Abstract
Chemotherapy induced peripheral neuropathy (CIPN) is a major challenge, with increasing impact as oncological treatments, using potentially neurotoxic chemotherapy, improve cancer cure and survival. Acute CIPN occurs during chemotherapy, sometimes requiring dose reduction or cessation, impacting on survival. Around 30% of patients will still have CIPN a year, or more, after finishing chemotherapy.
Accurate assessment is essential to improve knowledge around prevalence and incidence of CIPN. Consensus is needed to standardize assessment and diagnosis, with use of well validated tools, such as the EORTC-CIPN 20. Detailed phenotyping of the clinical syndrome moves towards a precision medicine approach, to individualize treatment. Understanding significant risk factors and pre-existing vulnerability may be used to improve strategies for CIPN prevention, or to use targeted treatment for established CIPN.
No preventive therapies have shown significant clinical efficacy, although there are promising novel agents such as histone deacetylase 6 (HDAC6) inhibitors, currently in early phase clinical trials for cancer treatment. Drug repurposing, e.g. metformin, may offer an alternative therapeutic avenue. Established treatment for painful CIPN is limited. Following recommendations for general neuropathic pain is logical, but evidence for agents such as gabapentinoids and amitriptyline is weak. The only agent currently recommended by the American Society of Clinical Oncology is duloxetine.
Mechanisms are complex with changes in ion channels (sodium, potassium and calcium), Transient Receptor Potential (TRP) channels, mitochondrial dysfunction, and immune cell interactions. Improved understanding is essential to advance CIPN management. On a positive note, there are many potential sites for modulation, with novel analgesic approaches.
Introduction
Chemotherapy induced peripheral neuropathy (CIPN) is a common and challenging complication arising from treatment with many commonly used anti-cancer agents. A number of factors have contributed to the increasing prevalence of CIPN, including an increased incidence of cancer, with improved survival and cancer cure rates. CIPN can occur acutely, during chemotherapy. If severe, it can require a reduction in the dose of chemotherapy, or even stopping prior to completing the planned course[34; 45]. Clearly, this may have implications for efficacy of oncological treatment and survival. Whilst in many cases, acute CIPN will resolve after finishing chemotherapy, in a number of cases, it will persist, resulting in chronic symptoms, months, or even years later. In some cases, CIPN can emerge shortly after finishing chemotherapy, a phenomenon known as “coasting” [34]. For neurotoxic chemotherapy overall, the prevalence of CIPN 1 month after finishing chemotherapy is around 68%, with this dropping to 60% at 3 months and 30% at 6 months or more [88]. The type of chemotherapy does influence the risk of developing CIPN, although even with single chemotherapeutic agents there is often a wide range of reported occurrence (see table1). Some potential confounders that may affect the reported incidence or prevalence include how CIPN was defined and assessed; differing dosing regimens, assessment time point(s), and exclusion of participants with pre-existing neuropathy.
Table 1. Common chemotherapeutics and incidence or prevalence of reported neuropathy.
| Chemotherapy | Approximate incidence/ prevalence of CIPN (%) |
|---|---|
| Oxaliplatin | Acute: 85-96; Chronic wide range: 40-93 |
| Cisplatin | 12-85 |
| Paclitaxel | 61-92 |
| Bortezomib | 47 |
| Vincristine | 20 |
| Combined cisplatin and paclitaxel | 69-76 |
The clinical syndrome
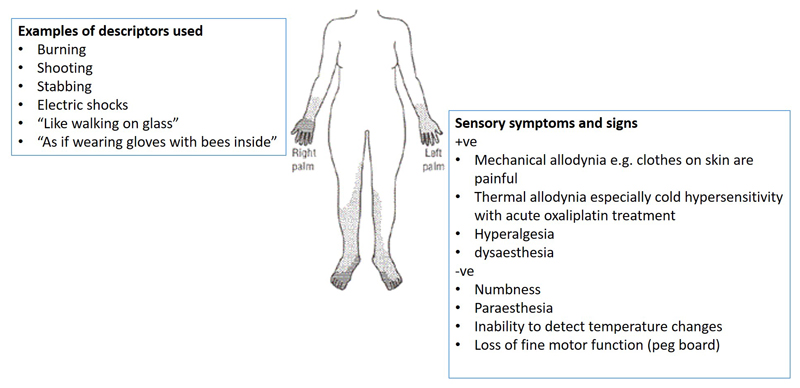
CIPN develops as a glove and stocking neuropathy, although in more severe cases it can spread proximally affecting most of the limbs. Whilst it is predominantly a sensory neuropathy, autonomic function can also be affected, as can fine motor function and proprioception, with evidence for loss of sensory fibres and reduced intraepidermal nerve fibre density (IENFD)[8; 13; 15] [106]. Sensory dysfunction is wide ranging, with both positive and negative sensory signs, some of which are more often associated with particular types of chemotherapy e.g, cold hypersensitivity during platinum-based therapy. Neuropathic descriptors such as burning, and shooting are often used, along with numbness and paraesthesia, although pain is not always a presenting feature[42]. CIPN can persist for many years, with a detailed assessment of long term survivors of childhood cancers finding ~48% of individuals having some evidence of neuropathy: predominantly sensory dysfunction and reduced quality of life[51] Some of the clinical features of CIPN are shown in figure 1.
Figure 1.
Clinical features of CIPN.
Assessment and diagnosis
Accurate assessment and diagnosis are not only the first steps in successful management, but are also important in understanding the epidemiology of CIPN. For painful CIPN, using a standard approach to neuropathic pain, the NeuPSIG guidelines for assessment and diagnosis of neuropathic pain can be applied. These guidelines recommend the use of screening questionnaires to identify potential patients, with a range of questionnaires available, many of which may not have been validated for CIPN [50]. Clinical examination is also an important part of assessment, but may have some challenges in non-specialist settings, particularly where using more detailed sensory profiling for definitive diagnosis[105] [44].
It could be argued, however, that painful CIPN is a particular case, with circumstances that require a more tailored approach than that used for general neuropathic pain:
There is predictable delivery of a known toxic, but necessary, insult (chemotherapy) given repeatedly over the course of a number of weeks to months.
Completing the chemotherapy dosing regimen maximizes the chances of cancer survival, with any dosage modification impacting on this;
Development of CIPN during treatment may necessitate dose reduction or cessation, with early identification of symptoms important in planning care;
CIPN mechanisms may be specific to the chemotherapy given.
The approach to CIPN may therefore require a more “bespoke” assessment process that is designed to identify the particular characteristics of CIPN, as early as possible, to allow appropriate management (including potential alteration to the chemotherapy regimen). This has been recognised with the development of a number of screening tools specific for CIPN (see below).
Some have been developed for specific chemotherapeutic agents, such as the Functional Assessment of Cancer Therapy (FACT)–Taxane. This has been extensively validated, and is sensitive to change[38]. The EORTC-CIPN 20 can be used for any type of neurotoxic chemotherapy and has been rigorously studied. Some of the questions in the CIPN20 may potentially be less reliable – particularly those in the autonomic subscale, with the potential for reducing the length of the questionnaire without impacting on its performance. Rasch analysis revealed some inconsistencies, with evidence of a floor effect, and some issues with item scaling[95–97]. A reduced version of the CIPN20 – the CIPN 15 may address some of these concerns, with the added benefit of a reduced questionnaire burden on patients [96]. The Total Neuropathy Score (TNSc(©)) performs reasonably well, with a mix of clinician-detected signs and patient self report, but it doesn’t specifically assess pain. Rasch analysis revealed some inconsistencies with the 7 item tool, and has suggested a shortened 5 item version may perform better[11; 20].
One of the widely used clinical tools for detecting neuropathy during chemotherapy is the National Cancer Institute – Common Terminology Criteria for Adverse Events (NCI-CTCAE), but it is was not specifically developed to assess pain, is not sensitive to change and has significant inter-rater variability[19]. While many studies have used this to identify CIPN, particularly older studies, it is not a reliable assessment tool for clinical research, although may still have some clinical utility[35].
This lack of consistency in assessing CIPN has implications for accurate epidemiological studies, including those of risk factors[98]. This issue has been identified in several systematic reviews, where many different primary outcome measures (or combinations) were identified, with almost half of studies not even clearly defining a primary outcome measure[39; 88]. The need for a more uniform approach to assessment and diagnosis, in future studies, has been highlighted, although debate remains about the ideal tool to use [18; 37]. In clinical practice, a simple tool that can be used by non-specialists, to detect abnormalities early is needed. For research purposes, there is a need for a tool that performs consistently in different settings, with good reliability, sensitivity to change, detects key early symptoms of development (see figure 2).
Figure 2.
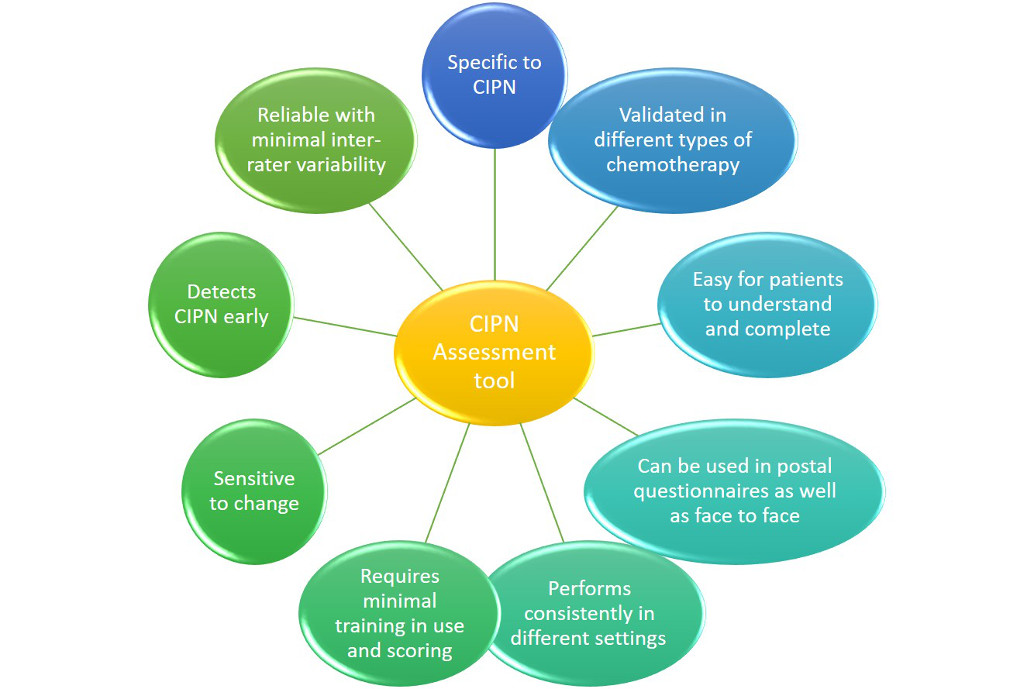
Important components for an ideal CIPN assessment tool.
Confirming a definitive diagnosis of CIPN does require more detailed phenotyping than a single assessment tool. Psychophysical testing is useful in the research setting, with the potential to use a modified approach for routine clinical use in the cancer setting [87; 107]. Nerve conduction studies are less sensitive at identifying CIPN than quantitative sensory testing (QST) with sensory fibres preferentially affected, although this may vary between chemotherapy type, and change as neuropathy develops. Decreases in IENFD correlated with altered QST, particularly for mechanical sensation, in patients with chronic CIPN after docetaxol or oxaliplatin [55]: After vincristine treatment, myelinated A-beta fibres were affected first, followed by A-delta and c fibres[29]. In patients with bortezomib induced CIPN no changes were identified in intraepidermal nerve fibre density, but there were clear reductions in subepidermal nerve fibre density of PGP9.5, with associated axonal swelling, and reduced sensory action potentials in the sural nerve[8]. Other studies of persistent CIPN after bortezomib, found a clear reduction in IENFD and Meissner’s corpuscles, with associated abnormalities in QST[14]. These differences may be due to time point of testing, dosing regimen, or other factors, and emphasise the need for consistent approaches to assessment to give a detailed and accurate understanding of the natural history of CIPN.
Risk factors and vulnerability
Identifying who is at higher risk of developing CIPN would be an important step forwards. Large scale population based studies combined with careful phenotyping is a potential approach to this. Are certain individuals more vulnerable to the toxic insult of chemotherapy than others… and if so, how can we identify them?
A number of factors have been identified, associated with an increased risk of developing CIPN, although causal links are less clear (see table 2). Some of these are potentially modifiable, giving opportunities for using approaches to reduce CIPN development. Perhaps the most obvious ones are type and cumulative dose of chemotherapy, although here the challenge is to understand how alterations in dosing regimes might impact on cancer survival. Better understanding of this might allow a more individualised approach to chemotherapy dosing. For example, if individual is generally more sensitive to chemotherapy (i.e both toxic and therapeutic effects), it may be that reducing the dose does not impact on individual survival: this needs further study.
Table 2. Some of the risk factors associated with an increased risk of developing CIPN.
| Risk factor | Comments | Reference |
|---|---|---|
| Older age | May be due to lower chance of recovery from acute CIPN | [17; 78] |
| Medication: cardiovascular especially beta blockers | Opportunity to modify medication prior to starting chemotherapy. | [85] |
| Co-morbid health conditions | Those where there may be associated increased risk of neuropathy e.g. diabetes, HIV, excess alcohol, smoking; decreased creatinine clearance | [28; 88] |
| Raised Body Mass Index (BMI) | Potential mechanism not well understood, but may be related to pro-inflammatory state associated with obesity | |
| Low serum albumin | May reflect lower general health status | [40] [54; 82] |
| Use of opioids | Prolonged use more likely in patients with CIPN. Need further work to understand if this is due to pain severity, or to a mechanistic interaction with opioids increasing CIPN risk (OR 2.0, 1.06-3.69) | [91] |
It may be possible to use detailed phenotyping to identify pre-existing vulnerability to developing CIPN. This approach is showing promise in some other areas, such as persistent post-surgical pain, [52; 61; 62; 100; 110]. QST has identified subclinical deficits in cancer patients prior to starting chemotherapy, compared to healthy controls, with those individuals showing the most marked deficits at higher risk of developing clinically significant CIPN. These findings were similar to a retrospective study of head and neck cancer patients, prior to starting chemotherapy, those with altered QST (and fine motor function) were at higher risk of developing CIPN [24; 83]. Predictors emerging during early chemotherapy have also been identified, with thermal hyperalgesia predicting development of severe oxaliplatin CIPN[5]. Our preliminary work, using functional neuroimaging, has also found structural and functional changes in the brain in pain processing areas, detected prior to starting chemotherapy, potentially indicating a pre-existing vulnerability to developing CIPN [89].
A number of studies have explored genetic factors in determining risk of developing CIPN. Studies using multivariate statistical modelling have identified several risk factors, including smoking, decreased creatinine clearance and baseline neuropathy, although there may be some statistical bias introduced by the techniques used[5; 28; 88]. Informed by other Genome Wide Association Studies (GWAS), a study in more than 1000 patients with multiple myeloma identified 13 Single Nucleotide Polymorphisms (SNPs) associated with CIPN development, of which 4 were relevant for neural function[65]. A GWAS in breast cancer patients found that severe taxane related CIPN was associated with genes involved in diabetes and diabetic neuropathy, with the G allele of rs1858826 in GNGT1 decreasing CIPN risk[98]. A recent review of predictive biomarkers for CIPN found 3 genetic biomarkers that have been consistently identified in a number of studies: ARHGEF10 rs9657362 (neuronal morphogenesis), CYP2C8 rs11572080/rs10509681 (drug metabolism) and FGD4 rs10771973 (mutations found in Charcot-Marie tooth disease)[26]. Some studies have also found associations with genes associated with mitochondrial dysfunction, which may link with preclinical evidence for underlying mechanisms of CIPN development (see section below)[54].
CIPN Prevention
To date there are no preventive treatments for CIPN. This lack of any effective neuroprotective agents is a key area of unmet clinical need. While a number of trials having studied agents that may potentially modify CIPN development by targeting the underlying mechanisms, there is as yet insufficient evidence to recommend any specific agent[46]. There have been quite a number of CPIN prevention trials, many with small sample size, none of which have yielded high quality evidence. Agents that have been studied in clinical trials, often based on postulated effects on underlying mechanisms, include: Acetyl-L-carnitine (ALC)(associated with worse outcomes), amifostine, N-acetylcysteine, amitriptyline, nimodipine, glutathione, carbamazepine, Vitamin E, omega-3 fatty acids, or oxycarbazepine [2; 4; 46–48; 111]. Prevention trials face a number of design challenges, including whether to treat all patients receiving neurotoxic chemotherapy, accepting that some of them would not develop CIPN anyway. Not only does this increase the required sample size, but also exposes patients to a novel therapy unnecessarily. This is one area where identification for pre-existing vulnerability could improve clinical trial design. An alternative approach to prevention trials is to look at intervening when there are early (or subclinical signs of CIPN), using a detailed phenotyping approach[30; 37; 39; 63].
Treatment strategies
There is a lack of good quality clinical trials focusing on treatment of established painful CIPN, with a pragmatic approach extrapolating from evidence and guidelines for treatment of other types of neuropathic pain. Duloxetine is one of the few agents where there is a positive RCT in CIPN[94]. This is reflected in Clinical Practice Guidelines from ASCO, where duloxetine is the only treatment where there is sufficient evidence to recommend its use. However, based on efficacy in other neuropathic pain syndromes, other agents can be trialled, including tri-cyclic anti-depressants, gabapentin and topical gel (baclofen (10 mg), amitriptyline HCL (40 mg), and ketamine (20 mg),) that had limited positive evidence from one trial[46].
Non-pharmacological treatments may also be useful, although again with a mixed evidence base. A recent review of pharmacological strategies identified a number of current clinical trials of non-drug strategies, including exercise, acupuncture, massage, and nutritional interventions, not all of which were based on strong underlying hypotheses[21] An RCT of a 6 week exercise programme showed small to moderate improvements in CIPN symptoms[53]. With the known benefits of exercise in chronic pain and cancer, combined with low risk of harm, supporting an increase in physical activity should be part of CIPN management[60; 108].
Mechanisms
By improving our understanding of the underlying mechanisms leading to the development and also factors contributing to chronicity, we can develop targeted treatments to reduce the significant impact that CIPN has on patients. The mechanism are complex, with peripheral, spinal and supraspinal changes, ranging from altered ion channel activity to changes in intracellular systems. A comprehensive analysis of these is beyond the scope of this review, and readers are referred to several recent reviews[15] [63].
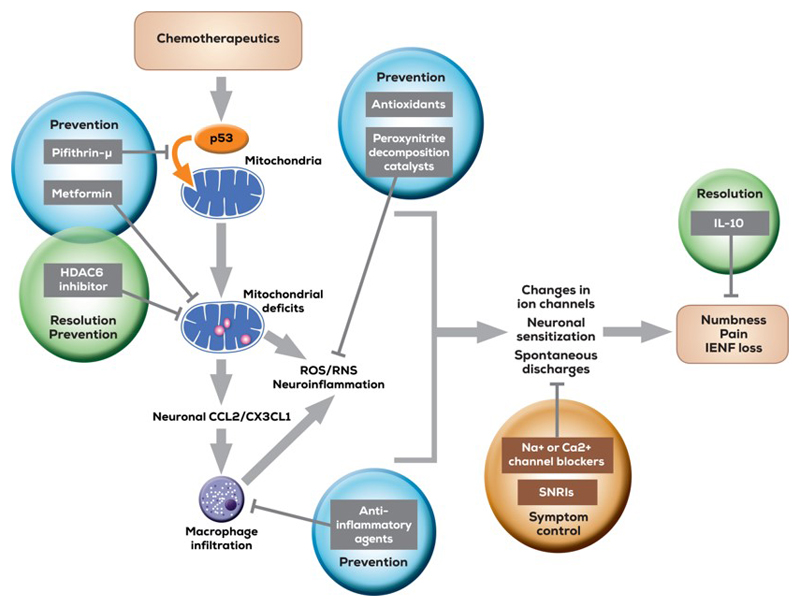
It is, however, worth considering some of these mechanisms and how they might translate into clinical benefit. An overview is given in figure 3.
Figure 3.
Schematic overview of mechanisms underlying chemotherapy-induced peripheral neuropathy and mechanism-based disease interventions. CCL2 indicates C-C motif chemokine 2; CX3CL1, C-X3-C motif chemokine ligand 1; HDAC6, histone deacetylase 6; IL-10, interleukin 10; IENF, intraepidermal nerve fiber; RNS, reactive nitrogen species; ROS, reactive oxygen species; SNRIs, serotonin-norepinephrine reuptake inhibitors. From: Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source, Ma et al. Cancer, Volume: 124, Issue: 11, Pages: 2289-2298, First published: 20 February 2018, DOI: (10.1002/cncr.31248).
Ion channels
Sodium channels: In CIPN, as in some other types of neuropathic pain, alterations in sodium channel type and activity may contribute to the development of CIPN[27]. Expression of Nav1.7 channel is increased in small fibre sensory neurones, in preclinical CIPN models, with increased spontaneous neuronal activity, with similar findings in human dorsal root ganglia in segments affected by CIPN[58]. An acute, Na channel mediated in neuronal excitability has been found in oxaliplatin treated patients who go on to develop chronic CIPN[77].
Potassium channels: Decreased potassium channel expression in primary sensory neurones has been found with several chemotherapy types, potentially leading to increased spontaneous neuronal firing[81; 112].
The combination of increased between Na channel and decreased K channel activity also predisposes towards hyper excitability, with agents that increase K channel hyperpolarization reducing CIPN features.[25; 72]
Calcium channels: Alterations in ion channel function and expressed subtypes have been widely described in neuropathic pain syndromes, including CIPN. Increase coupling of pre-synaptic calcium subunits (alpha-2 delta1) with the NMDA receptor has been shown in mice after paclitaxel, contributing to neuropathic pain features[22]. Type of chemotherapy may be important, with a decrease in Cav3.2 T type calcium channel was found with bortezomib, in mouse dorsal root ganglia[101]. However, in a rat model of paclitaxel neuropathy increased expression of Cav3.2 was found, associated with increase spontaneous activity in dorsal root ganglia neurones. Interestingly, this increased activity was found with infusion of lipopolysaccharide (an agonist at TLR4 receptors), and blocked by administration of a Cav3.2 inhibitor[59].
Transient Receptor Potential (TRP) channels: The TRP channels are known to be important in pain processing and thermal sensation, with demonstrated changes in neuropathic pain. Of these channels, the TRPVanilloid 1 (TRPV1) is activated by noxious heat, low pH, and by exogenous capsaicin [84; 90]. It is found on c fibres and is upregulated in neuropathic pain conditions. Several rodent models of CIPN (rat and mouse) have shown increases in TRPV1 expression and activity, with evidence of thermal hyperalgesia[41; 80; 99]. Clinically, a high dose 8% capsaicin patch has been used topically for focal neuropathic pain: while there is limited high quality evidence for its use specifically in CIPN, there have been some reports of efficacy[16; 32]. The TRPM8 channel, activated by non-noxious cool temperatures, is upregulated on a subset of c fibres in neuropathic pain. Activation of the TRPM8 receptors by topical agents, such as icilin and menthol (in percentages less than 10%), results in analgesia in rodent models of neuropathic pain, with some early clinical evidence of efficacy for menthol in CIPN[31; 79].
Endocannabinoid systems
There are a number of models showing involvement of endocannabinoids in CIPN, with potential for analgesia through agents with activity at CB1 and/or CB2 receptors, which may be peripheral or central[71; 109]. Inhibition of endocannabinoid metabolism is an alternative approach, via inhibition of fatty acid amide hydrolase (FAAH) [10; 74]. There have been a number of early phase clinical trials investigating this class of drugs. Unfortunately, in a phase 1 study of a FAAH inhibitor (BIA 10-2474), there were serious adverse events, resulting in the death of one participant, and 5 other hospitalised with neurological issues[67]. This led to all clinical trials using FAAH inhibitors being stopped at that point. Subsequent evidence indicates that the SAEs were related to wider “off target” effects of BIA 10-2474, rather than a class effect of FAAH inhibitors, with clinical trials now resuming in this area[104]
Mitochondrial dysfunction
Mitochondria are small membrane bound intracellular organelles that provide cellular energy mainly via oxidative phosphorylation to produce adenosine triphosphate (ATP). Neuronal cells have high energy requirements, and as such, are sensitive to process that disrupt mitochondrial function, such as chemotherapeutic agents[76]. Different agents appear to have different mechanism by which mitochondrial dysfunction occurs. For example, platinum based compounds may affect protein synthesis within mitochondria, whereas taxanes impact on membrane depolarization with changes in calcium release[63]. As a result, there is an increase in oxidative stress within mitochondria and consequent continued dysfunction in neuronal energy production. Reduced maximal respiration capacity and mitochondrial basal respiration as well as reduced ATP production are all seen, with an increase in Reactive Oxygen Species (ROS) overwhelming endogenous anti-oxidant systems [9; 33; 34; 113]. Understanding these mechanisms and targeting them is one potential preventive strategy[63].
Non neuronal mechanisms
Inflammatory processes induced in central glial cell, and peripheral immune cells, contribute to CIPN development[63; 70]. A number of mechanisms are involved in these processes. Paclitaxel-induced microglial activation, with an increase in calcium/ calmodulin- dependent protein kinase II (CAMKII) stimulated overexpression of Brain Derived Neurotrophic Factor (BDNF), produced high levels of the pro-inflammatory cytokine,IL-6, and increased expression of NR2B glutamate receptor subunits. All these changes were reduced by administration of a CB2 receptor agonist [109]. Another study found that paclitaxel induced significant upregulation in IL-1α, IL-1β, IL-6, TNF-α, INF-γ and MCP-1, which was attenuated by a TNF-alpha monoclonal antibody (etanercept) [1] In a vincristine CIPN model, the anti-inflammatory cytokine, IL-4, and associated STAT signalling was reduced, with an increase in pro-inflammatory cytokines such as IL-1-beta and TNF-alpha[92]. IL-10, an anti-inflammatory cytokine, may be important in recovery from CIPN, with preclinical evidence from both platinum and taxane-related CIPN [63].
Peripheral immune system cells such as macrophages and monocytes have been shown to be part of CIPN mechanisms. There may therefore be the potential for developing novel therapies with a unique mode of action, such as the chemokine CX3CL1 (fractalkine) and its receptor, expressed on monocytes, and important in communication with neuronal cells[70].
Matrix metalloproteinases (MMPs) are a class of enzymes involved in degradation of the extracellular matrix, with inhibitors showing some potential as anti-cancer agents [49]. An increase in MMP 2 and 9, with a decrease in the endogenous MMP inhibitor, TIMP1, was found in a paclitaxel mouse model, associated with the development of allodynia. If inhibited by intrathecal MMP9 monoclonal antibody, allodynia was reduced. In parallel, reductions in oxidative stress and inflammatory mediators were found in the dorsal root ganglia[102].
Central changes
There have been a few studies using neuroimaging in CIPN. There are clear changes in pain processing in response to a noxious stimulus compared to healthy volunteers, and to patients with cancer, but no CIPN. These altered responses are seen in areas of the brain involved in pain processing, such as the superior frontal gyrus, cingulate cortex and insula. Structural changes have also been seen with altered grey matter density[12; 73].
Discussion
In developing novel preventive or modifying treatment for CIPN, the complex interaction between cancer cells, immune system and neurons must be considered. In particular any novel therapies used during oncological treatment, must not interfere with the tumoricidal effects of chemotherapy. This creates an additional challenge compared to other types of neuropathic pain.
While there are some potential novel therapies to be explored for prevention and treatment of CIPN, there remains an urgent need to improve the efficiency of translation[93]. This will require true collaboration between clinicians, basic scientists and population health scientists. Lessons need to be learned from previous failures to translate promising compounds in preclinical models, and sometimes early phase clinical trials to clinical benefit. We need to consider how design of preclinical studies can be improved. As with clinical evidence, critical appraisal of the quality of preclinical studies, and risk of bias need to be considered. Issues such as proper sample size calculations, blinding and prospectively defined primary outcome measures should all be considered[3]. The relevance of the model to the clinical syndrome is also important, e.g. including behavioural assessment of spontaneous pain, studying male and female rodents[7; 69].
Developing the use of neuroimaging and QST to better understand CIPN mechanisms in the clinical syndrome should also help increase successful translation, allowing direct study of pharmacodynamic effects, stratification of clinical trials by mechanisms, and identification of placebo responses. Whilst there are not yet clear clinical biomarkers that can be used to identify patients who are inherently more vulnerable to developing CIPN, or more likely to respond to a particular targeted treatment, using this approach, with detailed phenotyping (including validated patient self report questionnaires, psychophysical testing, genetic testing and neuroimaging) is a definite step towards this. Although not practical for routine clinical use, it may then be possible to move towards simple bedside tests as surrogate markers, directing individual treatment, and improving clinical trials for CIPN. A recent consensus meeting (Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities and Networks (ACTTION)–Consortiium) has produced very useful recommendations about future trial design for CIPN, addressing many of these issues[37]. One area that needs to be explored further is whether or not sensitivity to chemotherapy is reflected in the efficacy of cancer treatment. If so, then it may be that more “personalised” chemotherapy regimens should be used, taking account of chemo sensitivity both in terms of efficacy and adverse effects, such as CIPN.
Summary
Using a strong translational approach to the problem of CIPN is most likely to be successful. Well-designed preclinical studies, reflecting the clinical situation as much as possible, combined with careful consideration of clinical trial design is needed. Working together to standardize assessment techniques and ensure that those used are robustly validated is important. Underlying CIPN mechanisms are wide ranging and therefore offer multiple targets for novel therapies. Whilst traditionally, pharmacological interventions have predominated, a holistic approach, with consideration of mechanistically driven non-pharmacological interventions is also needed. Collaboration will be the key to success, both between disciplines and countries – a challenge not to be underestimated, with many potential barriers and rewards.
Table 3. Some selected mechanisms by which chemotherapy may cause development and persistence of painful peripheral neuropathy.
| Potential mechanism of chemotherapy mitochondrial damage | Agent; action | References |
|---|---|---|
| Increased mitochondrial p53 (tumour suppressor molecule): Reduces mitochondrial membrane potential | Pifthrin-mu: prevents p53 accumulation in mitochondria without interfering with cancer killing effects of chemotherapy | [66] [57] |
| Disrupted axonal mitochondrial capacity via Histone diacetyl-6 (HDAC6) (deacetylates a range of substrates in the cytosol, such as tubulin and heat shock protein 90) | HDAC6 inhibitors: reverses/ prevents CIPN in preclinical models. Early stage clinical trials underway of safety in a variety of cancers, none focussing on CIPN (e.g.NCT02935790, NCT02632071, NCT02635061) | [56] [23; 86] |
| Reduced energy metabolism | Metformin: enhanced activation of carnitine palmitoyltransferase I, restoration of membrane potential; no clinical trials registered currently | [114] [43; 64; 75] |
| Increase in reactive oxygen species, overwhelming of endogenous mitochondrial anti-oxidant systems | Targeted mitochondrial anti-oxidants; accumulate in mitochondria and prevent CIPN related damage | [6; 36; 68] |
| Reduced mitochondrial respiration and energy production | Mitochondrial targeted peptide (SS 20): promotes mitochondrial respiration and positive effect on energy producing mechanisms | [103] |
Acknowledgments
Wellcome Trust funded grant for CIPN neuroimaging work: PhD Studentship (Marta Seretny) Collaboration with Irene Tracey, Marie Fallon.
Footnotes
Conflicts of Interest: Editor, British Journal of Anesthesia.
References
- [1].Al-Mazidi S, Alotaibi M, Nedjadi T, Chaudhary A, Alzoghaibi M, Djouhri L. Blocking of cytokines signalling attenuates evoked and spontaneous neuropathic pain behaviours in the paclitaxel rat model of chemotherapy-induced neuropathy. Eur J Pain. 2018;22(4):810–821. doi: 10.1002/ejp.1169. [DOI] [PubMed] [Google Scholar]
- [2].Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database of Systematic Reviews. 2014;(3) doi: 10.1002/14651858.CD005228.pub4. CD005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andrews NA, Latremoliere A, Basbaum AI, Mogil JS, Porreca F, Rice AS, Woolf CJ, Currie GL, Dworkin RH, Eisenach JC, Evans S, et al. Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. Pain. 2016;157(4):901–909. doi: 10.1097/j.pain.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anoushirvani AA, Poorsaadat L, Aghabozorgi R, Kasravi M. Comparison of the Effects of Omega 3 and Vitamin E on Palcitaxel-Induced Peripheral Neuropathy. Open access Macedonian journal of medical sciences. 2018;6(10):1857–1861. doi: 10.3889/oamjms.2018.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Attal N, Bouhassira D, Gautron M, Vaillant JN, Mitry E, Lepere C, Rougier P, Guirimand F. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain. 2009;144:245–252. doi: 10.1016/j.pain.2009.03.024. [DOI] [PubMed] [Google Scholar]
- [6].M B, Lowes DA, Torsney C, Colvin LA, Galley HF. Modulation of oxidative stress and mitochondrial health by mitochondrial-targeted antioxidants in an in vitro model of chemotherapy-induced neuropathic pain. British Journal of Anaesthesia. 2013;111:310. [Google Scholar]
- [7].Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. British Journal of Anaesthesia. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bechakra M, Nieuwenhoff MD, van Rosmalen J, Groeneveld GJ, Scheltens-de Boer M, Sonneveld P, van Doorn PA, de Zeeuw CI, Jongen JL. Clinical, electrophysiological, and cutaneous innervation changes in patients with bortezomib-induced peripheral neuropathy reveal insight into mechanisms of neuropathic pain. Mol Pain. 2018;14 doi: 10.1177/1744806918797042. 1744806918797042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bennett GJ. Pathophysiology and animal models of cancer-related painful peripheral neuropathy. Oncologist. 2010;15(Suppl 2):9–12. doi: 10.1634/theoncologist.2009-S503. [DOI] [PubMed] [Google Scholar]
- [10].Bhuniya D, Kharul RK, Hajare A, Shaikh N, Bhosale S, Balwe S, Begum F, De S, Athavankar S, Joshi D, Madgula V, et al. Discovery and evaluation of novel FAAH inhibitors in neuropathic pain model. Bioorg Med Chem Lett. 2019;29(2):238–243. doi: 10.1016/j.bmcl.2018.11.048. [DOI] [PubMed] [Google Scholar]
- [11].Binda D, Cavaletti G, Cornblath DR, Merkies IS, group C-Ps Rasch-Transformed Total Neuropathy Score clinical version (RT-TNSc(©) ) in patients with chemotherapy-induced peripheral neuropathy. Journal Of The Peripheral Nervous System. 2015;20:328–332. doi: 10.1111/jns.12140. [DOI] [PubMed] [Google Scholar]
- [12].Boland EG, Selvarajah D, Hunter M, Ezaydi Y, Tesfaye S, Ahmedzai SH, Snowden JA, Wilkinson ID. Central pain processing in chronic chemotherapy-induced peripheral neuropathy: a functional magnetic resonance imaging study. PLoS ONE. 2014;9:e96474. doi: 10.1371/journal.pone.0096474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boyette-Davis JA, Cata JP, Zhang H, Driver LC, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. Journal of Pain. 2011;12:1017–1024. doi: 10.1016/j.jpain.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boyette-Davis JA, Hou S, Abdi S, Dougherty PM. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag. 2018;8(5):363–375. doi: 10.2217/pmt-2018-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brozou V, Vadalouca A, Zis P. Pain in Platin-Induced Neuropathies: A Systematic Review and Meta-Analysis. Pain Ther. 2018;7(1):105–119. doi: 10.1007/s40122-017-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bulls HW, Hoogland AI, Kennedy B, James BW, Arboleda BL, Apte S, Chon HS, Small BJ, Gonzalez BD, Jim HSL. A longitudinal examination of associations between age and chemotherapy-induced peripheral neuropathy in patients with gynecologic cancer. Gynecol Oncol. 2018 doi: 10.1016/j.ygyno.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cavaletti G, Cornblath DR, Merkies IS, Postma TJ, Rossi E, Frigeni B, Alberti P, Bruna J, Velasco R, Argyriou AA, Kalofonos HP, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Annals of Oncology. 2013;24:454–462. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cavaletti G, Frigeni B, Lanzani F, Mattavelli L, Susani E, Alberti P, Cortinovis D, Bidoli P. Chemotherapy-Induced Peripheral Neurotoxicity assessment: a critical revision of the currently available tools. European Journal of Cancer. 2010;46:479–494. doi: 10.1016/j.ejca.2009.12.008. [DOI] [PubMed] [Google Scholar]
- [20].Cavaletti G, Frigeni B, Lanzani F, Piatti M, Rota S, Briani C, Zara G, Plasmati R, Pastorelli F, Caraceni A, Pace A, et al. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. Journal of the peripheral nervous system : JPNS. 2007;12(3):210–215. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- [21].Cavaletti G, Marmiroli P. Pharmacotherapy options for managing chemotherapy-induced peripheral neurotoxicity. Expert Opin Pharmacother. 2018;19(2):113–121. doi: 10.1080/14656566.2017.1415326. [DOI] [PubMed] [Google Scholar]
- [22].Chen Y, Chen SR, Chen H, Zhang J, Pan HL. Increased alpha2delta-1-NMDA receptor coupling potentiates glutamatergic input to spinal dorsal horn neurons in chemotherapy-induced neuropathic pain. J Neurochem. 2018 doi: 10.1111/jnc.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Choi H, Kim HJ, Kim J, Kim S, Yang J, Lee W, Park Y, Hyeon SJ, Lee DS, Ryu H, Chung J, et al. Increased acetylation of Peroxiredoxin1 by HDAC6 inhibition leads to recovery of Abeta-induced impaired axonal transport. Molecular neurodegeneration. 2017;12(1):23. doi: 10.1186/s13024-017-0164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de Carvalho BM, K AK, Eng C, Wendelschafer-Crabb G, Kennedy WR, Simone DA, W XS, Cleeland CS, Dougherty PM. A quantitative sensory analysis of peripheral neuropathy in colorectal cancer and its exacerbation by oxaliplatin chemotherapy. Cancer Research. 2014;74:5955–5962. doi: 10.1158/0008-5472.CAN-14-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Deuis JR, Lim YL, Rodrigues de Sousa S, Lewis RJ, Alewood PF, Cabot PJ, Vetter I. Analgesic effects of clinically used compounds in novel mouse models of polyneuropathy induced by oxaliplatin and cisplatin. Neuro Oncol. 2014;16(10):1324–1332. doi: 10.1093/neuonc/nou048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Diaz PL, Furfari A, Wan BA, Lam H, Charames G, Drost L, Fefekos A, Ohearn S, Blake A, Asthana R, Chow E, et al. Predictive biomarkers of chemotherapy-induced peripheral neuropathy: a review. Biomarkers in medicine. 2018;12(8):907–916. doi: 10.2217/bmm-2017-0427. [DOI] [PubMed] [Google Scholar]
- [27].Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nature Reviews Neuroscience. 2013;14:49–62. doi: 10.1038/nrn3404. [DOI] [PubMed] [Google Scholar]
- [28].Dimopoulos MA, Mateos MV, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kropff M, Spicka I, Palumbo A, Wu KL, Esseltine DL, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. Eur J Haematol. 2011;86(1):23–31. doi: 10.1111/j.1600-0609.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- [29].Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. Journal of Pain & Symptom Management. 2007;33:166–179. doi: 10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- [30].Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, Hansson P, Haroutounian S, Arendt-Nielsen L, Attal N, Baron R, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157(9):1851–1871. doi: 10.1097/j.pain.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fallon MT, S DJ, K A, W CJ, Mitchell R, F-W SM, Scott AC, Colvin LA. Cancer treatment-related neuropathic pain: proof of concept study with menthol--a TRPM8 agonist. Supportive Care in Cancer. 2015;23(9):2769–2777. doi: 10.1007/s00520-015-2642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Filipczak-Bryniarska I, Krzyzewski RM, Kucharz J, Michalowska-Kaczmarczyk A, Kleja J, Woron J, Strzepek K, Kazior L, Wordliczek J, Grodzicki T, Krzemieniecki K. High-dose 8% capsaicin patch in treatment of chemotherapy-induced peripheral neuropathy: single-center experience. Medical oncology (Northwood, London, England) 2017;34(9):162. doi: 10.1007/s12032-017-1015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review. Br J Anaesth. 2017;119(4):737–749. doi: 10.1093/bja/aex229. [DOI] [PubMed] [Google Scholar]
- [35].Frigeni B, Piatti M, Lanzani F, Alberti P, Villa P, Zanna C, Ceracchi M, Ildebrando M, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity can be misdiagnosed by the National Cancer Institute Common Toxicity scale. Journal Of The Peripheral Nervous System. 2011;16:228–236. doi: 10.1111/j.1529-8027.2011.00351.x. [DOI] [PubMed] [Google Scholar]
- [36].Galley HF, McCormick B, Wilson KL, Lowes DA, Colvin L, Torsney C. Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat. J Pineal Res. 2017;63(4) doi: 10.1111/jpi.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gewandter JS, Brell J, Cavaletti G, Dougherty PM, Evans S, Howie L, McDermott MP, O'Mara A, Smith AG, Dastros-Pitei D, Gauthier LR, et al. Trial designs for chemotherapy-induced peripheral neuropathy prevention: ACTTION recommendations. Neurology. 2018;91(9):403–413. doi: 10.1212/WNL.0000000000006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gewandter JS, Burke L, Cavaletti G, Dworkin RH, Gibbons C, Gover TD, Herrmann DN, McArthur JC, McDermott MP, Rappaport BA, Reeve BB, et al. Content validity of symptom-based measures for diabetic, chemotherapy, and HIV peripheral neuropathy. Muscle Nerve. 2017;55(3):366–372. doi: 10.1002/mus.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gewandter JS, Freeman R, Kitt RA, Cavaletti G, Gauthier LR, McDermott MP, Mohile NA, Mohlie SG, Smith AG, Tejani MA, Turk DC, et al. Chemotherapy-induced peripheral neuropathy clinical trials: Review and recommendations. Neurology. 2017;89(8):859–869. doi: 10.1212/WNL.0000000000004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ghoreishi Z, Keshavarz S, Asghari Jafarabadi M, Fathifar Z, Goodman KA, Esfahani A. Risk factors for paclitaxel-induced peripheral neuropathy in patients with breast cancer. BMC Cancer. 2018;18(1):958. doi: 10.1186/s12885-018-4869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Goswami C. TRPV1-tubulin complex: involvement of membrane tubulin in the regulation of chemotherapy-induced peripheral neuropathy. Journal Of Neurochemistry. 2012;123:1–13. doi: 10.1111/j.1471-4159.2012.07892.x. [Review] [DOI] [PubMed] [Google Scholar]
- [42].Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro-Oncology. 2012;14(Suppl 4):iv45–iv54. doi: 10.1093/neuonc/nos203. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Group DPPR. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. The lancet Diabetes & endocrinology. 2015;3(11):866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- [45].Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- [46].Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- [47].Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, Moinpour CM, Hansen L, Lew DL, Greenlee H, Fehrenbacher L, Wade JL, III, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. Journal of Clinical Oncology. 2013;31:2627–2633. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hu LY, Mi WL, Wu GC, Wang YQ, Mao-Ying QL. Prevention and Treatment for Chemotherapy-Induced Peripheral Neuropathy: Therapies Based on CIPN Mechanisms. Curr Neuropharmacol. 2019;17(2):184–196. doi: 10.2174/1570159X15666170915143217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jablonska-Trypuc A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. Journal of enzyme inhibition and medicinal chemistry. 2016;31(sup1):177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- [50].Jones RC, III, Backonja MM. Review of neuropathic pain screening and assessment tools. Current Pain & Headache Reports. 2013;17:363. doi: 10.1007/s11916-013-0363-6. [DOI] [PubMed] [Google Scholar]
- [51].Kandula T, Farrar MA, Cohn RJ, Mizrahi D, Carey K, Johnston K, Kiernan MC, Krishnan AV, Park SB. Chemotherapy-Induced Peripheral Neuropathy in Long-term Survivors of Childhood Cancer: Clinical, Neurophysiological, Functional, and Patient-Reported Outcomes. JAMA neurology. 2018;75(8):980–988. doi: 10.1001/jamaneurol.2018.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice ASC. Reliability of conditioned pain modulation: a systematic review. Pain. 2016;157(11):2410–2419. doi: 10.1097/j.pain.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kleckner IR, Kamen C, Gewandter JS, Mohile NA, Heckler CE, Culakova E, Fung C, Janelsins MC, Asare M, Lin PJ, Reddy PS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2018;26(4):1019–1028. doi: 10.1007/s00520-017-4013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kober KM, Olshen A, Conley YP, Schumacher M, Topp K, Smoot B, Mazor M, Chesney M, Hammer M, Paul SM, Levine JD, et al. Expression of mitochondrial dysfunction-related genes and pathways in paclitaxel-induced peripheral neuropathy in breast cancer survivors. Mol Pain. 2018;14 doi: 10.1177/1744806918816462. 1744806918816462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kroigard T, Schroder HD, Qvortrup C, Eckhoff L, Pfeiffer P, Gaist D, Sindrup SH. Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies. European Journal of Neurology. 2014;21:623–629. doi: 10.1111/ene.12353. [DOI] [PubMed] [Google Scholar]
- [56].Krukowski K, Ma J, Golonzhka O, Laumet GO, Gutti T, van Duzer JH, Mazitschek R, Jarpe MB, Heijnen CJ, Kavelaars A. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain. 2017;158(6):1126–1137. doi: 10.1097/j.pain.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Krukowski K, Nijboer CH, Huo X, Kavelaars A, Heijnen CJ. Prevention of chemotherapy-induced peripheral neuropathy by the small-molecule inhibitor pifithrin-mu. Pain. 2015;156:2184–2192. doi: 10.1097/j.pain.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li Y, North RY, Rhines LD, Tatsui CE, Rao G, Edwards DD, Cassidy RM, Harrison DS, Johansson CA, Zhang H, Dougherty PM. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018;38(5):1124–1136. doi: 10.1523/JNEUROSCI.0899-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Li Y, Tatsui CE, Rhines LD, North RY, Harrison DS, Cassidy RM, Johansson CA, Kosturakis AK, Edwards DD, Zhang H, Dougherty PM. Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain. 2017;158(3):417–429. doi: 10.1097/j.pain.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews. 2017;1 doi: 10.1002/14651858.CD011279.pub2. CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lotsch J, Sipila R, Tasmuth T, Kringel D, Estlander AM, Meretoja T, Kalso E, Ultsch A. Machine-learning-derived classifier predicts absence of persistent pain after breast cancer surgery with high accuracy. Breast cancer research and treatment. 2018;171(2):399–411. doi: 10.1007/s10549-018-4841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lotsch J, Ultsch A, Kalso E. Prediction of persistent post-surgery pain by preoperative cold pain sensitivity: biomarker development with machine-learning-derived analysis. Br J Anaesth. 2017;119(4):821–829. doi: 10.1093/bja/aex236. [DOI] [PubMed] [Google Scholar]
- [63].Ma J, Kavelaars A, Dougherty PM, Heijnen CJ. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer. 2018;124(11):2289–2298. doi: 10.1002/cncr.31248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ma J, Yu H, Liu J, Chen Y, Wang Q, Xiang L. Metformin attenuates hyperalgesia and allodynia in rats with painful diabetic neuropathy induced by streptozotocin. Eur J Pharmacol. 2015;764:599–606. doi: 10.1016/j.ejphar.2015.06.010. [DOI] [PubMed] [Google Scholar]
- [65].Mahmoudpour SH, Bandapalli OR, da Silva Filho MI, Campo C, Hemminki K, Goldschmidt H, Merz M, Forsti A. Chemotherapy-induced peripheral neuropathy: evidence from genome-wide association studies and replication within multiple myeloma patients. BMC Cancer. 2018;18(1):820. doi: 10.1186/s12885-018-4728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Maj MA, Ma J, Krukowski KN, Kavelaars A, Heijnen CJ. Inhibition of Mitochondrial p53 Accumulation by PFT-mu Prevents Cisplatin-Induced Peripheral Neuropathy. Frontiers in molecular neuroscience. 2017;10:108. doi: 10.3389/fnmol.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mallet C, Dubray C, Dualé C. FAAH inhibitors in the limelight, but regrettably. Int J Clin Pharmacol Ther. 2016;54(7):498–501. doi: 10.5414/CP202687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McCormick B, Lowes DA, Colvin L, Torsney C, Galley HF. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br J Anaesth. 2016;117(5):659–666. doi: 10.1093/bja/aew309. [DOI] [PubMed] [Google Scholar]
- [69].Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature Reviews Neuroscience. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- [70].Montague K, Malcangio M. The Therapeutic Potential of Monocyte/Macrophage Manipulation in the Treatment of Chemotherapy-Induced Painful Neuropathy. Frontiers in molecular neuroscience. 2017;10:397. doi: 10.3389/fnmol.2017.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mulpuri Y, Marty VN, Munier JJ, Mackie K, Schmidt BL, Seltzman HH, Spigelman I. Synthetic peripherally-restricted cannabinoid suppresses chemotherapy-induced peripheral neuropathy pain symptoms by CB1 receptor activation. Neuropharmacology. 2018;139:85–97. doi: 10.1016/j.neuropharm.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nodera H, Spieker A, Sung M, Rutkove S. Neuroprotective effects of Kv7 channel agonist, retigabine, for cisplatin-induced peripheral neuropathy. Neurosci Lett. 2011;505(3):223–227. doi: 10.1016/j.neulet.2011.09.013. [DOI] [PubMed] [Google Scholar]
- [73].Nudelman KN, McDonald BC, Wang Y, Smith DJ, West JD, O'Neill DP, Zanville NR, Champion VL, Schneider BP, Saykin AJ. Cerebral Perfusion and Gray Matter Changes Associated With Chemotherapy-Induced Peripheral Neuropathy. Journal of Clinical Oncology. 2016;34:677–683. doi: 10.1200/JCO.2015.62.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].O'Hearn S, Diaz P, Wan BA, DeAngelis C, Lao N, Malek L, Chow E, Blake A. Modulating the endocannabinoid pathway as treatment for peripheral neuropathic pain: a selected review of preclinical studies. Annals of palliative medicine. 2017;6(Suppl 2):S209–s214. doi: 10.21037/apm.2017.08.04. [DOI] [PubMed] [Google Scholar]
- [75].Oda SS. Metformin Protects against Experimental Acrylamide Neuropathy in Rats. Drug development research. 2017;78(7):349–359. doi: 10.1002/ddr.21400. [DOI] [PubMed] [Google Scholar]
- [76].Pareyson D, Piscosquito G, Moroni I, Salsano E, Zeviani M. Peripheral neuropathy in mitochondrial disorders. The Lancet Neurology. 2013;12(10):1011–1024. doi: 10.1016/S1474-4422(13)70158-3. [DOI] [PubMed] [Google Scholar]
- [77].Park SB, Goldstein D, Lin CS, Krishnan AV, Friedlander ML, Kiernan MC. Acute abnormalities of sensory nerve function associated with oxaliplatin-induced neurotoxicity. J Clin Oncol. 2009;27(8):1243–1249. doi: 10.1200/JCO.2008.19.3425. [DOI] [PubMed] [Google Scholar]
- [78].Park SB, Kwok JB, Asher R, Lee CK, Beale P, Selle F, Friedlander M. Clinical and genetic predictors of paclitaxel neurotoxicity based on patient- versus clinician-reported incidence and severity of neurotoxicity in the ICON7 trial. Ann Oncol. 2017;28(11):2733–2740. doi: 10.1093/annonc/mdx491. [DOI] [PubMed] [Google Scholar]
- [79].Proudfoot CJ, Garry EM, C DF, Rosie R, Anderson H, Roberston DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Current Biology. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- [80].Quartu M, Carozzi VA, Dorsey SG, Serra MP, Poddighe L, Picci C, Boi M, Melis T, Del Fiacco M, Meregalli C, Chiorazzi A, et al. Bortezomib treatment produces nocifensive behavior and changes in the expression of TRPV1, CGRP, and substance P in the rat DRG, spinal cord, and sciatic nerve. Biomed Res Int. 2014;2014 doi: 10.1155/2014/180428. 180428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wilson RH, Lehky T, Thomas RR, Quinn MG, Floeter MK, Grem JL. Acute oxaliplatin-induced peripheral nerve hyperexcitability. Journal of Clinical Oncology. 2002;20(7):1767–1774. doi: 10.1200/JCO.2002.07.056. [DOI] [PubMed] [Google Scholar]
- [82].Robertson J, Raizer J, Hodges JS, Gradishar W, Allen JA. Risk factors for the development of paclitaxel-induced neuropathy in breast cancer patients. Journal of the peripheral nervous system : JPNS. 2018;23(2):129–133. doi: 10.1111/jns.12271. [DOI] [PubMed] [Google Scholar]
- [83].Roldan CJ, Johnson C, Lee SO, Peng A, Dougherty PM, Huh B. Subclinical Peripheral Neuropathy in Patients with Head and Neck Cancer: A Quantitative Sensory Testing (QST) Study. Pain Physician. 2018;21(4):E419–e427. [PMC free article] [PubMed] [Google Scholar]
- [84].Salat K, Moniczewski A, Librowski T. Transient receptor potential channels - emerging novel drug targets for the treatment of pain. Current Medicinal Chemistry. 2013;20:1409–1436. doi: 10.2174/09298673113209990107. [DOI] [PubMed] [Google Scholar]
- [85].Sanchez-Barroso L, Apellaniz-Ruiz M, Gutierrez-Gutierrez G, Santos M, Roldan-Romero JM, Curras M, Remacha L, Calsina B, Calvo I, Sereno M, Merino M, et al. Concomitant Medications and Risk of Chemotherapy-Induced Peripheral Neuropathy. Oncologist. 2018 doi: 10.1634/theoncologist.2018-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Santo L, Hideshima T, Kung AL, Tseng JC, Tamang D, Yang M, Jarpe M, van Duzer JH, Mazitschek R, Ogier WC, Cirstea D, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119(11):2579–2589. doi: 10.1182/blood-2011-10-387365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Scott AC, S M, Laird B, Colvin L, F M. Quantitative Sensory Testing to assess the sensory characteristics of cancer-induced bone pain after radiotherapy and potential clinical biomarkers of response. European Journal of Pain. 2012;16:123–133. doi: 10.1016/j.ejpain.2011.05.002. [DOI] [PubMed] [Google Scholar]
- [88].Seretny M, Currie GL, Sena ES, R S, G R, M MR, Colvin LA, Fallon MT. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- [89].Seretny M, R L, W H, Warnaby CE, M J, R N, L SM, Colvin L, F M. Brainstem processing of peripheral punctate stimuli in patients with and without chemotherapy-induced peripheral neuropathy: a prospective cohort functional MRI study. Lancet. 2016;387:S15–S15. [Google Scholar]
- [90].Sexton JE, Vernon J, Wood JN. TRPs and pain. Handbook of Experimental Pharmacology. 2014;223:873–897. doi: 10.1007/978-3-319-05161-1_6. [Review] [DOI] [PubMed] [Google Scholar]
- [91].Shah A, Hoffman EM, Mauermann ML, Loprinzi CL, Windebank AJ, Klein CJ, Staff NP. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J Neurol Neurosurg Psychiatry. 2018;89(6):636–641. doi: 10.1136/jnnp-2017-317215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Shi Q, Cai X, Shi G, Lv X, Yu J, Wang F. Interleukin-4 protects from chemotherapy-induced peripheral neuropathy in mice modal via the stimulation of IL-4/STAT6 signaling. Acta cirurgica brasileira. 2018;33(6):491–498. doi: 10.1590/s0102-865020180060000003. [DOI] [PubMed] [Google Scholar]
- [93].Sikandar S, Dickenson AH., II No need for translation when the same language is spoken. British Journal of Anaesthesia. 2013;111:3–6. doi: 10.1093/bja/aet210. [DOI] [PubMed] [Google Scholar]
- [94].Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Smith EML, Banerjee T, Yang JJ, Bridges CM, Alberti P, Sloan JA, Loprinzi C. Psychometric Testing of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Chemotherapy-Induced Peripheral Neuropathy 20-Item Scale Using Pooled Chemotherapy-Induced Peripheral Neuropathy Outcome Measures Standardization and Alliance for Clinical Trials in Oncology A151408 Study Data. Cancer Nurs. 2018 doi: 10.1097/NCC.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Smith EML, Knoerl R, Yang JJ, Kanzawa-Lee G, Lee D, Bridges CM. In Search of a Gold Standard Patient-Reported Outcome Measure for Use in Chemotherapy- Induced Peripheral Neuropathy Clinical Trials. Cancer control : journal of the Moffitt Cancer Center. 2018;25(1) doi: 10.1177/1073274818756608. 1073274818756608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Smith EML, Zanville N, Kanzawa-Lee G, Donohoe C, Bridges C, Loprinzi C, Le-Rademacher J, Yang JJ. Rasch model-based testing of the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Chemotherapy-Induced Peripheral Neuropathy (QLQ-CIPN20) using Alliance for Clinical Trials in Oncology (Alliance) A151408 study data. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2018 doi: 10.1007/s00520-018-4553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sucheston-Campbell LE, Clay-Gilmour AI, Barlow WE, Budd GT, Stram DO, Haiman CA, Sheng X, Yan L, Zirpoli G, Yao S, Jiang C, et al. Genome-wide meta-analyses identifies novel taxane-induced peripheral neuropathy-associated loci. Pharmacogenet Genomics. 2018;28(2):49–55. doi: 10.1097/FPC.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Molecular Pain. 2010;6:15. doi: 10.1186/1744-8069-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Themistocleous AC, Crombez G, Baskozos G, Bennett DL. Using stratified medicine to understand, diagnose, and treat neuropathic pain. Pain. 2018;159(Suppl 1):S31–s42. doi: 10.1097/j.pain.0000000000001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tomita S, Sekiguchi F, Deguchi T, Miyazaki T, Ikeda Y, Tsubota M, Yoshida S, Nguyen HD, Okada T, Toyooka N, Kawabata A. Critical role of Cav3.2 T-type calcium channels in the peripheral neuropathy induced by bortezomib, a proteasome-inhibiting chemotherapeutic agent, in mice. Toxicology. 2019;413:33–39. doi: 10.1016/j.tox.2018.12.003. [DOI] [PubMed] [Google Scholar]
- [102].Tonello R, Lee SH, Berta T. Monoclonal Antibody Targeting the Matrix Metalloproteinase 9 Prevents and Reverses Paclitaxel-Induced Peripheral Neuropathy in Mice. J Pain. 2018 doi: 10.1016/j.jpain.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Toyama S, Shimoyama N, Szeto HH, Schiller PW, Shimoyama M. Protective Effect of a Mitochondria-Targeted Peptide against the Development of Chemotherapy-Induced Peripheral Neuropathy in Mice. ACS Chem Neurosci. 2018;9(7):1566–1571. doi: 10.1021/acschemneuro.8b00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].van Esbroeck ACM, Janssen APA, Cognetta AB, 3rd, Ogasawara D, Shpak G, van der Kroeg M, Kantae V, Baggelaar MP, de Vrij FMS, Deng H, Allara M, et al. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science. 2017;356(6342):1084–1087. doi: 10.1126/science.aaf7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].van Hecke O, Kamerman PR, Attal N, Baron R, Bjornsdottir G, Bennett DLH, Bennett MI, Bouhassira D, Diatchenko L, Freeman R, Freynhagen R, et al. Neuropathic pain phenotyping by international consensus (NeuroPPIC) for genetic studies: a NeuPSIG systematic review, Delphi survey, and expert panel recommendations. Pain. 2015;156(11):2337–2353. doi: 10.1097/j.pain.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ventzel L, Madsen CS, Karlsson P, Tankisi H, Isak B, Fuglsang-Frederiksen A, Jensen AB, Jensen AR, Jensen TS, Finnerup NB. Chronic Pain and Neuropathy Following Adjuvant Chemotherapy. Pain Med. 2018;19(9):1813–1824. doi: 10.1093/pm/pnx231. [DOI] [PubMed] [Google Scholar]
- [107].Wasner G, Baron R. Pain: clinical pain assessment: from bedside to better treatment. Nature Reviews Neuroscience. 2009;5:359–361. doi: 10.1038/nrneurol.2009.93. [DOI] [PubMed] [Google Scholar]
- [108].Wirtz P, Baumann FT. Physical Activity, Exercise and Breast Cancer - What Is the Evidence for Rehabilitation, Aftercare, and Survival? A Review. Breast care (Basel, Switzerland) 2018;13(2):93–101. doi: 10.1159/000488717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wu J, Hocevar M, Bie B, Foss JF, Naguib M. Cannabinoid Type 2 Receptor System Modulates Paclitaxel-Induced Microglial Dysregulation and Central Sensitization in Rats. J Pain. 2018 doi: 10.1016/j.jpain.2018.10.007. [DOI] [PubMed] [Google Scholar]
- [110].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- [111].Yehia R, Saleh S, El Abhar H, Saad AS, Schaalan M. L-Carnosine protects against Oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: A perspective on targeting Nrf-2 and NF-kappaB pathways. Toxicology and applied pharmacology. 2018;365:41–50. doi: 10.1016/j.taap.2018.12.015. [DOI] [PubMed] [Google Scholar]
- [112].Zhang H, Dougherty PM. Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology. 2014;120(6):1463–1475. doi: 10.1097/ALN.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Experimental Neurology. 2011 doi: 10.1016/j.expneurol.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of clinical investigation. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]