Abstract
Background
Self-management is intended to empower individuals in their recovery by providing the skills and confidence they need to take active steps in recognising and managing their own health problems. Evidence supports such interventions in a range of long-term physical health conditions, but a recent systematic synthesis is not available for people with severe mental health problems.
Aims
To evaluate the effectiveness of self-management interventions for adults with severe mental illness (SMI).
Method
A systematic review of randomised controlled trials was conducted. A meta-analysis of symptomatic, relapse, recovery, functioning and quality of life outcomes was conducted, using RevMan.
Results
A total of 37 trials were included with 5790 participants. From the meta-analysis, self-management interventions conferred benefits in terms of reducing symptoms and length of admission, and improving functioning and quality of life both at the end of treatment and at follow-up. Overall the effect size was small to medium. The evidence for self-management interventions on readmissions was mixed. However, self-management did have a significant effect compared with control on subjective measures of recovery such as hope and empowerment at follow-up, and self-rated recovery and self-efficacy at both time points.
Conclusion
There is evidence that the provision of self-management interventions alongside standard care improves outcomes for people with SMI. Self-management interventions should form part of the standard package of care provided to people with SMI and should be prioritised in guidelines: research on best methods of implementing such interventions in routine practice is needed.
Declaration of interests
None.
Keywords: Schizophrenia, bipolar affective disorders, community mental health teams, psychosocial interventions, psychotic disorders
Self-management broadly encompasses the tasks required to successfully live with and manage the physical, social and emotional impact of a chronic condition.1 Currently, there is no universally accepted classification of self-management, although it commonly involves the provision of information and education on a condition and its treatment, collaboratively creating an individualised treatment plan, developing skills for self-monitoring symptoms and strategies to support adherence to treatment including medication, psychological techniques, lifestyle and social support. A rapid synthesis1 of self-management interventions revealed a robust evidence base for improvement in outcomes of long-term conditions, such as diabetes and asthma, and some evidence for interventions in stroke, hypertension and depression, along with the potential for reducing healthcare resource use. The synthesis concluded that inclusion of self-management should be a requirement for high-quality care for all long-term conditions.
Background
A range of interventions badged as self-management are available for people with long-term conditions falling under the umbrella of severe mental illness (SMI)2 (schizophrenia spectrum disorders, bipolar disorder and major depression), but a recent systematic synthesis regarding their effectiveness is lacking. A 2002 review of interventions for this population identified four key elements that improved the course of illness of those with SMI: (a) providing psychoeducation about mental illness and its treatment, (b) behavioural tailoring to facilitate medication adherence, (c) developing a relapse prevention plan and (d) teaching coping strategies for persistent symptoms.3 More recently, an additional focus on patient-defined recovery and personal goals has been incorporated into self-management interventions. Through these elements, self-management interventions are thought to empower individuals by providing the knowledge and skills to enable them to make informed decisions to manage their own care,4 cope with symptoms and reduce susceptibility to relapse and reliance on services.3
Rationale for the review
To date, previous reviews of self-management interventions for SMI have focused on broad, nonspecific self-management interventions such as psychoeducation5,6 and self-help,7 or they have been confined to specific diagnoses within the SMI population8 – predominantly schizophrenia or psychosis. This limits the generalisability3 of the findings and results in the exclusion of studies focused on broad populations of mental health patients, even though self-management interventions are currently intended for use by a broad group. A comprehensive systematic review and meta-analysis of self-management interventions for people with SMI has not previously been available. Empowering mental health patients and supporting them in making choices about their care are increasingly given weight among the stated goals and values of mental health services and policies: self-management interventions have the potential to help achieve these goals.
The present review
The aim of the present study is to assess the effectiveness of self-management in the typical mixed populations of people with SMI, such as those found in National Health Service (NHS) secondary care settings and in community mental health services in many other systems. We look at the effect of self-management in both the short and longer term in relation to the following prespecified outcomes deemed important from both a commissioning and patient perspective: symptomatic recovery, relapse prevention, reduced need for admission to hospital, self-rated recovery, functioning and quality of life.
Method
A review protocol was developed following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines9 and was registered at PROSPERO (reference: CRD42017043048).
Inclusion criteria
The research question and inclusion criteria were formulated using the PICOS (Participant, Intervention, Comparison, Outcome and Study) design.9 This widely used framework supports formulation of focused and rigorous review questions.
Participants
Studies were included if participants were adults aged 18 years and over and diagnosed with a SMI,2 i.e. with a clinical diagnosis10 of schizophrenia spectrum disorders (schizoaffective disorder, delusional disorder and psychosis), bipolar disorder or major depression. Also included were studies with mixed populations of people with these diagnoses (which included those with personality disorder) who were using secondary care mental health services.
Intervention
Studies were included if patients directly received a self-management intervention that was designed to educate and equip individuals with the skills to manage symptoms, relapses and overall psychosocial functioning.11 Self-management interventions were delivered in conjunction with treatment as usual (TAU). To investigate the effectiveness of self-management itself, interventions with a broader focus that included self-management as only one of the intervention components were not included in the current review, unless it was possible to ascertain the specific impact of self-management. To be considered a self-management intervention for the purposes of this systematic review, the intervention had to include the following three (of the four) domains identified by Mueser and colleagues3 as effective areas of self-management:
-
(a)
psychoeducation about mental illness and its treatment (to make informed decisions about care);
-
(b)
recognition of early warning signs of relapse and development of a relapse prevention plan;
-
(c)
coping skills for dealing with persistent symptoms.
Additionally, the self-management intervention should include a recovery-focused element,11 such as setting personal goals based on an individual's own hopes for their recovery and learning how to effectively manage their illness in the context of pursuing those goals.
Strategies for medication management, the fourth domain identified by Mueser and colleagues,3 was not considered a necessary domain for a self-management intervention to be included in the current review. Making medication management a mandatory domain was considered at odds with a recovery-focused approach; however the majority of studies did include a medication management component.
Comparison
Studies employing either TAU, however defined, or active controls were included in this review.
Outcome
If studies reported on any of the following prespecified outcomes they were included in the meta-analyses:
-
(a)
symptom-focused outcomes;
-
(b)
relapse (or related service use outcomes: number and length of admissions);
-
(c)
recovery-focused outcomes (including measures of overall recovery processes and its components: self-empowerment and efficacy, social connectedness, hope, optimism and the pursuit of a meaningful life);12
-
(d)
functioning (global);
-
(e)
quality of life.
Study design
All randomised controlled trials (RCTs), including cluster RCTs and factorial RCTs, were considered for inclusion. Quasi-randomised studies were excluded.
Exclusion criteria
Studies were excluded for the following reasons.
-
(a)
The intervention had a therapeutic focus beyond that of improving an individual's self-management of their illness (e.g. cognitive remediation, cognitive–behavioural therapy, basic life skills or social skills), which prevented evaluating the specific efficacy of the self-management component.
-
(b)The intervention was delivered:
-
(i)to family members (either as the target recipients of the intervention or in addition to the patients);
- (ii)
-
(i)
Search strategy and selection criteria
A systematic search for all relevant literature was conducted using a PRISMA9 search strategy of the databases Medline, Embase, PsychINFO, DARE and CENTRAL, from their inception until 15 May 2018. The relevant parts of a published search strategy used for the National Institute for Health and Care Excellence (NICE) schizophrenia guidelines16 was used in the current study and details are included in Supplementary Appendix 1 available at https://doi.org/10.1192/bjp.2019.54. Abstracts were screened based on the review protocol (M.L.) and any uncertainties were reviewed to reach a consensus (M.L. and M.F.-A.). Twenty per cent of the full-text articles assessed for eligibility (n = 82) were blindly assessed to meet inclusion and exclusion criteria (M.F.-A. and A.M.). The few cases of disagreement were discussed and consensus was reached. Additionally, a hand search of reference lists was conducted.
All abstracts were retrieved and added to Mendeley referencing software (version 1.16.3 for MacOS).
Data extraction and quality assessment
Data were extracted and reviewed in Microsoft Excel. Characteristics of the study design, the intervention, participants and outcomes for all available data at all provided time points were extracted. Authors were contacted and asked to provide any missing data. Raw outcome data extracted from papers published before 2012 were kindly provided by the National Collaborating Centre for Mental Health from our group's previous work with them on the development of the NICE schizophrenia guidelines. The relevant studies and outcome data provided from the original search were then extracted according to this current review protocol and checked against the original manuscripts. When a study had three arms, we followed expert guidelines17 and combined both control groups into a single group to enable pairwise comparison. Mean values were multiplied by −1 to correct for differences in the direction of scales.
Assessment of bias
Assessment of bias was performed by two pairs of researchers (B.H.-S. and A.Y.-U.; M.L. and A.M.) using the Cochrane Collaboration Risk of Bias Tool.17 Each study was rated for risk of bias due to sequence generation, allocation concealment, blinding of assessors, selective outcome reporting and incomplete data. The blinding of participants in trials of complex interventions is problematic. As such, it is assumed that blinding of participants was at high risk for all studies. Risk of bias was rated as high (weakening confidence in results), low (unlikely to seriously alter results) or unclear. Funnel plots were generated to examine publication bias in analyses with more than ten studies.18
Statistical analysis
Review Manager (RevMan 5.2 for Windows) software was used to conduct the meta-analyses. When outcome data were reported for more than one follow-up point, the time point closest to 1-year post-intervention was used. Where more than one measure was used to report the same outcome in the same study, we prioritised the primary outcome of that study or included the outcome more commonly reported by other studies in the analysis. On the rare event that a study reported both symptomatic relapse and readmission data, we included the readmission data in the analysis. Studies with TAU and active control groups were analysed together.
Effect size calculation
Effect sizes for continuous data were calculated as standardised mean difference (SMD), Hedges' g, and studies were weighted using inverse variance.17 For dichotomous outcomes we calculated risk ratios and combined studies using the Mantel–Haenszel method.17 All outcomes are reported with 95% confidence intervals using random-effects modelling. If reported by studies, we used intention to treat data in our analysis.
Heterogeneity
Heterogeneity was assessed through visual inspection of forest plots, the P-value of the χ2 test (Q) and calculating the I2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than chance.19 A P-value <0.10 and an I2 >50% suggests substantial heterogeneity. Quantifying inconsistency across studies in this way allowed us to explore the possible reasons for heterogeneity through sensitivity analysis.
Sensitivity analyses were carried out using the one-study-removed method to examine the effect of a specific study on the pooled treatment effect. When a study was identified as substantially contributing to heterogeneity, the potential sources of clinical or methodological heterogeneity were reviewed and compared with the remaining studies to evaluate if their exclusion from the particular meta-analysis was warranted.
Results
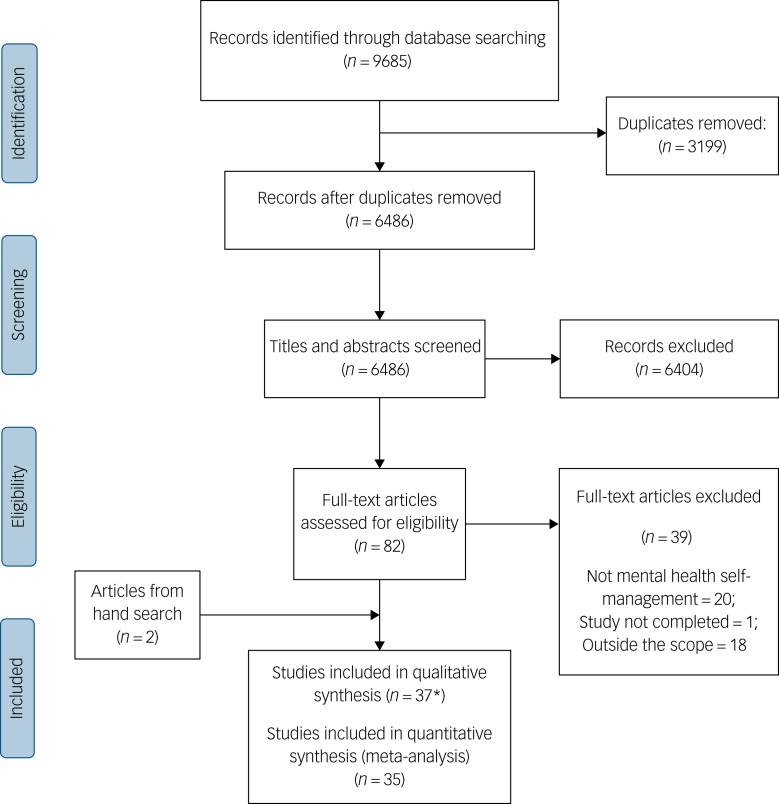
Of the 6486 potentially relevant citations, 82 papers were retrieved and assessed for inclusion (Fig. 1). Of these, 20 were excluded because they were not mental health self-management interventions (either they did not meet the three criteria for inclusion, or they covered social skills training only), one study was not completed (protocol paper only), and a further 18 papers were outside of the scope of this review (i.e. self-management was delivered as part of another intervention, or included family members in the intervention). Two papers were included from a reference hand search. A total of 37 RCTs (published across 45 full-text articles) were therefore included in the narrative synthesis. Two were not included in the meta-analyses20,21 as they did not report usable outcomes.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow chart.
*The 37 studies were from 45 full-text articles.
Study characteristics
A detailed breakdown of the characteristics of the studies included in this review20–64 can be found in Supplementary Table 1. Studies included in this review randomised 5790 participants with a median sample size of 107 (range 32–555). The majority of studies were conducted in high-income countries (k = 27), with a smaller but substantial proportion in lower- or middle-income countries (k = 10). The majority of studies (k = 29) included participants who were currently living in the community, with eight studies recruiting from in-patient settings.
The mean age of participants was 40 years and 44% were female. In relation to clinical diagnosis, 18 studies included only participants with schizophrenia spectrum disorder and 7 included only those with a diagnosis of bipolar disorder. The remaining 12 included mixed populations of participants with schizophrenia, psychosis, bipolar, major depressive disorder and personality disorder who were in contact with secondary mental health services.
Across the 37 studies, self-management interventions ranged broadly in duration from 1 to 52 weeks (median duration 12 weeks). Likewise, face-to-face/group contact time also ranged widely from 4 to 96 h (median 23 h). Most interventions were delivered in a group format and facilitated by clinicians (k = 25) or peers (k = 5). The remaining interventions were delivered to participants individually, either as an online, computer-based intervention (k = 2), by a clinician (k = 2) or by a peer (k = 1). Finally, two studies used a combination of group and individual sessions facilitated by a clinician. All interventions were delivered from a manualised protocol, however the depth, detail and fidelity of the intervention to the manual was not always reported in detail. All interventions were delivered in addition to TAU provided in the same respective settings.
Supplementary Table 2 provides a detailed breakdown of the studies reviewed, organised by a preliminary typology of self-management interventions developed as part of this review (further details in Supplementary Table 3).
Controls
Self-management interventions were compared with TAU in 19 studies; waiting list control conditions in 3 studies; and the remaining 12 had active control conditions such as group counselling, occupational therapy or psychoeducation (Supplementary Table 2). A further three were multi-arm studies with active and TAU control groups.
Outcome measures
Supplementary Table 4 outlines the continuous measures used in studies, categorised by outcome type. Dichotomous data were also reported. The outcome measures used across the studies were reported to be well-validated and reliable instruments. Symptom outcomes were reported on measures ranging from self-rated (the Internal State Scale) to those rated by caregivers (Psychosis Evaluation tool for Common use by Caregivers) and those requiring a clinical interview (Positive and Negative Syndrome Scale and Brief Psychiatric Rating Scale). In the majority of studies, relapse was measured as an admission to hospital. A small minority of trials additionally identified relapse in participants when a score reached a cut-off point on a scale, but admission data were given precedence in the present analysis. Measures of quality of life were self-rated, whereas functioning tended to be clinician rated. Measures of recovery which focused on personal recovery as opposed to clinical recovery were exclusively self-rated.
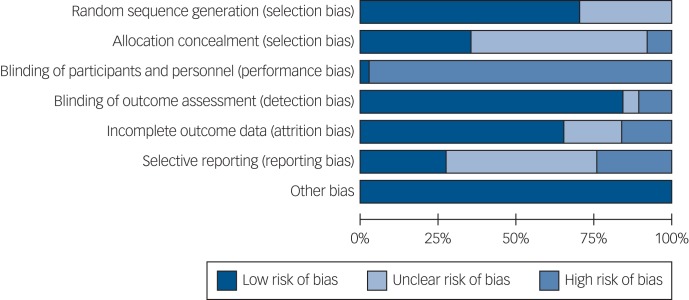
Risk of bias
The risk of bias summary is shown in Fig. 2 and the rating for each individual study can be found in Supplementary Fig. 1. Blinding of participants and personnel is generally considered to be challenging in complex interventions, so risk of bias in this respect was rated as high in all studies except for one.59 Of note, 9 studies were at a high risk of bias for selective reporting of outcomes measured and 18 were unclear. The ‘other bias’ category refers to whether any studies were discontinued due to adverse events, problems with the study design or acceptability of the intervention.
Fig. 2.
Cochrane Risk of Bias Summary.
Quantitative synthesis
Data were analysed at two time points: at the end of the treatment intervention (that is, immediately, or within 2 weeks) and at follow-up. The median follow-up length was 41 weeks (range 4–104 weeks) post-treatment; 52 weeks (range 7–130 weeks) post-randomisation. Summary results are outlined in Table 1 (forest plots in Supplementary Fig. 2).
Table 1.
Analysis of self-management intervention for people with severe mental illness compared with control (active or treatment as usual) (random-effects model)
| Outcome | Time of data collection | Trials (k) | Participants SM/control (n) | Estimate | Summary of estimate (95% CI) | Z, P | Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| Q-test | I2 (%) | |||||||
| Symptoms | ||||||||
| Total symptoms | End of treatment | 17 | 912/1067 | SMD | −0.43 (−0.63, −0.22) | 4.12, P < 0.0001* | Q = 72.84, P < 0.0001 | 78a |
| Follow-up | 13 | 676/844 | SMD | −0.88 (−1.19, −0.57) | 5.52, P < 0.0001* | Q = 82.69, P < 0.0001 | 87a | |
| Positive symptoms | End of treatment | 8 | 372/507 | SMD | −0.22 (−0.51, 0.07) | 1.50, P = 0.13 | Q = 26.09, P = 0.0005 | 73a |
| Follow-up | 6 | 312/459 | SMD | −0.61 (−1.03, −0.19) | 2.86, P = 0.004* | Q = 33.27, P < 0.0001 | 85a | |
| Negative symptoms | End of treatment | 9 | 457/590 | SMD | −0.26 (−0.47, −0.05) | 2.44, P = 0.01* | Q = 19.62, P = 0.01 | 59a |
| Follow-up | 7 | 378/523 | SMD | −0.51 (−0.82, −0.21) | 3.28, P = 0.001* | Q = 26.50, P = 0.0002 | 77a | |
| Affective symptoms (depression/anxiety) | End of treatment | 5 | 230/222 | SMD | −0.26 (−0.51, −0.01) | 2.04, P = 0.04* | Q = 6.69, P = 0.15 | 40 |
| Follow-up | 6 | 475/489 | SMD | −0.19 (−0.33, −0.04) | 2.43, P = 0.02* | Q = 5.91, P = 0.31 | 15 | |
| Relapse | ||||||||
| Mean number of readmissions to acute care | End of treatment | 5 | 315/456 | SMD | −0.39 (−0.89, 0.11) | 1.52, P = 0.13 | Q = 38.72, P < 0.0001 | 90a |
| Follow-up | 5 | 257/398 | SMD | −0.92 (−1.63, −0.21) | 2.53, P = 0.01* | Q = 57.74, P < 0.0001 | 93a | |
| Total number of patients in each group readmitted to acute care | End of treatment | 2 | 104/147 | RR | 0.84 (0.48, 1.46) | 0.63, P = 0.53 | Q = 0.72, p = 0.40 | 0 |
| Follow-up | 10 | 416/473 | RR | 0.75 (0.51, 1.08) | 1.54, P = 0.12 | Q = 15.05, P = 0.09a | 40 | |
| Length of admission to acute care | End of treatment | 6 | 359/543 | SMD | −0.26 (−0.50, −0.02) | 2.08, P = 0.04* | Q = 10.77, P = 0.03 | 63a |
| Follow-up | 7 | 350/558 | SMD | −0.68 (−1.10, −0.25) | 3.12, P = 0.002* | Q = 49.76, P < 0.0001 | 88a | |
| Recovery | ||||||||
| Total recovery | End of treatment | 11 | 507/506 | SMD | −0.62 (−1.03, −0.22) | 3.03, P = 0.002* | Q = 89.3, P < 0.0001 | 89a |
| Follow-up | 7 | 543/591 | SMD | −0.81 (−1.40, −0.22) | 2.68, P = 0.007* | Q = 105.09, P < 0.0001 | 94a | |
| Empowerment | End of treatment | 3 | 187/159 | SMD | −1.44 (−2.97, 0.08) | 1.86, P = 0.06 | Q = 44.89, P < 0.0001 | 96a |
| Follow-up | 2 | 278/260 | SMD | −0.25 (−0.43, −0.07) | 2.68, P = 0.007* | Q = 1.13, P = 0.29 | 12 | |
| Hope | End of treatment | 2 | 200/189 | SMD | −0.18 (−0.38, 0.01) | 1.81, P = 0.07 | Q = 0.52, P = 0.47 | 0 |
| Follow-up | 3 | 487/480 | SMD | −0.24 (−0.46, −0.02) | 2.16, P = 0.03* | Q = 5.74, P = 0.06 | 65a | |
| Self-efficacy | End of treatment | 4 | 322/279 | SMD | −0.38 (−0.62, −0.15) | 3.18, P = 0.001* | Q = 5.42, P = 0.14 | 45 |
| Follow-up | 1 | 121/100 | SMD | −0.34 (−0.61, −0.07) | 2.50, P = 0.01* | N/A | N/A | |
| Functioning | End of treatment | 15 | 884/1064 | SMD | −0.56 (−0.85, −0.28) | 3.90, P < 0.0001* | Q = 121.25, P < 0.0001 | 88a |
| Follow-up | 14 | 805/1000 | SMD | −0.90 (−1.34, −0.45) | 3.97, P < 0.0001* | Q = 237.9, P < 0.0001 | 95a | |
| Quality of life | End of treatment | 9 | 440/423 | SMD | −0.23 (−0.37, −0.10) | 3.38, P = 0.0007* | Q = 7.83, P = 0.45 | 0 |
| Follow-up | 7 | 491/489 | SMD | −0.25 (−0.37, −0.12) | 3.84, P = 0.0001* | Q = 3.07, P = 0.80 | 0 | |
SM, self-management intervention; SMD, standardised mean difference; RR, relative risk.
a. Indicates high heterogeneity: I2 >50% and/or P-value <0.10.
*P < 0.05.
Symptoms
A total of 17 studies (n = 1979) found a small but significant effect of self-management on total symptoms at post-treatment (SMD −0.43, 95% CI −0.63 to −0.22). At follow-up, 13 studies (n = 1520) demonstrated a marked effect of self-management on total symptoms (SMD −0.88, 95% CI −1.19 to −0.57). There was no significant effect on positive symptoms at post-treatment, however at follow-up (k = 6, n = 771) there was a moderate effect (SMD −0.61, 95% CI −1.03 to −0.19). Self-management had a small effect on negative symptoms at post-treatment (SMD −0.26, 95% CI −0.47 to −0.05) and a moderate effect at follow-up (SMD −0.51, 95% CI −0.82 to −0.21). When looking at symptoms of depression and anxiety, five studies (n = 452) favoured self-management both at end of treatment (SMD −0.26, 95% CI −0.51 to −0.01) and follow-up (SMD −0.19, 95% CI −0.33 to −0.04, k = 6, n = 964).
Relapse/readmission
Self-management did not have an effect on the total number of patients readmitted at either time point (SMD 0.84, 95% CI 0.48–1.46, and SMD 0.75, 95% CI 0.51–1.08, respectively), however there was an effect at follow-up on the mean number of readmissions (SMD −0.92, 95% CI −1.63 to −0.21). A small effect (SMD −0.26, 95% CI −0.50 to −0.02) was demonstrated on length of hospital admissions immediately following treatment (k = 6, n = 902), whereas a moderate effect (SMD −0.68, 95% CI −1.10 to −0.25) was found at follow-up (k = 7, n = 908).
Self-rated recovery
In relation to overall self-rated recovery, self-management was favoured over control at both time points with a moderate effect size (SMD −0.62, 95% CI −1.03 to −0.22) immediately following treatment (k = 11; n = 1013) and a large effect at follow-up (k = 7, n = 1134, SMD −0.81, 95% CI −1.40 to −0.22).
Empowerment
At the end of treatment (k = 3, n = 346) self-management interventions did not increase sense of empowerment (SMD −1.44, 95% CI −2.97 to 0.08), however at follow-up (k = 2, n = 538) there was a small but significant effect (SMD −0.25, 95% CI −0.43 to −0.07).
Hope
Self-management did not influence hope at end of treatment (k = 2, n = 389, SMD −0.18, 95% CI −0.38 to 0.01). At follow-up three studies with 967 participants showed a small but significant effect favouring self-management over control (SMD −0.24, 95% CI −0.46 to −0.02).
Self-efficacy
Four studies (n = 601) reporting on self-efficacy at end of treatment favoured self-management (SMD −0.38, 95% CI −0.62 to −0.15). One study provided data for self-efficacy at follow-up (n = 221), which also favoured self-management (SMD −0.34, 95% CI −0.61 to −0.07).
Functioning
At the end of treatment (k = 15, n = 1948), there was evidence of a moderate effect of self-management on functioning (SMD −0.56, 95% CI −0.85 to −0.28). At follow-up (k = 14, n = 1805) this increased to a large sized effect of self-management on social and functional disability (SMD −0.90, 95% CI −1.34 to −0.45).
Quality of life
Immediately following the end of the intervention, evidence from nine studies (n = 863) showed a small but significant effect of self-management on participants' self-rated quality of life (SMD −0.23, 95% CI −0.37 to −0.10) which was maintained at follow-up (k = 7, n = 980, SMD −0.25, 95% CI −0.37 to −0.12).
Heterogeneity and sensitivity analyses
Of the 22 meta-analyses, 17 had high levels of heterogeneity as assessed by an I2 >50% and/or a significant χ2 test. The one-study-removed method17 was used to explore sources of statistical heterogeneity. Although high heterogeneity was identified in a range of meta-analyses, it did not appear to be driven by just one study. An evaluation of clinical and methodological characteristics resulted in the decision to not remove any studies. A full account of the sensitivity analysis is in Supplementary Appendix 2.
Publication bias
Funnel plots were created for the six meta-analyses that had more than ten studies (see Supplementary Fig. 3). The small number of studies and participants across these studies meant that it was difficult to discern any evident publication bias.
Post hoc analysis
A post hoc subgroup analysis of TAU-only and active control-only studies was conducted (see results in Supplementary Table 4). No differential pattern of outcomes between the different comparators was found.
Discussion
This is the first comprehensive systematic review and meta-analysis evaluating self-management interventions for people with SMI. The reviewed evidence suggests that self-management does confer benefits across a broad range of outcomes. Specifically, self-management has a positive impact on total symptom severity, negative symptoms and the symptoms of depression and anxiety, both at end of treatment and at 1-year follow-up. Self-management was found to influence positive symptoms at follow-up only. The effect size for self-management on total symptom severity was comparable to or better than those found in recent meta-analyses of cognitive–behavioural therapy for psychosis (CBTp): pooled effect size −0.33 (95% CI −0.47 to −0.19)65 and 0.40 (95% CI 0.252–0.58).66 At longer-term follow-up (approximately 1 year post-intervention) self-management had a large effect (SMD −0.88, 95% CI −1.19 to −0.57), although the high heterogeneity should be noted.
Despite the positive effect on symptoms, the findings were inconsistent for variables related to relapse and readmission. This was in contrast to a previous meta-analysis of self-management interventions for those with schizophrenia only,8 which found a significant impact on relapse and readmission. In the present review, few studies reported relapse as an outcome and, of those that did, only a small number of participants experienced relapse events which may account for the lack of effect. The paucity of data impedes making any comment on the effect of self-management on relapse. However, self-management did demonstrate a small to moderate effect in terms of reducing the average length of hospital admissions, both at the end of treatment and 1 year follow-up.
Self-management did demonstrate a significant, medium-sized effect on global functioning and a small but significant effect on quality life at both end of treatment and 1 year follow-up. Furthermore, self-management seems to confer a benefit on outcomes valued especially highly by consumers,67 i.e. outcomes related to personal recovery and an individual's sense of empowerment, hope and self-efficacy. A moderate to large effect on overall recovery and self-efficacy was seen at both end of treatment and follow-up; the effect on the recovery-related concepts of empowerment and hope were significant at follow-up only.
Methodological limitations of primary studies
Although all studies included in this review were RCTs, there was variation in the reporting of sequence generation, allocation concealment and – as is common in complex interventions – blinding of participants and personnel was not always consistent. The greatest cause for concern was the selective reporting of outcomes which was noted or not clearly reported in two-thirds of the studies reviewed. Furthermore, the relatively small number of studies and participants in some studies meant that it was difficult to discern any evident publication bias. These limitations must be considered alongside the findings presented in this review to avoid an overestimate of the benefit of self-management.
Strengths and limitations of the review
This review gives a broad indication of the effectiveness and potential value of self-management interventions for people with SMI. A strength of this review is the generalisability of the findings to current practice. For instance, we included a diagnostically heterogeneous sample of people with SMI, representative of those on caseloads in secondary care mental health services and included samples from a wider range of countries and cultures.
Regarding limitations, heterogeneity was found to be high across many of the meta-analyses and, although a certain amount of heterogeneity is inevitable, we have tried to mitigate this through the use of random-effects modelling.17 A further potential limitation is from the risk of bias quality assessment of the studies included in this review. Interestingly, readmission rates and service use outcomes were infrequently measured by studies. We recommend the inclusion of this outcome in future studies of self-management. We also encourage collection and reporting of important sample characteristics such as participants' length of illness. Fewer than half of the included studies reported on length of illness – a potential mediator of the effectiveness of self-management interventions.
The choice to pool together comparisons of self-management against TAU or against active controls in the same analyses could be criticised. A post hoc subgroup analysis of TAU-only and active control-only studies showed no differential pattern of outcomes between the different comparators. Arguably, TAU varies hugely among the included studies and all of the active controls are treatments which might be available from a multidisciplinary community mental health team. Thus, irrespective of whether TAU and active controls are combined or not, the analysis is evaluating the addition of self-management to highly varied care.
The absence of patient and public involvement in this review is a limitation. Its inclusion would have been particularly useful in developing the operationalisation of self-management, as well as contributing to the interpretation and implications of findings from a patient's perspective. A final limitation in conducting this review was the lack of consensus of how to define the concept known as self-management. Our review is based on a clear operationalisation of self-management, however there is still substantial variation in such interventions.
Implications for practice
Although self-management for this population has been previously recommended at a guideline level,16,68 it remains to be routinely implemented at a service level. On the basis of this review, there is a strong case for including self-management as a high priority for psychosis services and generic community mental health services, alongside interventions such as CBTp or employment support. The diagnostically mixed populations in many studies may have been an impediment to identification of self-management as a high priority in guidance focused on specific groups, but our study supports recommendations from policy bodies and patient groups which state that self-management should be at the core of care for all long-term health conditions, physical and mental.69,70 Self-management interventions are relatively straightforward compared with other psychotherapeutic interventions and can be delivered across settings and in a variety of ways (including group, individual or digital therapies, bibliotherapy or a combination of these), increasing potential for wide implementation. In this population they are often supported: support may be from clinicians, but also from peers. One may hypothesise that peer support could be especially effective in empowering patients and increasing self-efficacy to manage their illness. Effective implementation of these interventions has the potential to alter the long-term course of both the mental and physical health of people with SMI.
Research implications
In terms of future research, demonstrating whether there are clear effects on relapse and readmission is likely to require large, methodologically robust trials that include these outcomes along with cost-effectiveness analysis. The high heterogeneity in this review suggests there are important differences in the content and implementation or context of self-management interventions which influence how effective they may be. There are likely a number of potential contributors: length of intervention, contact time, facilitator (clinician or peer) and type of self-management intervention (from proposed subtypes). Future intervention studies would also benefit from the inclusion of measures of potential mediators and moderators: for instance, the addition of cognitive outcomes will be important for assessing the role of cognitive factors in mediating improvements in functioning. Additionally, structured development of future self-management programmes in conjunction with patients is recommended.71
Accordingly, there is a need to explore what forms of self-management are most effective, feasible and acceptable, and for whom. Possible study paradigms include realist evaluation of what works for whom, mechanistic studies or a broader systematic review that would have in its scope naturalistic studies using a variety of methods to look at experiences and outcomes of delivering self-management in various ways. Nevertheless, the evidence that self-management already has positive effects on a range of important outcomes is already substantial: thus research is now needed on how to overcome implementation barriers and embed self-management in a sustained and widespread way to routine care for people with long-term mental health conditions, and how to evaluate the effect of this. Implementation–evaluation designs have potential to address these questions.
Funding
This paper reports work undertaken as part of the CORE Study, which is funded by the National Institute for Health Research under its Programme Grants for Applied Research programme (reference RP-PG-0109-10078). The views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1192/bjp.2019.54.
click here to view supplementary material
References
- 1.Taylor SJ, Pinnock H, Epiphaniou E, Pearce G, Parke HL, Schwappach A, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS – Practical systematic Review of Self-Management Support for long-term conditions. Heal Serv Deliv Res 2014; 2: 1–580. [PubMed] [Google Scholar]
- 2.Interdepartmental Serious Mental Illness Coordinating Committee. The Way Forward: Federal Action for a System That Works for All People Living With SMI and SED and Their Families and Caregivers – Full Report Substance Abuse and Mental Health Services Administration (SAMHSA), 2017.
- 3.Mueser KT, Corrigan PW, Hilton DW, Tanzman B, Schaub A, Gingerich S, et al. Illness Management and Recovery: a review of the research. Psychiatr Serv 2002; 53: 1272–84. [DOI] [PubMed] [Google Scholar]
- 4.Mueser KT, McGurk SR. Schizophrenia. Lancet 2004; 363: 2063–72. [DOI] [PubMed] [Google Scholar]
- 5.Zhao S, Sampson S, Xia J, Jayaram MB. Psychoeducation (brief) for people with serious mental illness. Cochrane Database Syst Rev 2015; 4: 1–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lincoln TM, Wilhelm K, Nestoriuc Y. Effectiveness of psychoeducation for relapse, symptoms, knowledge, adherence and functioning in psychotic disorders: a meta-analysis. Schizophr Res 2007; 96: 232–45. [DOI] [PubMed] [Google Scholar]
- 7.Scott AJ, Webb TL, Rowse G. Self-help interventions for psychosis: a meta-analysis. Clin Psychol Rev 2015; 39: 96–112. [DOI] [PubMed] [Google Scholar]
- 8.Zou H, Li Z, Nolan MT, Arthur D, Wang H, Hu L. Self-management education interventions for persons with schizophrenia: a meta-analysis. Int J Ment Health Nurs 2013; 22: 256–71. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th edn, text rev.). American Psychiatric Association, 2000. [Google Scholar]
- 11.Mueser KT, Deavers F, Penn DL, Cassisi JE. Psychosocial treatments for schizophrenia. Annu Rev Clin Psychol 2013; 9: 465–97. [DOI] [PubMed] [Google Scholar]
- 12.Leamy M, Bird V, LeBoutillier C, Williams J, Slade M. Conceptual framework for personal recovery in mental health: systematic review and narrative synthesis. Br J Psychiatry 2011; 199: 445–52. [DOI] [PubMed] [Google Scholar]
- 13.Bauer MS, McBride L, Williford WO, Glick H, Kinosian B, Altshuler L, et al. Collaborative care for bipolar disorder: part I. Intervention and implementation in a randomized effectiveness trial. Psychiatr Serv 2006; 57: 927–36. [DOI] [PubMed] [Google Scholar]
- 14.Bauer MS, McBride L, Williford WO, Glick H, Kinosian B, Altshuler L, et al. Collaborative care for bipolar disorder: part II. Impact on clinical outcome, function, and costs. Psychiatr Serv 2006; 57: 937–45. [DOI] [PubMed] [Google Scholar]
- 15.Bauer MS, Biswas K, Kilbourne AM. Enhancing multiyear guideline concordance for bipolar disorder through collaborative care. Am J Psychiatry 2009; 166: 1244–50. [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence (NICE). Psychosis and Schizophrenia in Adults: Prevention and Management. NICE, 2014. (https://www.nice.org.uk/guidance/cg178). [PubMed] [Google Scholar]
- 17.Higgins J, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
- 18.Sterne JAC, Egger M, Smith GD. Systematic Reviews in Health Care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001; 323: 101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuijpers P. Meta-Analyses in Mental Health Research: A Practical Guide. Vrije Universiteit Amsterdam, 2016. [Google Scholar]
- 20.Eckman T, Wirshing W, Marder S, Liberman R, Johnston-Cronk M, Zimmerman K, et al. Techniques for training schizophrenic patients in illness self-management: a controlled trial. Am J Psychiatry 1992; 149: 1549–55. [DOI] [PubMed] [Google Scholar]
- 21.Kopelowicz A, Wallace C, Zarate R. Teaching psychiatric inpatients to re-enter the community: a brief method of improving the continuity of care. Psychiatr Serv 1998; 49: 1313–6. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson JM, Coia DA, Gilmour WH, Harper JP. The impact of education groups for people with schizophrenia on social functioning and quality of life. Br J Psychiatry 1996; 168: 199–204. [DOI] [PubMed] [Google Scholar]
- 23.Barbic S, Krupa T, Armstrong I. A randomized controlled trial of the effectiveness of a modified recovery workbook program: preliminary findings. Psychiatr Serv 2009; 60: 491–7. [DOI] [PubMed] [Google Scholar]
- 24.Chien WT, Lee IYM. The mindfulness-based psychoeducation program for chinese patients with schizophrenia. Psychiatr Serv 2013; 64: 376–9. [DOI] [PubMed] [Google Scholar]
- 25.Chien WT, Thompson DR. Effects of a mindfulness-based psychoeducation programme for Chinese patients with schizophrenia: 2-year follow-up. Br J Psychiatry 2014; 205: 52–9. [DOI] [PubMed] [Google Scholar]
- 26.Chien WT, Bressington D, Yip A, Karatzias T. An international multi-site, randomized controlled trial of a mindfulness-based psychoeducation group programme for people with schizophrenia. Psychol Med 2017; 47: 2081–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook JA, Copeland ME, Jonikas JA, Hamilton MM, Razzano LA, Grey DD, et al. Results of a randomized controlled trial of mental illness self-management using wellness recovery action planning. Schizophr Bull 2011; 38: 881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook JA, Copeland ME, Floyd CB, Jonikas JA, Hamilton MM, Razzano LA, et al. A randomized controlled trial of effects of wellness recovery action planning on depression, anxiety, and recovery. Psychiatr Serv 2012; 63: 541–7. [DOI] [PubMed] [Google Scholar]
- 29.Jonikas JA, Grey DD, Copeland ME, Razzano LA, Hamilton MM, Floyd CB, et al. Improving propensity for patient self-advocacy through wellness recovery action planning: results of a randomized controlled trial. Community Ment Health J 2013; 49: 260–9. [DOI] [PubMed] [Google Scholar]
- 30.Cook JA, Steigman P, Pickett S, Diehl S, Fox A, Shipley P, et al. Randomized controlled trial of peer-led recovery education using Building Recovery of Individual Dreams and Goals through Education and Support (BRIDGES). Schizophr Res 2012; 136: 36–42. [DOI] [PubMed] [Google Scholar]
- 31.Pickett SA, Diehl SM, Steigman PJ, Prater JD, Fox A, Shipley P, et al. Consumer empowerment and self-advocacy outcomes in a randomized study of peer-led education. Community Ment Health J 2012; 48: 420–30. [DOI] [PubMed] [Google Scholar]
- 32.Dalum HS, Waldemar AK, Korsbek L, Hjorthøj C, Mikkelsen JH, Thomsen K, et al. Illness management and recovery: clinical outcomes of a randomized clinical trial in community mental health centers. PLoS One 2018; 13: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalum HS, Waldemar AK, Korsbek L, Hjorthoj C, Mikkelsen JH, Thomsen K, et al. Participants’ and staffs’ evaluation of the Illness Management and Recovery program: a randomized clinical trial. J Ment Heal 2018; 27: 30–7. [DOI] [PubMed] [Google Scholar]
- 34.Färdig R, Lewander T, Melin L, Folke F, Fredriksson A. A randomized controlled trial of the illness management and recovery program for persons with schizophrenia. Psychiatr Serv 2011; 62: 606–12. [DOI] [PubMed] [Google Scholar]
- 35.Hasson-Ohayon I, Roe D, Kravetz S. A randomized controlled trial of the effectiveness of the illness management and recovery program. Psychiatr Serv 2007; 58: 1461–6. [DOI] [PubMed] [Google Scholar]
- 36.Levitt AJ, Mueser KT, Degenova J, Lorenzo J, Bradford-Watt D, Barbosa A, et al. Randomized controlled trial of illness management and recovery in multiple-unit supportive housing. Psychiatr Serv 2009; 60: 1629–36. [DOI] [PubMed] [Google Scholar]
- 37.Lin EC-L, Chin Hong C, Wen-Chuan S, Mei-Feng L, Shujen S, Mueser KT, et al. A randomized controlled trial of an adapted Illness Management and Recovery program for people with Schizophrenia awaiting discharge from a psychiatric hospital. Psychiatr Rehabil J 2013; 36: 243–9. [DOI] [PubMed] [Google Scholar]
- 38.Monroe-DeVita M, Morse G, Mueser KT, McHugo GJ, Xie H, Hallgren KA, et al. Implementing illness management and recovery within assertive community treatment: a pilot trial of feasibility and effectiveness. Psychiatr Serv 2018; 69: 562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry A, Tarrier N, Morriss R, Mccarthy E, Limb K. Randomised controlled trial of efficacy of teaching patients with bipolar disorder to identify early symptoms of relapse and obtain treatment. BMJ 1999; 318: 149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sajatovic M, Davies MA, Ganocy SJ, Bauer MS, Cassidy KA, Hays RW, et al. A comparison of the life goals program and treatment as usual for individuals with bipolar disorder. Psychiatr Serv 2009; 60: 1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salyers MP, McGuire AB, Rollins AL, Bond GR, Mueser KT, MacY VR. Integrating assertive community treatment and illness management and recovery for consumers with severe mental illness. Community Ment Health J 2010; 46: 319–29. [DOI] [PubMed] [Google Scholar]
- 42.Shon K-H, Park S-S. Medication and symptom management education program for the rehabilitation of psychiatric patients in Korea: the effects of promoting schedule on self-efficacy theory. Yonsei Med J 2002; 43: 579–89. [DOI] [PubMed] [Google Scholar]
- 43.Smith DJ, Griffiths E, Poole R, di Florio A, Barnes E, Kelly MJ, et al. Beating Bipolar: exploratory trial of a novel internet-based psychoeducational treatment for bipolar disorder. Bipolar Disord 2011; 13: 571–7. [DOI] [PubMed] [Google Scholar]
- 44.Tan CHS, Ishak RB, Lim TXG, Marimuthusamy P, Kaurss K, Leong JYJ. Illness management and recovery program for mental health problems: reducing symptoms and increasing social functioning. J Clin Nurs 2017; 26: 3471–85. [DOI] [PubMed] [Google Scholar]
- 45.Todd NJ, Solis-Trapala I, Jones SH, Lobban FA. An online randomised controlled trial to assess the feasibility, acceptability and potential effectiveness of ‘Living with Bipolar’: a web-based self-management intervention for Bipolar Disorder. Trial design and protocol. Contemp Clin Trials 2012; 33: 679–88. [DOI] [PubMed] [Google Scholar]
- 46.Todd NJ, Jones SH, Hart A, Lobban FA. A web-based self-management intervention for Bipolar Disorder ‘living with bipolar’: a feasibility randomised controlled trial. J Affect Disord 2014; 169: 21–9. [DOI] [PubMed] [Google Scholar]
- 47.Torrent C, Bonnin CdM, Martínez-Arán A, Valle J, Amann BL, González-Pinto A, et al. Efficacy of functional remediation in bipolar disorder: a multicenter randomized controlled study. Am J Psychiatry 2013; 170: 852–9. [DOI] [PubMed] [Google Scholar]
- 48.Van Gestel-Timmermans H, Brouwers EPM, van Assen MaL, van Nieuwenhuizen C. Effects of a peer-run course on recovery from serious mental illness: a randomized controlled trial. Psychiatr Serv 2012; 63: 54–60. [DOI] [PubMed] [Google Scholar]
- 49.Vreeland B, Minsky S, Yanos PT, Menza M, Gara M, Kim E, et al. Efficacy of the team solutions program for educating patients about illness management and treatment. Psychiatr Serv 2006; 57: 822–8. [DOI] [PubMed] [Google Scholar]
- 50.Wang LQ, Chien WT, Yip LK, Karatzias T. A randomized controlled trial of a mindfulness-based intervention program for people with schizophrenia: 6-month follow-up. Neuropsychiatr Dis Treat 2016; 12: 3097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou B, Zhang P, Gu Y. Effectiveness of self-management training in community residents with chronic schizophrenia: a single-blind randomized controlled trial in Shanghai, China. Shanghai Arch Psychiatry 2014; 26: 81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anzai N, Yoneda S, Kumagai N, Nakamura Y, Ikebuchi E, Liberman RP. Training persons with Schizophrenia in illness self-management: a randomized controlled trial in Japan. Psychiatr Serv 2002; 53: 545–7. [DOI] [PubMed] [Google Scholar]
- 53.Chan SH-W, Lee SW-K, Chan IW-M. TRIP: a psycho-educational programme in Hong Kong for people with schizophrenia. Occup Ther Int 2007; 14: 86–98. [DOI] [PubMed] [Google Scholar]
- 54.Colom F, Vieta E, Martinez-Aran A, Reinares M, Goikolea JM, Benabarre A, et al. A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry 2003; 60: 402–7. [DOI] [PubMed] [Google Scholar]
- 55.Colom F, Vieta E, Sánchez-Moreno J, Palomino-Otiniano R, Reinares M, Goikolea JM, et al. Group psychoeducation for stabilised bipolar disorders: 5-Year outcome of a randomised clinical trial. Br J Psychiatry 2009; 194: 260–5. [DOI] [PubMed] [Google Scholar]
- 56.Cook JA, Jonikas JA, Hamilton MM, Goldrick V, Steigman PJ, Grey DD, et al. Impact of Wellness Recovery Action Planning on service utilization and need in a randomized controlled trial. Psychiatr Rehabil J 2013; 36: 250–7. [DOI] [PubMed] [Google Scholar]
- 57.Mackain SJ, Smith TE, Wallace CW, Kopelowicz A. Evaluation of a community re-entry program. Int Rev Psychiatry 1998; 10: 76–83. [Google Scholar]
- 58.Proudfoot J, Parker G, Hyett M, Manicavasagar V, Smith M, Grdovic S, et al. Web-based bipolar disorder program. Aust N Z J Psychiatry 2007; 41: 903–9. [DOI] [PubMed] [Google Scholar]
- 59.Proudfoot J, Parker G, Manicavasagar V, Hadzi-Pavlovic D, Whitton A, Nicholas J, et al. Effects of adjunctive peer support on perceptions of illness control and understanding in an online psychoeducation program for bipolar disorder: a randomised controlled trial. J Affect Disord 2012; 142: 98–105. [DOI] [PubMed] [Google Scholar]
- 60.Salyers MP, McGuire AB, Kukla M, Fukui S, Lysaker PH, Mueser KT. A randomized controlled trial of illness management and recovery with an active control group. Psychiatr Serv 2014; 65: 1005–11. [DOI] [PubMed] [Google Scholar]
- 61.Schaub A, Mueser KT, von Werder T, Engel R, Moller HJ, Falkai P. A randomized controlled trial of group Coping-Oriented Therapy vs Supportive Therapy in Schizophrenia: results of a 2-year follow-up. Schizophr Bull 2016; 42: S71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wirshing DA, Guzik LH, Zorick TS, Pierre JM, Resnick SA, Goldstein D, et al. Community re-entry program training module for schizophrenic inpatients improves treatment outcomes. Schizophr Res 2006; 87: 338–9. [DOI] [PubMed] [Google Scholar]
- 63.Xiang YT, Weng YZ, Li WY, Gao L, Chen GL, Xie L, et al. Training patients with schizophrenia with the community re-entry module: a controlled study. Soc Psychiatry Psychiatr Epidemiol 2006; 41: 464–9. [DOI] [PubMed] [Google Scholar]
- 64.Xiang YT, Weng YZ, Li WY, Gao L, Chen GL, Xie L, et al. Efficacy of the community re-entry module for patients with schizophrenia in Beijing, China: outcome at 2-year follow-up. Br J Psychiatry 2007; 190: 49–56. [DOI] [PubMed] [Google Scholar]
- 65.Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry 2014; 204: 20–9. [DOI] [PubMed] [Google Scholar]
- 66.Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 2008; 34: 523–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slade M, Longden E. The Empirical Evidence About Mental Health and Recovery: How Likely, How Long, What Helps? MI Fellowship, 2015. [Google Scholar]
- 68.National Institute for Health and Care Excellence (NICE). Bipolar Disorder: Assessment and Management: Clinical guideline 185. NICE, 2014. [Google Scholar]
- 69.National Voices. Supporting Self-Management. National Voices, 2014. (https://www.nationalvoices.org.uk/sites/default/files/public/publications/supporting_self-management.pdf). [Google Scholar]
- 70.Foot C, Gilburt H, Dunn P, Jabbal J, Seale B, Goodrich J, et al. People in Control of Their Own Health and Care: the State of Involvement. The King's Fund, 2014. (https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/people-in-control-of-their-own-health-and-care-the-state-of-involvement-november-2014.pdf). [Google Scholar]
- 71.Milton A, Lloyd-Evans B, Fullarton K, Morant N, Paterson B, Hindle D, et al. Development of a peer-supported, self-management intervention for people following mental health crisis. BMC Res Notes 2017; 10: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1192/bjp.2019.54.
click here to view supplementary material