Abstract
BACKGROUND:
Enteral iron supplementation and red blood cell (RBC) transfusions are routinely administered to very low birth weight infants (VLBW), although the potential risks of these exposures have not been adequately quantified. This study evaluated the association between the cumulative dose of enteral iron supplementation, total volume of RBC transfused and risk of bronchopulmonary dysplasia (BPD) in VLBW infants.
STUDY DESIGN AND METHODS:
Retrospective, multicenter observational cohort study in Atlanta, GA. Cumulative supplemental enteral iron exposure and total volume or RBC transfused were measured until the age at assessment of BPD. Multivariable generalized linear models used to control for confounding and the reliability of the factors assessed in 1000 bootstrap models.
RESULTS:
A total of 598 VLBW infants were studied. In multivariable analysis, a greater cumulative dose of supplemental enteral iron exposure was associated with an increased risk of BPD (adjusted relative risk per 50mg increase: 1.07, 95% CI 1.02–1.11; P=0.002). Similarly, a greater volume of RBC transfused was associated with a higher risk of BPD (adjusted relative risk per 20 ml increase: 1.05, 95% CI 1.02–1.07; P <0.001). Both factors were reliably associated with BPD (>50%). Volume of RBC transfused was similar to gestational age in reliability as a risk factor for BPD (present in 100% of models) and was more reliable than mechanical ventilation at 1 week of age.
CONCLUSION:
The cumulative dose of supplemental enteral iron exposure and total volume of RBC transfusion are both independently associated with an increased risk of BPD in VLBW infants.
Keywords: blood transfusion, neonate, preterm, chronic lung disease, epidemiology, outcome
INTRODUCTION
Iron is an essential micronutrient for infant brain development and erythropoiesis1. The American Academy of Pediatrics currently recommends that all preterm infants should receive at least 2 mg/kg per day of enteral iron for the first year of life,2 although data from one randomized trial does not support supplementation of more than 2 mg/kg per dayfor very low birth weight (VLBW) infants3. Over half of VLBW infants will receive additional iron from red blood cell (RBC) transfusions4. Although short-term exposure to high-doses of iron have not been associated with oxidative stress,5 the cumulative chronic exposure to both enteral iron supplementation and RBC transfusions may potentially place infants at risk of iron overload6. Several reports have shown an association between RBC transfusion and a higher risk of BPD7,8, although it is unclear whether the risk is due to the transfusion itself or the likelihood that infants who have worse respiratory disease receive more RBC transfusion. The mechanistic role of iron and oxidative stress in the development of BPD has been postulated,9 although no clear relationship has been identified10. Importantly, none of these studies have evaluated the contribution of iron from enteral supplementation on the risk of BPD.
In this study, we examined the association between cumulative supplemental enteral iron exposure and the risk of BPD in VLBW infants. As a secondary aim, we evaluated the independent association between the cumulative volume of transfused RBCs and the risk of BPD.
METHODS
Study population
We conducted a secondary, retrospective analysis using prospectively collected data from a multicenter observational birth-cohort study investigating the transfusion-transmission of cytomegalovirus in preterm infants (TT-CMV study)11,12. We included VLBW infants born at three neonatal intensive care units (NICUs) in Atlanta, Georgia with coverage provided by separate groups (two with coverage by an academic division and 1 with coverage by a private practice group). All enrolled VLBW infants were followed from birth to 90 days, hospital discharge, transfer to a non-study affiliated hospital, or death (whichever was sooner). Our inclusion criteria were: 1) birth weight ≤ 1500 grams and 2) postnatal age ≤ 5 days. Our exclusion criteria included: 1) infant not expected to survive beyond 7 days of life based on the assessment by the treating neonatologist; 2) severe congenital abnormality; 3) transfusion before enrollment; or 4) maternal refusal to participate. This study was approved by the Institutional Review Board and/or Research Oversight Committees at all participating centers. This study was reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement13.
Definitions
All study data were collected in standardized case report forms (CRFs) using DataFax (DF/Net Research, Inc., Seattle, WA). The dose (in mg) and duration (in days and number of doses) of enteral iron supplementation was systematically recorded. Changes in absolute doses of iron and body weight were updated at scheduled assessments each week. The cumulative dose was determined by multiplying the absolute iron dose by the total number of doses with updating as the dose of iron changed up through the age of assessment of BPD (e.g. 36 weeks postmenstrual age). Iron supplementation was given as ferrous sulfate or multivitamin with iron with initiation and dosing adjustments at the discretion of the treating team. No infants received parenteral iron dextran or iron sucrose. Each RBC transfusion, including the volume transfused, was recorded. All RBC transfusions administered were leukoreduced, citrate phosphate dextrose adenine (CPDA-1) units from cytomegalovirus-seronegative donors. The primary outcome was any severity of BPD, defined according to the consensus definition by the National Institutes of Health14. Additional analyses were limited to only moderate-to-severe BPD. Study exposures were measured up until the postnatal age at assessment of BPD. Baseline illness severity was ascertained using the score for neonatal acute physiology (SNAP)15. Race and ethnicity were determined by maternal self-report using options defined by federally funded study guidelines.
Statistical Analysis
The sample size was fixed based on enrollment into the primary study. Any missing data was quantified by reporting of denominators or in footnotes. Relative risks were calculated to measure the degree of association between individual risk factors and BPD by fitting a modified Poisson regression model using a robust error variance16. We used SAS GENMOD Procedure (SAS Institute, Version 9.4, Cary, North Carolina) to implement the regression model as previously described16.
Two-stage modeling was used to control for confounding. In the first stage, longitudinal weight measurements were modeled using a random coefficients model to estimate infant-specific weight gain17, which allowed us to account for doses of iron exposure specific to an infant’s weight over time. This was used to avoid bias from higher iron doses given to infants with greater birth weight and because the study did not record weight each study day. Weight gain was estimated using a mixed effects model specifying that weight follows a linear regression over time (between days 7–90), with random slope and intercept for each infant. The infant-specific coefficient deviations (random effects) were added to the overall coefficient (fixed effects) to obtain an infant-specific weight gain, which was then included in multivariable modified Poisson models in the second stage of modeling. Control for confounding was performed based on available knowledge of factors thought to be associated with the risk of BPD or respiratory illness severity that were measured within the 1st week of life18. We included adjustment for total volume of RBCs transfused (also a secondary exposure of interest), gestational age, small for gestational age, receipt of any antenatal steroids, illness severity (SNAP), positive airway pressure on the day of birth (including both continuous positive airway pressure and mechanical ventilation), surfactant treatment on the day of birth, caffeine therapy on the day of birth, mechanical ventilation at 1 week of age, days until birth weight regained, weight gain, ever fed breast milk and clinical center. We used the same model to evaluate the relationship between the rate of iron exposure and BPD by replacing the variable for cumulative supplemental iron exposure with median rate of iron exposure (cumulative iron dose, in mg, divided by time-at risk, measured from birth up to the age of assessment of BPD, in days). Next, we evaluated the dose exposure-outcome relationships by replacing the continuous exposure variable in the above model with parameters for quartiles of cumulative enteral iron exposure. We also assessed a model that only included variables reliably associated with BPD that appeared in at least 50% of 1000 bootstrapped models. Each model fit was evaluated using a modified Akaike Information criterion goodness-of-fit statistic19. In addition, we evaluated for heterogeneity in the relationship between enteral iron exposure and BPD among infants fed any breast milk compared to no breast milk using interaction terms in a multivariable model and also evaluated the outcome of moderate-to-severe BPD. To avoid overfitting, the latter two models were reduced and included adjustment for volume of RBC transfusion, weight gain (with patient-specific intercept used as a measure of birth weight), days until birth weight regained, receipt of antenatal steroids, illness severity (SNAP), positive airway pressure on the day of birth, surfactant treatment on the day of birth, caffeine therapy on the day of birth, ever fed breast milk and center. A two-sided P value of <0.05 indicated statistical significance.
RESULTS
We evaluated a total of 598 VLBW infants. The median birth weight and gestational age of the cohort was 1024 grams (interquartile range [IQR] 790–1233) and 28 weeks (IQR 26–30), respectively (Table 1). Two infants received erythropoiesis-stimulating agents. A total of 495 (82.8%) infants received enteral iron supplementation. The cumulative supplemental enteral iron dose was 69 mg (18–186) overall and 101 mg (IQR 40–221) among those receiving supplementation. The median duration of iron supplementation was 29 days (IQR 17–41). The median rate of enteral iron supplementation was 1.2 mg/day (IQR 0.4–3.6) overall and 2.0 mg/day (IQR 0.8–4.0) among those infants receiving supplementation. The majority of infants were fed breast milk (87.0%). Baseline characteristics among only infants receiving enteral iron supplementation were similar to the full cohort of infants (Supplementary Table 1).
Table 1.
Characteristics of the study cohort
| Patient characteristics | All infants (N=598) |
|---|---|
| Birth weight, median g (IQR) | 1024 (790-1233) |
| Gestational age, median wk (IQR) | 28 (26-30) |
| Small for gestational age | 138/598 (23.1%) |
| Male gender | 302/598 (50.5%) |
| Race | |
| Black | 346/598 (57.9%) |
| White | 200/598 (33.4%) |
| Othera | 52/598 (8.7%) |
| Antenatal steroids given | 501/598 (83.8%) |
| SNAP, median (IQR) | 11 (6-14) |
| Positive airway pressure on DOB | 532/598 (89.0%) |
| Surfactant on DOB | 248/598 (41.5%) |
| Caffeine on DOB | 313/598 (52.3%) |
| Days until birth weight regained, median d (IQR) | 7 (5-10) |
| Ever fed breast milk | 520/598 (87.0%) |
| Receipt of an erythropoiesis stimulating agent | 2 (0.3%) |
| Total enteral iron exposure, median mg (IQR)b,c | 69 (18-186) |
| Rate of enteral iron supplementation, median mg/day (IQR)b,c | 1.2 (0.4, 3.6) |
| Receipt of any enteral iron supplementation | 495/598 (82.8%) |
| Total enteral iron exposure among infants receiving supplementation, median mg (IQR)c,d |
101 (40-221) |
| Duration of supplementation, median d (IQR)c,d | 29 (17-41) |
| Death during hospitalization | 32/598 (5.4%) |
Values are n (%), unless indicated otherwise. Abbreviations: IQR, interquartile range; DOB, day of birth; BPD, bronchopulmonary dysplasia.
Indicates Asian, American Indian, Alaska Native, Native Hawaiian or other Pacific Islander, more than one race or other unidentified race.
Among all 598 infants, including those who did not receive iron supplementation.
Evaluated from birth until postnatal age of BPD assessment.
Among 495 infants receiving enteral iron supplementation.
BPD of any severity developed in 240 infants (40.1%) and moderate-to-severe BPD occurred in 136 infants (22.7%). The median dose of supplemental iron exposure among infants with BPD was 70 mg (IQR 24–194), compared to 59 mg (IQR 11–172) among those without BPD (Table 2). Infants with BPD received more RBC transfusion (median 56ml vs. 0 ml), had lower daily weight gain (21 g/d vs 27 g/d), received more surfactant (63% vs. 27%) and had higher SNAP scores on the day of birth (12 vs. 9). The incidence of any severity of BPD among RBC transfused infants was 64% compared to 11% among infants not RBC transfused (Supplementary Table 2).
Table 2.
Univariable Analysis of Risk factors for BPD (n=598)
| Factor | BPD (n=240) |
No BPD (n=358) |
Relative Risk (95%) CI |
Relative risk per |
|---|---|---|---|---|
| Supplemental enteral iron, median mg (IQR) | 70 (24-194) | 59 (11-172) | 1.02 (0.98, 1.06) | 50 mg increase |
| Age at first enteral iron dose, median d (IQR)a | 24 (15, 42) | 14 (11, 25) | 1.16 (1.12, 1.19) | Per 7 day increase |
| Volume of RBC transfused, median ml (IQR) | 56 (24-105) | 0 (0-22) | 1.14 (1.11, 1.18) | 20 ml increase |
| Gestational age at birth, median weeks (IQR) | 26 (25, 27) | 29 (28, 30) | 0.73 (0.71, 0.76) | 1 week increase |
| Small for gestational age, n (%) | 44 (18%) | 94 (26%) | 0.74 (0.57, 0.98) | yes vs. no |
| Birth weight, median g (IQR) | 800 (682-979) | 1158 (983-1325) | 0.78 (0.75, 0.81) | 100 g increase |
| Received ≥ 1 dose of antenatal steroids, n (%) | 200 (83%) | 301 (84%) | 0.98 (0.75, 1.27) | yes vs. no |
| Score for Neonatal Acute Physiology, median (IQR) | 12 (10-15) | 9 (4-13) | 1.07 (1.05, 1.08) | 1 unit increase |
| Positive airway pressure on DOB, n (%) | 222 (93%) | 310 (87%) | 1.53 (1.02, 2.30) | yes vs. no |
| Surfactant on DOB, n (%) | 151 (63%) | 97 (27%) | 2.39 (1.95, 2.94) | yes vs. no |
| Caffeine on DOB, n (%) | 136 (57%) | 177 (49%) | 1.19 (0.98, 1.45) | yes vs. no |
| Mechanical ventilation at 1 week of age, n (%) | 194 (81%) | 120 (34%) | 3.81 (2.89, 5.04) | yes vs. no |
| Days until birth weight regained, median (IQR)c | 8 (5-11) | 7 (5-10) | 1.15 (1.05, 1.25) | Per 7 day increase |
| Mean weight gain, g/day (SE)b | 21.4 (0.4) | 27.3 (0.4) | 0.90 (0.88, 0.91) | 1 g/d increase |
| Ever fed breast milk, n (%) | 215 (90%) | 309 (86%) | 1.21 (0.87, 1.70) | yes vs. no |
Abbreviations: BPD, bronchopulmonary dysplasia; IQR, interquartile range; DOB, day of birth; SE, standard error.
Among the 495 infants receiving enteral iron supplementation.
Results from mixed-effects linear model, with random intercept and slope for each infant.
N=594. Four infants died before birth weight regained.
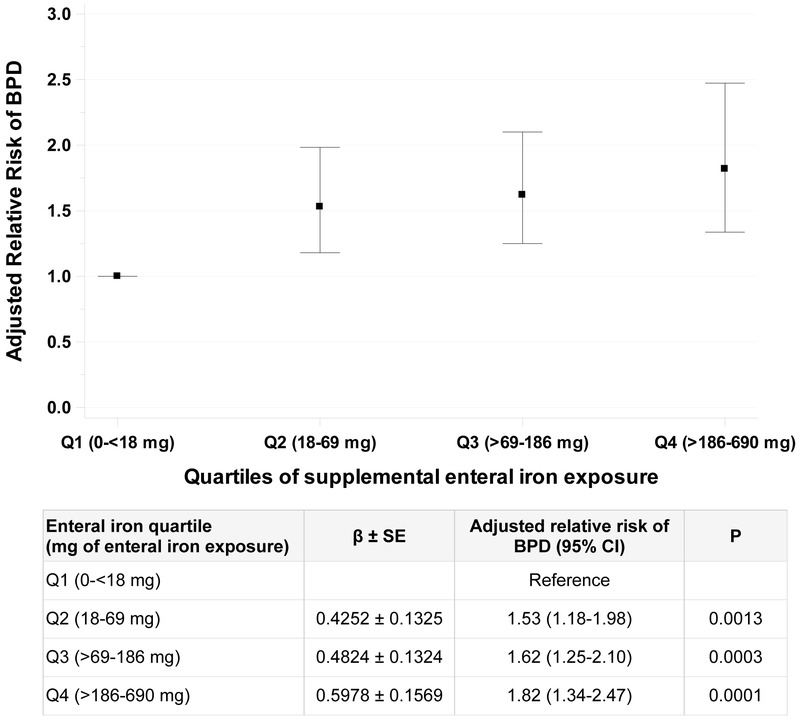
In multivariable analysis, a greater cumulative dose of enteral iron exposure was associated with a higher risk of BPD (adjusted relative risk [RR] per 50 mg increase: 1.07, 95% CI 1.02–1.11; P=0.002) (Table 3). Similarly, a greater total volume of RBC transfusion was independently associated with a higher risk of BPD (adjusted RR per 20 ml increase in RBCs transfused: 1.05, 95% CI 1.02–1.07; P<0.001). Both of these exposures were reliably associated with BPD (present in ≥ 50% of 1000 bootstrap models). In regression modelling, infants with BPD had slower weight gain compared to those without BPD (21.4 ± 0.4 g/d vs. 27.3 ± 0.4 g/d; P<0.0001) and lower weight at 30 days of age (1260 ± 21 g vs 1726 ± 17 g; P<0.0001). The association between enteral iron exposure and BPD was similar when only including covariates that were reliably associated with BPD (Table 4). When evaluating the outcome of moderate-to-severe BPD, we found similar point estimates of the association with enteral iron exposure as prior analyses, although the association was not statistically significant (adjusted RR per 50 mg increase 1.05; 95% CI 0.97–1.14). In this analysis, RBC transfusion was associated with moderate-to-severe BPD (adjusted RR per 20 ml increase 1.14; 95% CI 1.10–1.19). Infants in the highest quartile of iron exposure had a 1.8 times (95% CI 1.34–2.47) greater risk of BPD compared to infants in the lowest quartile (Figure 1). Findings were consistent, although diminished in magnitude, when limiting the analysis to only infants receiving enteral iron supplementation (Supplementary Figure 1). When evaluating the rate, instead of dose, of iron exposure in the primary model, with adjustment for other confounders, to account for potential differences in the time at risk iron supplementation, we found no significant association between each 1 mg/day increase in iron and risk of BPD (adjusted RR 1.04; 95% CI 0.99–1.10). In subgroup analyses by feeding type, no difference in the relationship between cumulative enteral iron exposure and BPD was detected among those infants fed any breastmilk (RR per 50 mg increase 1.05; 95% CI 1.01–1.09) and those infants not fed breast milk (1.27; 95% CI 1.13–1.42; interaction P=0.06).
Table 3.
Multivariable Analysis of Risk Factors Associated with BPD (n=592)
| Risk Factora | Estimated β (SE) | Relative Risk of BPD (95% CI) |
P | Reliability (%)b |
|---|---|---|---|---|
| Primary exposure | ||||
| Enteral iron (per 50 mg increase)c | 0.0013 (0.0004) | 1.07 (1.02, 1.11) | 0.002 | 69 |
| Secondary exposure | ||||
| RBC transfusion (per 20 ml increase)c | 0.0022 (0.0006) | 1.05 (1.02, 1.07) | <0.001 | 100 |
| Other factors in multivariable model | ||||
| Gestational age (per 1 week increase) | −0.1633 (0.0350) | 0.85 (0.79, 0.91) | <0.001 | 100 |
| Small for gestational age | 0.2062 (0.1294) | 1.23 (0.95, 1.58) | 0.111 | 48 |
| Antenatal steroids | 0.0455 (0.1029) | 1.05 (0.86, 1.28) | 0.658 | 4 |
| SNAP (per 1 point increase) | 0.0109 (0.0088) | 1.01 (0.99, 1.03) | 0.215 | 6 |
| Positive airway pressure on DOB | −0.1495 (0.1772) | 0.86 (0.61, 1.22) | 0.399 | 6 |
| Surfactant on DOB | 0.1970 (0.0963) | 1.22 (1.01, 1.47) | 0.041 | 54 |
| Caffeine on DOB | 0.0721 (0.1091) | 1.07 (0.87, 1.33) | 0.509 | 6 |
| Mechanical ventilation at 1 week of age | 0.5727 (0.1582) | 1.77 (1.30, 2.42) | <0.001 | 90 |
| Days until birth weight regained (per 7 day increase) | 0.0053 (0.0058) | 1.04 (0.96, 1.12) | 0.358 | 30 |
| Weight gain (g/day) | −0.0218 (0.0121) | 0.98 (0.96, 1.00) | 0.072 | 18 |
| Ever fed breast milk | 0.1763 (0.1449) | 1.19 (0.90, 1.58) | 0.224 | 14 |
Abbreviations: BPD, bronchopulmonary dysplasia; SE, standard error; CI, confidence interval. RBC, red blood cell; SNAP, Score for Neonatal Acute Physiology; DOB, day of birth.
Model fitted to outcome of BPD (n=240 events) and includes adjustment for center (not shown). Reference category is no, unless indicated otherwise. Four infants died before birth weight regained and 2 infants with missing weight gain data were excluded from the analysis.
Percentage of time risk factors appears in 1000 bootstrap models (factors ≥ 50% are reliable).
Cumulative exposure was evaluated from birth until postnatal age of BPD assessment.
Table 4.
Multivariable Analysis of Risk Factors for BPD with Reliability ≥ 50% (n=592)
| Risk Factora | Estimated β (SE) | Relative Risk of BPD (95% CI) |
P | Reliability (%)b |
|---|---|---|---|---|
| Primary exposure | ||||
| Enteral iron (per 50 mg increase)c | 0.0010 (0.0004) | 1.05 (1.01, 1.09) | 0.01 | 69 |
| Secondary exposure | ||||
| RBC transfusion (per 20 ml increase)c | 0.0025 (0.0006) | 1.05 (1.03, 1.08) | <0.001 | 100 |
| Other factors in multivariable model | ||||
| Gestational age (per 1 week increase) | −0.1853 (0.0288) | 0.83 (0.79, 0.88) | <0.001 | 100 |
| Surfactant on DOB (yes vs. no) | 0.2396 (0.0951) | 1.27 (1.05, 1.53) | 0.01 | 54 |
| Mechanical ventilation at 1 week of age (yes vs. no) | 0.6262 (0.1551) | 1.87 (1.38, 2.53) | <0.001 | 90 |
Abbreviations: BPD, bronchopulmonary dysplasia; SE, standard error; CI, confidence interval. RBC, red blood cell; DOB, day of birth.
Model fitted to outcome of BPD (n=240 events) and includes adjustment for center (not shown, P=0.0005). Four infants died before birth weight regained and 2 infants with missing weight gain data were excluded from the analysis.
Percentage of time risk factors appears in 1000 bootstrap models (factors ≥ 50% are reliable).
Cumulative exposure was evaluated from birth until postnatal age of BPD assessment.
Figure 1. Association between enteral iron supplementation and BPD by quartiles of iron exposure.
Relative risk estimates derived from multivariable model that includes 592 infants, with the 1st (lowest) quartile as reference. Error bars reflect 95% confidence interval.
DISCUSSION
In this observational study, we demonstrate that a greater cumulative dose of supplemental enteral iron exposure is associated with a higher risk of BPD in VLBW infants. To our knowledge, this is the first study reporting this finding, which is hypothesis generating. Potential biological explanations underlying the observed relationship between enteral iron exposure and BPD were not assessed in this epidemiologic study. In neonatal murine studies, enteral iron intake has been associated with increases in oxidative stress, although the relevance of this murine model to preterm infants may be limited20. In two prior studies in preterm infants, no association between short-term high dose enteral iron supplementation and oxidative stress markers in preterm infants was found. In a study of 16 infants, short term enteral iron supplementation beginning at a postmenstrual age of 30 weeks in doses ranging from 3 to 12 mg/kg/day with treatment for 2 to 3 weeks had no effect on markers of oxidative injury such as urine and blood isoprostanes21. In another study, 21 healthy VLBW infants were given high-doses (18 mg/daily) of iron for 1 week and no changes in urine 8-isoprostane or plasma total hydroperoxides, both markers of oxidant injury, were observed within 1 week of treatment5. Both of these studies suggest that short-term exposure to high doses of enteral iron are unlikely to mediate oxidant injury in preterm infants. However, neither study evaluated the effects of long-term cumulative supplemental exposure in which the risk of iron overload may be increased6. As this was an epidemiologic study, we did not obtain serum markers of iron status or measures of oxidative injury, which prevented us from determining if these factors have any role in mediating the relationship between cumulative supplemental enteral iron exposure and BPD or whether any enrolled patient exhibited any laboratory findings to support iron overload.
We assessed for heterogeneity in the relationship between enteral iron exposure and BPD depending on the type of feeding. Two recent studies have demonstrated formula to be a risk factor for BPD, while breast milk to be protective in a dose-dependent manner22,23. We found potential differences in the relationship between enteral iron exposure and BPD depending on if an infant received any breast milk feeding. However, these subgroup analyses should be viewed cautiously as they were exploratory.
We found an association between a greater total volume of RBCs transfused and higher risk of BPD. This finding has been previously reported in prior observational studies, including a prospective observational study in the UK24 and more recent single-center observational studies from China7,25. A study from Korea found similar associations between volume and number of RBC transfusions and BPD in a univariable analysis, but no association with ferritin levels or average iron supplementation during hospitalization and BPD26. By contrast, a recent prospective study from Japan found hyperferritenemia (ferritin ≥ 500ng/mL) to be associated with a higher risk of BPD27. However, ferritin is an acute phase reactant and, as BPD has an associated inflammatory component, the relationship may reflect inflammation that influences the risk of BPD instead of iron status. RBC transfusion has been shown to increase non-transferrin bound iron in preterm infants, which exists partly in the ferrous form and may increase the generation of reactive oxygen species28. In addition, in the setting of hyperoxia, iron in the redox-active state may accumulate in the lung without raising serum ferritin in mice29. However, the relationship between free iron and oxidative injury measured by lipid peroxidation is not clear10 and the relationship between oxidative stress and BPD may depend on antioxidant capacity30,31. Furthermore, a systematic-review and meta-analysis of randomized trials comparing liberal vs. restrictive RBC transfusion approaches have not demonstrated a higher risk of BPD among preterm infants that receive more liberal RBC transfusions compared to more restrictive RBC transfusions (relative risk 0.99; 95% CI 0.92–1.06)32. Therefore, the association between RBC transfusion and BPD may be confounded by indication for RBC transfusion, with the decision to transfuse RBCs dependent on the level of respiratory support an infant is receiving.
The strengths of our study include the systematic measurement of iron exposure from enteral supplementation and RBC transfusion and the systematic assessment of BPD. In addition, we were able to measure and adjust for multiple potential confounders, including baseline illness severity and respiratory support at 1 week of age, a major determinant of BPD. We also assessed the interaction between the type of enteral feeding and the risk of BPD. Finally, we were able to account for changes in weight gain over time and, therefore, could control for a weight-based dosing of iron given dose changes of iron often do not occur on a daily basis with changes in weight and the effective weight-based dose of iron could change as an infant gains weight.
Our study has several limitations. We could not measure all sources of iron exposure, such as enteral iron from routine feeding due to the challenges of daily measurement of all enteral feeding amounts. However, we evaluated the interaction between feeding type and the relationship between iron exposure and BPD. Furthermore, iron supplementation was not standardized. While this allowed us to assess the exposure-outcome relationship across variable approaches to enteral supplementation, the external validity of our findings to centers with differing supplementation practices or routine use of erythropoiesis stimulating agents may be limited. We acknowledge the limitations of any particular definition of BPD and the possibility that our study definition may have led to a higher incidence of BPD than if we had used alternative definitions33, evidenced by the results from our analysis evaluating moderate-to-severe BPD. However, we used a validated definition of BPD14 that has been shown to identify infants with higher risk of adverse pulmonary outcomes in early childhood34 and the fewer events of moderate-to-severe BPD could have limited statistical estimation. In addition, we did not evaluate a composite outcome of death or BPD because overall mortality was low in this cohort and infants who died may not have been eligible for enteral iron supplementation, potentially leading to a biased assessment of the outcome of death or BPD. It is possible, but unlikely, that our results were biased by reverse causation, as most infants initiated enteral iron supplementation in the first month of life, before BPD could be diagnosed and we did not include any iron exposure after 36 weeks PMA. Finally, we could not account for all potential confounding variables, including early oxygen exposure, and, therefore, our findings are intended to be hypothesis generating.
In conclusion, higher amounts of cumulative supplemental enteral iron exposure may potentially be associated with an increased risk of BPD in VLBW infants. Therefore, additional studies are needed to compare the risks and benefits of routine enteral iron supplementation, including the optimal dose and timing of initiation, given iron is an essential micronutrient for neurodevelopment and hematopoiesis. Until such studies are conducted, we believe it is reasonable to follow the recommendation of the American Academy of Pediatrics and provide 2 mg/kg/day of supplemental iron to human milk fed infants starting by 1 month of age2, but suggest caution with higher doses of iron or the routine supplementation of iron to formula-fed preterm infants. In addition, the strength of association between volume of RBC transfusion and risk of BPD in our study supports the need for further studies into the potential role of RBC transfusion in lung injury in preterm infants. Ongoing randomized trials comparing liberal and restrictive RBC transfusion thresholds will provide additional data on the effect of RBC transfusion on the risk of BPD in extremely preterm infants. Until these trial data are available, we recommend transfusing RBCs within the approaches, including specific hemoglobin threshold triggers that have been studied in randomized trials to date35.
Supplementary Material
ACKNOWLEDGEMENTS
The National Institutes of Health supported this study under awards UL1 TR000454, KL2 TR000455 and K23 HL128942. The parent study was funded under award P01 HL086773. The findings and conclusions in this abstract are those of the authors and do not necessarily represent the views of the NIH.
Statement of Financial Support: This study was supported by the National Institutes of Health (NIH). R.M.P. received salary support from the NIH National Center for Advancing Translational Sciences (NCATS) under awards UL1 TR000454 and KL2 TR000455 and from the National Heart Lung Blood Institute (NHLBI) under award K23 HL128942. The parent study was funded by the NHLBI under award P01 HL086773. The NIH had no role in: (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication. The findings and conclusions in this abstract are those of the authors and do not necessarily represent the views of the NIH.
Footnotes
CONFLICT OF INTEREST: The authors have no conflicts of interest to disclose.
Disclosure Statement: The authors have no conflicts of interest or financial relationships to disclose.
REFERENCES
- 1.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev 2011;69 Suppl 1: S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker RD, Greer FR, Committee on Nutrition American Academy of P. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 2010;126: 1040–50. [DOI] [PubMed] [Google Scholar]
- 3.Taylor TA, Kennedy KA. Randomized trial of iron supplementation versus routine iron intake in VLBW infants. Pediatrics 2013;131: e433–8. [DOI] [PubMed] [Google Scholar]
- 4.Strauss RG. Practical issues in neonatal transfusion practice. Am J Clin Pathol 1997;107: S57–63. [PubMed] [Google Scholar]
- 5.Braekke K, Bechensteen AG, Halvorsen BL, Blomhoff R, Haaland K, Staff AC. Oxidative stress markers and antioxidant status after oral iron supplementation to very low birth weight infants. J Pediatr 2007;151: 23–8. [DOI] [PubMed] [Google Scholar]
- 6.Rao R, Georgieff MK. Iron therapy for preterm infants. Clin Perinatol 2009;36: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Huang X, Lu H. Association between red blood cell transfusion and bronchopulmonary dysplasia in preterm infants. Sci Rep 2014;4: 4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr 2009;155: 331–37 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses 2006;66: 355–64. [DOI] [PubMed] [Google Scholar]
- 10.Cooke RW, Drury JA, Yoxall CW, James C. Blood transfusion and chronic lung disease in preterm infants. Eur J Pediatr 1997;156: 47–50. [DOI] [PubMed] [Google Scholar]
- 11.Josephson CD, Castillejo MI, Caliendo AM, Waller EK, Zimring J, Easley KA, Kutner M, Hillyer CD, Roback JD. Prevention of transfusion-transmitted cytomegalovirus in low-birth weight infants (</=1500 g) using cytomegalovirus-seronegative and leukoreduced transfusions. Transfus Med Rev 2011;25: 125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josephson CD, Caliendo AM, Easley KA, Knezevic A, Shenvi N, Hinkes MT, Patel RM, Hillyer CD, Roback JD. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr 2014;168: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335: 806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163: 1723–9. [DOI] [PubMed] [Google Scholar]
- 15.Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics 1993;91: 617–23. [PubMed] [Google Scholar]
- 16.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159: 702–6. [DOI] [PubMed] [Google Scholar]
- 17.Brown H, Prescott R. Applied mixed models in medicine. 2nd ed. Chichester, England ; Hoboken, NJ: John Wiley, 2006. [Google Scholar]
- 18.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, Stoll BJ, Buchter S, Laptook AR, Ehrenkranz RA, Cotten CM, Wilson-Costello DE, Shankaran S, Van Meurs KP, Davis AS, Gantz MG, Finer NN, Yoder BA, Faix RG, Carlo WA, Schibler KR, Newman NS, Rich W, Das A, Higgins RD, Walsh MC, Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med 2011;183: 1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan W Akaike’s information criterion in generalized estimating equations. Biometrics 2001;57: 120–5. [DOI] [PubMed] [Google Scholar]
- 20.Berggren KL, Chen J, Fox J, Miller J, Dodds L, Dugas B, Vargas L, Lothian A, McAllum E, Volitakis I, Roberts B, Bush AI, Fox JH. Neonatal iron supplementation potentiates oxidative stress, energetic dysfunction and neurodegeneration in the R6/2 mouse model of Huntington’s disease. Redox Biol 2015;4: 363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller SM, McPherson RJ, Juul SE. Iron sulfate supplementation decreases zinc protoporphyrin to heme ratio in premature infants. J Pediatr 2006;148: 44–8. [DOI] [PubMed] [Google Scholar]
- 22.Fonseca LT, Senna DC, Silveira RC, Procianoy RS. Association between Breast Milk and Bronchopulmonary Dysplasia: A Single Center Observational Study. Am J Perinatol 2016. [DOI] [PubMed] [Google Scholar]
- 23.Spiegler J, Preuss M, Gebauer C, Bendiks M, Herting E, Gopel W, German Neonatal N, German Neonatal Network GNN. Does Breastmilk Influence the Development of Bronchopulmonary Dysplasia? J Pediatr 2016;169: 76–80 e4. [DOI] [PubMed] [Google Scholar]
- 24.Silvers KM, Gibson AT, Russell JM, Powers HJ. Antioxidant activity, packed cell transfusions, and outcome in premature infants. Arch Dis Child Fetal Neonatal Ed 1998;78: F214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan J, Kong X, Li Q, Hua S, Zhang S, Zhang X, Feng Z. Association between anemia and bronchopulmonary dysplasia in preterm infants. Sci Rep 2016;6: 22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SH, Kim HM. The Iron Status of Very Low Birth Weight Infants Receiving Multiple Erythrocyte Transfusions during Hospitalization in the Neonatal Intensive Care Unit. Pediatr Gastroenterol Hepatol Nutr 2015;18: 100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochiai M, Kurata H, Inoue H, Tanaka K, Matsushita Y, Fujiyoshi J, Wakata Y, Kato K, Taguchi T, Takada H. An Elevation of Serum Ferritin Level Might Increase Clinical Risk for the Persistence of Patent Ductus Arteriosus, Sepsis and Bronchopulmonary Dysplasia in Erythropoietin-Treated Very-Low-Birth-Weight Infants. Neonatology 2016;111: 68–75. [DOI] [PubMed] [Google Scholar]
- 28.Hirano K, Morinobu T, Kim H, Hiroi M, Ban R, Ogawa S, Ogihara H, Tamai H, Ogihara T. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch Dis Child Fetal Neonatal Ed 2001;84: F188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dennery PA, Spitz DR, Yang G, Tatarov A, Lee CS, Shegog ML, Poss KD. Oxygen toxicity and iron accumulation in the lungs of mice lacking heme oxygenase-2. J Clin Invest 1998;101: 1001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrone S, Tataranno ML, Buonocore G. Oxidative stress and bronchopulmonary dysplasia. J Clin Neonatol 2012;1: 109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saugstad OD. Oxygen and oxidative stress in bronchopulmonary dysplasia. J Perinat Med 2010;38: 571–7. [DOI] [PubMed] [Google Scholar]
- 32.Whyte R, Kirpalani H. Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database Syst Rev 2011: CD000512. [DOI] [PubMed] [Google Scholar]
- 33.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, Auten K, Newman N, Rowan G, Grisby C, Arnell K, Miller L, Ball B, McDavid G, National Institute of Child H, Human Development Neonatal Research N. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114: 1305–11. [DOI] [PubMed] [Google Scholar]
- 34.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K, National Institutes of Child H, Human Development Neonatal Research N. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116: 1353–60. [DOI] [PubMed] [Google Scholar]
- 35.Patel RM, Josephson CD. Neonatal Transfusion Avery’s Diseases of the Newborn (Tenth Edition): Elsevier, 2018:1180–6. e3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.