Abstract
Introduction/objectives:
Pneumocystis jirovecii pneumonia (PJP) is a rare but potentially fatal opportunistic infection; however, consensus varies around which conditions or medications confer a level of risk sufficient to justify antibiotic prophylaxis for PJP. We used electronic health record (EHR) data to assess the current patterns of PJP prophylaxis, PJP outcomes, and prophylaxis-related adverse events among patients with rheumatic diseases who were receiving high-risk immunosuppressant drugs.
Methods:
Data derive from the EHR of a large health system. We included new immunosuppressant users with diagnoses of vasculitis, myositis, or systemic lupus erythematosus. We calculated the proportion of patients who received PJP prophylaxis for each diagnosis and drug combination. We also calculated the number of PJP infections and the number of antibiotic adverse drug events (ADEs) per patient-year of exposure.
Results:
We followed 316 patients for 23.2 +/− 14.2 months. Overall, 124 (39%) of patients received prophylactic antibiotics for PJP. At least 25% of patients with the highest risk conditions (e.g. vasculitis) or highest risk immunosuppressants (e.g. cyclophosphamide) did not receive PJP prophylaxis. We found no cases of PJP infection over 640 patient-years of follow up, including among those not receiving prophylaxis, and an overall incidence rate of ADEs of 2.2% per patient-year.
Conclusions:
PJP prophylaxis for patients with rheumatic conditions is inconsistent, with one quarter of patients who have high risk conditions or high risk immunosuppressants not receiving prophylaxis. However, given extremely low rates of PJP infection, but detectable ADEs to prophylactic antibiotics, our findings suggest that evidence to guide more personalized risk assessments are needed to inform PJP prophylaxis.
Keywords: vasculitis, immunosuppression, infection
INTRODUCTION
Pneumocystis jirovecii pneumonia (PJP) is a rare but potentially fatal opportunistic infection with a 30–60% mortality rate among immunocompromised (non-HIV) patients.[1] Prior epidemiologic studies have described the highest risk populations as those with a combination of specific diseases and drugs, for example patients with granulomatosis with polyangiitis, microscopic polyangiitis, inflammatory myopathies with interstitial lung disease, and those who are receiving high dose steroids or cyclophosphamide.[2,3,4,5,6,7,8,9] Although a recent Cochrane review recommended that PJP prophylaxis should be considered in non-HIV immunocompromised patients when the risk of PJP is greater than 6.2% per person-year, opinions vary around which conditions or medications confer this level of risk.[10]
The decision to use antimicrobial prophylaxis for PJP includes not only weighing the risks associated with PJP infection, but also the risks of adverse events associated with the regimen used for prophylaxis, which may be rare but are potentially life-threatening. Trimethoprim-sulfamethoxazole (TMP-SMX) is the recommended first-line prophylactic agent for PJP, is effective and usually well-tolerated, although serious adverse reactions to the sulfa moiety can occur.[10,11] Alternative antibiotics that can be used for PJP prophylaxis include dapsone, atovaquone, and aerosolized pentamidine, although these medications are more expensive and less effective than TMP-SMX.[2] Although some studies have examined the risk of PJP in well-defined cohorts using a single type of immunosuppression, or in claims data where PJP prophylaxis was not clinically confirmed, no studies have examined a real-world cohort of patients and the relationship of high-risk diagnoses and high-risk immunosuppressants with patterns of PJP prophylaxis and risk of subsequent infection.
In this study, we used explored practice patterns of PJP prophylaxis in a large tertiary healthcare system. We also calculated the incidence of PJP infection and the incidence of adverse drug events related to antibiotic prophylaxis among patients with rheumatic diseases who were receiving high risk immunosuppressant drugs.
METHODS
Data sources:
Data derive from the EHR of as tertiary care referral university health system (University of California – San Francisco) with over 750,000 outpatient visits per year. The catchment area is large, and includes much of northern California. All EHR data were available, including demographics, diagnosis codes, problem lists, medications, laboratory studies, procedures, clinical encounter notes, and scanned documents. Variables were initially extracted electronically via back-end access to our Epic EHR data warehouses, using structured fields and keyword searches of clinical notes. Following the automated data extraction, a chart review by at least 2 authors (GS, KJ, JY, MG, SP, ZI, IA) was performed to ensure the validity of all extracted variables, including rheumatologic diagnoses, medications, PJP infections and antibiotic-associated adverse events.
Study population:
We defined a cohort of patients based on diagnoses and immunosuppressant drug use. Eligible patients had at least 1 encounter (inpatient or outpatient) in our healthcare system between June 1, 2012 and September 30, 2016. Patients were included in the study if they had at least 2 inpatient or face-to-face ambulatory encounters 30 days apart for one of the following diagnoses (granulomatosis with polyangiitis, microscopic polyagiitis, dermatomyositis, polymyositis, or systemic lupus erythematosus). We chose to limit our analysis to patients with these conditions because they are those for which rheumatologists commonly make clinical decisions regarding PJP prophylaxis when starting high-risk immunosuppressive drugs. Patient under age 18 years, or with any diagnosis of HIV, AIDS, pregnancy, active malignancy, or solid organ transplant during the study period were excluded.
Drug exposure:
We identified a cohort of new users of at least one of the following immunosuppressants - azathioprine (AZA), cyclophosphamide, methotrexate, mycophenolate mofetil (MMF), rituximab, or high dose glucocorticoids (GC), defined as prednisone with a dose of at least 20 mg daily, or equivalent. The index date was defined as the date of the first prescription for any of the above drugs following at least 6 months of no use. Patients were required to have at least 2 diagnoses for their autoimmune condition prior to the index date and 1 encounter at least 30 days after the index date. Patients were followed for at least 30 days after their index date; they were censored from the study when they developed PJP pneumonia, died, had > 60-day gap their immunosuppressant use, were loss to follow up (indicated by no encounter, lab, note, or medication order for > 180 days), or on October 31, 2016, whichever came first.
Because many of these drugs are used in combination, we assessed all concomitant immunosuppressants patients received alongside the drug started on the index date, or any immunosuppressant started ≤ 45 days after the index date. For example, if high dose GC were newly initiated and cyclophosphamide was administered two weeks later, these drugs were considered to be part of a single treatment decision and defined as a drug combination of “cyclophosphamide + high dose GC.” If a patient’s additional immunosuppressant drugs started more than 45 days after the index date, these were not included in this analysis. We created a hierarchy of drugs in order to categorize drug combinations according to risk of drug-conferred risk of PJP in the following order: cyclophosphamide (highest risk), rituximab, high-dose GC, MMF, AZA, and MTX (lowest risk).[6,7,8,12]
Outcomes:
The primary outcome was prescription of an antibiotic used for PJP prophylaxis after the index date. We required a 30-day supply of the antibiotic in order to identify orders for antibiotics that were intended to be taken chronically versus orders for antibiotics used to treat acute infections. Qualifying antibiotics included trimethoprim-sulfamethoxazole (TMP-SMX), dapsone, atovaquone, or pentamidine. Patients for whom qualifying antibiotics were ordered for less than a 30-day supply were considered non-prophylaxed. Orders for drugs with intermittent dosing (e.g., TMP-SMX given three times per week, where only 15 pills might be dispensed), still appeared as a 30-day supply of drug and would therefore qualify as prophylaxis.
As an adjunct analysis, we searched diagnosis codes (ICD-9 code 136.3 and ICD-10 code B59) or keywords in clinical notes (“PJP;” “PCP;” “pneumocystis;” or “pneumocystosis”) for any PJP infections among included patients and confirmed them via chart review, including review of clinical notes and microbiology results.
Because we were interested in potential adverse consequences of PJP antibiotic prophylaxis, we assessed for adverse drug events (ADEs) associated with PJP prophylactic antibiotics. ADEs were extracted from the EHR allergy tables and confirmed by chart review, and coded as fatal, life-threatening, serious, or significant according to established rating frameworks.[13] Patient-time of exposure was calculated from to the first date of antibiotic exposure until a relevant ADE was documented, death, loss to follow up (indicated by no encounter, lab, note, or medication order for > 180 days), or on October 31, 2016, whichever came first. All patients included in this analysis had at least 30 days of follow up following prescription of the prophylactic antibiotic. ADEs could occur in the prophylaxed or non-prophylaxed groups, since patients for whom an order for a qualifying antibiotic was written for less than 30 days could have experienced an ADE but would not have been included in the prophylaxis group.
Additional variables:
Demographic variables including age, sex, and race were extracted from the EHR. To identify comorbidities, we extracted diagnoses from encounters, problem lists, invoices, claims, hospital accounts, hospital admissions, and surgical cases at any time leading up to the index date. A modified Charlson score was calculated according to the Deyo protocol. [14] Lung disease was defined based on the Charlson score definition and calculated using any codes for 416.8, 416.9, 490.x-505.x, 506.4, 508.1, or 508.8 prior to the index date. Primary diagnosis requiring immunosuppression was extracted from encounter diagnoses or clinical notes on the index date and confirmed via chart review. These were classified as “high risk” diagnoses (GPA, MPA) and “intermediate risk” diagnoses (myositis, SLE) with regards to PJP infection.[2,6,7,8,9]
Statistical analysis:
We used chi-squared and t-test tests to assess the bivariate association between baseline characteristics, disease and drug category, and receipt of PJP prophylaxis. We used multiple logistic regression to assess the independent effects of disease and anchor drug category after adjusting for sex, age, and race. Included variables were tested for collinearity. An estimated annual rate of detected PJP infections is also presented with an exact Poisson 95% confidence interval.[15] We compared incidence rates for ADEs among patients who received PJP prophylaxis compared to those who did not.
The study was approved by the Committee on Human Research at the University of California, San Francisco.
RESULTS
We included 316 patients, followed for an average of 23.1 (14.1) months. Additional patient characteristics are shown in Table 1. The majority of patients were receiving immunosuppressant drugs for a diagnosis of SLE (56%). High-dose GC and MMF were the most common anchor medications (30% and 22%, respectively).
Table 1.
Baseline characteristics, diagnoses, and immunosuppressant medications among included patients, by prophylaxis use.
| Total Cohort (N=316) |
Yes prophylaxis (N=124) |
No prophylaxis (N=192) |
|||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value* | |
| Female | 252 | 80% | 93 | 75% | 159 | 83% | 0.12 |
| Age (mean (SD)) | 43.3 (17.3) | 44.7 (16.6) | 42.3 (17.7) | 0.13 | |||
| <18 | 12 | 4% | 2 | 2% | 10 | 5% | |
| 18–50 | 184 | 58% | 68 | 55% | 116 | 60% | |
| 51–75 | 110 | 35% | 51 | 41% | 59 | 31% | |
| >75 | 10 | 3% | 3 | 2% | 7 | 4% | |
| Race | 0.25 | ||||||
| White | 118 | 37% | 54 | 44% | 64 | 33% | |
| African American | 39 | 12% | 16 | 13% | 23 | 12% | |
| Asian | 61 | 19% | 19 | 15% | 42 | 22% | |
| Hispanic | 60 | 19% | 19 | 15% | 41 | 21% | |
| Other/multiple | 38 | 12% | 16 | 13% | 22 | 11% | |
| Comorbid conditions and clinical parameters | |||||||
| Charlson score (mean (SD)) | 3.3 (2.9) | 3.6 (3.1) | 3.2 (2.7) | 0.22 | |||
| Lung disease* | 44 | 14% | 31 | 16% | 13 | 10% | 0.21 |
| White blood cell count (1000/mL, mean (SD)) | 7.4 (3.6) | 8.5 (4.0) | 6.7 (3.1) | <0.001 | |||
| Lymphocyte count (1000/mL, mean (SD))† | 1.2 (0.7) | 1.2 (0.7) | 1.3 (0.7) | 0.08 | |||
| Neutrophil count (1000/mL, mean (SD))‡ | 5.6 (3.4) | 6.7 (3.7) | 4.8 (3.0) | <0.001 | |||
| Diagnosis requiring immunosuppression | <0.001 | ||||||
| High Risk | |||||||
| Granulomatosis with polyangiitis | 47 | 15% | 30 | 24% | 17 | 9% | |
| Microscopic polyangiitis | 21 | 7% | 13 | 10% | 8 | 4% | |
| Intermediate Risk | |||||||
| Dermatomyositis | 55 | 17% | 30 | 24% | 25 | 13% | |
| Polymyositis | 17 | 5% | 6 | 5% | 11 | 6% | |
| Systemic Lupus Erythematosus | 176 | 56% | 45 | 36% | 131 | 68% | |
| Anchor immunosuppressant drug in drug bundle, among patients with an index drug | <0.001 | ||||||
| Highest Risk | |||||||
| Cyclophosphamide | 30 | 9% | 23 | 19% | 7 | 4% | |
| Intermediate Risk | |||||||
| Rituximab | 41 | 13% | 28 | 23% | 13 | 7% | |
| High-dose glucocorticoids | 94 | 30% | 37 | 30% | 57 | 30% | |
| Lower Risk | |||||||
| Mycophenolate | 71 | 22% | 15 | 12% | 56 | 29% | |
| Azathioprine | 33 | 10% | 11 | 9% | 22 | 11% | |
| Methotrexate | 47 | 15% | 10 | 8% | 37 | 19% | |
p-value for chi square or t-test, as appropriate
Lung disease: defined as any code for 416.8, 416.9, 490.x-505.x, 506.4, 508.1, or 508.8 prior to the index date
: N = 296
: N=296
: N=296
Practice patterns around PJP prophylaxis:
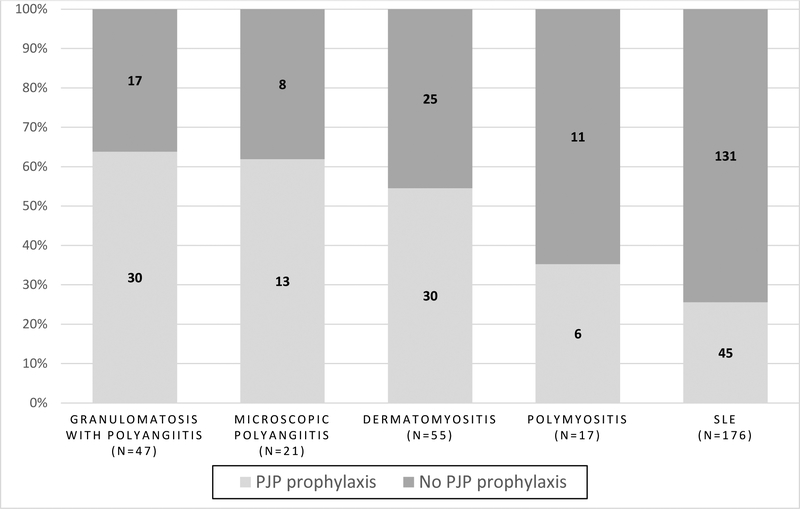
Overall, 124 (39%) of patients received prophylactic antibiotics for PJP at any time during the follow-up period. There were 29 patients who received an eligible antibiotic for < 30 days who were considered non-prophylaxed. Figure 1 shows PJP prophylaxis patterns stratified by diagnosis. Patients with the highest risk diagnoses (vasculitis) were most likely to receive PJP prophylaxis (75%, vs. 60% for patients with dermatomyositis, 35% for patients with polymyositis, and 36% for patients with SLE; p < 0.001).
Figure 1.
PJP prophylaxis patterns, by underlying diagnosis.
Table 2 shows the proportion of patients who received PJP prophylaxis stratified by anchor immunosuppressant drug. Patients receiving drug combinations with cyclophosphamide as the anchor drug were most likely to receive PJP prophylaxis (77%, vs. 68% for rituximab, 39% for high-dose GC, 33% for AZA, 21% for MMF, and 21% for MTX; p<0.001). Prophylaxis patterns did not differ based on the comorbidity index or existing diagnosis of lung disease. The mean WBC count was slightly lower among patients who did not receive prophylaxis; however, lower WBC and neutrophil counts were associated with a diagnosis of SLE (p=0.004 and p=0.02 respectively), and likely represent a manifestation of SLE.
Table 2.
PJP prophylaxis patterns among patients with new immunosuppressant drug starts, stratified by anchor immunosuppressant.
| Anchor immunossupressant |
Specific drug bundle | N | Yes prophylaxis |
% | No prophylaxis |
% | |
|---|---|---|---|---|---|---|---|
| Highest risk | Cyclophosphamide (CYC) | 30 | 23 | 77% | 7 | 23% | |
| CYC alone | 8 | 5 | 3 | ||||
| CYC + high-dose GC | 13 | 9 | 4 | ||||
| CYC + Rituximab | 9 | 9 | 0 | ||||
| Rituximab | 41 | 28 | 68% | 13 | 32% | ||
| Intermediate risk | Rituximab alone | 13 | 11 | 2 | |||
| Rituximab + GC | 23 | 16 | 7 | ||||
| Rituximab + other(s) | 5 | 1 | 4 | ||||
| High-dose glucocorticoids (GC) | 94 | 37 | 39% | 57 | 61% | ||
| High-dose GC alone | 27 | 5 | 22 | ||||
| High-dose GC + MMF | 51 | 21 | 30 | ||||
| High-dose GC + other(s) | 16 | 11 | 5 | ||||
| Lower risk | Mycophenolate mofetil (MMF) | 71 | 15 | 21% | 56 | 79% | |
| MMFalone | 19 | 1 | 18 | ||||
| MMF + GC | 48 | 14 | 34 | ||||
| MMF + other(s) | 4 | 0 | 4 | ||||
| Azathioprine (AZA) | 33 | 11 | 33% | 22 | 67% | ||
| AZA alone | 6 | 2 | 4 | ||||
| AZA + GC | 27 | 9 | 18 | ||||
| Methotrexate (MTX) | 47 | 10 | 21% | 37 | 79% | ||
| MTX alone | 14 | 1 | 13 | ||||
| MTX + GC | 33 | 9 | 24 | ||||
Anchor immunosuppresant: we created a hierarchy of drugs in order to categorize drug combinations according to risk of drug-conferred risk of PJP in the following order: cyclophosphamide (highest risk), rituximab, high-dose GC, MMF, AZA, and MTX (lowest risk).
In a multivariable regression model that included sex, age, race, diagnosis, and anchor drug, only diagnosis and anchor drug were independently associated with receipt of PJP prophylaxis (Table 3). Four of the 5 patients (80%) with vasculitis and receiving cyclophosphamide-based regimens received PJP prophylaxis.
Table 3.
Multivariate logistic regression model predicting use of PJP prophylaxis among patients with rheumatic conditions.
| Characteristic | Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
|---|---|---|
| Sex (Male vs. Female) | 1.61 (0.92,2.79) | 0.84 (0.43,1.64) |
| Age (per 1 year) | 1.01 (0.99,1.02) | 0.99 (0.98,1.01) |
| Race (Non-white vs. White) | 0.65 (0.41,1.03) | 1.12 (0.62,2.03) |
| Diagnosis | ||
| Granulomatosis with polyangiitis | 5.14 (2.59,10.19) | 5.74 (2.41,13.70) |
| Microscopic polyangiitis | 4.73 (1.84,12.15) | 6.59 (2.10,20.73) |
| Dermatomyositis | 3.49 (1.86, 6.56) | 5.04 (2.46,10.29) |
| Polymyositis | 1.59 (0.55,4.54) | 2.74 (0.87, 8.59) |
| SLE | referent | referent |
| Anchor immunosuppressant | ||
| Cyclophosphamide (CYC) | 12.16 (4.06,36.42) | 18.90 (5.77, 61.91) |
| Rituximab | 7.97 (3.05, 20.80) | 5.62 (1.99,15.93) |
| High-dose glucocorticoids (GC) | 2.40 (1.07, 5.41) | 2.57 (1.07,6.17) |
| Mycophenolate mofetil (MMF) | 0.99 (0.40, 2.44) | 1.14 (0.43,2.97) |
| Azathioprine (AZA) | 1.85 (0.68, 5.06) | 1.24 (0.41, 3.74) |
| Methotrexate (MTX) | referent | referent |
Model c-statistic = 0.789
PJP infection:
Overall, we found zero cases of PJP infection among patients over 640 patient-years of observation. Applying an exact Poisson 95% confidence interval to this finding produced a 95% confidence interval of (0.0 – 3.7) cases per 640 patient-years. Stratification by PJP prophylaxis status (Y/N) yielded similar 95% CI: for the prophylaxed group, (0.0, 3.7) cases per 265 patient-years; for the non-prophylaxed group, (0.0, 3.7) cases per 375 patient-years.
ADEs to prophylactic antibiotics:
Trimethoprim-sulfamethoxazole (TMP-SMX) was the most commonly used prophylactic antibiotic (73%), followed by dapsone (16%), atovaquone (10%), and pentamidine (1%). Overall, we detected 14 ADEs to prophylactic antibiotics during the study period, during 640 person-years of observation, for an overall incidence rate of 2.2% per person-year. Among 124 patients who received PJP prophylaxis (with mean (SD) 26 (18.0) months of follow up), 12 (9.7%) had a newly reported adverse event to a prophylactic antibiotic during the study period. Among 192 patients not receiving PJP prophylaxis (with a mean 23 (14.5) months of follow-up), 2 (1.0%) developed an ADE to a relevant antibiotic (which was presumably given for purposes other than PJP prophylaxis, such as infection). The specific ADEs and their severity are listed in Table 4. Dapsone conferred the highest risk of ADE (6 events in 65 person-years or 9% per person-year); interestingly, 4 of the 6 patients with ADE to dapsone were Asian. TMP/SMX conferred a risk ADE of 3% per person-year (8 events in 275 person-years). We detected no ADEs to atovaquone or pentamidine (see Table 3).
Table 4.
Adverse drug events associated with PJP prophylactic antibiotic use among patients with new immunosuppressant starts.
| Prophylactic antibiotic |
Number of patients |
Person-years of observation |
Number of ADEs |
Severity of ADE | Example ADE |
|---|---|---|---|---|---|
| Bactrim | 129 | 275 | 8 | Total | |
| 4 | Significant | Rash | |||
| 4 | Serious | Creatinine elevation; AKI and thrombocytopenia | |||
| 0 | Life-threatening | ||||
| Dapsone | 28 | 65 | 6 | Total | |
| 1 | Significant | Rash | |||
| 4 | Serious | Methemoglobinemia; hemolytic anemia; neutropenia | |||
| 1 | Life-threatening | Hypersensitivity syndrome requiring ICU stay | |||
| Atovaquone | 17 | 41 | 0 | Total | |
| 0 | Significant | ||||
| 0 | Serious | ||||
| 0 | Life-threatening | ||||
| Pentamidine | 2 | 2 | 0 | Total | |
| 0 | Significant | ||||
| 0 | Serious | ||||
| 0 | Life-threatening |
DISCUSSION
In this study using EHR data from a large tertiary care center, we found that both diagnosis and drug combination were the drivers of PJP prophylaxis, and that at least 75% of patients with either high risk conditions (such as vasculitis) or high risk immunosuppressants (such as cyclophosphamide) did receive PJP prophylaxis. Prophylaxis patterns for patients at intermediate or lower risks was less consistent. To our knowledge, this is the first study to examine PJP prophylaxis patterns for patients exposed to common combinations of immunosuppressant drugs, as well as the consequences of prophylaxis, including both PJP infections and antibiotic ADEs.
Few studies have explored practice patterns around PJP prophylaxis. One very large, claims-based study of children receiving glucocorticoids found PJP prophylaxis was prescribed in 10% of cases.[16] Similar studies have not been performed in adults. One survey-based study of rheumatologists found that rheumatologists early in their careers and those with academic and US-based practices were more likely to prescribe prophylaxis.[17] Prescribers reported that the most important determinant for issuing prophylaxis was medication regimen, in addition to underlying diagnosis and medication dosage. Our study is consistent with this survey, with diagnosis and drug emerging as the key drivers of prophylaxis.
Our data indicate that clinicians are prophylaxing the highest risk patients most of the time in an environment where non-prophylaxed patients almost never develop PJP infection: interestingly, we found no cases of PJP infection in this cohort of patients that included patients with high risk diagnoses and patients receiving high risk immunosuppressants. Even at the upper bound of our 95% confidence interval, the rate of PJP infection in the non-prophylaxed group is 3.7/375 person-years, or less than 1% per year, which falls below the recommendation for prophylaxis in cases where risk of PJP is greater than 6.2% per year.[10] Previously published estimates showed that PJP incidence rates in patients with granulomatosis with polyangiitis and those with polymyositis/dermatomyositis were 71.9 per 100,000 patient-year (5 out of 339 patients) and 53.6 per 100,000 patient-year (2 out of 182 patients), respectively, still well below this threshold.[18]
However, our findings may also indicate that the selective use of PJP prophylaxis for patients with high-risk diagnoses and those receiving high-risk immunosuppressants was effective in preventing the occurrence of PJP among patients in this cohort. Of note, one recent study found that use TMP/SMX prophylaxis reduced the risk of any type of severe infection among patients with vasculitis receiving rituximab.[19] Future work should address whether for some patients, the benefits of antibiotic prophylaxis outweigh the risks if all infections are considered, not just PJP.
Our overall rate of ADEs to TMP/SMX of 3% per person-year are consistent with ADEs in a recent meta-analysis, which found severe adverse events to TMP/SMX requiring permanent discontinuation, including leukopenia, thrombocytopenia or severe dermatological reactions, occurred in 3.1% of adults.[10] A significant proportion of patients in this study received second-line prophylactic agents for PJP, which are known to be less effective, more costly, have their own side effect profiles. Presumably, these second line agents were chosen because of a prior history of ADE to TMP/SMX. Interestingly, we found a surprisingly high incidence of ADEs to dapsone (0.09/person-year), including the only life-threatening ADE in the entire study. Four out of 6 of these patients were Asian, who have been reported to have higher rates of ADEs to this drug.[20]
Our study had several limitations. First, we may have underestimated the proportion of patients who received PJP prophylaxis because we assumed that an order for < 30 days of antibiotic was not intended for PJP prophylaxis. However, there were only 29 patients who had any short-term exposure to eligible antibiotics, and most of these were ordered for < 14 days, increasing our confidence that they were not intended for long-term use. Second, among patients with GPA, TMP/SMX has been associated with decreased rates of disease relapse,[21] and so among these patients, we may have over-estimated the rates of deliberate PJP prophylaxis. Third, ADEs were likely under-ascertained because we relied on the allergy table in the EHR to detect these; this would tend to make PJP prophylaxis seem more favorable and erroneously increase any estimate of number needed to harm.[22] Fourth, data from our large university healthcare system (with a catchment of over 750,000 patients) did not produce many events (PJP infections or ADEs to antibiotics). It is unknown whether data from a multi-institutional source such as a national patient registry, would indicate higher rates of infection or ADEs. [23] Robust estimates of the risk-benefit ratio of PJP prophylaxis in immunosuppressed patients may depend on such sources.
PJP remains a dreaded complication of immunosuppressive therapy. However, there may be unintended consequences to universal PJP prophylaxis with limited benefit. Our findings reveal that despite uneven PJP prophylaxis in our health system, the incidence of PJP was extremely low, and that ADEs from antibiotics occur at a low but detectable rate. Use of second-line agents for PJP prophylaxis was significant. Future work should develop evidence to guide more personalized risk assessments to inform PJP prophylaxis. It is possible that our understanding of PJP infection risk does not take into account other important factors, such as patient-level differences in drug metabolism or the burden of PJP in the community or treating clinic; perhaps it is only the patients with recent exposures to PJP who need to be prophylaxed at all. [24,25] In addition, health systems should aim to improve documentation of ADEs, including improved infrastructure in the EHR that includes fields for historical vs. witnessed events, and severity of reactions.[26] Lack of sufficient ADE documentation has been associated with unnecessary use of second-line agents, which are less effective and may be less well tolerated.[27] More work is also needed on communication around medication risks and benefits, since it is these areas of equipoise that benefit most from frank discussions and shared decision making with patients.[28] Finally, we need automated methods to identify patients at highest risk for PJP, so that in the future, clinical decision support tools can be used to target prophylaxis effectively.
ACKNOWLEDGEMENTS
We would like to acknowledge Chris Tonner MPH, who assisted in technical aspects of data acquisition for this project.
This work is supported by AHRQ R01 HS024412 and K23 AR063770 (GS). Drs. Yazdany and Schmajuk are also supported by the Russell/Engleman Medical Research Center for Arthritis. Dr. Sarkar is supported by AHRQ P30HS023558. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- 1.Thomas CF Jr., Limper AH. Pneumocystis pneumonia. N Engl J Med 2004, 350(24):2487–2498. [DOI] [PubMed] [Google Scholar]
- 2.Mecoli CA, Saylor D, Gelber AC, Christopher-Stine L. Pneumocystis jiroveci pneumonia in rheumatic disease: a 20-year single-centre experience. Clin Exp Rheumatol. 2017. Jul-Aug;35(4):671–673. [PubMed] [Google Scholar]
- 3.Singer NG, McCune WJ. Prevention of infectious complications in rheumatic disease patients: immunization, Pneumocystis carinii prophylaxis, and screening for latent infections. Current Opinion in Rheumatology 1999;11(3): 173–8. [DOI] [PubMed] [Google Scholar]
- 4.Roux A, Gonzalez F, Roux M, Mehrad M, Menotti J, Zahar JR, et al. Groupe de recherche respiratoire en reanimation en onco-hématologie (Grrr-OH). Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Médecine et Maladies Infectieuses 2014;44(5):185–98. [DOI] [PubMed] [Google Scholar]
- 5.Stamp LK, Hurst M. Is there a role for consensus guidelines for P. jiroveci pneumonia prophylaxis in immunosuppressed patients with rheumatic diseases? J Rheumatol 2010, 37(4):686–688. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Manta KG, Betsi GI, Pappas G. Infection-related morbidity and mortality in patients with connective tissue diseases: a systematic review. Clin Rheumatol 2007, 26(5):663–670. [DOI] [PubMed] [Google Scholar]
- 7.Kadoya A, Okada J, Iikuni Y, Kondo H. Risk factors for Pneumocystis carinii pneumonia in patients with polymyositis/dermatomyositis or systemic lupus erythematosus. J Rheumatol 1996, 23(7):1186–1188. [PubMed] [Google Scholar]
- 8.Sepkowitz KA. Opportunistic infections in patients with and patients without Acquired Immunodeficiency Syndrome. Clin Infect Dis 2002, 34(8):1098–1107. [DOI] [PubMed] [Google Scholar]
- 9.Suryaprasad A, Stone JH. When is it safe to stop Pneumocystis jiroveci pneumonia prophylaxis? Insights from three cases complicating autoimmune diseases. Arthritis Rheum 2008, 59(7):1034–1039. [DOI] [PubMed] [Google Scholar]
- 10.Stern A, Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 2014(10):CD005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis. 2017. November 1 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ennishi D, Terui Y, Yokoyama M, Mishima Y, Takahashi S, Takeuchi K, et al. Increased incidence of interstitial pneumonia by CHOP combined with rituximab. Int J Hematol. 2008. May; 87(4):393–7. [DOI] [PubMed] [Google Scholar]
- 13.Sawarkar A, Keohane CA, Maviglia S, Gandhi TK, Poon EG. Adverse Drug Events caused by Serious Medication Administration Errors. BMJ quality & safety. 2012;21(11):933–938. doi: 10.1136/bmjqs-2012-000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 15.Garwood F, “Fiducial Limits for the Poisson Distribution” Biometrica 28:437–442, 1936. [Google Scholar]
- 16.Basiaga ML, Ross ME, Gerber J, Ogdie A. Incidence of Pneumocystis jirovecii and Adverse Events Associated With Pneumocystis Prophylaxis in Children Receiving Glucocorticoids. J Pediatric Infect Dis Soc. 2017. July 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cettomai D, Gelber AC, Christopher-Stine L: A survey of rheumatologists’ practice for prescribing pneumocystis prophylaxis. J Rheumatol 2010, 37(4):792–799. [DOI] [PubMed] [Google Scholar]
- 18.Fillatre P, Decaux O, Jouneau S, Revest M, Gacouin A, Robert-Gangneux F, et al. Incidence of Pneumocystis jiroveci pneumonia among groups at risk in HIV-negative patients. Am J Med. 2014. December;127(12):1242.e11–7. [DOI] [PubMed] [Google Scholar]
- 19.Kronbichler A, Kerschbaum J, Gopaluni S, Tieu J, Alberici F, Jones RB, et al. Trimethoprim-sulfamethoxazole prophylaxis prevents severe/life-threatening infections following rituximab in antineutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis. 2018. June 27 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tangamornsuksan W, Lohitnavy M. Association Between HLA-B*1301 and Dapsone-Induced Cutaneous Adverse Drug Reactions: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018. March 14 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stegeman C, Tervaert J, de Jong P, Kallenberg C. Trimethoprim-sulfamethoxazole (Co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. New Engl J Med 1996;335:16–20 [DOI] [PubMed] [Google Scholar]
- 22.Gandhi TK, Seger AC, Overhage JM, et al. Outpatient adverse drug events identified by screening electronic health records. J Patient Saf. 2010. June;6(2):91–6. [DOI] [PubMed] [Google Scholar]
- 23.Yazdany J, Bansback N, Clowse M, et al. Rheumatology Informatics System for Effectiveness: A National Informatics-Enabled Registry for Quality Improvement. Arthritis Care Res (Hoboken). 2016. December;68(12):1866–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori S, Cho I, Sugimoto M. A followup study of asymptomatic carriers of Pneumocystis jiroveci during immunosuppressive therapy for rheumatoid arthritis. J Rheumatol 2009;36:1600–5. [DOI] [PubMed] [Google Scholar]
- 25.Mulpuru S, Knoll G, Weir C, et al. Pneumocystis pneumonia outbreak among renal transplant recipients at a North American transplant center: Risk factors and implications for infection control. Am J Infect Control. 2016. Apr 1;44(4):425–31. [DOI] [PubMed] [Google Scholar]
- 26.Parès Y, Declerck G, Hussain S, Ng R, Jaulent MC. Building a time-saving and adaptable tool to report adverse drug events. Stud Health Technol Inform. 2013;192:903–7. [PubMed] [Google Scholar]
- 27.Vaisman A, McCready J, Powis J. Clarifying a “Penicillin” AllergyA Teachable Moment. JAMA Intern Med. 2017;177(2):269–270. [DOI] [PubMed] [Google Scholar]
- 28.Elwyn G, Edwards A, Kinnersley P, Grol R. Shared decision making and the concept of equipoise: the competencies of involving patients in health care choices. Br J Gen Pract. 2000;50:892–9. [PMC free article] [PubMed] [Google Scholar]