Abstract
Background:
High school students 16–18 years-old (yo) contribute 10% of the United States blood supply. Mitigating iron depletion in these donors is important because they continue to undergo physical and neurocognitive development.
Study design and methods:
Study objectives were to determine the prevalence of iron depletion in 16–18 yo donors and whether their risk for iron depletion was greater than adult donors. Successful, age-eligible donors were enrolled from high school blood drives at two large US blood centers. Plasma ferritin testing was performed with ferritin <12 ng/mL our primary measure of iron depletion and ferritin <26 ng/mL a secondary measure. Multivariable repeated-measures logistic regression models evaluated the role of age and other demographic/donation factors.
Results:
Ferritin was measured from 4,265 enrollment donations September to November 2015 and 1,954 follow-up donations through May 2016. At enrollment, prevalence of ferritin <12 ng/mL in teenagers was 1% in males and 18% in females making their first blood donation, and 8% in males and 33% in females with prior donations. Adjusted odds for ferritin <12 ng/mL were 2.1 to 2.8 times greater in 16–18 yo than in 19–49 yo, and for ferritin <26 ng/ml were 3.3- to 4.7-fold higher in 16–18 yo. Progression to hemoglobin deferral was twice as likely in 16–18 yo vs 19–49 yo females.
Conclusion:
Age 16–18 yo is an independent risk factor for iron deficiency in blood donors at any donation frequency. Blood centers should implement alternate eligibility criteria or additional safety measures to protect teenage donors from iron depletion.
Keywords: Blood donation, Iron depletion, Iron deficiency, young donors
INTRODUCTION
Iron depletion is common in blood donors, estimated to be present in 33% of US donors.1 The prevalence of iron depletion is directly related to donation intensity and, therefore, is present in approximately two-thirds of female and half of male donors who donate frequently.2 Hemoglobin testing prevents donation by those with iron deficiency anemia (IDA) by establishing a minimum level of 12.5 g/dL for female and 13.0 g/dL for male donors, but has poor sensitivity to detect non-anemic iron deficiency (NAID).2 Since 2006, the number of states in the US allowing donation by 16-year-olds (yo) has risen from 6 to 37.3 Consequently, high school-aged donors now contribute over 10% of the national supply, even as total collections have dropped from 17 to 12 million units of blood annually.4
Teenagers are undergoing continued physical growth and neurocognitive development, physiological processes that require iron. Teenage females are adjusting to iron losses from the onset of menses, and may also have inadequate dietary intake.5 Population-based studies in the US6 and elsewhere7 show lower iron stores in teenagers compared to adults, measured as serum or plasma ferritin. Thus, teenage blood donors may face more serious health consequences from donation than adult counterparts. To determine whether teenagers face higher risk for blood donation-associated iron depletion than adult donors, we assessed the iron status of persons donating at high school blood drives over the course of a single academic year.
METHODS
Enrollment in the Comparison of donation History and Iron LeveLs in teenage blood donors study (CHILL) took place between September 1 and November 13, 2015 at two US blood centers participating in the National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) program: American Red Cross Blood Services in Farmington, Connecticut, and BloodCenter of Wisconsin in Milwaukee, Wisconsin. Enrollment occurred at blood drives hosted by high school sponsors during the first drive of the 2015–16 academic year. Eligibility criteria included: age 16–49 years old, successful donation, availability of residual blood samples (collected routinely for laboratory testing), and negative infectious disease test results. For donors age 16–18, a minimum estimated blood volume of 3.5L was also required for donation eligibility; this measure was instituted to reduce vasovagal reaction rates in young, inexperienced donors.8 Routine donation consent procedures were followed at both blood centers, where informed consent for donation includes language authorizing attendant research. Accordingly, written information about CHILL was included with standard informational materials provided to donors and parents or legal guardians prior to enrollment; donors could opt out of CHILL by donating at a different venue than the high school drive. With no study-specific consent or assent required beyond that for blood donation, enrollment was passive, with no research staff involved or recruitment at any drives. Institutional Review Boards at both blood centers, the REDS-III central laboratory (Blood Systems Research Institute, San Francisco, California) and the REDS-III data coordinating center (RTI International, Research Triangle Park, North Carolina) approved the study.
Donors were followed through May 31, 2016. Residual blood samples were retrieved at enrollment and subsequent donations at the same high school. The enrollment period transitioned to follow-up when the expected sum of enrollment plus follow-up donations surpassed 3,000 at each blood center, anticipating a ratio of enrollment to return donations of 2:1. Donations made at other locations fell outside study tracking procedures and were not available for ferritin testing. Donor demographic characteristics were obtained from operational databases for all donor visits, including those from which ferritin values were not available.
Laboratory testing and donor notification
Residual blood samples collected in EDTA tubes were retrieved from blood center labs, electronically cross-referenced with Donation Identification Numbers from eligible donors, and stored at 4oC up to 5 days until centrifugation to separate plasma as previously validated.9 Plasma was stored at −80oC until shipment on dry ice for batch ferritin testing by the central laboratory after the final blood drive of the school year for each participating high school (Beckman-Coulter AU680 chemistry analyzer, Sacramento, California; linear range 8–450 ng/mL).
A notification letter was sent to each CHILL subject. The letter provided a quantitative result from their most recent ferritin test, the date of the corresponding donation, and messaging based on whether their iron stores were “low” (<26 ng/mL), “adequate” (26 to 550 ng/mL) or “high” (≥ 550 ng/mL) on the date of their donation. Subjects were provided a research office phone number for questions about the study, the results provided, and the recommendations contained therein.
Study objectives and analytical approach
The primary objectives were to estimate the prevalence of iron depletion in a cohort of teenage blood donors (16–18 yo) and to assess whether their risk for iron depletion was higher than adult controls (19–49 yo) after controlling for known risk factors including donation frequency and demographics. Initiating enrollment in September maximized the opportunity for longitudinal sample collection during a single academic year. Integration of CHILL into routine operations reduced the potential for selection bias in the case of differential rates of participation across subjects. Except for enrichment for high schools with multiple drives during the academic year, study donors represent an unselected population of age-eligible high school donors. Teenage donors at high school drives are mostly students who attend the school, whereas adults include staff, parents, and other community members and, hence, represent a convenience sample. Blood drive scheduling was unaffected by the study. The overwhelming majority of return donations were made at subsequent high school drives, though donors were able to donate elsewhere as long as the minimum 8-week interval between donations and other eligibility criteria were met.
A secondary objective was to assess whether risk for progression to IDA differed by age, after controlling for donation frequency and demographics.
Sample size
Monte Carlo simulations of a hypothetical study cohort were developed to determine sample size and power for estimation of risk for ferritin <12 ng/mL in 16–18 yo compared to 19–49 yo donors. Simulations were based on high school donor demographics and donation frequency sourced from the longitudinal REDS-III donation database (July 2012 - December 2016),10 estimated ferritin values in first-time donors from the National Health and Nutrition Examination Survey (NHANES) 2001–2002,6 and incremental risk for ferritin <12 ng/mL with progressing donation frequency from published longitudinal cohort data on risk for iron depletion by age, sex and donation behavior.2 A sample size of 4,000 donors was estimated to provide 80% power to detect a risk difference for ferritin <12 ng/mL of 16-percentage-points and 90% power for a 20-point difference. Accordingly, the enrollment window was scheduled to close after approximately 2,100 subjects were enrolled at each blood center, from whom were expected an additional 900 donations during the study period.
Statistical methods
Baseline demographics were summarized for eight age-sex cohorts (16-, 17-, 18-, and 19–49 yo by males and females). Enrollment hemoglobin and ferritin were stratified by first-time versus repeat blood donor and compared across age groups by sex. Mean ferritin for first-time donors was compared to NHANES 2001–02 for all eight age-sex groups.6 Repeated measures, multivariable logistic regression assessed whether young age was an independent predictor of low iron stores after controlling for donation frequency and recency, body weight, and sex. The impact of donation on ferritin in teenage versus adult donors was evaluated with interaction terms between age and prior two-year donation frequency and between age and time since last donation. Specific comparisons were made between 16-, 17- and 18 yo in logistic regression, with multiple comparison handled by Bonferroni adjustment. A priori candidate interactions were age by sex and weight by sex,2,11 although data-driven models evaluated all possible interactions using automated backwards elimination.
Estimates for the aggregate prevalence of ferritin <12 or <26 ng/mL in the two blood centers’ overall high school donor populations were developed by standardizing regression results to the entire high school donor population at both blood centers, thereby controlling for variation in donation frequency across high schools.
In exploratory analysis, risk for progression from NAID to IDA was assessed using exclusion from donation for low hemoglobin as a proxy measure (<12.5 g/dL) and was limited to female donors given the ten-fold difference in risk by sex.12 Unadjusted rates of deferral were compiled by age and donation frequency. Adjusted risk for low hemoglobin deferral was estimated using repeated measures logistic regression controlling for demographic characteristics and donation behavior.
Data analysis took place in SAS Enterprise Guide Version 7.13 using SUDAAN Release 11.0.1.
RESULTS
Study population:
The study population included 4,265 blood donors enrolled at 121 high schools hosting one or more blood drives during the study period: 694 were 16 yo, 2,595 were 17 yo, 425 were 18 yo and 551 were 19–49 yo at time of their donation at the first high school drive between September and November 2015 (Figure 1). Donors were evenly split by sex overall, but 61% of the 16 yo were female, while 65% of the 18 yo were male. Approximately 40% of the donors provided one to four additional donations, totaling 2,290 follow-up donations. During the nine-month study period, 8.2% of teenagers (n=305) donated three or more times. The cohort was majority Caucasian (61%), with 8% African-American, 5% multi-racial or another race, and 26% unreported. Approximately 15% reported Hispanic ethnicity (Table 1).
Figure 1: CHILL study flow diagram.
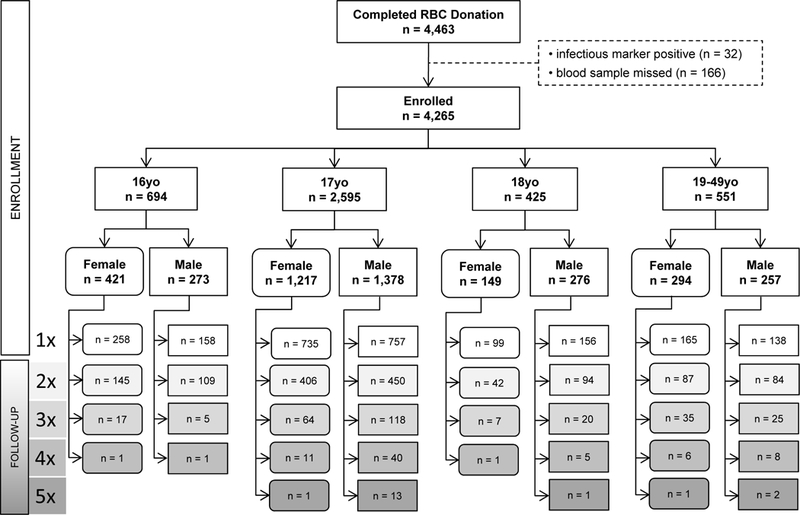
Number of eligible donors by age (years completed) and sex at enrollment, with total donation count during CHILL (where “Nx” represents N successful donation procedures with 1 or 2 red cell units donated per procedure). Ferritin values were available from all 4,265 enrollment donations and from 1,954 of 2,290 follow-up donations.
Table 1:
Baseline characteristics of study donors by sex and age
| FEMALES | MALES | TOTAL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16yo | 17yo | 18yo | 19–49yo | 16yo | 17yo | 18yo | 19–49yo | FEMALE | MALE | ||
| N | 421 | 1,217 | 149 | 294 | 273 | 1,378 | 276 | 257 | 2,081 | 2,184 | |
| BLOOD CENTER* N | |||||||||||
| BCW | 421 | 529 | 82 | 111 | 273 | 477 | 111 | 93 | 1,143 | 954 | |
| ARC-CT | 0 | 688 | 67 | 183 | 0 | 901 | 165 | 164 | 938 | 1,230 | |
| RACE N (%) | |||||||||||
| Caucasian | 234 (56) | 754 (62) | 97 (65) | 220 (75) | 144 (53) | 832 (60) | 136 (49) | 180 (70) | 1,305 (63) | 1,292 (59) | |
| African-American | 19 (4.5) | 89 (7.3) | 10 (6.7) | 11 (3.7) | 15 (5.5) | 144 (10) | 36 (13) | 13 (5.1) | 129 (6.2) | 208 (9.5) | |
| Other / multi-race | 6 (1.4) | 65 (5.3) | 6 (4.0) | 3 (1.0) | 5 (1.8) | 91 (6.6) | 29 (11) | 6 (2.3) | 80 (3.8) | 131 (6.0) | |
| Not reported | 162 (39) | 309 (25) | 36 (24) | 60 (20) | 109 (40) | 311 (23) | 75 (27) | 58 (23) | 567 (27) | 553 (25) | |
| ETHNICITY (any race) N (%) | |||||||||||
| Hispanic | 45 (11) | 196 (16) | 30 (20) | 28 (9.5) | 23 (8.4) | 275 (20) | 55 (20) | 18 (7.0) | 299 (14) | 371 (17) | |
| Non-Hispanic | 227 (54) | 784 (64) | 91 (61) | 212 (72) | 143 (52) | 884 (64) | 178 (65) | 190 (74) | 1,314 (63) | 1,396 (64) | |
| Not reported | 149 (35) | 237 (20) | 28 (19) | 54 (18) | 107 (39) | 218 (16) | 43 (16) | 49 (19) | 468 (23) | 417 (19) | |
| WEIGHT N (%) | |||||||||||
| 110–149 lbs | 241 (57) | 701 (58) | 78 (53) | 124 (43) | 84 (31) | 385 (28) | 756 (28) | 11 (4.3) | 1,146 (55) | 556 (26) | |
| 150–174 lbs | 109 (26) | 290 (24) | 36 (24) | 83 (28) | 90 (33) | 420 (31) | 82 (30) | 46 (18) | 518 (25) | 638 (29) | |
| 175–199 lbs | 40 (9.5) | 137 (11) | 17 (11) | 46 (16) | 44 (16) | 319 (23) | 63 (23) | 63 (25) | 240 (12) | 488 (22) | |
| 200+ lbs | 30 (7.1) | 89 (7.3) | 18 (12) | 39 (13) | 55 (20) | 254 (18) | 56 (20) | 137 (53) | 176 (8.5) | 503 (23) | |
| Not reported | 1 (0.2) | 1 (0.0) | |||||||||
| PRIOR DONATION HISTORY AT ENROLLMENT | |||||||||||
| First-time donor, N (%) | 333 (79) |
838 (69) |
66 (44) |
128 (44) |
230 (84) |
1,011 (73) |
125 (45) |
100 (39) |
1,365 (66) |
1,466 (67) |
|
| Repeat donor, Donations last 12m† |
1.15 (0.36) |
1.24 (0.65) |
1.24 (0.86) |
1.45 (1.11) |
1.14 (0.41) |
1.37 (0.68) |
1.57 (1.04) |
1.68 (1.24) |
1.28 (0.79) |
1.46 (0.91) |
|
| Repeat donor, Donations last 24m† |
1.15 (0.36) |
1.50 (0.77) |
1.86 (1.04) |
2.70 (1.89) |
1.14 (0.41) |
1.52 (0.77) |
2.03 (1.25) |
3.10 (2.13) |
1.78 (1.25) |
1.95 (1.43) |
|
BCW = BloodCenter of Wisconsin; ARC-CT = American Red Cross, Connecticut Blood Services Region
mean (SD)
Baseline hemoglobin and iron status:
Most donors (66%) were first-time donors at enrollment (Table 1). Older donors were more likely to have previously donated. Among repeat donors, increasing age was associated with higher number of previous donations. Hemoglobin was similar across age groups for first-time male and female donors, and for repeat female donors (Figure 2A). In contrast, ferritin varied by age, sex, and prior donation history (Figure 2B). Male and female first-time donors had ferritin values comparable to age-based population surveys (Figure 3), with the prevalence of ferritin <12 and ferritin <26 ng/ml in 16–18 yo females approximately 18% and 50%, respectively, nearly double that of first-time 19 to 49 yo females (Figure 2C, p<0.002 by ANOVA). The share of first-time males below either ferritin cut-off value was far lower than first-time females, but 16–18 yo males were more likely to have low ferritin than 19 to 49 yo first time males (Figure 2D, p<0.0001 by ANOVA for both 12- and 26 ng/mL). The proportion of repeat donors of all ages with ferritin <12 ng/mL at enrollment was higher than first-time donors, ranging from 5–8% in males to 25–34% in females while ferritin <26 ng/mL ranged from 13–31% in males and 52–73% in females.
Figure 2: Hemoglobin and ferritin values at enrollment.
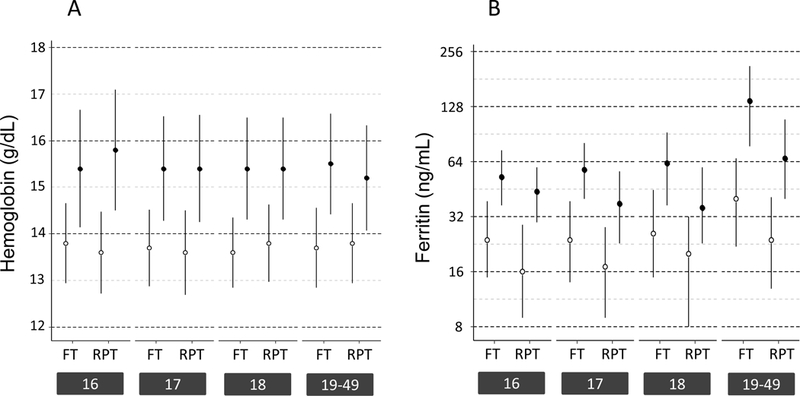
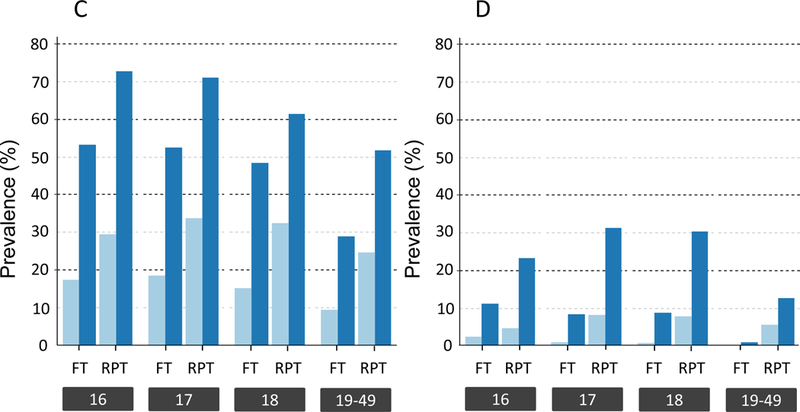
A) Mean hemoglobin (± 1SD) of first-time (FT) and repeat (RPT) donors at enrollment stratified by age and sex (○ females, ● males). B) Median ferritin (IQR) of FT and RPT donors at enrollment stratified by age and sex, plotted on log 2 scale (○ females, ● males). C) Prevalence of ferritin <12 ng/mL (light blue) and <26 ng/mL (dark blue) among FT and RPT female donors at enrollment stratified by age. D) Prevalence of ferritin <12 ng/mL (light blue) and <26 ng/mL (dark blue) among FT and RPT male donors at enrollment stratified by age.
Figure 3: Mean ferritin at enrollment in CHILL first-time donors vs NHANES subjects from 2001–2002.
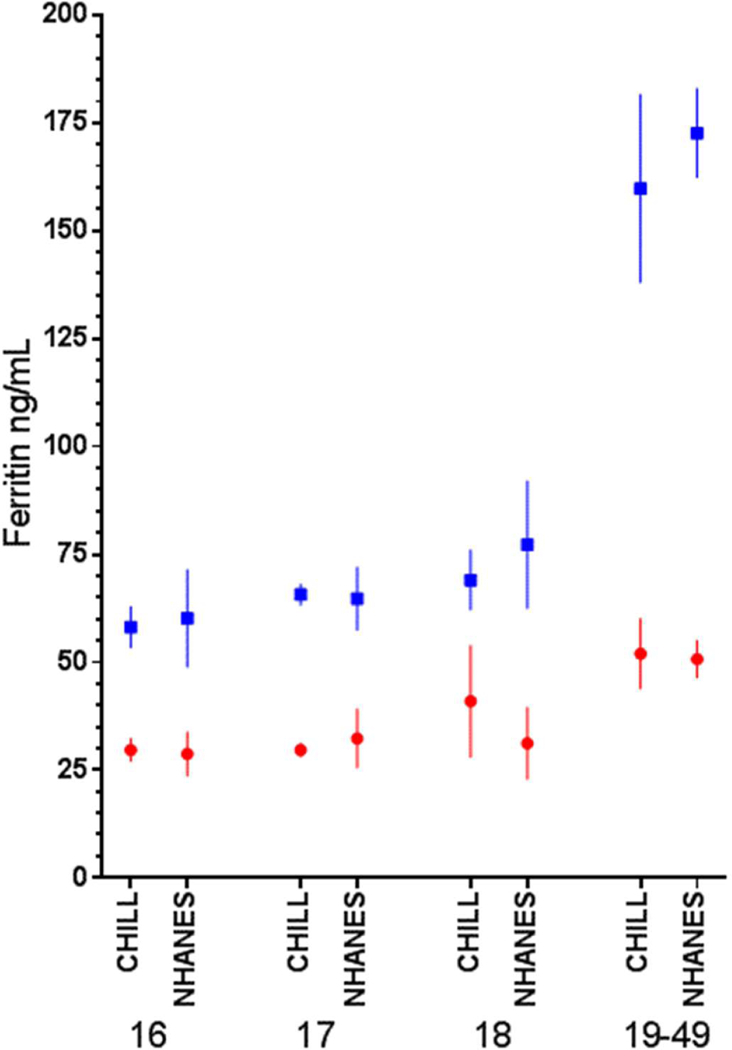
Mean and 95% C.I. of the mean ferritin for CHILL first time donors at enrollment are plotted alongside age-sex counterparts from NHANES 2001–2002. Red denotes female values; blue denotes male values.
Age 16 to 18 yo is an independent risk factor for iron deficiency:
Overall, 17% of 6,219 study donations were associated with ferritin <12 ng/mL and 41% with ferritin <26 ng/mL (Figure 4A). In unadjusted analyses, younger age, female sex, lower weight, higher 24-month donation count and shorter interval since last donation were all associated with higher odds for either low ferritin outcome (Figure 4A). Race and ethnicity were not associated with iron status at enrollment or in longitudinal analysis. The elevated risk for iron deficiency associated with age 16–18 yo became stronger after controlling for other covariates. Donors 16 −18 yo at enrollment had odds for ferritin <12 ng/mL more than twice that of adults (OR 2.1, 95% C.I. 1.4, 3.2 for 18 vs 19–49 yo), and odds for ferritin <26 ng/mL at least 3-fold higher than adults (OR 3.3, 95% C.I. 2.4, 4.4 in 18 yo; Figure 4B). Evidence for differences in risk for low ferritin when comparing 16-, 17-, and 18 yo was not demonstrated. Notably, the risk for low ferritin following donation persisted for at least one year. In subjects across all age groups, individuals donating at 8 to 16-week intervals had the largest risk, with odds for ferritin <12 or <26 ng/mL 5–6 times greater than those donating at intervals of one year or longer (Figure 4B); intervals of 24 to 52 weeks were associated with an OR of 2.1 (95% C.I. 1.1, 4.4) for ferritin <12 and comparable values for <26 ng/mL.
Figure 4: Prevalence of and risk for ferritin <12 ng/mL or <26 ng/mL across 6219 study visits.
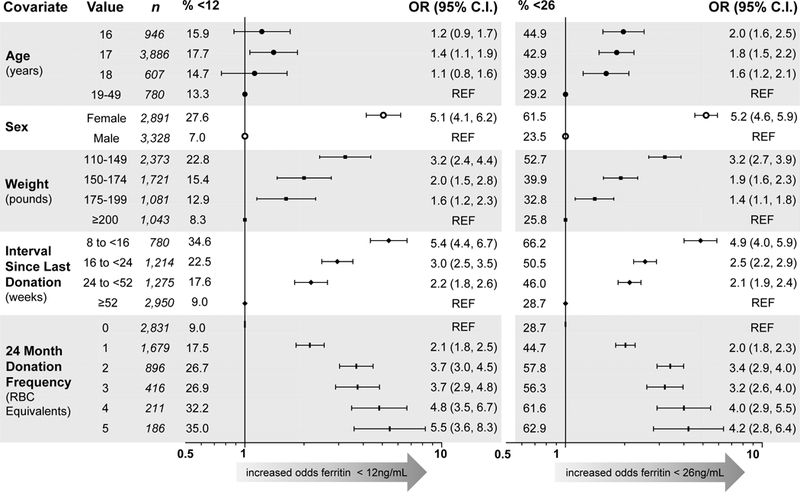
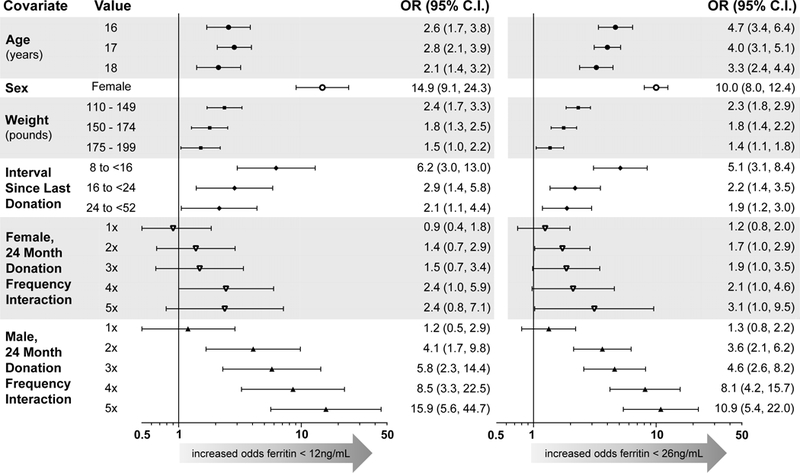
A) Unadjusted odds ratios for ferritin <12 ng/mL (left) and <26 ng/mL (right). Reference for Age is 19–49; for Sex is male; for Weight is ≥200 pounds; for Interval Since Last Donation is ≥52 weeks; for 24-Month Donation Frequency is 0. “n” represents the number of donations from subjects at each level of the modeled covariates, and “% <12” and “% <26” represent the proportion of donations at each level below the respective ferritin cutoff. B) Adjusted odds ratios for ferritin <12 ng/mL (left) and <26 ng/mL (right) in repeated measures multivariable logistic regression. Reference group is 19–49 yo male with 0 RBC donations in 24 months and weight ≥200 pounds; prevalence of ferritin <12 ng/mL is 0% and <26 ng/mL is 2%. Double red blood cell donation as most recent phlebotomy and blood center were also included in both models, but were not statistically significant and are not shown.
The impact of blood donation on iron status is mediated by sex:
Most interaction terms defined a priori did not improve model fit, but the automated data-driven selection identified sex by donation frequency as an important predictor of low ferritin (Figure 4B). The odds for ferritin <12 or <26 ng/mL were 14.9 (95% C.I. 9.1, 24.3) and 10.0 (95% C.I. 8.0, 12.4) greater, respectively, in females than males with 0 donations in the last 24 months. However, the increase in risk with successive donations was sharper in males, with odds for ferritin <12 ng/mL of 15.9 in males who donated ≥ five units (95% C.I., 5.6, 44.7) versus 2.4 (95% C.I. 0.8, 7.1) in females, a difference that derives in part from the low prevalence of ferritin <12 ng/mL in males with zero donations and measurement on a ratio scale. On an additive scale, the increased risk in males still outpaced that in females with increasing donation intensity, but the estimated risk for low ferritin was greater in females than in males at any given donation frequency (Figures 5A-B).
Figure 5: The impact of donation frequency on iron status is mediated by sex and by age.
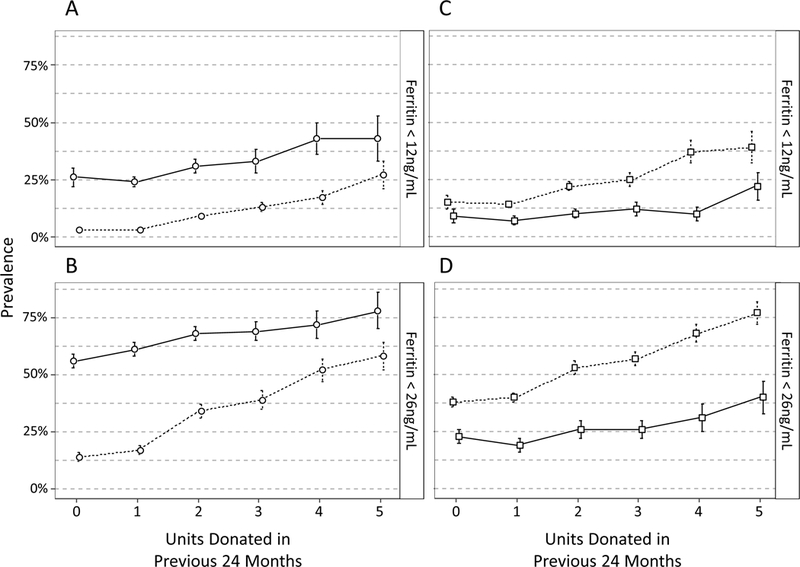
In separate longitudinal regression models, interaction terms indicate that the increase in risk for low ferritin with increasing donation frequency varies by sex (A, B) and by age (C, D). 5A plots the predicted prevalence (± 1 SE) of ferritin <12 ng/mL by sex and RBC units donated in 24 months if all 6,219 donation visits were made by subjects of the indicated sex, assuming all other covariate distributions were unchanged (solid line = females, dashed line = males). 5B plots expected prevalence of ferritin <26 ng/mL for the sex by donation frequency interaction. A separate model with an interaction term for age group (16–18 vs 19–49 years old) and donation frequency shows that donors 16 to 18 yo are more likely to have ferritin <12 ng/mL (C) and <26 ng/mL (D) with no donations and that the gap between younger and older donors grows with successive donations (solid line = 19–49 yo, dashed line = 16–18 yo).
The impact of blood donation on iron status is mediated by age:
In a separate model analyzing donors 16–18 yo combined rather than as separate age groups, subjects age 16–18 yo with zero donations had an OR for ferritin <26 ng/mL of 2.6 (95 %.C.I 1.8, 3.8) when compared to 19–49 yo. This risk further increased more quickly in 16–18 yo compared to 19–49 yo with each additional donation in the prior 24 months (Figures 5C-D), indicating that high school age donors begin their donation career at higher risk than adults 19–49 yo and that subsequent donations exacerbate the disparity.
Study donors were broadly representative of the larger high school donor pool at the two blood centers:
Standardization of modeled risk for ferritin <12 or <26 ng/mL to the overall high school donor population at the two blood centers found the iron status of study donors broadly representative of that of the larger high school donor pools. This extrapolation was feasible because the prediction models for low ferritin were based on routine donor information, with no additional data (aside from ferritin measurements) collected from CHILL donors than were collected from other high school donors at the two blood centers. In aggregate, approximately 25% of teen female high school donors were estimated to have ferritin <12 ng/mL at the time of donation compared to 18% of adult females 19–49 yo; 60% of teen female and 40% of adult female high school donors were estimated to have ferritin <26 ng/mL. Approximately 5% of male high school donors were estimated to have ferritin <12 ng/mL and 20% to have ferritin <26 ng/mL, with little variation between teenagers and adults. In sum, the estimated prevalence of low ferritin in the overall high school donor population was essentially equivalent to that observed in CHILL subjects, suggesting that our findings were not influenced by the study enrollment procedures.
Teenage females had higher risk for low hemoglobin deferral than adults:
Enrollment hemoglobin distributions did not vary in females by age or donation history (Figure 2A). However, following their enrollment donation, unadjusted rates of deferral for low hemoglobin appeared higher in 16-, 17-, and 18-yo compared to 19–49 yo females (Table 2A). After controlling for demographic factors and donation behavior (Table 2B), the adjusted risk for low hemoglobin deferral remained higher in 16 yo females than 19–49 yo at subsequent visits (OR 2.3, 95% C.I. 1.2, 4.4). Iron status at enrollment was a strong predictor of the onset of low hemoglobin with subsequent donations. Donors with ferritin <12 ng/mL at enrollment had 5.9 times the odds of being deferred for a low hemoglobin (95% C.I. 3.9, 8.8) and those with ferritin 12 to 25 ng/mL 2.9 times the odds (95% C.I. 1.9, 4.5) compared to donors with ferritin 26 ng/mL or higher at enrollment. Low hemoglobin deferral is much less frequent in males, and an effect of age and risk for hemoglobin deferral in males was not observed.
Table 2A:
Low hemoglobin deferral rate in female donors by count of units donated over trailing 24 months
| Number of units of red cells donated over prior 24 months | |||||
|---|---|---|---|---|---|
| 1 unit | 2 units | 3 units | 4 units | ≥ 5 units | |
| 16 yo | 11.3% (238) |
17.0% (88) |
33.3% (21) |
NS* | NS* |
| 17 yo | 12.8% (665) |
17.2% (348) |
18.8% (128) |
9.5% (42) |
12.0% (25) |
| 18 yo | 9.0% (67) |
13.5% (52) |
15.2% (33) |
NS* | NS* |
| 19 – 49 yo | 5.9% (101) |
8.2% (85) |
8.8% (68) |
4.0% (50) |
5.0% (80) |
Rate of deferral for low hemoglobin estimated as (Number of low hemoglobin deferrals)/(Number of donor presentations to donate). Shown as deferral rate % (N donor presentations).
NS: Not shown (fewer than 20 donor visits at that combination of donation frequency and age).
Table 2B:
Adjusted risk for low hemoglobin deferral at follow-up visits in female donors by age and iron status at enrollment visit
| Adjusted ORs* | 95% C.I. | |
|---|---|---|
| Age | ||
| 16yo vs 19–49yo | 2.3 | 1.2, 4.4 |
| 17yo vs 19–49yo | 1.5 | 0.9, 2.5 |
| 18yo vs 19–49yo | 1.6 | 0.8, 3.3 |
| Ferritin at enrollment visit | ||
| < 12 vs ≥ 26 ng/mL | 5.9 | 3.9, 8.8 |
| 12 to 25 vs ≥ 26 ng/mL | 2.9 | 1.9, 4.5 |
Adjusted ORs from repeated measures logistic regression model also controlling for race, weight, donation frequency, donation interval, and blood center.
DISCUSSION
Frequent blood donation by adults produces iron deficiency in both males and females. In this longitudinal cohort of 3,714 teenage (16–18 yo) and 551 adult (19–49 yo) blood donors, male and female teenagers were more likely to be iron deficient at their first donation and more likely to become iron deficient with repeated donation than adults. The risk for iron deficiency lasted up to a year following donation, longer than previously documented.3 Teenage females presenting for repeated donation were more susceptible than adult females to progress to hemoglobin below 12.5 g/dL, producing a low hemoglobin deferral, a result strongly associated with low ferritin at baseline.
These findings extend the results from the REDS-II RISE study, which demonstrated a high prevalence of intermediate or advanced iron depletion in adult blood donors, only 5% of whom were younger than 20 yo.3,11 The accelerated depletion of iron stores and hemoglobin observed in teenagers occurred despite their meeting eligibility criteria for blood donation on their initial study entry, indicating that current safety standards inadequately protect teenagers from donation-induced iron deficiency and iron deficiency anemia. Overall, more than 40% of teenage donations came from individuals with ferritin <26 ng/mL and 17% from those with ferritin <12 ng/mL, raising potential public health concerns. Though data were not collected on clinical manifestations of NAID or IDA, a precautionary approach should be considered given our current understanding of iron deficiency. Neuroimaging studies have demonstrated that brain development continues into early adulthood,13 and iron depletion between ages 12 and 16 has been associated with altered brain structure 8 to 12 years later in healthy young adults.14 In the latter study, both white matter integrity in the brain and serial assessments of iron status (measured as serum transferrin) were shown to be genetically-mediated, so the causal relationships appear to be complex and multifactorial. In women of reproductive age, alterations to cognitive function have been associated with iron deficiency without anemia.15,16 The recently published INTERVAL study, a randomized trial that assigned 45,000 UK donors to different levels of donation frequency, found no difference in cognitive function by donation frequency, but the study was powered for other outcomes and did not include subjects 16- or 17-years old.17 Any clinical impact from donation-associated iron depletion may be subtle and challenging to detect, and this issue would benefit from further scientific inquiry.
For US blood centers, our findings indicate that stronger measures than existing educational materials are needed to protect younger blood donors, a conclusion recently endorsed by an expert committee.18 Alternate approaches for mitigating this issue include discontinuing collections from some or all teenagers, limiting donation frequency in this age group, replacing iron by facilitating access to iron supplements, and testing donations for ferritin with appropriate deferral from future donation for those with iron deficiency. Outside the US, many jurisdictions report one or more of these measures to protect against iron depletion in younger blood donors, including setting a minimum age of 17 or 18.19 The US is not unique in applying the same eligibility criteria to 16- and 17 yo donors as adults, and in fact many countries rely heavily on younger donors up to age 25 compared to their share of the general population.20 Most countries, however, require longer interdonation intervals or allow fewer donations from female and often male donors compared to US regulations (56-day interval, 6 to 7 donations/year), and some require a higher minimum hemoglobin for male donors.19,21 The risk for iron depletion in blood donors is not a new concern,22 and the recent expansion of eligibility for 16 yo has occurred in parallel with increasing industry and regulatory scrutiny of the potential vulnerability of younger donors.18,23 Two large US blood collectors recently initiated ferritin testing of all 16–18 yo donors, with extended deferral of 6- (male) and 12-months (female) for ferritin values below prescribed cutoffs.24,25 Given the variation in geography, donor populations, and blood center logistics, it is unlikely any single mitigation approach would be optimal for all blood collectors. The impact on blood availability of new restrictions remains unknown, but it should be measured as data accumulate and meanwhile should be assessed with predictive models or simulations.
The large number of teenagers donating blood has implications for clinicians who care for them. Caregivers should consider routinely asking 16–18 yo patients if they have donated blood and consider screening both males and females who have done so for iron deficiency. Clinicians also may be consulted by teenage donors or their parents about the results of blood center ferritin screening, offering the opportunity to discuss the risk for blood donation-induced iron deficiency and how it can be mitigated by use of oral iron supplements or less frequent donation.
One limitation of CHILL is that dietary iron consumption and use of multivitamins and iron supplements were not measured. One previous study found donors age 17–25 were less than one-half as likely to report use of supplemental iron as older age groups (8% versus 18–30%), 26 a finding consistent with population-based surveys.27 It may be that younger donors in CHILL were less likely to take iron supplementation, putting them at increased risk of NAID or IDA, and such behavioral differences might partly confound the reported associations with age. The comparisons between teen and adult donors could also be influenced by any enrichment of the adult control population for relatively robust donors. If those who respond poorly to donation –whether by experiencing side effects or being more likely to receive a deferral—discontinue donating, that creates the potential for selection bias in making comparisons by age of fitness for donation. CHILL did not match teen and adult donors by lifetime donation history, so this possibility cannot be ruled out. The potential phenomenon of adverse outcomes leading to donor dropout might be consistent with the reversal of female predominance of 16 yo donors at enrollment (61%) to male predominance (65%) in 18 yo donors. In any case, the theoretical possibility that teenage donors would compare more favorably to an adult population that includes lapsed donors does not diminish the importance or strength of the finding that current practice allows for onset of iron deficiency more readily in donors age 16 to 18 than in older donors, and that the youngest females are less capable of recovering the post-donation decrement in hemoglobin.
The CHILL study, in turn, had several strengths. With 96% of age-eligible donors enrolled (4,265 of 4,463 successful donors), selection bias due to differential participation rates is not a factor. The longitudinal design, together with availability of ferritin values from 95% of study visits (6,219 of 6,555), provides for robust data and analytical power. Although the study was conducted in only 2 blood centers, we believe the results are generalizable to other teenage blood donor populations for the following reasons: First-time donors had similar ferritin values to those reported in national surveys (Figure 3) and, thus, appear representative of the general population; and the prevalence of iron depletion in first-time adult donors was similar to that in first-time donors in the RISE study.11 (Tab1) Thus, the available evidence supports our findings of a high prevalence of iron depletion in teens and greater risk for iron depletion at any given donation frequency in teens compared to adult donors.
ACKNOWLEDGEMENTS
The authors wish to acknowledge NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III), which was supported by NHLBI contracts NHLBI HHSN2682011–00001I, −00002I, −00003I, −00004I, −00005I, −00006I, −00007I, −00008I, and −00009I. The authors would like to express their deep gratitude to the CHILL research staff at the participating blood centers and testing lab for its exceptional performance and contribution to this project.
Financial support: This work was supported by NHLBI contracts HHSN268201100001I, HHSN268201100002I, HHSN268201100003I, HHSN268201100004I, HHSN268201100005I, and HHSN268201100006I
Footnotes
Conflict of Interest: AEM receives research grant funding from Novo Nordisk. The other authors have no competing interests.
The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), domestic component, is the responsibility of the following persons:
Hubs:
A.E. Mast and J.L. Gottschall, BloodCenter of Wisconsin (BCW), Milwaukee, Wisconsin
D.J. Triulzi and J.E. Kiss, The Institute for Transfusion Medicine (ITXM), Pittsburgh, Pennsylvania
E.L. Murphy and E.M. St. Lezin, University of California, San Francisco (UCSF), and Laboratory Medicine,
Department of Veterans Affairs Medical Center, San Francisco, California
E.L. Snyder, Yale University School of Medicine, New Haven, Connecticut R.G. Cable, American Red Cross Blood Services, Farmington, Connecticut
Data coordinating center:
D.J. Brambilla and M.T. Sullivan, Research Triangle International, Rockville, Maryland
Central laboratory:
M.P. Busch and P.J. Norris, Blood Systems Research Institute, San Francisco, California
Publication committee chairman:
R.Y. Dodd, American Red Cross, Holland Laboratory, Rockville, Maryland
Steering committee chairman:
S.H. Kleinman, University of British Columbia, Victoria, British Columbia, Canada
National Heart, Lung, and Blood Institute, National Institutes of Health:
S.A. Glynn, K.B. Malkin, and A.M. Cristman, Bethesda, Maryland
References
- 1.Spencer BR, Johnson B, Wright DJ, et al. Potential impact on blood availability and donor iron status of changes to donor hemoglobin cutoff and interdonation intervals. Transfusion. 2016; 56(8);1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012;52(4):702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eder AF, Crowder LA, Steele WR. Teenage blood donation: demographic trends, adverse reactions, and iron balance. ISBT Sci Series. 2017;12:395–400. [Google Scholar]
- 4.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States - 2015. Transfusion 2017;57(Suppl 2): 1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. National Institutes of Health, Office of Dietary Supplements. Iron fact sheet for health professionals. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional. Accessed 13 August 2018.
- 6.Centers for Disease Control. NHANES 2001–2002. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2001. Accessed 13 August 2018.
- 7.Milman N Serum ferritin in Danes: studies of iron status from infancy to old age, during blood donation and pregnancy. Int J Hematology. 1996;63(2);103–135. [DOI] [PubMed] [Google Scholar]
- 8.Eder A, Dy B, Kennedy J, et al. Improved safety for young whole blood donors with new selection criteria for estimated blood volume. Transfusion. 2011; 51(7):1522–1531. [DOI] [PubMed] [Google Scholar]
- 9.Stone M, Brambilla D, Murcia K, et al. Feasibility of routine ferritin testing for donor management: validation of delayed processing and demonstration of within donor reproducibility over time. Transfusion. 2016;56(10):2422–2425. [DOI] [PubMed] [Google Scholar]
- 10.National Heart, Lung and Blood Institute. U.S. linked donor component recipient databases. https://reds-iii.rti.org/ResearchStudies/DonorRecipientDatabase.aspx. Accessed 13 August 2018.
- 11.Cable RG, Glynn SA, Kiss JE, et al. NHLBI Retrovirus Epidemiology Donor Study-II. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51(3):511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mast AE, Schlumpf KS, Wright DJ, et al. Demographic correlates of low hemoglobin deferral among prospective whole blood donors. Transfusion. 2010;50(8): 1794–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004;101(21):8174–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahanshad N, Kohannim O, Hibar DP, Stein JL, McMahon KL, et al. Brain structure in healthy adults is related to serum transferrin and the H63D polymorphism in the HFE gene. PNAS. 2012;109(14):E851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott SP, Murray-Kolb LE. Iron status is associated with performance on executive functioning tasks in nonanemic young women. J Nutr. 2016;146(1):30–7. [DOI] [PubMed] [Google Scholar]
- 16.Blanton CA, Green MW, Kretsch MJ. Body iron is associated with cognitive executive planning function in college women. Br J Nutr. 2013;109(5):906–13. [DOI] [PubMed] [Google Scholar]
- 17.Di Angelantonio E, Thompson SG, Kaptoge S, et al. Efficiency and safety of varying the frequency of whole blood donation (INTERVAL): a randomised trial of 45000 donors. Lancet. 2017;390(10110):2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AABB. Updated strategies to limit or prevent iron deficiency in blood donors. Association Bulletin #17–02, March 16, 2017. http://www.aabb.org/programs/publications/bulletins/Docs/ab17-02.pdf. Accessed 13 August 2018.
- 19.Goldman M, Magnussen K, Gorlin J, et al. International forum regarding practices related to donor haemoglobin and iron. Vox Sang. 2016;111(4):449–473. [DOI] [PubMed] [Google Scholar]
- 20.Goldman M, Steele WR, Di Angelantonio E, et al. Comparison of donor and general population demographics over time: a BEST Collaborative group study. Transfusion. 2017;57(10):2469–2476. [DOI] [PubMed] [Google Scholar]
- 21.Vuk T, Magnussen K, de Kort W, et al. International forum: an investigation of iron status in blood donors. Blood Transfus. 2017;15(1):20–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianco C, Brittenham G, Gilcher RO, et al. Maintaining iron balance in women blood donors of childbearing age: summary of a workshop. Transfusion. 2002;42(6):798–805. [DOI] [PubMed] [Google Scholar]
- 23.Center for Biologics Evaluation and Research, US Food and Drug Administration. Blood Products Advisory Committee, November 17, 2016. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/ucm501664.htm. Accessed 13 August 2018.
- 24.Kiss JE, Vassallo RR. How do we manage iron deficiency after blood donation? British Journal of Haematology. 2018;181(5):590–603. [DOI] [PubMed] [Google Scholar]
- 25.American Red Cross. Young blood donors. https://www.redcrossblood.org/donate-blood/blood-donation-process/before-during-after/iron-blood-donation/young-blood-donors.html. Accessed 13 August 2018.
- 26.Cable RG, Papallo CT, Spencer BR. Self-initiated iron supplementation (IS) in blood donors: demographics, patterns of use, and motivation. Transfusion 2016;56(suppl): 98A. [DOI] [PubMed] [Google Scholar]
- 27.Cogswell ME, Kettel-Khan L, Ramakrishnan U. Iron supplement use among women in the United States: science, policy and practice. J Nutr. 2003;133(6):1974S–1977S. [DOI] [PubMed] [Google Scholar]


