant effect of Connectivity as well as a Connectivity × Time interaction (all ps>0.05; see Supplementary Results). Taken together, these results specifically highlight theta-band rACC-rAI connectivity as a predictor of depression improvement.
rACC connectivity as a moderator or mediator of the effect of baseline rACC activity on depression improvement
For theta-band rACC-rAI connectivity, neither the Connectivity × rACC theta (B=3.30, 95% CI=−8.47, 15.06, p=0.58) nor the Connectivity × rACC theta × Time (B=0.61, 95% CI=−1.54, 2.75, p=0.58) interaction was significant, indicating no moderation. We also found no evidence for theta-band rACC-rAI connectivity acting as a mediator. The two mediation models tested are described in the Supplementary Results and shown in Fig. S2.
rACC connectivity as a predictor of depression remission
Theta-band rACC-rAI connectivity changes from baseline to week 1 predicted remission status after controlling for baseline HRSD scores (odds ratio=2.90, 95% CI=1.11, 7.58, p=0.03). Specifically, as theta rACC-rAI connectivity changes from baseline to week 1 increased by one unit, a participant was 2.9 times more likely to achieve symptom remission by week 8 [connectivity change in remitters (n=73): M=0.44, SD=0.34; change in non-remitters (n=122): M=0.32, SD=0.31)]. Theta-band rACC-rAI connectivity changes in remitters and non-remitters are shown in Fig. 2, with tests of potential confounds reported in the Supplementary Results.
Figure 2.
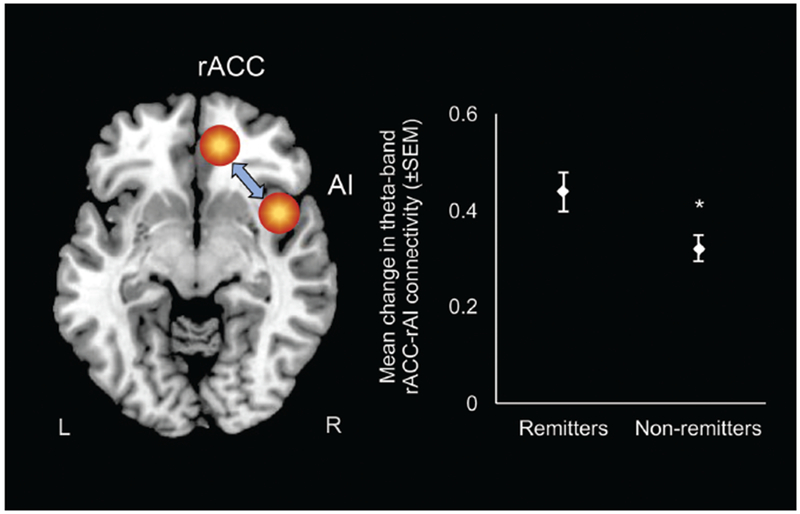
Early changes (baseline to week 1) in theta-band connectivity between the rostral anterior cingulate cortex (rACC) and the right anterior insula (rAI) – a major region in the salience network (SN) – as a function of depression remission status. Remission was defined as a Hamilton Depression Rating Scale score of ≤7 at week 8.
Discussion
Baseline theta rACC activity has emerged as an important indicator of clinical response to a range of depression interventions, including antidepressants, electroconvulsive therapy, sleep deprivation, and also placebo (13), and – in combination with known clinical/demographic predictors of depression prognosis – could be used to identify patients who require careful monitoring and more intensive intervention. As the rACC has rich anatomical connections with large-scale functional networks involved in attention, emotion regulation and cognitive control (2), we hypothesized that rACC connectivity with other brain systems may play a mechanistic role in depression recovery. Several key findings emerged. First, greater theta-band connectivity between the rACC and rAI – a key region within the SN – predicted greater reduction in depression severity across treatment conditions, controlling for demographic/clinical covariates and baseline rACC activity. Second, adding theta-band rACC-rAI connectivity as a predictor provided an improved model fit compared to a model containing only the demographic/clinical covariates and rACC activity. Importantly, in this final model, rACC activity remained a significant predictor of depression improvement. Combined with the lack of evidence for rACC connectivity moderating or mediating the link between baseline rACC activity and symptom improvement, this suggests that rACC activity and rACC connectivity are independent predictors of depression improvement. Third, baseline theta-band rACC-rAI connectivity did not interact with treatment group, indicating that it represents a non-specific “prognostic” predictor of depression improvement (as previously found for baseline rACC activity; 13). Fourth, increases in theta-band rACC-rAI connectivity from baseline to week 1 predicted a greater likelihood of achieving remission by week 8, indicating that early connectivity changes may be a useful marker of clinically meaningful outcomes.
Prior work has shown that rACC activity increases under conditions involving emotional conflict (37), or inhibiting attention to irrelevant emotional information (38). Consequently, elevated rACC activity may reflect a greater ability to modulate emotional responding using top-down control (2), and this may in turn promote better outcomes. Our findings extend this by showing that communication between the rACC and a region involved in the detection of personally salient events and which regulates communication between the DMN and FPN (25), may be another important predictor of future symptom improvement. One explanation is that rACC-rAI synchronization may aid in DMN downregulation in response to emotionally salient events, and this may be a mechanism that facilitates depression recovery. Support for this comes from a study in healthy individuals, which showed that ignoring task-irrelevant, unpleasant words was associated with task-evoked increases in rACC-AI functional connectivity (39). Furthermore, disruption of this functional coupling via brain injury-related damage to the white matter tract linking the rAI to the ACC, results in difficulty deactivating the DMN under conditions requiring external task focus (40).
Communication between the rACC and rAI may also be implicated in monitoring the salience of one’s emotions and interoceptive states, and this may partially explain the link between rACC-rAI connectivity and clinical response to placebo observed in our study, and in other work (22). For example, rAI and ACC co-activation has been observed when subjects view pictures of their body (41), and connectivity between these regions has been found to be negatively correlated with impairments in social- and self-awareness in healthy adults (42). This hints at the role of rACC-rAI connectivity in adaptive self-related processes, which may play an important role in both antidepressant and placebo effects. Furthermore, our observations that rACC connectivity with the DMN (the PCC region) or the FPN (the left DLPFC region) were not predictors of depression improvement, suggests that the integrity of systems that coordinate DMN-FPN switching (i.e., the SN), rather than the integrity of the DMN or FPN per se, may be more closely associated with the responsiveness of the depressed state to intervention. Moreover, the specificity of our findings to the theta band may reflect the putative role that the ACC (including the rACC) has in generating frontal midline theta frequency synchronization (e.g., see 15).
Our finding that early changes (i.e., after 1 week of treatment) in theta-band rACC-rAI connectivity predicted depressive symptom improvement aligns with prior findings showing that changes in rACC cortical thickness after one week of sertraline treatment (potentially reflecting increased 5-HT1A receptor concentrations; 28) predicted greater depressive symptom improvement. Furthermore, involvement of the rAI is consistent with prior studies showing that changes in activity among a set of brain regions (including the insula) following one week of treatment with an SSRI, were predictive of greater therapeutic response (22). However, in the current study, early changes in theta-band rACC-rAI connectivity (and the relationship between these early changes and better depression improvement) cannot be entirely attributed to the effects of sertraline, since theta-band rACC-rAI connectivity predicted better response to both sertraline and placebo. Future research is needed to determine what neuromodulatory processes may influence early changes in functional connectivity in individuals undergoing treatment with placebo. In the context of our findings, enhanced baseline theta-band rACC-rAI connectivity and early changes in this connectivity may be an indicator of the degree to which an individual’s depressive symptoms are responsive to intervention more generally. An important avenue for future studies will be to examine whether this reflects a) a unique subtype of depression characterized by early response to treatment, or b) a marker that is indicative of remission that is currently/already in progress. Examining changes in theta-band rACC-rAI connectivity over a longer time course during treatment (e.g., from baseline to week 8) would allow for these competing interpretations to be tested. Furthermore, it will be important to link this marker to previously reported depression endophenotypes (43).
We initially hypothesized that rACC-outcome associations observed in prior work (e.g., 32) may be driven by rACC connectivity, however, we found no evidence for rACC connectivity acting as a moderator or mediator. Although we cannot infer directionality from our analysis, the link between rACC-rAI connectivity and depression improvement may be driven by inputs coming from the rAI. Support for this comes from dynamic causal modeling (DCM) research showing that the rAI acts as a ‘causal outflow hub’ within the SN that triggers FPN modulation of the DMN in accordance with salient events (24). Another DCM study points to the relevance of excitatory rAI signaling in depression, showing weaker excitatory input from the rAI to the middle frontal gyrus in MDD patients, compared to controls (44). In the context of our study, coordinated input from the rAI to the DMN (via the rACC) may facilitate adaptive processing of emotionally salient events, which may in turn promote treatment responsiveness.
An important next step is to determine whether malleability of theta-band rACC-rAI connectivity identifies patients whose depression is likely to spontaneously remit, or whether it indicates patients who show greater susceptibility to placebo effects. Although these two processes are likely to be closely related, links between rACC-rAI connectivity and greater placebo response will have important implications for clinical trials. For example, if the mechanism by which elevated baseline theta-band rACC-rAI connectivity facilitates greater symptom improvement is via greater susceptibility to placebo effects, then this may be used to identify individuals for whom treatment non-specific factors are likely to play a larger role in determining treatment outcome. This, in turn, might allow for a better estimation of treatment-specific effects.
Some limitations must be emphasized. First, although EEG source functional connectivity has high temporal resolution for examining connectivity at discrete frequencies, lagged phase synchronization quantifies only synchronization strength (ranging from 0 to 1) and does not indicate synchronization direction. Studies using metrics that assess both connectivity strength and direction are needed to confirm whether greater positive or greater anticorrelated theta-band rACC-rAI connectivity predicts depression improvement. Causal links between rACC-rAI connectivity and depression improvement should also be probed using neurostimulation techniques that modulate fronto-insula connectivity (e.g., prefrontal theta-burst stimulation; 45). Second, source localization techniques cannot estimate connectivity involving subcortical regions. Subcortical dysfunction is critical to MDD pathophysiology, therefore fMRI-based connectivity studies must examine relationships between rACC-subcortical connectivity, and depression improvement. Third, in addition to showing significant main effects and interactions involving theta-band rACC-rAI connectivity, the final model also revealed a number of unanticipated significant effects that warrant further investigation. These include a main effect of Site, and a Site × Time interaction, both of which were unanticipated due to standardization of treatment across study sites. The significant Treatment × Age interaction was also unanticipated, as there is little evidence to suggest that the effects of sertraline (relative to placebo) are moderated by patient age in adults aged 18-65. Finally, since our sample was comprised of individuals with chronic or recurrent MDD with onset before age 30, further research is needed to assess the generalizability of our findings to individuals with milder or later onset depression.
In sum, our findings suggest that in MDD patients undergoing treatment with sertraline or placebo, elevated baseline theta-band connectivity between the rACC and rAI – a key region of the SN – is an important prognostic, treatment non-specific indicator of depression improvement, and early changes in this connectivity may be useful for identifying patients likely to achieve remission. In conjunction with recent findings (13), our results indicate that lower pre-treatment rACC activity and reduced rACC-rAI connectivity at baseline may be useful markers for identifying MDD patients who would benefit from more careful monitoring or intensive intervention.
Supplementary Material
Acknowledgements
We would like to acknowledge the important contribution of Dr. Craig Tenke, who sadly passed away on the 19th of December 2017. Through his expertise in electrophysiology, Dr. Tenke spearheaded the development of the EEG acquisition protocol and made a major contribution through his creation of a standardized EEG preprocessing pipeline. It is to Dr. Tenke that we wish to dedicate this manuscript.
Funding: The EMBARC study was supported by the National Institute of Mental Health under award numbers U01MH092221 (Trivedi, M.H.) and U01MH092250 (McGrath, P.J., Parsey, R.V., Weissman, M.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the EMBARC National Coordinating Center at UT Southwestern Medical Center, Madhukar H. Trivedi, M.D., Coordinating PI, and the Data Center at Columbia and Stony Brook Universities.
Role of Funder: The funder has no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclosures
In the last three years, the authors report the following financial disclosures, for activities unrelated to the current research:
Dr. Carmody: honorarium from the University of Texas, San Antonio.
Dr. Dillon: funding from NIMH, consulting fees from Pfizer.
Dr. Fava: Dr. Fava reports the following lifetime disclosures: http://mghcme.org/faculty/faculty-detail/maurizio_fava
Dr. Kayser: funding from NIMH and the Templeton Foundation.
Dr. McInnis: funding from NIMH; consulting fees from Janssen and Otsuka Pharmaceuticals.
Dr. Oquendo: funding from NIMH; royalties for the commercial use of the Columbia-Suicide Severity Rating Scale. Her family owns stock in Bristol Myers Squibb.
Dr. Pizzagalli: funding from NIMH, Brain and Behavior Research Foundation, and the Dana Foundation; consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehreinger Ingelheim, Posit Science, and Takeda Pharmaceuticals.
Dr. Trivedi: Dr. Trivedi reports the following lifetime disclosures: research support from the Agency for Healthcare Research and Quality, Cyberonics Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Diabetes and Digestive and Kidney Diseases, Johnson & Johnson, and consulting and speaker fees from Abbott Laboratories Inc., Akzo (Organon Pharmaceuticals Inc.), Allergan Sales LLC, Alkermes, AstraZeneca, Axon Advisors, Brintellix, Bristol-Myers Squibb Company, Cephalon Inc., Cerecor, Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals Inc., Forest Pharmaceuticals, GlaxoSmithKline, Health Research Associates, Johnson & Johnson, Lundbeck, MedAvante Medscape, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America Inc., MSI Methylation Sciences Inc., Nestle Health Science-PamLab Inc., Naurex, Neuronetics, One Carbon Therapeutics Ltd., Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories.
Dr. Trombello: Dr. Trombello currently owns stock in Gilead Sciences and Merck and within the last 36 months owned stock in Johnson & Johnson.
Dr. Weissman: funding from NIMH, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Sackler Foundation, and the Templeton Foundation; royalties from the Oxford University Press, Perseus Press, the American Psychiatric Association Press, and MultiHealth Systems.
All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: clinicaltrials.gov; http://clinicaltrials.gov; NCT01407094.
References
- 1.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. (2006): Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163: 28–40. [DOI] [PubMed] [Google Scholar]
- 2.Pizzagalli DA (2011): Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology 36: 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. (1997): Cingulate function in depression: a potential predictor of treatment response. Neuroreport 8: 1057–1061. [DOI] [PubMed] [Google Scholar]
- 4.Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR Jr (2003): Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry 160: 522–532. [DOI] [PubMed] [Google Scholar]
- 5.Pizzagalli DA, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. (2001): Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 158: 405–415. [DOI] [PubMed] [Google Scholar]
- 6.Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, et al. (2007): Rostral anterior cingulate cortex activity in the theta band predicts response to antidepressive medication. Clin EEG Neurosci 38: 78–81. [DOI] [PubMed] [Google Scholar]
- 7.Korb AS, Hunter AM, Cook IA, Leuchter AF (2009): Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clin Neurophysiol 120: 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korb AS, Hunter AM, Cook IA, Leuchter AF (2011): Rostral anterior cingulate cortex activity and early symptom improvement during treatment for major depressive disorder. Psychiatry Res Neuroim 192: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody AL, Saxena S, Silverman DH, Fairbanks LA, Phelps ME, Huang S-C, et al. (1999): Brain metabolic changes in major depressive disorder from pre-to post-treatment with paroxetine. Psychiatry Res 91: 127–139. [DOI] [PubMed] [Google Scholar]
- 10.Little JT, Ketter TA, Kimbrell TA, Dunn RT, Benson BE, Willis MW, et al. (2005): Bupropion and venlafaxine responders differ in pretreatment regional cerebral metabolism in unipolar depression. Biol Psychiatry 57: 220–228. [DOI] [PubMed] [Google Scholar]
- 11.Teneback CC, Nahas Z, Speer AM, Molloy M, Stallings LE, Spicer KM, et al. (1999): Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci 11: 426–435. [DOI] [PubMed] [Google Scholar]
- 12.Arns M, Etkin A, Hegerl U, Williams LM, DeBattista C, Palmer DM, et al. (2015): Frontal and rostral anterior cingulate (rACC) theta EEG in depression: implications for treatment outcome? Eur Neuropsychopharmacol 25: 1190–1200. [DOI] [PubMed] [Google Scholar]
- 13.Pizzagalli DA, Webb C, Dillon D, Tenke C, Kayser J, Goer F, et al. (2018): Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: A randomized clinical trial. JAMA Psychiatry 75: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, et al. (2016): Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. Psychiatry Res 78: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzagalli DA, Oakes TR, Davidson RJ. (2003): Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: an EEG/PET study of normal and depressed subjects. Psychophysiology 40: 939–949. [DOI] [PubMed] [Google Scholar]
- 16.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. (2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. (2015): Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. (2008): A dual-networks architecture of top-down control. Trends Cogn Sci 12: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou Z-W, Williams LM, et al. (2013): Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci USA 110: 19944–19949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitton AE, Deccy S, Ironside ML, Kumar P, Beltzer M, Pizzagalli DA. (2018): Electroencephalography source functional connectivity reveals abnormal high-frequency communication among large-scale functional networks in depression. Biol Psychiatry Cogn Neurosci Neuroimaging 3: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, et al. (2010): Glutamatergic and resting-state functional connectivity correlates of severity in major depression-the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci 4: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sikora M, Heffernan J, Avery ET, Mickey BJ, Zubieta J-K, Pecina M. (2016): Salience network functional connectivity predicts placebo effects in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruder GE, Stewart JW, McGrath PJ. (2017): Right brain, left brain in depressive disorders: clinical and theoretical implications of behavioral, electrophysiological and neuroimaging findings. Neurosci Biobehav Rev 78: 178–191. [DOI] [PubMed] [Google Scholar]
- 24.Sridharan D, Levitin DJ, Menon V (2008): A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 105: 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon V, Uddin LQ. (2010): Saliency, switching, attention and control: a network model of insula function. Brain Struct Func 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riedl V, Bienkowska K, Strobel C, Tahmasian M, Grimmer T, Förster S, et al. (2014): Local activity determines functional connectivity in the resting human brain: a simultaneous FDG-PET/fMRI study. Journal of neuroscience 34: 6260–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartlett E, DeLorenzo C, Sharma P, Yang J, Zhang M, Petkova E, et al. Pre- and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacology: 10.1038/s41386-41018-40122041389). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton M. (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JB. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- 31.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. (2003): The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54: 573–583. [DOI] [PubMed] [Google Scholar]
- 32.Tenke CE, Kayser J, Pechtel P, Webb CA, Dillon DG, Goer F, et al. (2017): Demonstrating test-retest reliability of electrophysiological measures for healthy adults in a multisite study of biomarkers of antidepressant treatment response. Psychophysiology 54: 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascual-Marqui RD, Lehmann D, Koukkou M, Kochi K, Anderer P, Saletu B, et al. (2011): Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos Trans A Math Phys Eng Sci 369: 3768–3784. [DOI] [PubMed] [Google Scholar]
- 34.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104: 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etkin A, Egner T, Kalisch R. (2011): Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH Ringo, Banich MT, et al. (2007): Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 44: 343–351. [DOI] [PubMed] [Google Scholar]
- 39.Szekely A, Silton RL, Heller W, Miller GA, Mohanty A. (2017): Differential functional connectivity of rostral anterior cingulate cortex during emotional interference. Soc Cogn Affect Neurosci 12: 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, et al. (2012): Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci USA 109: 4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, et al. (2007): Here I am: the cortical correlates of visual self-recognition. Brain Res 1143: 169–182. [DOI] [PubMed] [Google Scholar]
- 42.Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, et al. (2009): Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry 166: 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJ, et al. (2016): Neural correlates of three promising endophenotypes of depression: evidence from the EMBARC study. Neuropsychopharmacology 41: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kandilarova S, Stoyanov D, Kostianev S, Specht K. (2018): Altered resting state effective connectivity of anterior insula in depression. Front Psychiatry 9: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwabuchi SJ, Raschke F, Auer DP, Liddle PF, Lankappa ST, Palaniyappan L. (2017): Targeted transcranial theta-burst stimulation alters fronto-insular network and prefrontal GABA. Neuroimage 146: 395–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


