Abstract
Background:
Early childhood depression is associated with anhedonia and reduced ERP responses to rewarding or pleasant stimuli. Whether these neural measures are indicators of target engagement or treatment outcome is not yet known.
Methods:
We measured ERP responses to win and loss feedback in a guessing task and to pleasant versus neutral pictures in young (4.0 to 6.9 years) depressed children before and after randomization to either 18 weeks of Parent Child Interaction Therapy – Emotion Development (PCIT-ED) or waitlist (WL).
Results:
Analyses included RewP data from 118 children randomized to PCIT-ED (60) or WL (58) at baseline and LPP data from 99 (44 PCIT-ED vs. 55 WL) at baseline. Children in PCIT-ED showed a greater reduction in anhedonia, F(1,103)=10.32, p=.002, partial η2=.09. RewP reward responses increased more (F(1,87)=5.45, p=.02, partial η2=.06) for PCIT-ED and a greater change in RewP was associated with a greater reduction in MDD symptoms (r=−.24, p=.05). Baseline RewP did not predict treatment change. LPPs to positive pictures did not change across treatment, but greater Baseline LPPs to positive pictures predicted a higher likelihood of remission from MDD in PCIT-ED (B=0.14; SE=0.07; OR=1.15; p=0.03).
Conclusions:
The ERP reward response improved in young children with depression during a treatment designed to enhance emotion development, providing evidence of target engagement of the neural systems associated with reward. Further, greater baseline LPP responses to positive pictures was associated with a greater reduction in depression, suggesting that this ERP measure can predict which children are most likely to respond to treatment.
Keywords: depression, preschool, reward, ERP, Clinical Trial, anhedonia
Major depressive disorder (MDD) is often characterized by impairments in the ability to experience reward and pleasure (1, 2). These disruptions contribute to functional impairment, are associated with deficits in specific neural systems (2, 3), and are a central target of treatments (4). MDD has been validated to manifest as early as age 3 (5–9), with prevalence rates similar to those found at school age (1-2%) (9–12). Young children with MDD show many of the same clinical features as adults (8, 13), as well as disruptions in associated neural systems (14, 15). The absence of positive affect, especially to rewarding events, is a salient feature of early childhood MDD (7, 8, 13, 16, 17) and is referred to as anhedonia (18, 19). Reduced positive affect at age 3 predicts depressive cognitive styles at age 7 (20). Further, the specificity of anhedonia for preschool MDD is evidenced by depressed preschoolers being 17 times more likely than healthy peers, 7 times more likely than disruptive disordered peers, and 5 times more likely than anxiety disordered peers to exhibit behavioral manifestations of anhedonia (8). Reductions in positive affect associated with MDD may be particularly important in younger children, given that joyful play is a central activity known to facilitate multiple dimensions of development (21, 22).
We recently completed a randomized controlled trial of a parent-child psychotherapy for early childhood depression, using a modified Parent-Child Interaction Therapy with an added novel “Emotion Development” module (PCIT-ED) (23). This treatment targeted emotion development, including enhancing the ability to sustain joy and regulate loss and sadness, and was highly effective for remediating depression and general impairment (23). In our RCT of PCIT-ED, significant reductions in depression were found in depressed children post treatment (23), providing motivation for the investigation of both reductions in anhedonia symptoms and modulation of the neural correlates of anhedonia. Here we examine whether two event related potential (ERP) components that are neural indicators of either response to reward, the Reward Positivity (RewP), or affective stimuli (including but not limited to positive stimuli), the Late Positive Potential (LPP), predict clinical response to PCIT-ED or index modulation of the neural systems targeted by the treatment.
Consistent evidence in adults and adolescents with, or at risk for, children with depression demonstrates impaired responses to reward using behavioral assessments and both ERP and fMRI measures of brain function (24–28). A number of studies have examined the RewP, an ERP component elicited by feedback indicating rewards versus losses, that is thought to reflect activity of the reward circuit (i.e., the ventral striatum, caudate and dACC) (29–31). Depressed adults show decreased RewPs (32, 33) and increased depression in children/adolescents is related to reduced RewP (26, 34, 35). A reduced RewP predicts later depression in adolescents (25, 34) and is related to the severity of anhedonia in adults (36). We have shown that preschool-aged children with MDD also show decreased RewPs (15), with the effects being driven by reduced response to win feedback. Further, a reduced RewP among adults with anxiety and depression predicted a greater response to cognitive behavioral therapy (4). Together these findings suggest the reduced RewP is an important biomarker of anhedonia in MDD. If reductions in the RewP are more of a trait related marker of anhedonia or risk for depression, it may serve to predict who might respond to treatment targeting anhedonia, with either those most impaired showing a greater response to treatment, or those least impaired best able to respond to PCIT-ED. Alternatively, if reduced RewP is more of a state related maker of current anhedonia and/or depression, it may improve as a function of treatment and serve as a measure of “target engagement” or modulation of the neural systems associated with hedonic processing by treatment.
Another neural indicator of processing of positive stimuli is the LPP, which is larger to arousing stimuli capable of eliciting emotional responses (pleasant and unpleasant) compared to neutral stimuli (37–40). Adolescents and adults with depression demonstrate a reduced LPP to pleasant stimuli (41–43). Further, children and adolescents at risk for depression show reduced LPP to pleasant stimuli (44, 45), and a reduced LPP to pleasant pictures is associated with the experience of depression following stressful events (46). We have also shown that young children with depression show a reduced LPP to pleasant pictures (47). Thus, LPP responses to pleasant stimuli may be another neural correlate of anhedonia. As with the RewP, if reductions in the LPP are more of a trait related marker of depression risk, it may predict who responds to treatment. Alternatively, if it is more state related, then it may be modulated by treatment and serve as a measure of target engagement of relevant neural systems.
The goal of the current study was to use ERPs to examine neural responses to reward and pleasant pictures among children with MDD prior to and following PCIT-ED treatment. We predicted that compared to the waitlist control, children undergoing PCIT-ED treatment would show an increase in the RewP to wins and an increase in the LPP to pleasant pictures, and that the magnitude of the increase would correlate with the degree of depression and anhedonia reduction. We also predicted that children who showed lower RewP and LPP responses at baseline would show a greater response to treatment.
Method
Participants
Children (aged 3.0-6.9) were participants in an RCT of PCIT-ED compared to waitlist (WL). Analyses of the depression outcome measures are reported elsewhere (23). Details about recruitment, study design, and inclusion/exclusion are provided in the Supplemental Materials, which also includes a Consort Diagram. Study materials and procedures were approved by the WUSM institutional review board, and written informed consent was obtained from caregivers with verbal assent obtained from children. The trial was registered with clinicaltrials.gov (NCT02076425).
The ERP component was added 18 months after trial initiation (see Figure S1 for Consort Diagram), and 194/216 children approached agreed to participate (ages 4.0 to 6.9). 156 were randomized to treatment, and 124 completed at least one ERP task. There were no significant demographic or clinical differences between those randomized children who did or did not complete at least one ERP task (Table S1). Of the children completing at least one task, 118 had data that survived quality control (Supplemental Materials) for the RewP analyses (60 randomized to PCIT and 58 to WL), of which 47 in the PCIT and 45 in the WL group completed the Post Treatment assessment. Ninety-nine children had data that survived quality control for the LPP (44 PCIT and 55 WL), of which 44 in the PCIT and 41 in the WL group completed the post assessment. Children who did not have usable ERP data (Table S2) were younger (RewP and LPP), more likely to have a co-morbid externalizing disorder (RewP, not LPP) and more likely to be on a medication (LPP, not RewP), though children on any antidepressant were excluded at baseline. The comparison of baseline data between depressed children and a healthy control sample have already been reported for the RewP (15) and the LPP (47).
Parent Child Interaction Therapy-Emotion Development (PCIT-ED)
This treatment is a dyadic parent-child psychotherapy expanded and adapted from the well-validated Parent Child Interaction Therapy (PCIT) (48). A novel Emotion Development (ED) module (8 sessions) was added after the standard PCIT modules (12 sessions). The ED modules build on empirical findings in emotional development utilizing the basic techniques of PCIT (teaching of parent followed by coaching the parent in interactions with the child in vivo using a bug-in-the-ear device) to focus on enhancing the child’s emotional experience, emotional competence (49) and emotion regulation (50). This approach addresses early childhood depression impairments in the ability to recognize, understand, and regulate emotions in self and others, as well as helping the child/parent increase reactivity to positive stimuli, and decrease reactivity to negative stimuli.
Measures
Psychopathology
The K-SADS-early childhood (EC), a semi-structured clinical interview for DSM-5 disorders adapted for use in children aged 3.0-6.11, was used to assess severity of MDD and other Axis I comorbidities at baseline and post treatment or WL. This measure has good test retest reliability and construct validity and generates both categorical and dimensional measures of DSM-5 Axis I disorders (14, 51). The MDD score was the number of core MDD symptoms endorsed on the K-SADS-EC. All K-SADS-EC interviews were conducted by master’s level clinicians, videotaped, reviewed for reliability, and calibrated for accuracy. Satisfactory inter-rater reliability was maintained on a monthly basis with overall kappas of K=0.74 for MDD; all diagnoses K=0.88 achieved during the study period. The anhedonia score was the sum of boredom, anhedonia, and amotivation items from the KSADS-EC (Cronbach’s alpha = .48).
Preschool Feelings Checklist (PFC)
The Preschool Feelings Checklist Scale (52), a 23-item Likert scale, adapted from the PFC, was administered at baseline and post assessments to measure depression severity via caregiver report (23, 53).
Tasks
Children were given practice trials on both tasks to assure that they understood how to do the tasks. Tasks were administered on a computer, using Presentation (Neurobehavioral Systems, Inc., Albany, California, USA) software and children used a Logitech Gamepad F310 game controller to respond.
“Doors” Guessing Game to assess the Reward Positivity
Children completed a guessing task – the Doors Guessing Task (see Figure S2)– used in numerous previous studies of older children, adolescents, and adults with depression (25, 27, 45, 54, 55). Participants were shown a graphic displaying two adjacent doors and told to select a door to win or lose points to pick a prize. Following each choice, a feedback stimulus (green up arrow or red down arrow) appeared on the screen informing the children whether they lost or gained points. See Supplemental Materials for details.
Picture Task to assess the Late Positive Potential
Children were told that they would see lots of different pictures and that we wanted them to simply look at all of the pictures that showed up on the screen. Children were told that that an arrow pointing to the left or the right would appear on the screen after each picture (see Figure S3). Children were instructed to press the left or right button on the game controller that matched the direction of the arrow on the screen. Forty developmentally appropriate pictures were selected from the International Affective Picture System (56): 20 depicted pleasant/affectively positive scenes (e.g., smiling faces and candy) and 20 depicted neutral scenes (e.g., neutral faces and household object such as a towel). Each picture was displayed twice in a random order in color and occupied the entirety of a 20-inch monitor. See Supplemental Materials for details.
Psychophysiological Recording and Data Reduction
See Supplemental Materials for details.
Data Analysis
Reward Positivity
The 200-millisecond window before feedback onset served as the baseline. We measured the mean amplitude between 300 and 500 milliseconds at electrode site Pz separately for win and loss trials, focusing on Pz because of our prior work showing reduced Reward-related amplitudes at Pz in depressed preschool children (15). To be included in analyses children had to have at least 20 usable ERP segments per condition (Win versus Loss outcomes). 6 children were excluded for this reason. Based on recommendations in the literature (58–60), we used linear regression to create residualized scores for both baseline and post-treatment that allowed us to examine treatment effects for wins, partialing out the effect of loss. Such scores have good internal consistency and reliability (61). These residual scores reflected variation in the response to wins not accounted for by loss responses (Winresid). We used these residual scores in three analyses: 1) whether response to Reward changed as a function of treatment, using an ANCOVA with treatment groups as a between subject factor (PCIT-ED vs. WL) and Winresid posttreatment as the dependent measure, with baseline ERP response, age, and baseline PFC score as covariates (intent-to-treat analyses are in Supplemental Materials); 2) whether changes in Winresid from pre- to post- treatment in PCIT-ED were correlated with depressive or anhedonia symptom change, using partial correlations of difference scores (post-baseline), controlling for age; and 3) whether baseline ERP responses to Winresid predicted treatment response, either reduction in MDD or anhedonia scores (linear regression) or remission from MDD (binary logistic regression). The same analyses with raw scores are in Supplemental Materials.
Late Positive Potential
The 200-millisecond window before picture onset served as the baseline. We focused on 250-600 ms at the average of the electrode sites O1, Oz, and O2 for pleasant and neutral pictures, as our prior work demonstrated that children with early childhood depression showed reduced LPPs to pleasant pictures in this time window (47). The LPP has good internal reliability (62). To be included in LPP analyses children had to have at least 20 usable ERP segments per condition (positive, neutral pictures). Second, children had to press an arrow that matched the direction of an arrow presented on the screen in order to keep children engaged and attentive to the task and stimuli presentation. Thus, to be included in the analyses children had to have responded to at least half of the trials. Of the total 124 children who completed the LPP assessment, n = 25 children (PCIT = 19; Waitlist =6) were excluded because either: (1) they had less than 20 usable ERP segments in one condition (N = 7) or did not press enough buttons during the stimulus presentation (N = 18). We again used regression to create residual scores (i.e., Pleasantresid, reflecting variation in the neural response to pleasant stimuli not accounted for by the response to neutral stimuli) separately for Baseline and Post Treatment. We conducted parallel analyses to those described above looking at change as a function of treatment and prediction of treatment outcome.
Results
Demographic and Clinical Characteristics
The PCIT-ED and waitlist groups did not differ on sex or gender for the RewP or LPP (Table 1), but the PCIT-ED group was slightly younger and had lower baseline PFC scores. Therefore, age and PFC scale scores were used as covariates.
Table 1:
Demographic Characteristics of Participants at Baseline with Usable ERP data
| Reward Positivity Analyses | Late Positive Potential Analyses | |||||
|---|---|---|---|---|---|---|
| Waitlist (N = 58) | Treatment (N = 60) | Group Comparison | Waitlist (N = 55) | Treatment (N = 44) | Group Comparison | |
| Sex (% male) | 64 | 65 | X21= 0.02, p=0.89 | 67 | 64 | X21= 0.14, p=0.71 |
| Race | ||||||
| % White | 80 | 78 | X22= 0.39, p=0.82 | 79 | 80 | X22= 0.42, p=0.81 |
| % African American | 9 | 12 | 9 | 11 | ||
| % Other | 12 | 10 | 13 | 9 | ||
| Age y, mean (SD) | 5.83 (0.80) | 5.46 (0.84) | t116= 2.44, p=0.02 | 5.79 (0.84) | 5.64 (0.81) | t97= 0.83, p=0.41 |
| Preschool Feelings Checklist (PFC) scale score, mean (SD) | 41.76 (11.82) | 37.73 (9.17) | t116= 2.07, p=0.04 | 42.04 (11.45) | 37.32 (10.22) | t97= 2.14, p=0.04 |
| Anhedonia sum score | 1.40(1.11) | 1.38(1.01) | t116= .07, p=0.95 | 1.45(1.12) | 1.32(1.05) | t97= .62, p=0.54 |
| % Co-morbid externalizing disorders | 55 | 47 | X21= 0.85, p=0.36 | 60 | 46 | X21= 2.08, p=0.15 |
| % on non-antidepressant medications | 2 | 7 | X21= 1.77, p=0.18 | 2 | 2 | X21= 0.03, p=0.87 |
| Income-to-Needs, mean (SD) | 2.85 (1.38) | 2.99 (1.26) | t116= −0.57, p=0.57 | 2.86 (1.34) | 3.04 (1.25) | t97= −0.68, p=0.50 |
Abbreviations: SD = standard deviation; % = percent; t=t-test test statistic; p=p-value; X2=Chi-Square test statistic
Change in Anhedonia
The analyses of the primary outcome measures have been reported (23), with the same primary analysis for the ERP subsample in Table S3, but did not include anhedonia symptoms specifically. General linear model analysis of anhedonia Post Treatment, controlling for baseline anhedonia, indicated the PCIT-ED group (M=0.13, SD=0.44) exhibited significantly fewer anhedonia symptoms compared with WL (M=0.51, SD=.67), F(1,103)=10.32, p=.002, partial η2=.09.
Reward Positivity
Response to Treatment
The grand average ERP waveforms for Win and Loss feedback across groups and assessment points are shown in Figure S4, Figure S5 is a headmap for Win responses from 300 to 500 ms, ERPs for Win response pre and post treatment for PCIT-ED and WL are shown in Figure 1, and means and SDs for all ERP measures are in Table 2. The Reward ANCOVA showed a significant effect of treatment group on the Winresid score Post Assessment, after controlling for Age, PFC score, Winresid at Baseline, and Lossresid at Post Assessment, F(1,86)=5.449, p=.02, partial η2=.06. Follow up repeated measures ANOVAs (pre to post treatment change) within each treatment group separately were not significant (ps> .4), though the effect size of the positive change in the PCIT-ED group (η2=.015) was larger than the negative change in the WAITLIST group (η2=.001). Further, the mean residualized score for the treatment group (M=0.77, SE=0.47) was significantly more positive than the waitlist group (M=−0.81, SE=0.48). These results held if we also included the LPP to positive pictures post treatment as an additional covariate (η2=.051). The same results in intent-to-treat analyses are provided in Supplemental Materials.
Figure 1. Reward Positivity at Baseline and Post Treatment:
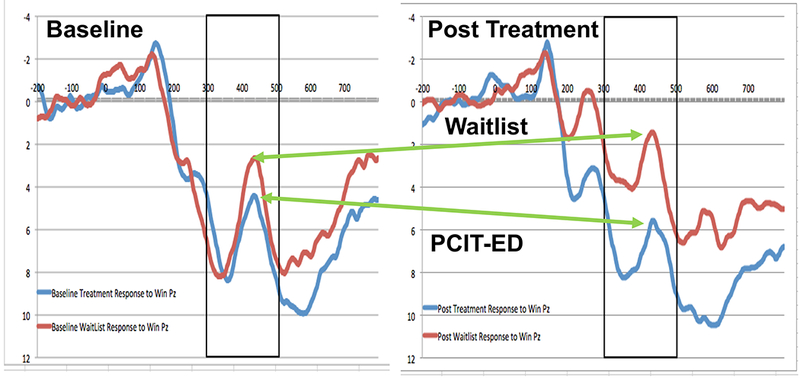
ERP waveforms at Pz to win outcomes at baseline (graph on the left) and post treatment (graph on the right). The blue lines are responses in the PCIT-ED treatment condition and the red lines are responses in the Wait List Control condition. Voltages are plotted with the more negative values at the top of the graph, as is the frequent convention in ERP reports.
Table 2:
Means and Standard Deviations for ERP measures
| Reward Positivity Analyses | Late Positive Potential Analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Win | Loss | Pleasant Picture | Neutral Picture | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Raw Components | ||||||||
| Baseline | ||||||||
| PCIT-ED | 5.74 | 9.68 | 4.84 | 9.27 | 48.43 | 13.69 | 48.48 | 14.42 |
| Waitlist Control | 5.62 | 7.65 | 4.68 | 7.44 | 47.61 | 14.11 | 45.13 | 13.34 |
| Post Treatment | ||||||||
| PCIT-ED | 6.63 | 8.78 | 4.52 | 8.82 | 47.58 | 14.49 | 43.44 | 13.30 |
| Waitlist Control | 3.30 | 7.38 | 1.71 | 8.32 | 47.22 | 11.84 | 42.75 | 12.18 |
| Residualized Components | ||||||||
| Baseline | ||||||||
| PCIT-ED | −0.03 | 5.79 | 0.12 | 5.52 | −1.59 | 6.04 | 1.84 | 6.43 |
| Waitlist Control | −0.03 | 5.34 | 0.05 | 5.21 | 0.43 | 5.92 | −0.79 | 5.38 |
| Post Treatment | ||||||||
| PCIT-ED | 0.65 | 5.83 | 0.09 | 5.86 | −0.14 | 6.15 | 0.18 | 5.61 |
| Waitlist Control | −0.67 | 5.01 | −0.10 | 5.64 | 0.14 | 5.63 | −0.19 | 5.75 |
Does Change in ERP response to Reward or Loss Relate to Change in Depressive Symptoms?
Partial correlations controlling for age demonstrated that in the treatment group, a greater increase in Winresid from Baseline to Post Treatment (Figure 2) was marginally associated with a greater decrease in MDD symptoms (r=−.24, p=.05, 95% CI −.45 to −.002), though not with change in PFC score (r=−.12, p=.21, 95% CI −.35 to .12) or anhedonia (r=−.11, p=.23, 95% CI −.34 to .13), though both were in the same direction.
Figure 2. Association Between Change in Depression Symptoms and Change in Reward Positivity:
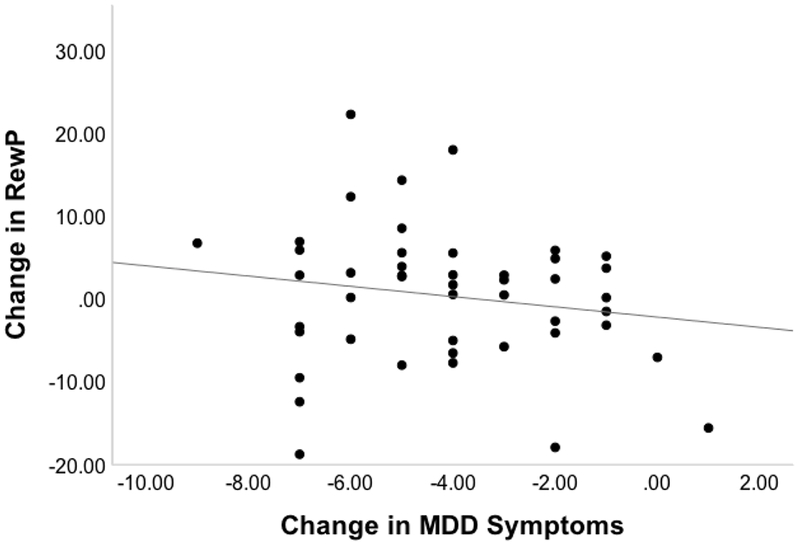
Graph illustrating the relationship between change in Reward Positivity (RewP) at Pz and change in Depression. The RewP score is the difference between Post Treatment Winresid and Baseline Winresid and the MDD score is the difference between Post Treatment and Baseline MDD symptom scores.
Do Baseline Reward or Loss Responses Predict Treatment Outcome
Baseline Winresid did not predict change in MDD symptoms or PFC scores, change in Anhedonia, or remission from MDD (all ps > .19).
Late Positive Potential
Response to Treatment
The grand average ERP waveforms for the LPP are shown in Figure S6, Figure S7 is a headmap for positive picture responses from 250 to 600 ms, and means and SD are in Table 2. One-way ANCOVAs on Pleasantresid at Post Treatment, with age, PFC scale-scores, and Baseline Pleasantresid as covariates indicated no significant treatment group differences in the residualized responses to pleasant pictures (F(1,79)=0.16, p=.69, partial η2=.002). The same results in intent-to-treat analyses are in Supplemental Materials.
Does Change in LPP response to Pleasant Pictures Relate to Change in Depressive Symptoms?
Partial correlations controlling for age demonstrated that in the treatment group, Pleasantresid from Baseline to Post Treatment was not associated with change in MDD symptoms (r=.07, p=.34, 95% CI −.18 to .31), PFC scale-scores (r=−.02, p=.45, 95% CI −.27 to .23), or anhedonia (r=−.04, p=.40, 95% CI −.28 to 21).
Do Baseline LPP Responses to Pleasant Pictures Predict Treatment Outcome
Pleasantresid did not predict change in MDD symptoms, PFC scores or anhedonia symptoms (all ps > .12). However, baseline Pleasantresid did predict remission from depression (B=0.14; SE=0.07; OR=1.15; p=0.03), as shown in Figure 3.
Figure 3. Baseline Late Positive Potential and Remission:
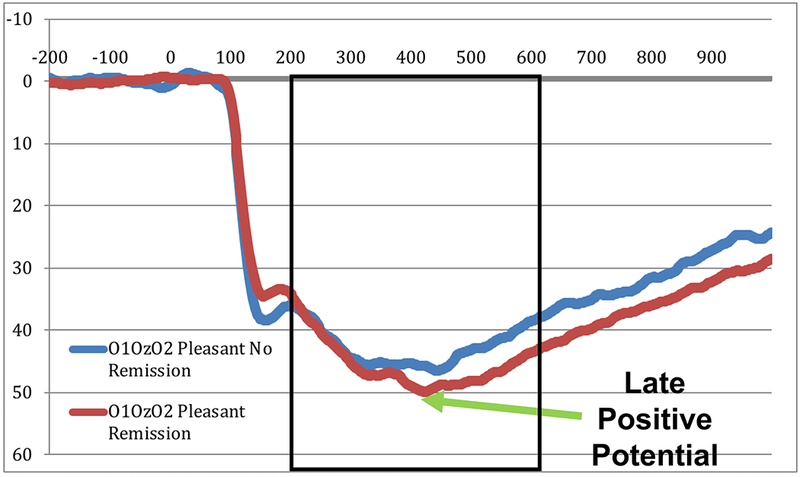
ERP waveforms at the average of O1, Oz, and O2 to pleasant pictures at Baseline in children who did not remit from depression (blue lines) and those who did remit from depression (red lines). Voltages are plotted with the more negative values at the top of the graph, as is the frequent convention in ERP reports.
Discussion
Children in PCIT-ED treatment compared to WL showed a greater reduction in anhedonia. Further, as predicted, children in the PCIT-ED group showed a greater increase in the RewP to rewards from baseline to post treatment, and the magnitude of this increase correlated with the magnitude of reduction in MDD symptoms. In contrast, the LPP to pleasant pictures did not change as a function of treatment, though greater baseline LPP to pleasant pictures predicted likelihood of remission from depression in PCIT-ED. These data provide novel evidence that a treatment designed to enhance the ability to experience joy and pleasure can enhance a neural indicator of hedonic response. The intriguing finding that baseline LPP responses to pleasant pictures predicted treatment response, but did not change as a function of treatment, suggests important dissociations among indicators of response to reward and emotional pictures, and their role in treatment evaluation.
Numerous studies have shown that the RewP is reduced in depression (26, 32–35) and predicts the likelihood of developing depression (25, 34). The current findings add to this literature by showing that the RewP can be enhanced through a treatment designed to augment response to reward and pleasure. These findings contribute to the literature on biomarkers of treatment response in depression (63, 64), providing the first evidence of a biomarker that can be used effectively even in very young children. However, we should note that while the effect size of improvement in the treatment group was significantly larger than in the waitlist group, change when analyzed within the treatment group alone was not significant. In future work it will be important to determine whether the degree of RewP change during treatment predicts subsequent outcomes, such as maintenance of treatment gains or likelihood of depression reemergence, including whether such prediction has power over and above clinical assessments of depression change. If so, the RewP could have utility in helping to predict who needs further intervention or “booster” sessions to help maintain treatment response.
The LPP to pleasant pictures did not change with treatment, though higher pleasant picture LPP at baseline predicted a greater likelihood of response to PCIT-ED. RewP and the LPP have different neural generators, with the RewP more reflective of reward circuit functioning (29–31) commonly found to be altered in depression in relation to hedonic processing (28) while the LPP is more reflective of activity in occipital, inferotemporal and parietal regions involved in emotion processing (65–67). In addition, the RewP is a response to feedback about an active choice made by an individual, while the LPP is thought to index more general attention to salient stimuli, both pleasant and unpleasant (68). It is possible that the more attentional nature of processes driving the LPP may make it less sensitive to change as a function of treatment, as PCIT-ED focused more on changing the child’s active responding in both positive and negative emotion processing during interactions with the caregiver than on their general attention to salient stimuli. At the same time, this characteristic of the LPP might make it a more sensitive indicator of emotion responsivity in an individual, such that those with greater attention to salient stimuli are more available to benefit from treatment. Another possibility is that not all children understood or evaluated the differences between the positive and neutral stimuli. We did not have the children make explicit ratings of the emotional content of the stimuli, and some of the children may not have perceived a difference between the two, though this would be unusual in children aged 4 and older. If so, another intriguing possibility is that those children at baseline who were more sensitive to the difference between positive and neutral stimuli had more intact emotional processing that allowed them to benefit more from the PCIT-ED treatment. This hypothesis can be tested in future studies by having children make explicit ratings of the stimuli.
These findings need to be interpreted in the context of several limitations. The control condition was WL, which is not the most stringent comparator. There are no other empirically proven treatments for young children with depression, and thus using a WL control is an important first step. Future studies will need to dismantle PCIT-ED and determine the active ingredients compared to active control conditions. These data are also from a relatively short follow-up. While promising, it will be important to determine whether findings of increased RewP are maintained over time. Further, the measure of anhedonia was based on the sum of three clinically rated symptoms and may have had some limitation in range that might have reduced the magnitude of individual difference relationships. In addition, it may be that parent report of anhedonia in a child may miss variance that might be captured by self-report in older samples. We also do not have information about the temporal relationships between improvements in depression and increases in the RewP. Future studies using dense sampling designs will allow for analyzing leading and lagging relationships. In addition, the LPP task only included responses to pleasant and neutral pictures, and did not include responses to negative pictures because of time constraints. As such, we cannot tell whether the relationship between treatment response and LPP that was specific to positive stimuli or reflected responses to emotionally evocative stimuli in general. Further, this study has a smaller N than the parent treatment study (23) as the ERPs were added after the parent study started. Lastly, we did not include multiple comparison correction given our a priori hypotheses.
In summary, the current study makes a novel and clinically relevant contribution to the literature on treatment of early childhood depression by demonstrating that both clinical ratings of anhedonia and RewP responses improved in depressed children during a PCIT-ED treatment designed to enhance emotion development. These findings are particularly novel given that they are in very young children, where the speculative hope is that plasticity is greater and thus the impact may be more enduring. Further, greater baseline LPP responses to pleasant pictures was associated with a greater reduction in depression during PCIT-ED, consistent with LPP being a useful tool to predict which children are most likely to respond to treatment. Further, the fact that we saw these results with ERP measures of responses to reward and pleasant stimuli respectively has clinical relevance, as ERP measures may end up being more feasible to implement in a clinical setting that other measures of neural responding, such as functional magnetic resonance imaging.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by the National Institute of Mental Health, Grant # 5R01MH098454-04 and K23MH115074-01
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Additional Contributions: We thank the families participating in this study and the staff who helped make the project a success.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: Dr. Barch consults for Pfizer. Dr. Luby receives royalties from Guildford Press. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Luking KR, Pagliaccio D, Luby JL, Barch DM (2016): Depression Risk Predicts Blunted Neural Responses to Gains and Enhanced Responses to Losses in Healthy Children. J Am Acad Child Adolesc Psychiatry. 55:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barch DM, Pagliaccio D, Luking K (2016): Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. Curr Top Behav Neurosci. 27:411–449. [DOI] [PubMed] [Google Scholar]
- 3.Luking KR, Pagliaccio D, Luby JL, Barch DM (2016): Reward Processing and Risk for Depression Across Development. Trends Cogn Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, et al. (2016): Neural Reactivity to Reward as a Predictor of Cognitive Behavioral Therapy Response in Anxiety and Depression. Depress Anxiety. 33:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luby JL, Heffelfinger AK, Mrakotsky C, Hessler MJ, Brown KM, Hildebrand T (2002): Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Adolesc Psychiatry. 41:928–937. [DOI] [PubMed] [Google Scholar]
- 6.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Hessler M, Spitznagel E (2003): Modification of DSM-IV criteria for depressed preschool children. The American journal of psychiatry. 160:1169–1172. [DOI] [PubMed] [Google Scholar]
- 7.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E (2004): Characteristics of depressed preschoolers with and without anhedonia: evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 161:1998–2004. [DOI] [PubMed] [Google Scholar]
- 8.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E (2009): The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disord. 112:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egger HL, Angold A (2006): Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. Journal of child psychology and psychiatry, and allied disciplines. 47:313–337. [DOI] [PubMed] [Google Scholar]
- 10.Gleason MM, Zamfirescu A, Egger HL, Nelson CA 3rd, Fox NA, Zeanah CH (2011): Epidemiology of psychiatric disorders in very young children in a Romanian pediatric setting. Eur Child Adolesc Psychiatry. 20:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavigne JV, Lebailly SA, Hopkins J, Gouze KR, Binns HJ (2009): The prevalence of ADHD, ODD, depression, and anxiety in a community sample of 4-year-olds. J Clin Child Adolesc Psychol. 38:315–328. [DOI] [PubMed] [Google Scholar]
- 12.Wichstrom L, Berg-Nielsen TS, Angold A, Egger HL, Solheim E, Sveen TH (2012): Prevalence of psychiatric disorders in preschoolers. J Child Psychol Psychiatry. 53:695–705. [DOI] [PubMed] [Google Scholar]
- 13.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E (2009): Preschool depression: homotypic continuity and course over 24 months. Archives of General Psychiatry. 66:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaffrey MS, Barch DM, Bogdan R, Farris K, Petersen SE, Luby JL (2017): Amygdala Reward Reactivity Mediates the Association Between Preschool Stress Response and Depression Severity. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, et al. (2016): Neural Correlates of Reward Processing in Depressed and Healthy Preschool-Age Children. J Am Acad Child Adolesc Psychiatry. 55:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luby J (2007): Depression in preschool-age children: current evidence. The Brown University CHild and Adolescent Behavior Letter. 23. [Google Scholar]
- 17.Tandon M, Cardeli E, Luby J (2009): Internalizing disorders in early childhood: a review of depressive and anxiety disorders. Child and adolescent psychiatric clinics of North America. 18:593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.APA (2013): Diagnostic and statistical manual of mental disorders, Fifth Edition. 5th ed ed. Washington, D.C.: American Psychiatric Association. [Google Scholar]
- 19.Belden AC, Luby JL (2006): Preschoolers’ depression severity and behaviors during dyadic interactions: the mediating role of parental support. J Am Acad Child Adolesc Psychiatry. 45:213–222. [DOI] [PubMed] [Google Scholar]
- 20.Hayden EP, Klein DN, Durbin CE, Olino TM (2006): Positive emotionality at age 3 predicts cognitive styles in 7-year-old children. Development and psychopathology. 18:409–423. [DOI] [PubMed] [Google Scholar]
- 21.Erickson RJ (1985): Play contributes to the full emotional development of the child. Education. 105:261–263. [Google Scholar]
- 22.Ginsburg KR (2007): The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics. 119:182–191. [DOI] [PubMed] [Google Scholar]
- 23.Luby JL, Barch DM, Whalen D, Tillman R, Freedland KE (in press): A Randomized Controlled Trial of Parent-Child Psychotherapy Targeting Emotion Development for Early Childhood Depression. American Journal of Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foti D, Hajcak G (2009): Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological psychology. 81:1–8. [DOI] [PubMed] [Google Scholar]
- 25.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G (2013): Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 50:74–81. [DOI] [PubMed] [Google Scholar]
- 26.Bress JN, Smith E, Foti D, Klein DN, Hajcak G (2012): Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological psychology. 89:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foti D, Kotov R, Klein DN, Hajcak G (2011): Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. Journal of abnormal child psychology. 39:913–924. [DOI] [PubMed] [Google Scholar]
- 28.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J (2013): The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. Journal of affective disorders. 151:531–539. [DOI] [PubMed] [Google Scholar]
- 29.Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G (2011): Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. NeuroImage. 57:1608–1616. [DOI] [PubMed] [Google Scholar]
- 30.Foti D, Weinberg A, Dien J, Hajcak G (2011): Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: response to commentary. Human brain mapping. 32:2267–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foti D, Weinberg A, Dien J, Hajcak G (2011): Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Human brain mapping. 32:2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foti D, Carlson JM, Sauder CL, Hajcak-Proudfit G (in submission): Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burkhouse KL, Gorka SM, Afshar K, Phan KL (2017): Neural reactivity to reward and internalizing symptom dimensions. J Affect Disord. 217:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G (2016): Blunted Neural Response to Rewards as a Prospective Predictor of the Development of Depression in Adolescent Girls. Am J Psychiatry.appiajp201615121524. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg A, Liu H, Hajcak G, Shankman SA (2015): Blunted neural response to rewards as a vulnerability factor for depression: Results from a family study. J Abnorm Psychol. 124:878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, et al. (2014): The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 53:213–220. [DOI] [PubMed] [Google Scholar]
- 37.Foti D, Hajcak G, Dien J (2009): Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 46:521–530. [DOI] [PubMed] [Google Scholar]
- 38.Dillon DG, Cooper JJ, Grent-’t-Jong T, Woldorff MG, LaBar KS (2006): Dissociation of event-related potentials indexing arousal and semantic cohesion during emotional word encoding. Brain Cogn. 62:43–57. [DOI] [PubMed] [Google Scholar]
- 39.Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ (2000): Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 37:257–261. [PubMed] [Google Scholar]
- 40.Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ (2000): Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 52:95–111. [DOI] [PubMed] [Google Scholar]
- 41.Weinberg A, Perlman G, Kotov R, Hajcak G (2016): Depression and reduced neural response to emotional images: Distinction from anxiety, and importance of symptom dimensions and age of onset. J Abnorm Psychol. 125:26–39. [DOI] [PubMed] [Google Scholar]
- 42.Webb CA, Auerbach RP, Bondy E, Stanton CH, Foti D, Pizzagalli DA (2017): Abnormal neural responses to feedback in depressed adolescents. J Abnorm Psychol. 126:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grunewald M, Dohnert M, Brandeis D, Klein AM, von Klitzing K, Matuschek T, et al. (2018): Attenuated LPP to Emotional Face Stimuli Associated with Parent- and Self-Reported Depression in Children and Adolescents. J Abnorm Child Psychol. [DOI] [PubMed] [Google Scholar]
- 44.Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN (2012): Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of child psychology and psychiatry, and allied disciplines. 53:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson BD, Perlman G, Hajcak G, Klein DN, Kotov R (2015): Familial risk for distress and fear disorders and emotional reactivity in adolescence: an event-related potential investigation. Psychol Med. 45:2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levinson AR, Speed BC, Hajcak G (2018): Neural Response to Pleasant Pictures Moderates Prospective Relationship Between Stress and Depressive Symptoms in Adolescent Girls. J Clin Child Adolesc Psychol.1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whalen D, Gilbert KE, Belden AC, Kelly D, Hajcak G, Kappenman ES, et al. (in submission): Early childhood onset major depressive disorder is characterized by electrocortical deficits in processing pleasant emotional pictures. [Google Scholar]
- 48.Eyberg SM, Funderburk BW, Hembree-Kigin TL, McNeil CB, Querido JG, Hood KK (2001): Parent-Child Interaction Therapy with behavior problem children: One and two year maintenance of treatment effects in the family. Child Fam Behav Ther. 23:1–20. [Google Scholar]
- 49.Saarni C (1999): The Development of Emotional Competence. New York: Guildford Press. [Google Scholar]
- 50.Lenze SN, Pautsch J, Luby J (2011): Parent-child interaction therapy emotion development: a novel treatment for depression in preschool children. Depress Anxiety. 28:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaffrey MS, Luby JL (2012): Kiddie Schedule for Affective Disorders and Schizophrenia - Early Childhood Version (K-SADS-EC). St. Louis: Washington University School of Medicine. [Google Scholar]
- 52.Luby JL, Heffelfinger A, Koenig-McNaught AL, Brown K, Spitznagel E (2004): The Preschool Feelings Checklist: a brief and sensitive screening measure for depression in young children. J Am Acad Child Adolesc Psychiatry. 43:708–717. [DOI] [PubMed] [Google Scholar]
- 53.Luby J, Lenze S, Tillman R (2012): A novel early intervention for preschool depression: findings from a pilot randomized controlled trial. Journal of child psychology and psychiatry, and allied disciplines. 53:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bress JN, Meyer A, Hajcak G (2015): Differentiating anxiety and depression in children and adolescents: evidence from event-related brain potentials. J Clin Child Adolesc Psychol. 44:238–249. [DOI] [PubMed] [Google Scholar]
- 55.Foti D, Carlson JM, Sauder CL, Proudfit GH (2014): Reward dysfunction in major depression: multimodal neuroimaging evidence for refining the melancholic phenotype. Neuroimage. 101:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lang PJ, Bradley MM, Cuthbert BN (1999): International affective picture system (IAPS): Technical manual and affective ratings. Gainesville: University of Florida. [Google Scholar]
- 57.Gratton G, Coles MG, Donchin E (1983): A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology. 55:468–484. [DOI] [PubMed] [Google Scholar]
- 58.Hajcak G, Moser JS, Holroyd CB, Simons RF (2007): It’s worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 44:905–912. [DOI] [PubMed] [Google Scholar]
- 59.Dunning JP, Hajcak G (2007): Error-related negativities elicited by monetary loss and cues that predict loss. Neuroreport. 18:1875–1878. [DOI] [PubMed] [Google Scholar]
- 60.Holroyd CB, Larsen JT, Cohen JD (2004): Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology. 41:245–253. [DOI] [PubMed] [Google Scholar]
- 61.Levinson AR, Speed BC, Infantolino ZP, Hajcak G (2017): Reliability of the electrocortical response to gains and losses in the doors task. Psychophysiology. [DOI] [PubMed] [Google Scholar]
- 62.Bondy E, Stewart JG, Hajcak G, Weinberg A, Tarlow N, Mittal VA, et al. (2018): Emotion processing in female youth: Testing the stability of the late positive potential. Psychophysiology. 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fonseka TM, MacQueen GM, Kennedy SH (2018): Neuroimaging biomarkers as predictors of treatment outcome in Major Depressive Disorder. J Affect Disord. 233:21–35. [DOI] [PubMed] [Google Scholar]
- 64.Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JR, Deckersbach T, et al. (2015): Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry. 172:124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kayser J, Tenke CE, Abraham KS, Alschuler DM, Alvarenga JE, Skipper J, et al. (2016): Neuronal generator patterns at scalp elicited by lateralized aversive pictures reveal consecutive stages of motivated attention. Neuroimage. 142:337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabatinelli D, Keil A, Frank DW, Lang PJ (2013): Emotional perception: correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biol Psychol. 92:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sabatinelli D, Lang PJ, Keil A, Bradley MM (2007): Emotional perception: correlation of functional MRI and event-related potentials. Cereb Cortex. 17:1085–1091. [DOI] [PubMed] [Google Scholar]
- 68.Proudfit GH, Bress JN, Foti D, Kujawa A, Klein DN (2015): Depression and Event-related Potentials: Emotional disengagement and reward insensitivity. Curr Opin Psychol. 4:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


