Abstract
Cells need to cope with toxic or reactive intermediates formed during metabolism. One strategy is to sequester reactions that produce such intermediates within specialized compartments or tunnels connecting different active sites. Here we show that propionyl-CoA synthase (PCS), a ~400 kDa homodimer, three-domain fusion protein and the key enzyme of the 3-hydroxypropionate bi-cycle for CO2-fixation, sequesters its reactive intermediate acrylyl-CoA. Structural analysis showed that PCS forms a multi-catalytic reaction chamber. Kinetic analysis suggests that access to the reaction chamber and catalysis are synchronized by interdomain communication. The reaction chamber of PCS features three active sites and has a volume of only 33 nm3. As one of the smallest multi-reaction chambers described in biology, PCS could inspire the engineering of a new class of dynamically regulated nanoreactors.
Introduction
Biological systems face the challenging task of efficiently catalyzing hundreds to thousands of different chemical reactions in one “pot”, the cytoplasm. Diffusion is relatively fast compared to biochemical reactions, which leads to uniform concentrations of metabolites in the cytoplasm, in particular when considering the size and structural organization of microbial cells1. The free diffusion of pathway intermediates can result in “cross-talk” between metabolic pathways and cause cross-inhibition, inactivation or even irreparable damage to metabolism, especially when the respective intermediates are instable or reactive2. Nature has evolved several strategies to ensure that problematic pathway intermediates are not released into the cytoplasm, but directly transferred to the next enzyme or active site. These strategies include encapsulation of intermediates in membrane- or protein-delimited organelles (compartmentalization), covalent attachment of intermediates to multi-domain enzyme complexes or carrier proteins, electrostatic guidance of intermediates from one active site to the next, or formation of direct intramolecular tunnels between two active sites3.
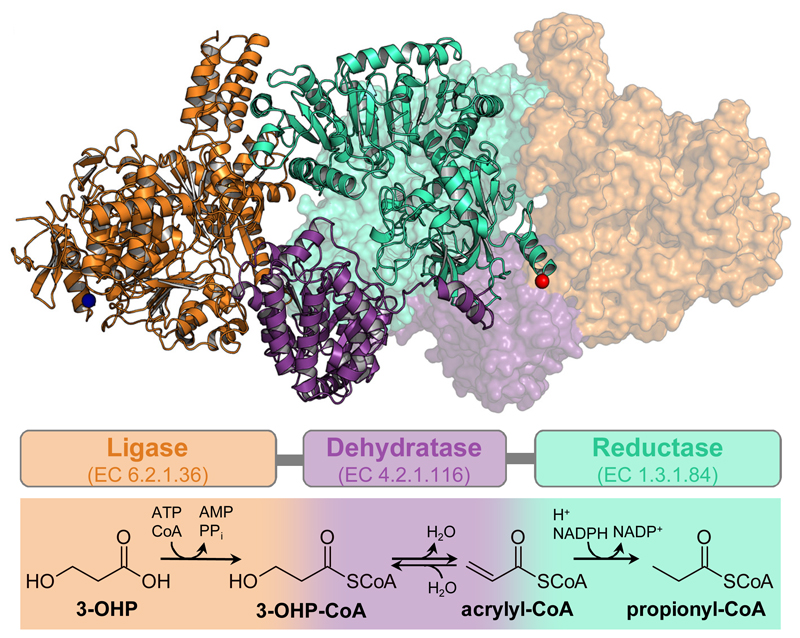
Here, we report on the enzyme reaction cascade from 3-hydroxypropionate to propionyl-CoA, which is the key sequence in the 3-hydroxypropionate bi-cycle for autotrophic CO2 assimilation4,5. The overall reaction sequence comprises three enzymatic steps, during which a highly reactive, toxic and unstable intermediate, acrylyl-CoA, is formed6. In autotrophic Sulfolobales the three reactions are catalyzed by individual enzymes7,8. However, in several phyla (e.g. Proteobacteria and Chloroflexi, Supplementary Fig. 1) the three reactions are catalyzed by a fusion enzyme of about 1850 amino acids, that comprises three catalytic domains, the propionyl-CoA synthase (PCS; Fig. 1 and Supplementary Figure 2)4, suggesting that PCS specifically evolved as a fusion enzyme to overcome the free diffusion of reactive acrylyl-CoA. Here we show that the PCS from Erythrobacter sp. NAP1 (GenBank accession no. EAQ29651) is a multi-catalytic “nanoreactor” that features a central reaction chamber to sequester its reaction intermediates acrylyl-CoA during catalysis. Biochemical experiments show that access to the reaction chamber is dynamically controlled during the catalytic cycle and that catalysis is synchronized through interdomain communication.
Figure 1. Trifunctional PCS: Structure and reaction sequence.
Dimeric structure of PCS from Erythrobacter sp. NAP1 (PDB 6EQO). One protomer is depicted in cartoon and one in surface representation. The multi-domain organization is highlighted by different colors: orange, ligase domain; purple, dehydratase domain; cyan, reductase domain; blue sphere, N-terminus; red sphere, C-terminus. Schematic arrangement of the three domains and their individual reactions are shown using the same color code.
Results
PCS sequesters the reactive intermediate acrylyl-CoA
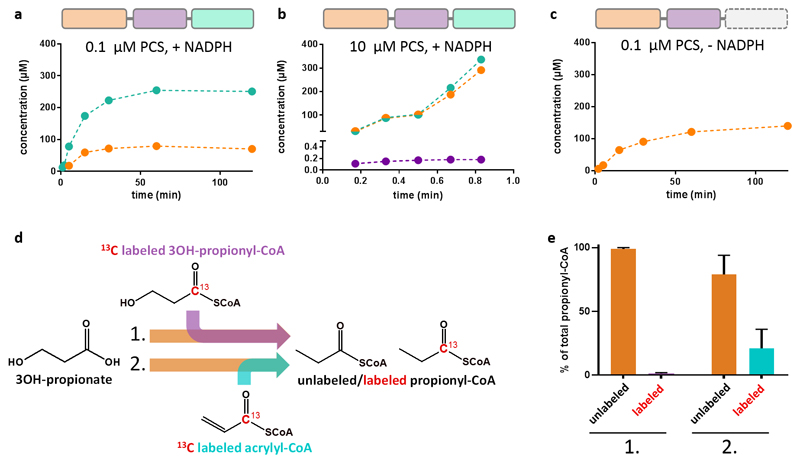
To study the catalytic cycle of PCS from 3-hydroxypropionyl-CoA to propionyl-CoA (Fig. 1), we produced PCS from Erythrobacter sp. NAP1 (GenBank accession no. EAQ29651) and characterized the enzyme biochemically. When we followed the overall reaction of PCS, we could not detect acrylyl-CoA in the assay mixture, while the other intermediates 3-hydroxypropionyl-CoA and propionyl-CoA accumulated over time (Fig. 2a). Only when we increased the concentration of the enzyme by two orders of magnitude, acrylyl-CoA became detectable, albeit at very low levels (Fig. 2b). The concentration of acrylyl-CoA corresponded to 1.8 ± 0.1 % of PCS monomers and stayed constant during steady-state. This demonstrated that acrylyl-CoA was formed in situ by the dehydratase domain, but presumably stayed sequestered within PCS, where it was quickly consumed by the reductase domain. Notably, when the reduction reaction was prevented (by omitting NADPH), acrylyl-CoA did not accumulate, while 3-hydroxypropionyl-CoA still did (Fig. 2c). This indicated that even when interrupting the catalytic sequence, acrylyl-CoA remained effectively sequestered within PCS. We could also show that externally added intermediates were not or only to a minor extent converted by the enzyme (Fig. 2d,e), as discussed in detail later in this manuscript.
Figure 2. PCS sequesters the reactive intermediate acrylyl-CoA.
a, Time course of the overall reaction with 0.1 µM PCS, 800 µM CoA, 500 µM 3-hydroxypropionate, 800 µM ATP and 300 µM NADPH. Production of the 3-hydroxypropionyl-CoA intermediate (orange) and the final product propionyl-CoA (cyan) was observed. In contrast no free acrylyl-CoA was detectable. b, Time course of the reaction containing 10 µM PCS, 5 mM CoA, 5 mM 3-hydroxypropionate, 5 mM ATP and 5 mM NADPH. At these high enzyme concentrations acrylyl-CoA (purple) was detected at 0.18 µM during steady-state corresponding to 1.8% occupancy of reductase active sites. 3-Hydroxypropionyl-CoA und propionyl-CoA accumulate over time. c, as in a, but without NADPH. Again, formation of 3-hydroxypropionyl-CoA was observed, but not of free acrylyl-CoA. d, Isotopic labeling competition experiment containing unlabeled 3-hydroxypropionate and either 13C-labeled 3-hydroxypropionyl-CoA (experiment 1) or acrylyl-CoA (experiment 2). The reaction was started by the addition of PCS. Products were analyzed by LC-MS (see Supplementary Table 4 for detailed assay conditions). e, Results of the isotopic labeling competition experiment. Only 0.8 ± 0.4 % of propionyl-CoA was produced from exogenous 13C-labeled 3-hydroxypropionyl-CoA during steady state (experiment 1). Approximately every fifth propionyl-CoA (21 ± 15%) was formed from exogenous 13C-labeled acrylyl-CoA during steady-state (experiment 2). a – c, data of a representative single experiment. e, data mean ± s.d. (n=3).
PCS forms a multi-catalytic reaction chamber
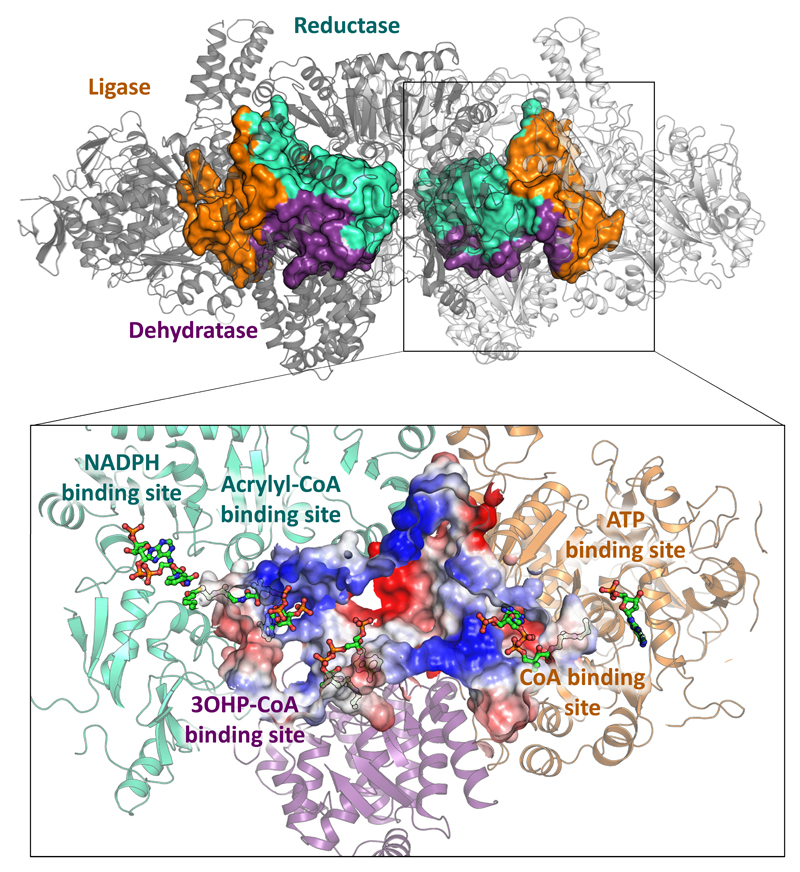
To understand the structural basis of acrylyl-CoA sequestration in PCS, we solved the crystal structure of the enzyme in the presence of CoA, NADP+ and an ATP analog (phosphomethylphosphonic acid adenylate ester) using a new phasing compound9 (PDB 6EQO, Supplementary Table 1 and Supplementary Figs. 3 and 4). PCS forms a dimer of ~400 kDa around a central core of reductase domains (Fig. 1). The ligase and dehydratase domains extend to both sides, enclosing spherical compartments (‘reaction chambers’, Supplementary Fig. 5) that each feature three internal active sites (Fig. 3). The active sites are not connected through individual tunnels but all open into the central cavity of the reaction chamber. The surface of the central cavity is positively charged, which may help in retaining the CoA-ester intermediates during catalysis or even guide them between active sites as shown in the example of the malate dehydrogenase–citrate synthase cascade10–12. Escape of intermediates from the reaction chamber is presumably disfavored by negative charges surrounding any small openings.
Figure 3. Multi-catalytic reaction chamber of PCS.
Volume filling representation17 of the reaction chambers enclosed by PCS. The central catalytic reaction chamber of each protomer is formed through the contribution of all three domains. Orange, contribution of the ligase domain; purple, contribution of the dehydratase domain; cyan, contribution of the reductase domain. The close up shows a cross section through the reaction chamber. Electrostatic charge distribution is shown as a gradient from negatively charged (red) to positively charged (blue). The three active sites are well connected within the reaction chamber. Large positively charged patches may help in retaining the CoA-ester intermediates inside during catalysis or even guide them between active sites. Negative charges around the small openings may also prevent leakage of the negatively charged CoA-derivatives. The PCS structure co-crystallized with CoA (no density), an ATP analog and NADP+ is depicted in cartoon showing the ligase domain in orange, the dehydratase domain in purple and the reductase domain in cyan. CoA binding sites have been modelled based on the superposition of the structures of lone-standing CoA ligase (PDB 2P2F)18, dehydratase (PDB 5JBX)19 and reductase (PDB 4A0S)20 onto PCS (compare Supplementary Figs. 6, 7 and 8). Distances between the active sites have been determined by measuring the distance between sulfur atoms of modelled CoA moieties: ligase–dehydratase, 42.5 Å; dehydratase–reductase, 33.7 Å; ligase–reductase, 63.5 Å.
The inner diameter of the reaction chamber is between 3.5–5.5 nm with a total volume of 33 nm3. This volume is between three and six orders of magnitude smaller than that of bacterial microcompartments13 (14 × 103 to ~10 × 107 nm3, calculated from inner diameters ranging from 30 to 600 nm)14, two orders of magnitude smaller than of described nanocompartments, such as encapsulins (5 × 103 nm3, calculated from inner diameter of 22 nm)15, and even half of that of proteasomes (59 and 84 nm3)16. Thus, PCS forms one of the smallest multi-catalytic reaction chambers observed in nature.
Structural elements in PCS mimic protomer contributions
The active sites of the acyl-CoA ligase, dehydratase and reductase domain of PCS align well with those of their corresponding lone-standing homologues (i.e., individual acyl-CoA ligase, dehydratase and reductase enzymes; Supplementary Figs. 6, 7 and 8). However, these lone-standing enzyme pendants are organized as homo-oligomers that require contributions from neighboring protomers in the oligomeric complex, whereas the individual domains of PCS seem to be organized as functional monomers within the enzyme18–20. Notably, in PCS, the individual “monomeric” domains carry additional structural elements that apparently compensate (or “mimic”) the essential contributions from the missing neighboring protomers in the lone-standing pendants (Supplementary Figs. 9, 10 and 11).
As an example, compared to lone-standing dehydratases, the dehydratase domain of PCS features additional helices (Supplementary Fig. 10) containing two highly conserved residues (Phe1220 and Lys1223, ref. 19) that are involved in stabilization of the CoA-ester. In the lone-standing dehydratases, these residues usually protrude from one protomeric subunit helix into the active site of a neighboring protomeric subunit of the homo-trimeric complex. Another example is the reductase domain of PCS that carries a structural extension compensating the part of the CoA binding site, which is provided by a neighboring protomeric subunit in lone-standing homo-tetrameric enoyl-CoA reductase/carboxylase homologues (Supplementary Fig. 11). Although the additional elements in the monomeric PCS domains structurally mimic the missing protomeric contributions from the lone-standing counterparts, they seem not to be related on the primary sequence level, which raises questions about the evolutionary history and importance of these additional structural elements.
In addition to these protomer-mimicking structural elements, the ligase domain of PCS carries structural extensions that are absent in any lone-standing acyl-CoA synthetase homologues (Supplementary Fig. 9), most prominently, an additional four-helix bundle. This four-helix bundle appears to be unique to PCS and is exclusively found in PCS homologues (based on BLASTP analysis). The four-helix bundle caps one side of the reaction chamber. Its absence would leave the reaction compartment wide open (Supplementary Fig. 14). Taken together, PCS is more than just a simple fusion of individual catalytic domains, but contains structural extensions that allow it to form a highly organized multi-functional enzyme compartment.
Access to the PCS reaction chamber is controlled
How is the sequence of reactions orchestrated within the compartment? The three enzyme reactions of PCS can be measured individually, when the appropriate substrates and cofactors are provided, demonstrating that all active sites are in principle accessible for their respective substrates4. We determined the kinetic parameters for the overall reaction of PCS as well as for each catalytic domain. While the ligase and the dehydratase domains had apparent turnover frequencies (kcat) comparable to the overall reaction of PCS, the kcat of the reductase domain was almost 30-fold higher (Supplementary Fig. 12, Supplementary Tables 2 and 3). This suggests that acrylyl-CoA is immediately consumed upon its formation in situ. To study whether externally provided intermediates can access the reaction chamber of PCS during steady-state, we performed an isotopic labeling competition experiment21,22. When starting from 3-hydroxypropionate, PCS preferentially catalyzed the overall reaction. Externally added 3-hydroxypropionyl-CoA was unable to enter PCS under these conditions, while some externally added acrylyl-CoA was converted to propionyl-CoA. However, the amount of isotopically labeled propionyl-CoA corresponded well to the amount of acrylyl-CoA expected to be turned over in the pre-steady-state of PCS (i.e., within the first 0.6 s before 3-hydroxypropionyl-CoA is formed by the ligase domain; Fig. 2d,e and Supplementary Table 4). In other words: despite the high catalytic efficiency of the reductase domain, only a minor amount of externally added acrylyl-CoA was reduced by PCS in the steady-state. These results demonstrated that catalysis in PCS is consecutive, and that internally produced 3-hydroxypropionyl-CoA and acrylyl-CoA are effectively channeled within the enzyme.
Interdomain communication regulates chamber access
Apparently, external acrylyl-CoA is prevented from entering the reaction chamber of PCS, indicating that the enzyme assumes a “closed state” during catalysis. Therefore, we wondered if any of the substrates (CoA, ATP, 3-hydroxypropionate) or products (AMP) would restrict access of exogenous acrylyl-CoA to the reductase domain during steady-state. Indeed, CoA had a strong effect on both the KM and the kcat of acrylyl-CoA consumption by the reductase domain. Most notably, binding of CoA to the ligase domain directly lowered activity of the reductase domain. We showed that Lys783 plays a crucial role in conferring this interdomain communication (Supplementary Figs. 12, 13 and Supplementary Table 2). The synchronization of domains in PCS was additionally supported by the fact that NADPH (the co-substrate of the third reaction) had a strong effect on the kinetic parameters of the first reaction. Notably, this effect was independent from an active reductase domain. NADPH had an ever stronger effect on the kinetic parameters of the ligase reaction when the cofactor was added to a dehydratase mutant of PCS (E1027Q variant), where the reductase domain is still functional but not provided with substrate due to the inactive dehydratase domain. These observations suggested that interdomain communication in PCS works in both directions (Supplementary Fig. 12 and Supplementary Table 2) and that the individual domains in PCS do not act independently from each other, but catalyze the three-step reaction sequence in a concerted fashion.
Taken together, our experiments demonstrated a functional coupling of the last reaction step in PCS to the first one and vice versa. Apparently, PCS undergoes synchronized conformational changes during catalysis allowing substrates and products to enter and leave the reaction chamber. The gatekeeper to the reaction chamber of PCS is presumably the ligase domain. Stand-alone CoA ligases undergo substantial conformational changes between “open” and “closed” states during catalysis18,23. When we superposed a “closed-state” Salmonella enterica ligase (PDB 2P2F)18 with the PCS ligase domain, the structures aligned almost perfectly (rmsd of 0.932 Å over 441 Cα-atoms) with the exception of above mentioned additional four-helix bundle extension that is only present in PCS (Supplementary Figure 9). An “open-state” ligase of Saccharomyces cerevisiae (PDB 1RY2)23 still aligned well to the PCS ligase domain with its N-terminal domain (rmsd of 0.955 Å over 374 Cα-atoms), while the C-terminal domain (~130 residues) appeared to be rotated outwards. Modelling a corresponding conformational change onto PCS resulted in the exposure of a “hole” that would provide access to the interior of an “open state” compartment (Supplementary Figure 14). Upon binding of CoA and formation of 3-hydroxypropionyl-CoA the ligase domain would switch back to the closed state, sealing the reaction chamber and sequestering the CoA-bound intermediates.
We performed limited proteolysis on PCS with trypsin in the absence and presence of different substrates and products to directly test for conformational changes of PCS during catalysis (Supplementary Figure 15). When incubated in the presence of CoA, which restricts access to the active site of the reductase and forces the enzyme in the “closed” confirmation, PCS was fragmented within 90 min. In contrast, the simultaneous addition of 3-hydroxypropionate, NADP+ and ATP protected PCS from total proteolysis (Supplementary Figure 16). Peptide fragment analysis localized the changes in proteolysis to the flexible parts of the ligase domain that become solvent exposed in the “closed” formation and presumably buried in the “open” confirmation of the enzyme. In summary, limited proteolysis confirmed that the enzyme assumed different conformations depending on the presence of different substrates or products. Additional SAXS analyses of PCS in the presence of different substrates supported this conclusion (Supplementary Table 5).
Discussion
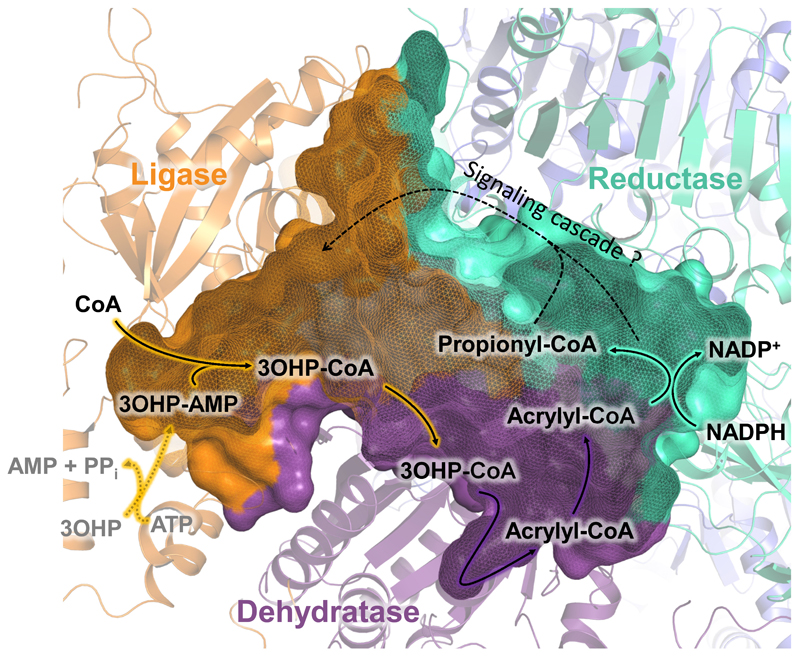
PCS, a key enzyme in the 3-hydroxypropionate bi-cycle for CO2 fixation, catalyzes the three-step reaction sequence from 3-hydroxypropionate to propionyl-CoA. The enclosed reaction chamber, the observed interdomain communication and the proposed conformational changes suggest a highly complex and synchronized catalytic mechanism according to the following model (Fig. 4). First, the ligase forms 3-hydroxypropionyl-AMP from ATP and 3-hydroxypropionate in the open conformation. Binding of CoA then closes the reaction chamber, leading to the formation of 3-hydroxypropionyl-CoA inside the enzyme. CoA binding is facilitated by NADPH, which is consistent with the lowered KM,CoA observed upon NADPH addition and might ensure that the enzyme contains all necessary cofactors to catalyze the complete reaction sequence after closing the chamber. The formed 3-hydroxypropionyl-CoA is subsequently dehydrated to acrylyl-CoA within the closed reaction chamber, isolated from the external environment. The final reduction reaction triggers the re-opening of the reaction chamber for product release and prepares the enzyme for the next catalytic cycle. This mechanism fits well with the observation that the kcat of the ligase reaction alone drops substantially in the presence of NADPH, indicating that NADPH stabilizes the closed conformation and limits the catalytic rate of the ligase domain.
Figure 4. Proposed catalytic cycle of PCS.
In the open conformation, 3-hydroxypropionate (3OHP) and ATP are converted to 3-hydroxypropionyl-AMP (3OHP-AMP) through the ligase domain (orange). The binding of CoA induces closing of the enzyme and the formation of 3-hydroxypropionyl-CoA (3OHP-CoA). 3OHP-CoA is released into the reaction chamber, where it is converted by the dehydratase domain (purple) to acrylyl-CoA. Acrylyl-CoA then enters the active site of the reductase domain (cyan). Following the reduction of acrylyl-CoA to propionyl-CoA, the reaction chamber reopens to release propionyl-CoA, which leaves PCS ready for the next catalytic cycle.
Our experiments show that the three domains of PCS follow a synchronized reaction mechanism to sequester and channel the toxic intermediate acrylyl-CoA between active sites. Thus, PCS is not the simple fusion product of three individual enzymes, but represents a sophisticated three-dimensional arrangement of three different domains enclosing a central reaction chamber connecting all three active sites. The volume of the reaction chamber of PCS (33 nm3) is several orders of magnitude smaller than those of known bacterial micro- or nanocompartments. Usually proteinaceous compartments consist of self-assembling shell proteins that encapsulate their enzymes24–27. In PCS, these elements are integrated into one polypeptide, which fulfills both the structural role of forming the reaction chamber, as well as the catalytic role of driving the multi-reaction sequence. This minimizes biosynthetic costs, because no additional proteins are required to build the compartment.
The three-reaction sequence in PCS is orchestrated through interdomain communication and conformational changes. Access to the reaction chamber is catalytically controlled by the ligase domain that is the only entry and exit site. This makes PCS a complex ‘nanoreactor’ and differentiates the enzyme from canonical channeling enzymes (e.g. tryptophane synthase28,29, amidotransferases30,31), which usually connect two active sites through a narrow channel where conformational changes of single residues suffice to gate separate entry and exit sites30,32,33. Compared to dimethylglycine oxidase (DMGO) that has also been described to possess an internal cavity and a single entry and exit funnel, PCS appears to be more complex, featuring a reaction chamber of three active sites and a more complex opening and closing mechanism34,35.
PCS catalyzes three very fundamental chemical reactions in CoA-ester biochemistry and is able to retain free CoA-esters within its reaction chamber. Known CoA-ester enzyme cascades, such as polyketide, fatty acid or HMG-CoA synthases, require covalently attached CoA-intermediates or shared binding sites of the CoA moiety between two active sites to direct intermediates along a defined multi-reaction sequence36–38. Compared to those examples, PCS employs an intriguingly simple design principle to catalyze a consecutive reaction sequence within a controlled environment. The natural example of a minimal self-assembling nanoreactor that is dynamically regulated could serve as a model for the engineering of spatially and temporally controlled reaction sequences39–44, especially such that proceed via toxic, reactive or unstable intermediates.
Online Methods
a. Chemicals
Chemicals were obtained from Sigma-Aldrich (Munich, Germany) and CARL ROTH GmbH (Karlsruhe, Germany). 3-hydroxypropionate was bought from TCI Deutschland GmbH (Eschborn, Germany). Coenzyme A was purchased from Roche Diagnostics. 1-13C-propionate sodium salt was purchased from Cambridge Isotope Laboratories Inc. (Tewksbury, USA). Biochemicals and materials for cloning and expression were obtained from Thermo Fisher Scientific (St. Leon-Rot, Germany), New England Biolabs GmbH (Frankfurt am Main, Germany) and Macherey-Nagel GmbH (Düren, Germany). Carbonic anhydrase was bought from MP Biomedicals (Illkirch, France). Primers or synthesized genes were obtained from Eurofins MWG GmbH (Ebersberg, Germany) or the DOE Joint Genome Institute (California, USA), respectively. Materials and equipments for protein purification were obtained from GE Healthcare (Freiburg, Germany), Bio Rad (Munich, Germany) or Merck Millipore GmbH (Schwalbach, Germany).
b. Synthesis of 3-hydroxypropionyl-CoA, 13C-acrylyl-CoA and 13C-3-hydroxypropionyl-CoA
For the synthesis of unlabeled 3-hydroxypropionyl-CoA a previously described method using carbonyldiimidazole coupling of the precursor acid with coenzyme A was used 45. Unlabeled acrylyl-CoA was synthesized using a previously described mixed anhydride coupling via ethylchloroformate45. 13C-acrylyl-CoA and 13C-3-hydroxypropionyl-CoA were synthesized in two steps from 13C-propionate. In the first step the CDI coupling method was adapted for the synthesis of 13C-propionyl-CoA by a protonation step. 13C-propionate (0.156 mmol, 4.8 eq.) was dissolved into 1 mL THF containing pTsOH (0.156 mmol, 4.8 eq.) for 15 min, the mixture was centrifuged and CDI (0.130 mmol, 4 eq.) added to the supernatant. The mixture was stirred at RT for 1 h. CoA (0.0325 mmol, 1 eq.) dissolved in 250 µL 0.5M NaHCO3 was added and stirred for 1h. The mixture was lyophilized, HPLC purified and again lyophilized. 13C-acrylyl-CoA was synthesized enzymatically using the acyl-CoA oxidase Acx4 from Arabidopsis thaliana 46. A 1 mL assay contained 100 µL 1 M KHPO4 200 µL 30 mM 13C-propiony-CoA and 600 µL 1mg/mL Acx4. The reaction was quenched after 1 h by adding 20 µL 50% formic acid and directly injected into the HPLC-MS for purification using a previously described purification protocol 45. In case of 13C-3-hydroxypropionyl-CoA the assay contained additionally 50 µL of the dehydratase PhaJ from Pseudomonas aeruginosa for direct hydration of the in situ generated acrlyl-CoA.
c. Bacterial strains and growth conditions
E. coli DH5α (Thermo Scientific™) strains were used for cloning and grown in LB medium 47. For protein expression E. coli BL21-AI™ (Invitrogen) or Arctic-Express (DE3) RIL (Agilent Technologies) were grown in TB medium48. Incubation temperature was 37°C. Antibiotics for selection purposes were used accordingly: 100 µg/ml ampicillin, 15 µg/ml gentamycin.
Cloning. All in silico cloning was performed with Clone Manager 9 (Scientific & Educational Software). For purification, preparation, cloning, transformation and amplification of DNA, standard protocols were used 49. Plasmid isolation and PCR product purification was performed with kits from Macherey Nagel (Düren, Germany) according to the manufacturer’s protocols.
The gene encoding for PCS (GenBank accession no. EAQ29651) with an N-terminal 10x His tag was synthesized by the DOE Joint Genome Institute. The construct was cloned into the expression backbone pET-16b by restriction cloning, resulting in the plasmid pTE1005. Point mutants were generated by QuickChange® Site-Directed mutagenesis (Stratagene, La Jolla, USA). Following primers were used: forward primer (5’-CGT TTC GGT CAA CCA CAA ATC AAT CTT CGC-3’) and reverse primer (5’-GCG AAG ATT GAT TTG TGG TTG ACC GAA ACG-3’) for the E1027Q variant; forward primer (5’-CGG AAA TTT TTG GCA CAG CGC TGT GCA ATG CTT ATG AG-3’) and reverse primer (5’-CTC ATA AGC ATT GCA CAG CGC TGT GCC AAA AAT TTC CG-3’) for the H1769A variant; forward primer (5’-CCT CAC AGC CAG ATG GGT GTA ACT CC-3’) and reverse primer (5’-GGA GTT ACA CCC ATC TGG CTG TGA GG-3’) for the K783M variant.
d. Expression and purification of PCS
PCS was expressed from the plasmid pTE1005 using E.coli ArcticExpress (DE3) RIL as expression host. The cells were transformed with the expression plasmid and plated on LB agar containing selective antibiotic and grown overnight. The colonies were used to inoculate 1 L TB medium. The expression culture was incubated at 37°C while shaking at 110 rpm until an OD600 of 0.7 – 0.9 was reached. The E.coli ArcticExpress (DE3) RIL culture was cooled down to 14°C before induction. Expression was induced by adding 0.25 mM IPTG. The culture was incubated for 16-20 h. The cells were harvested by centrifugation at 5000 x g for 10 min. The pellet was stored at -20°C, optionally. Cells were resuspended in a 1:3 ratio (w/w) in Buffer A (50 mM Tris-HCl pH 7.9, 500 mM NaCl) containing SIGMAFAST™ protease inhibitor (Sigma-Aldrich, Munich, Germany) and lysed by ultrasonication. The lysate was cleared by ultracentrifugation at 50’000 x g for 45 min at 4°C followed by filtration through a 0.45 μm syringe filter. The lysate was loaded onto a 1 mL His-Trap (GE Healthcare). Unspecifically bound proteins were washed off with 15 mL of 5 % Buffer B (50 mM Tris-HCl pH 7.9, 500 mM NaCl, 500 mM imidazole). To wash away the E.coli ArcticExpress (DE3) RIL Cpn60 chaperone, an additional wash step was performed with 15 mL removal buffer (50 mM Tris-HCl pH 7.5, 50 mM KCl, 20 mM MgCl2, 5 mM ATP). PCS was eluted with 100% buffer B and applied to a pre-equilibrated HiLoad 16/60 200 pg superdex (GE Life Science) column (150 mM NaCl, 20 mM Tris HCl pH 7.9). The purity of the PCS was tested by SDS-PAGE (Supplementary Figure 17).
e. Kinetic characterization of PCS
Spectrophotometric assays were set up to measure the activity of PCS. The assays were performed in 10 mm quartz cuvettes (Hellma Analytics) on a Cary-60 UV/Vis spectrometer (Agilent Technologies Inc. Santa Clara, CA, USA). The assay temperature was set to 30 °C. The parameters for the CoA ligase domain alone were measured using a coupling assay via myokinase (purified from ASKA JW1375), pyruvate kinase and lactate dehydrogenase (SigmaAldrich P02694). To probe the influence of acrylyl-CoA on the ligase reaction, the assay was repeated using the PCS E1027Q variant deficient in the enoyl-CoA hydratase reaction to avoid back reaction of acrylyl-CoA to 3-hydroxypropionyl-CoA. In this assay PCS and acrylyl-CoA were added to the reaction and incubated for 5 min at 30°C before starting the reaction with the addition of CoA. The effect of NADPH on the ligase reaction was also tested with the PCS E1027Q variant to avoid overall reaction. The reaction catalyzed by the dehydratase domain was assayed using the PCS H1769A variant that is deficient in the reductase reaction. Acrylyl-CoA formation was coupled to its reduction by a stand-alone reductase (Etr1p from Saccharomyces cerevisiae). The PCS reductase reaction was measured using the E1027Q variant that is deficient in the enoyl-CoA hydratase reaction to avoid the back reaction of acrylyl-CoA to 3-hydroxypropionyl-CoA. The assay was repeated in presence of different concentrations of free CoA. PCS and CoA were added to the reaction mixture and incubated for 10 min at 30°C before starting the reaction with the addition of acrylyl-CoA.
All reactions were measured by following the consumption of NADPH or NADH (ligase coupling assay) at 340 nm (εNAD(P)H = 6.22 mM-1 cm-1) or at 365 nm (εNADH = 3.4 mM-1 cm-1). The detailed conditions for all assays can be found in Supplementary Table 2.
f. Crystallization of PCS
All crystallization was performed at 18°C using the sitting drop method in 96-well 2-drop MRC Crystallization Plates in polystyrene (Molecular Dimensions, Suffolk, UK). Crystallization drops (1.4 – 2 µL) contained PCS at 10 mg/ml premixed with 2mM of CoA, NADP+ and phosphomethylphosphonic acid adenylate ester each mixed with reservoir solution in a 1:1 ratio. First thin needle-shaped crystals appeared after several weeks in 100 mM BisTris pH 6.5, 200 mM NaAc, 25 % (w/v) polyethylene glycol (PEG) 3350 supplemented with 3 % (w/v) trimethylamine N-oxide dihydrate as additive (condition 1). These crystals had a C2 symmetry and the best resolution obtained was 2.7 Å, which was used for the final structure model. Increasing the additive trimethylamine N-oxide dihydrate to 6 % still lead to crystal formation (condition 2) but exhibited strong twinning. Crystals could also be reproduced in the same condition replacing the additive with 100 mM D-(-)-fructose (condition 3) or 4 % (v/v) tert-butanol (condition 4). Crystals of the space group P 21 21 2 were obtained in the same condition supplemented with 2 % (w/v) benzamidine hydrochloride as additive (condition 5). Phasing was achieved by soaking crystals of condition 3 in 100 mM Xo4 9 for 4 minutes. All crystals were cryo-protected with the respective crystallization solution supplemented with 20 – 30 % ethylene glycol.
g. X-ray crystallography analysis
Numerous heavy atom derivatives have been tested attempting to solve the structure of PCS such as: Potassium tetrachloroplatinate(II), organic mercury derivatives, 5-amino-2,4,6-triiodoisophthalic acid and lanthanide phasing compounds (NatX-ray SAS, Saint Martin d'Hères, France). These derivatives resulted in either very low occupancy of the heavy atoms or a substantial decrease in diffraction. A recently developed lanthanide complex, Xo4, containing a terbium ion 9 gave the best results with high anomalous signal not interfering with diffraction quality for short time soakings. We solved PCS in the C2 crystalline form using the single-wavelength anomalous scattering method. Datasets were collected at beamline Proxima-2A of the SOLEIL synchrotron (Paris, France) at the Tb LIII absorption edge (wavelength of 1.649165 Å) on two different crystals (condition 3, see above) soaked in Tb-Xo4. Merging provided a 3.45 Å resolution data set with high redundancy facilitating the location of 18 terbium sites using the SHELX 50 software and PHASER 51. After multiple cycles of phasing, electron density modification, and secondary structure building using AUTOSOL from the PHENIX package 52, the electron density quality was sufficient to build a model with Buccaneer from the CCP4 package 51. The initial model was then used as template for molecular replacement with a dataset of a native crystal (condition 1, see above) using PHASER-MR. The native dataset was collected (wavelength of 0.97625 Å) at beamline ID29 of the ESRF (Grenoble, France). Manual extension of the model was done using COOT 53. Several rounds of manual and automatic refinements were performed using COOT and PHENIX-Refine. The model (PDB 6EQO) structure was refined with Ramachandran statistics of 94.93 % favored, 4.82 % allowed, and 0.25 % outliers.
h. Limited proteolysis
PCS at 0.5 mg/mL was forced into a supposedly closed or open state by adding 3 mM CoA or a combination of 3.4 mM ATP, 2 mM 3-hydroxypropionate and 2 mM NADP+, respectively. A zero time sample of 10 μL was taken. Trypsin (Promega, diluted in 25 mM Tris-HCl pH 7.5, 10 mM CaCl2) was added in a protein:protease ratio of 200:1. Samples of 10 µL were taken at different time points. The sample was quenched with 10 μL 4x SDS buffer and heated at 90°C for 10 min. Samples were applied onto an SDS-PAGE gel. For peptide quantification, the limited proteolysis was repeated as described. However, the samples were quenched by adding PMSF protease inhibitor (dissolved in 2-propanol) to a final concentration of 1 mM. The propylation was performed overnight in the dark (at RT) in 100 mM HEPES buffer pH 7.5, 400 mM sodium cyanoborohydride and 5 % (v/v) acetone 54. The open state PCS samples were treated with unlabeled acetone while the closed state PCS samples were incubated with D6-labeled acetone. The reaction was stopped with 0.07 % TFA. Samples were concentrated and dried in a Speedvac. The open and closed state samples of each time point were combined and purified over a C18 membrane (cut from Empore™ SPE disks). Peptides were eluted with 0.1 % trifluoroacetic acid (TFA) in 50 % acetonitrile (ACN). Samples were dried in the Speedvac. Peptides were resuspended in 50 µl 0.1 % TFA. 1 µl of the peptide sample was mixed with 1 µl solution of 3 g/L alpha-Cyano-4-hydroxycinnamic acid in 80 % ACN (v/v) containing 0.3 % TFA onto a MALDI plate. The dried spots were measured automatically for MS and MSMS in a MALDI TOF/TOF analyzer (Applied Biosystems/MDS Sciex, Framingham, MA, USA) and the 4800 Series Explorer Software.
i. Time course assays
The time course assays with 0.1 µM PCS contained 0.8 mM CoA, 0.5 mM 3-hydroxypropionate, 0.8 mM ATP, 4 mM MgCl2, 40 mM KCl, 50 mM KHCO3 and 0.3 mM NADPH, if stated, in 100 mM potassium phosphate buffer pH 8. At specific time points 20 µL of the assay were quenched with 20 µL of 50 % formic acid. The samples were centrifuged at 17´000 × g and frozen in liquid nitrogen. Samples were immediately thawed before application to hrLC-MS. The time course assay with 10 µM PCS contained 5 mM CoA, 5 mM 3-hydroxypropionate, 5 mM ATP, 6 mM MgCl2, 60 mM KCl and 2 mM NADPH in 100 mM Tris-HCl pH 7.8. At specific time points 10 µL of the assay were quenched with 10 µL acetonitrile and 10 % formic acid and directly injected into the hrLC-MS. Standard curves (0.05 µM to 500 µM) for quantification for 3-hydroxypropionyl-CoA, acrylyl-CoA and propionyl-CoA were prepared in the corresponding buffer conditions and treated in parallel to the samples. Acrylyl-CoA concentrations in PCS samples were too low for UV/Vis quantification, therefore MS based quantification using the extracted ion count of the standard curve the was used. Acrylyl-CoA could only be detected via MS in the samples containing high amounts of PCS (acrylyl-CoA detection limit was 5nM).
j. Isotopic labeling competition experiment
The competition contained 3 mM CoA, 2 mM 3-hydroxypropionate, 200 µM NADPH, 5 mM ATP, 7.5 mM Mg2Cl, 60 mM KCl, 100 mM KHCO3 and 100 mM Tris-HCl pH 7.8. For the competition either 100 µM 13C-3-hydroxypropionyl-CoA or 100 µM 13C-acrylyl-CoA was added. The assay was started with 2 µL of 1.28 mg/mL PCS wt and the reaction monitored photospectrometrically at 340 nm using a Cary-60 UV/Vis spectrometer (Agilent Technologies Inc. Santa Clara, CA, USA) at 30°C using quartz cuvettes (10-mm path-length; Hellma® (Germany)). The assay was quenched after a ΔAbs of 0.36 that corresponds to a turnover of 60 µM. The isotopic pattern of the produced propionyl-CoA was analyzed by hrLC-MS.
k. High resolution LC-MS (hrLC-MS)
3-hydroxypropionyl-Coa, acrylyl-CoA and propionyl-CoA were analyzed using an Agilent 6550 iFunnel Q-TOF LC-MS system equipped with an electrospray ionization source set to positive ionization mode through a 1290 Infinity UPLC (Agilent Technologies Inc. Santa Clara, CA, USA). Compounds were separated on a RP-18 column (50 mm x 2.1 mm, particle size 1.7 µm, Kinetex XB-C18, Phenomenex, Aschaffenburg, Germany) using a mobile phase system comprised of 50 mM ammonium formate pH 8.1 (A) and methanol (B). Chromatographic separation was carried out using the following gradient condition at a flow rate of 250 µl/min: 0 min 0% B; 1 min 0% B, 3 min 2.5% B; 9 min 23% B; 14 min 80 %B; 16 min 80%; 17 min 0 % B; 18 min 0 % B.
Capillary voltage was set at 3.5 kV and nitrogen gas was used as nebulizing (20 psig), drying (13 l/min, 225 °C) and sheath gas (12 l/min, 400°C). The TOF was calibrated using an ESI-L Low Concentration Tuning Mix (Agilent Technologies Inc. Santa Clara, CA, USA) before measurement (residuals less than 2 ppm for five reference ions). MS data were acquired with a scan range of 750-1200 m/z.
CoA-thioesters were additionally detected by UV absorbance at 260 nm using a diode array detector (1290 Infinity II, Agilent Technologies Inc. Santa Clara, CA, USA)
LC-MS data were analyzed using MassHunter Qualitative Analysis software (Agilent).
l. SAXS analysis
PCS was freshly purified as described above two days before SAXS analysis was performed. The protein was stored on ice until measurements. Gel filtration buffer for dilutions and blank measurements was treated equally. SAXS data were recorded at the European Synchrotron Radiation Facility (Grenoble, France) on beamline BM29. The protein was up-concentrated at the beamline. If stated, cofactors were added to the concentrated protein at following concentrations: 3-hydroxypropionate, 2mM; CoA, 3 mM; ATP, 3.4 mM. Two-fold dilution series (4 mg/mL to 0.125 mg/ml) were prepared by dilution with gel filtration buffer containing the corresponding cofactors. The different dilutions were measured to investigate sample quality. Sample storage and measurement temperature was set to 20°C. The ESRF BM29 online software was employed for primary data reduction. PrimusQt (version 4.8.1 55) was used for data analysis.
Supplementary Material
Acknowledgements
We thank Jörg Kahnt for help with the limited proteolysis experiments and Patrick Pausch for his help in collecting SAXS data. We acknowledge support from the European Synchrotron Radiation (ESRF, beamlines ID29, BM30 and BM29), Grenoble, France and the Synchrotron SOLEIL (beamline PX-2A), Paris, France. The work conducted by the U.S. Department of Energy Joint Genome Institute, a DOE Office of Science User Facility, is supported under Contract No. DE-AC02-05CH11231. This work was funded by the Deutsche Forschungsgemeinschaft through Collaborative Research Centre SFB 987, the European Research Council (ERC 637675 ‘SYBORG’), a FET-Open Grant 686330 (‘FutureAgriculture’), the Gebert-Rüf-Stiftung (GRS 062-12), and the Max-Planck-Society.
Footnotes
Data availability. The coordinates and structure factors of the crystal structure generated from this research are available at the Protein Data Bank under accession number 6EQO. All other relevant data are available in this article and its Supplementary Information files, or from the corresponding author upon request.
Author contributions I.B, B.V, D.M.P, J.Z, and T.J.E conceived the project. I.B, B.V, T.W., J.Z, and T.J.E designed and performed experiments and analyzed the data. E.G, F.R, and O.M designed and prepared the phasing compound Tb-Xo4. I.B, B.V, S.E, E.G, T.W and J.Z collected X-ray data sets, T.W and J.Z solved crystal structures. E.G, G.B, and S.S oversaw crystallography and SAXS experiments, provided equipment and beam-time. N.S.C. performed mass spectrometry and analyzed the data. I.B, B.V, T.W, J.Z, and T.J.E wrote the manuscript with contributions from all other authors.
Competing interests The authors declare no competing financial interests.
References
- 1.Wheeldon I, et al. Substrate channelling as an approach to cascade reactions. Nat Chem. 2016;8:299–309. doi: 10.1038/nchem.2459. [DOI] [PubMed] [Google Scholar]
- 2.Linster CL, Van Schaftingen E, Hanson AD. Metabolite damage and its repair or pre-emption. Nat Chem Biol. 2013;9:72–80. doi: 10.1038/nchembio.1141. [DOI] [PubMed] [Google Scholar]
- 3.Wheeldon I, et al. Substrate channelling as an approach to cascade reactions. Nature chemistry. 2016;8:299. doi: 10.1038/nchem.2459. [DOI] [PubMed] [Google Scholar]
- 4.Alber BE, Fuchs G. Propionyl-coenzyme A synthase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation. J Biol Chem. 2002;277:12137–12143. doi: 10.1074/jbc.M110802200. [DOI] [PubMed] [Google Scholar]
- 5.Zarzycki J, Brecht V, Müller M, Fuchs G. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proc Natl Acad Sci USA. 2009;106:21317–21322. doi: 10.1073/pnas.0908356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd JD, Curson ARJ, Sullivan MJ, Kirkwood M, Johnston AWB. The Ruegeria pomeroyi acuI Gene Has a Role in DMSP Catabolism and Resembles yhdH of E. coli and Other Bacteria in Conferring Resistance to Acrylate. Plos One. 2012;7:e35947. doi: 10.1371/journal.pone.0035947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teufel R, Kung JW, Kockelkorn D, Alber BE, Fuchs G. 3-hydroxypropionyl-coenzyme A dehydratase and acryloyl-coenzyme A reductase, enzymes of the autotrophic 3-hydroxypropionate/4-hydroxybutyrate cycle in the Sulfolobales. J Bacteriol. 2009;191:4572–4581. doi: 10.1128/JB.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg IA, Kockelkorn D, Buckel W, Fuchs G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in archaea. Science. 2007;318:1782–1786. doi: 10.1126/science.1149976. [DOI] [PubMed] [Google Scholar]
- 9.Engilberge S, et al. Crystallophore: a versatile lanthanide complex for protein crystallography combining nucleating effect, phasing properties and luminescence. Chem Sci. 2017;8:5909–5917. doi: 10.1039/c7sc00758b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindbladh C, et al. Preparation and kinetic characterization of a fusion protein of yeast mitochondrial citrate synthase and malate dehydrogenase. Biochemistry. 1994;33:11692–11698. doi: 10.1021/bi00205a004. [DOI] [PubMed] [Google Scholar]
- 11.Shatalin K, Lebreton S, Rault-Leonardon M, Vélot C, Srere PA. Electrostatic channeling of oxaloacetate in a fusion protein of porcine citrate synthase and porcine mitochondrial malate dehydrogenase. Biochemistry. 1999;38:881–889. doi: 10.1021/bi982195h. [DOI] [PubMed] [Google Scholar]
- 12.Datta A, Merz JM, Spivey HO. Substrate channeling of oxalacetate in solid-state complexes of malate dehydrogenase and citrate synthase. J Biol Chem. 1985;260:15008–15012. [PubMed] [Google Scholar]
- 13.Chowdhury C, Sinha S, Chun S, Yeates TO, Bobik TA. Diverse Bacterial Microcompartment Organelles. Microbiol Mol Biol Rev. 2014;78:438–468. doi: 10.1128/MMBR.00009-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutter M, Greber B, Aussignargues C, Kerfeld CA. Assembly principles and structure of a 6.5-MDa bacterial microcompartment shell. Science. 2017;356:1293–1297. doi: 10.1126/science.aan3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutter M, et al. Structural basis of enzyme encapsulation into a bacterial nanocompartment. Nat Struct Mol Biol. 2008;15:939–947. doi: 10.1038/nsmb.1473. [DOI] [PubMed] [Google Scholar]
- 16.Jung T, Grune T. The proteasome and the degradation of oxidized proteins: part I—structure of proteasomes. Redox Biol. 2013;1:178–182. doi: 10.1016/j.redox.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho BK, Gruswitz F. HOLLOW: generating accurate representations of channel and interior surfaces in molecular structures. BMC Struct Biol. 2008;8:49. doi: 10.1186/1472-6807-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reger AS, Carney JM, Gulick AM. Biochemical and Crystallographic Analysis of Substrate Binding and Conformational Changes in Acetyl-CoA Synthetase. Biochemistry. 2007;46:6536–6546. doi: 10.1021/bi6026506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bock T, Reichelt J, Müller R, Blankenfeldt W. The Structure of LiuC, a 3-Hydroxy-3-Methylglutaconyl CoA Dehydratase Involved in Isovaleryl-CoA Biosynthesis in Myxococcus xanthus, Reveals Insights into Specificity and Catalysis. Chembiochem. 2016;17:1658–1664. doi: 10.1002/cbic.201600225. [DOI] [PubMed] [Google Scholar]
- 20.Quade N, Huo L, Rachid S, Heinz DW, Müller R. Unusual carbon fixation gives rise to diverse polyketide extender units. Nat Chem Biol. 2012;8:117–124. doi: 10.1038/nchembio.734. [DOI] [PubMed] [Google Scholar]
- 21.Spivey HO, Ovadi J. Substrate channeling. Methods. 1999;19:306–321. doi: 10.1006/meth.1999.0858. [DOI] [PubMed] [Google Scholar]
- 22.Lyle S, Ozeran JD, Stanczak J, Westley J, Schwartz NB. Intermediate Channeling between ATP Sulfurylase and Adenosine 5'-Phosphosulfate Kinase from Rat Chondrosarcoma. Biochemistry. 1994;33:6822–6827. doi: 10.1021/bi00188a010. [DOI] [PubMed] [Google Scholar]
- 23.Jogl G, Tong L. Crystal structure of yeast acetyl-coenzyme A synthetase in complex with AMP. Biochemistry. 2004;43:1425–1431. doi: 10.1021/bi035911a. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Sawaya MR, Yeates TO. Structure and Mechanisms of a Protein-Based Organelle in Escherichia coli. Science. 2010;327:81–84. doi: 10.1126/science.1179513. [DOI] [PubMed] [Google Scholar]
- 25.Giessen TW, Silver PA. Widespread distribution of encapsulin nanocompartments reveals functional diversity. Nat Microbiol. 2017;2:17029. doi: 10.1038/nmicrobiol.2017.29. [DOI] [PubMed] [Google Scholar]
- 26.Fan CG, Cheng SQ, Sinha S, Bobik TA. Interactions between the termini of lumen enzymes and shell proteins mediate enzyme encapsulation into bacterial microcompartments. PNAS. 2012;109:14995–15000. doi: 10.1073/pnas.1207516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerfeld CA, et al. Protein structures forming the shell of primitive bacterial organelles. Science. 2005;309:936–938. doi: 10.1126/science.1113397. [DOI] [PubMed] [Google Scholar]
- 28.Pan P, Woehl E, Dunn MF. Protein architecture, dynamics and allostery in tryptophan synthase channeling. Trends Biochem Sci. 1997;22:22–27. doi: 10.1016/s0968-0004(96)10066-9. [DOI] [PubMed] [Google Scholar]
- 29.Hyde C, Ahmed S, Padlan E, Miles EW, Davies D. Three-dimensional structure of the tryptophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium . J Biol Chem. 1988;263:17857–17871. [PubMed] [Google Scholar]
- 30.Mouilleron S, Badet-Denisot M-A, Golinelli-Pimpaneau B. Glutamine binding opens the ammonia channel and activates glucosamine-6P synthase. J Biol Chem. 2006;281:4404–4412. doi: 10.1074/jbc.M511689200. [DOI] [PubMed] [Google Scholar]
- 31.Thoden JB, Holden HM, Wesenberg G, Raushel FM, Rayment I. Structure of carbamoyl phosphate synthetase: a journey of 96 Å from substrate to product. Biochemistry. 1997;36:6305–6316. doi: 10.1021/bi970503q. [DOI] [PubMed] [Google Scholar]
- 32.Singh H, Arentson BW, Becker DF, Tanner JJ. Structures of the PutA peripheral membrane flavoenzyme reveal a dynamic substrate-channeling tunnel and the quinone-binding site. PNAS. 2014;111:3389–3394. doi: 10.1073/pnas.1321621111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith NE, Vrielink A, Attwood PV, Corry B. Biological channeling of a reactive intermediate in the bifunctional enzyme DmpFG. Biophys J. 2012;102:868–877. doi: 10.1016/j.bpj.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leys D, Basran J, Scrutton NS. Channelling and formation of ‘active’formaldehyde in dimethylglycine oxidase. EMBO J. 2003;22:4038–4048. doi: 10.1093/emboj/cdg395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tralau T, et al. An internal reaction chamber in dimethylglycine oxidase provides efficient protection from exposure to toxic formaldehyde. J Biol Chem. 2009;284:17826–17834. doi: 10.1074/jbc.M109.006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa M, Tsuchiya D, Oyama T, Tsunaka Y, Morikawa K. Structural basis for channelling mechanism of a fatty acid β-oxidation multienzyme complex. EMBO J. 2004;23:2745–2754. doi: 10.1038/sj.emboj.7600298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith S, Tsai S-C. The type I fatty acid and polyketide synthases: a tale of two megasynthases. Nat Prod Rep. 2007;24:1041–1072. doi: 10.1039/b603600g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vögeli B, et al. Archaeal acetoacetyl-CoA thiolase/HMG-CoA synthase complex channels the intermediate via a fused CoA-binding site. PNAS. 2018 doi: 10.1073/pnas.1718649115. 201718649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bale JB, et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science. 2016;353:389–394. doi: 10.1126/science.aaf8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aussignargues C, et al. Structure and Function of a Bacterial Microcompartment Shell Protein Engineered to Bind a [4Fe-4S] Cluster. J Am Chem Soc. 2016;138:5262–5270. doi: 10.1021/jacs.5b11734. [DOI] [PubMed] [Google Scholar]
- 41.Giessen TW, Silver PA. A Catalytic Nanoreactor Based on in Vivo Encapsulation of Multiple Enzymes in an Engineered Protein Nanocompartment. Chembiochem. 2016;17:1931–1935. doi: 10.1002/cbic.201600431. [DOI] [PubMed] [Google Scholar]
- 42.Azuma Y, Zschoche R, Tinzl M, Hilvert D. Quantitative Packaging of Active Enzymes into a Protein Cage. Angew Chem. 2016;55:1531–1534. doi: 10.1002/anie.201508414. [DOI] [PubMed] [Google Scholar]
- 43.Burton AJ, Thomson AR, Dawson WM, Brady RL, Woolfson DN. Installing hydrolytic activity into a completely de novo protein framework. Nat Chem. 2016;8:837–844. doi: 10.1038/nchem.2555. [DOI] [PubMed] [Google Scholar]
- 44.Brasch M, et al. Assembling Enzymatic Cascade Pathways inside Virus-Based Nanocages Using Dual-Tasking Nucleic Acid Tags. J Am Chem Soc. 2017;139:1512–1519. doi: 10.1021/jacs.6b10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peter DM, Vögeli B, Cortina NS, Erb TJ. A chemo-enzymatic road map to the synthesis of CoA esters. Molecules. 2016;21:517. doi: 10.3390/molecules21040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwander T, von Borzyskowski LS, Burgener S, Cortina NS, Erb TJ. A synthetic pathway for the fixation of carbon dioxide in vitro. Science. 2016;354:900–904. doi: 10.1126/science.aah5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertani G. STUDIES ON LYSOGENESIS I.: The Mode of Phage Liberation by Lysogenic Escherichia coli. J Bacteriol. 1951;62:293. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tartof K, Hobbs C. Improved media for growing plasmid and cosmid clones. Focus. 1987;9:12. [Google Scholar]
- 49.Sambrook J, Russel D. Molecular cloning: a laboratory manual. 3 edn. Cold Spring Laboratory Press; New York: 2001. [Google Scholar]
- 50.Sheldrick GM. A short history of SHELX. Acta Crystallogr A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 51.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 54.Eichacker L, Granvogl B, Gruber P. Method for quantitative comparison of two or more proteins. Germany patent EP1947461. 2008
- 55.Konarev PV, Volkov VV, Sokolova AV, Koch MH, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J Appl Crystallogr. 2003;36:1277–1282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.