Abstract
HIV stigma continues to be a barrier to physical and mental health among people living with HIV globally, especially in vulnerable populations. We examined how stigma is associated with health outcomes and quality of life among rural women living with HIV in South India (N=600). Interviewer-administered measures assessed multiple dimensions of stigma, as well as loneliness, social support, ART adherence, time since diagnosis, and quality of life. Internalized stigma and a lack of social support were associated with a lower quality of life, while the association between internalized stigma and adherence was mediated by the use of stigma-avoidant coping strategies, suggesting that keeping one’s diagnosis a secret may make it more difficult to take one’s medications. These findings suggest that these women constitute a vulnerable population who need additional services to optimize their health and who might benefit from peer support interventions and stigma-reduction programs for family and community members.
Keywords: HIV Stigma, rural women, India, adherence, quality of life
INTRODUCTION
The role of HIV stigma and discrimination in health care settings
HIV-related stigma and discrimination continue to have a major worldwide impact on the lives of people living with HIV (PLWH). HIV-related stigma refers to the devalued status that society attaches to those living with HIV (1, 2) and manifests as discrimination toward PLWH by their communities, their own families and relatives, as well as health care providers (3–5). Stigma within the general population has been shown to be driven largely by lack of awareness, fear of casual transmission due to lack of knowledge or misperceptions about HIV, prejudices toward groups associated with HIV, and the attitude that HIV-positive individuals deserved their fate (3, 6–9).
Stigma and negative attitudes toward PLWH by health care providers can manifest in poor patient-provider relationships and denial of proper care (8, 10–15), and continue to be a focus of stigma interventions (16–22). Stigma and discrimination are also directed toward providers who treat HIV patients (23) and can perpetuate an unwillingness to treat PLWH.
Health consequences of stigma
Perceived fears of stigma have been significantly associated with depression in Nigeria and Ethiopia (24, 25) as well as with suicide attempts (26). Stigma and discrimination has also been found associated with a decreased willingness to disclose one’s HIV status, which in turn may lead to depression and anxiety (2, 24–27). Stigma and discrimination in health care settings lead to PLWH hiding their HIV status from providers (8, 27, 28), creating a primary barrier to HIV testing (7, 12, 29, 30) and care-seeking (7, 10, 31). A meta-analysis of global studies published between 1996 and 2013 found that people who had experienced HIV-related stigma were at greater risk of depression, lower social support, and “were 21% less likely to access or use health and social services” (32).
Internalized stigma
While general accounts of stigma and discrimination are clearly detrimental, studies examining the specific impact of perceived and internalized stigma highlight the depth of the problem. A South African study, using the PLHIV Stigma Index, found that internalized stigma frequently manifested in self-isolation, avoidance of care, fear of physical assault, and withdrawal from employment (33). Similarly, a Ugandan study showed the greater the internalized stigma, the less likely PLWH were to disclose their HIV status (34). This internalization can apply to family members as well. In Vietnam, family caregivers described a perceived need to keep their loved one’s status a secret to avoid stigma and discrimination, causing great fear, anxiety, and frustration, and limiting social support (35).
HIV stigma and health in India
Numerous studies show similar consequences of stigma in India. A qualitative study from 2015 described a context of highly internalized stigma, characterized by common feelings of guilt, shame, worthlessness, and fear of rejection (36). In our previous work, we developed an India-specific model of stigma, which showed perceived and internalized stigma to be associated with higher levels of depression (2) which has been replicated by other India studies (37, 38). We have also shown in previous work how stigma in India delays health care-seeking (31), limits disclosure, and impacts mental health outcomes over time (27).
Recent studies in India have also linked depression to delayed HIV testing (39) and delayed treatment (40), with internalized stigma a common theme described by participants. Stigma also continues to act as a common barrier to timely care-seeking, treatment, and prevention in India (36, 41, 42), though studies have shown mixed results in terms of stigma’s relationship to ART adherence once on treatment (43, 44). While disclosure varies among studies, the main reasons given for non-disclosure were shame and fear of discrimination and isolation (36, 45–47). Within the health care setting, PLWH in India have reported experiences of provider discrimination, purposeful delay or even denial of treatment (47), and breach of confidentiality following their diagnosis (48). These PLWH-reported experiences of provider stigma appear to be validated in our previous work examining stigma among Indian health care workers (3, 7)
Impact of HIV stigma on women living with HIV
Women may be particularly affected by HIV-related stigma (49). In the U.S., fear of stigma from providers led women to decline HIV testing and to not disclose their status during pregnancy; keeping them from accessing preventive measures for Prevention of Mother-To-Child-Transmission (PMTCT) or counseling (15). In Belgium and Lebanon, provider attitudes and discrimination toward HIV-positive women, as well as stigmatizing policies, kept women from seeking HIV testing or proper and timely care, and limited disclosure (8, 12). Women living with HIV (WLHIV) in Chile, South Africa, and Namibia experienced forced sterilization by providers wanting to limit mother-to-child transmission, highlighting widespread discrimination (50). A study of HIV-positive pregnant women in Uganda described high levels of perceived stigma and personal shame, which interfered with HIV care engagement and, coupled with fear of intimate partner violence and lack of family support, led to high levels of distress and limited disclosure (51).
Our previous work in Andra Pradesh, India echoes this trend by documenting the particularly challenging experience of WLHIV and stigma (52–54). The women not only experienced overt discrimination by health care providers, but also commonly witnessed or heard stories of discrimination in their communities and experienced high levels of internalized stigma, which was associated with a lower quality of life. Other papers have also highlighted important gender differences in the experience and impact of stigma in India (55–58).
As far as we know, there has been no previous research among rural Indian women living with HIV that describes the association between the dimensions of stigma and health previously identified in our India-specific conceptual model (2, 27) Given the poor rates of adherence (41) and quality of life (41, 59) in this population, it is vitally important that we examine the role that stigma may play in these outcomes. The present paper aims to accomplish this, by examining the stigma fears, vicarious stigma, internalized stigma and the disclosure avoidant coping strategies reported by rural Indian women living with HIV as well as the association between these factors and the self-reported antiretroviral medication adherence and their perceived quality of life.
METHODS
Design
The analyses are based on baseline interviews from 600 WLHIV in the South Indian state of Andhra Pradesh, who were enrolled in a 2×2 factorial clinical trial designed to assess the impact of support by Asha community health workers, with and without food supplementation and nutrition education, on adherence to ART and improved health outcomes for the women and their children. In the rural district of Nellore, in the state of Andhra Pradesh, four comparable high HIV prevalence sites were selected, based on ease of access and randomly assigned to one of the four conditions of the trial. Participants were followed quarterly for a year. Additional details of the trial design have been reported previously (41). Human Subjects Protection Committee clearances were obtained both in the US and in India.
Participants
Women needed to meet the following inclusion criteria for study enrollment: 1) 18–50 years of age; 2) diagnosed with HIV and receiving ART for at least three months; 3) have CD4 levels above 100; and 4) reporting to have a child aged 3–8 living with them. Only one child per woman was included in the study.
Screening Procedures
Study flyers were developed in collaboration with our community partners, and posted in selected Primary Health Clinics (PHCs), targeting local WLHIV. Those who indicated interest were given a screening appointment with study staff in a private area at the PHC to assess age, HIV and ART status and having a child (3–8 years) to determine eligibility. Eligible and interested women completed the interviewer-administered baseline questionnaire in Telugu (the local language), using a tablet format.
Measures
Sociodemographic factors included age, education, employment status, religion, marital status and number of children.
Medical history was self-reported and included the following:
Time Living with HIV was calculated based on a woman’s self-report of the month and year that she was diagnosed with HIV.
History of Opportunistic Infections (OI) was assessed by asking what kind of opportunistic infections out of a list of eight had been experienced within the last six months. The number of OI endorsed was then counted.
Perceived ART Symptoms and Side Effects.
Participants were asked if they had experienced any of 18 specific symptoms, such as nausea, skin rashes, or numbness around the mouth in the past six months and responses for each were recorded as a “yes” (1) or “no” (0) and summed.
Loneliness.
A single item asked how frequently the participant had felt lonely in the past week, with four response options ranging from ‘less than 1 day’ to ‘5–7 days’ (60).
Stigma Fears.
This scale was developed by our team, based on formative research with PLWH participating in an earlier study. Participants indicated on a 4-point Likert-type scale, how worried (from 0, “Not at all worried” to 3 “Very worried”) they were of stigmatizing or discriminatory reactions by family members (6 items), friends (6 items), health care workers (HCW, 5 items), people at work (4 items) and community members (12 items) if they were to disclose their HIV status to members of these social groups. A mean score was taken for each of the 5 subgroups. Cronbach’s alpha ranged from 0.77 for “family” to 0.92 for “work.”
Internalized Stigma was computed as the mean of 8 items from an Internalized Stigma Scale, which has been used in our previous research with Indian PLWH (7, 61, 62). Response options range from 0, “Not at all” to 3, “A great deal”. Alpha for the eight-item scale was 0.79.
Vicarious Stigma was the mean of 10 items assessing how often (0 “Never” to 3 “Frequently”) the respondent had heard of or witnessed other PLWH experiencing discrimination such as denial of care, or being ostracized by their family or village. Reliability in this sample was α=0.82.
Stigma-Avoidant Coping Strategies were assessed by asking how often the WLHIV used strategies such as lying about the reason for medical visits to try to keep others from knowing that they had HIV/AIDS (0 “Never” to 3 “Often”) and was based on a scale used in our previous research in the region (2). A scale score was constructed by calculating the mean over all 9 items (α =0.81).
Social Support was assessed by the RAND Corporation’s Medical Outcomes Study (MOS) Social Support Scale (63), modified for India. The 18-item scale assesses frequency of support from friends and (extended) family or partners on a scale ranging from 1, “None of the time” to 5 “All of the time”. In addition, one item asked the participant to estimate her number of close friends or relatives. Since the majority of participants scored the minimum on the MOS scale and reported no close friends/relatives, we dichotomized social support as none (MOS score = 1 and 0 close friends/family) vs. any (MOS score > 1 and/or ≥1 close friend/relative).
Adherence to ART was assessed via self-report using a Visual Analogue Scale (VAS) used in our previous studies, to assess proportion of prescribed pills taken in the past month (64, 65).
Quality of Life during the past week was assessed by asking the WLHIV how satisfied they were with 10 aspects of life such as health, work, mood, family relationships, etc. (66). Responses were on a 4-point Likert-type scale ranging from 0 “Very unsatisfied” to 3 “Very satisfied”. We calculated the mean score over all items (α =0.81).
Data Analytic Considerations
Descriptive statistics consisted of frequency tabulations for categorical variables and mean and SD, or median and interquartile range (IQR) for continuous variables, depending on their distribution. Given the skewed and kurtotic nature of several of the variables, bivariate associations were assessed via Spearman’s correlation coefficient.
We next ran linear regression analyses to assess the relationships between the HIV stigma variables and the outcomes of (1) ART adherence in the past month (VAS) and (2) QOL. We used cluster-robust standard errors to account for potential non-independence of the observations from participants recruited at the same site. There were four sites in the Nellore district of Andhra Pradesh (n=140 each) and four in the district of Prakasam (n=10 each, hence combined into one cluster). To improve normality of the distribution of the outcome variables, they were transformed by taking their natural logarithm (ln) (QOL was rescaled to a 1–4 scale prior to transformation, to avoid values of 0). After the regression analyses, we exponentiated the regression coefficients to allow interpretation as the percent change in the outcome (in its original metric) associated with a 1-unit increase in a given predictor (67).
Potential covariates in regression models for both outcomes included the demographic variables marital status (currently married vs. other), age, education (any formal education vs. none) and religion (Hindu vs. other). Based on our previous work (41), we also considered number of months since HIV diagnosis (natural-log transformed), number of side effects and number of Opportunistic Infections (linear and quadratic term) when VAS was the outcome. For the QOL outcome we considered as additional covariates social support and loneliness (dichotomized at <3 vs. ≥3 days in past week) (59). All potential covariates with unadjusted regression coefficients with a p-value ≤ 0.10 were included in multivariable regression analyses. There were no problems with multicollinearity or influential outliers. Non-significant predictors were subsequently removed via backward elimination until all remaining predictors were significant.
Upon noticing that the coefficient for internalized stigma in the multivariable regression with the VAS outcome changed sign in the presence of stigma-avoidant coping, we performed a mediation analysis to test if stigma-avoidant coping mediated the relation between internalized stigma and adherence, using Hayes’ DIRECT macro for SPSS (68), with 5000 bootstrap resamples and seed=110271. We report percentile bootstrap confidence intervals for the indirect effect (69).
Descriptive and mediation analyses were performed in SPSS v24, regression analyses in Stata v15.
RESULTS
As shown in Table 1, though mean (SD) age was only 34.3 (7.0), about half the women in the sample were already widows. Almost half had received no formal education and nearly all were employed as casual or day laborers. Most (73.2%) were Hindu, followed by Christian (19.5%). The average time since HIV-diagnosis was 4 years. Percent of ART pills taken in the past month averaged only 30.4% (SD=13.2). None of the participants was optimally adherent based on the usual cut-off of 95%. Sixty percent of the participants reported having no social support or any friends or relatives to whom they felt close and 15.9% expressed feeling lonely at least 3 days in the past week (Table 2). The overall quality of life reported was very low, with a median QOL of 0.3 out of 3. Table 2 also summarizes the levels of stigma reported by these WLHIV. On a 0 – 3 scale, the median level of stigma reported on the various measures ranged from 2.6 for vicarious stigma to 3 on the stigma fear subscales. Use of stigma-avoidant coping strategies was equally high (median=2.6). As can be seen from the lower limits of the IQRs, the majority of respondents fell in the upper half of the distribution on all stigma-related variables, and in the case of stigma fears related to friends and work, over 75% of the sample had the maximum score. As can be seen in Table 3, this also reflected itself in moderate to high Spearman correlations (rho) among the stigma fear subscales, ranging from rho = .21 for stigma fears related to work and community, to rho = .55 for stigma fears related to family and health care workers (all p < .001). The stigma fear variables were also significantly correlated with avoidant coping (rho = .17 to .31; p < .001) and internalized stigma (rho = .22 to .38, p < .001). Internalized stigma and avoidant coping were correlated at rho = .47 (p < .001). The correlations with social support and loneliness were smaller (rho = −.16 to .01) and not always statistically significant.
Table 1:
Sample characteristics
| Frequency | Percent | |
|---|---|---|
| Marital Status: | ||
| Married | 238 | 39.7 |
| Widowed | 308 | 51.3 |
| Divorced/Separated | 54 | 9.0 |
| Number of children | ||
| 1 | 207 | 34.5 |
| 2 | 296 | 49.3 |
| ≥3 | 97 | 16.2 |
| Occupation | ||
| Casual/day labor | 579 | 96.5 |
| Other | 20 | 3.3 |
| None | 1 | 0.2 |
| Religion | ||
| Hindu | 439 | 73.2 |
| Christian | 117 | 19.5 |
| Muslim | 44 | 7.3 |
| Education | ||
| None | 292 | 48.7 |
| < 5 yrs | 98 | 16.3 |
| 5–9 yrs | 123 | 20.5 |
| ≥10 yrs | 87 | 14.5 |
| Mean | (SD) | |
| Age | 34.3 | (7.0) |
| Mo. since HIV diagnosis | 48.2 | (34.6) |
| % ART pills taken in past mo. | 30.4 | (13.2) |
| Number of side effects past 6 mo. | 13.4 | (1.5) |
| Number of opportunistic infections past 6 mo. | 4.6 | (1.2) |
Table 2:
Baseline stigma scores and psychological well-being
| Median | IQR | |
|---|---|---|
| Vicarious stigma (0–3) | 2.60 | (2.30 – 2.80) |
| Internalized stigma (0–3) | 2.88 | (2.75 – 3) |
| Stigma-avoidant coping (0–3) | 2.56 | (2.33 – 2.67) |
| Stigma fears family (0–3) | 2.67 | (2.33 – 3) |
| Stigma fears friends (0–3) | 3 | (3 – 3) |
| Stigma fears HCW (0–3) | 3 | (2.60 – 3) |
| Stigma fears work (0–3) | 3 | (3 – 3) |
| Stigma fears community (0–3) | 3 | (2.75 – 3) |
| Quality of life (0–3) | 0.30 | (0 – 0.40) |
| Frequency | Percent | |
| Any close friends/relatives or social support | 238 | 39.7 |
| Loneliness past 7 days | ||
| < 1day | 194 | 32.3 |
| 1–2 days | 311 | 51.8 |
| 3–4 days | 76 | 12.7 |
| 5–7 days | 19 | 3.2 |
Table 3.
Spearman’s correlation between stigma-related variables, social support and loneliness
| VS | AC | IS | SF fam | SF frn | SF hcw | SF work | SF comm. | SS | Lone- liness |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Vicarious stigma (VS) | 1 | |||||||||
| Avoidant coping (AC) | .29*** | 1 | ||||||||
| Internalized stigma (IS) | .34*** | .47*** | 1 | |||||||
| Stigma fears (SF) family | .39*** | .31*** | .34*** | 1 | ||||||
| Stigma fears friends | .19*** | .23*** | .30*** | .40*** | 1 | |||||
| Stigma fears HCW | .32*** | .31*** | .34*** | .55*** | .41*** | 1 | ||||
| Stigma fears work | .33*** | .17*** | .22*** | .32*** | .25*** | .39*** | 1 | |||
| Stigma fears comm | .24*** | .31*** | .38*** | .43*** | .53*** | .50*** | .21*** | 1 | ||
| Social support (SS) | −.16*** | −.16*** | −.09* | −.13** | −.06 | −.09* | −.02 | −.20*** | 1 | |
| Loneliness | .02 | −.10* | .03 | −.03 | −.13** | −.09* | .01 | .02 | −.09* | 1 |
comm community; fam family; frn friends; hcw health care workers; wrk people at work
p<.05;
p<.01;
p<.001
The results of the regression analyses of QOL (ln transformed) on HIV-stigma variables and other covariates are presented in Table 4. Given that for four of the five stigma fear variables the median was the maximum score, we dichotomized them for the regression analyses at <3 vs. 3. All stigma variables except the stigma fears related to HCW were negatively associated with QOL with at least marginal significance level in the unadjusted analyses, as were being unmarried and lack of social support. But in multivariable analyses, only internalized stigma remained significant (b = −0.23, 95% CI: −0.39 to −0.08, p =.013). Exponentiating the coefficient shows that a 1-unit increase on the internalized stigma scale was associated with, on average, a 21% decrease in the QOL score. Of the other variables, only social support was significant in the multivariable analyses (b = 0.11, 95% CI: 0.0002 to 0.21, p = .049). Those with social support on average had 11% higher QOL scores than those without.
Table 4.
Linear regression analyses for quality of life outcome
| Predictor | Unadjusted coefficient |
p un- adjusted |
Adjusted coefficient | 95% CI | p adjusted |
|---|---|---|---|---|---|
| Vicarious stigma | −0.13 | .015 | |||
| Avoidant coping | −0.20 | .007 | |||
| Intern. stigma | −0.26 | .005 | −0.23 | −0.39 to −0.08 | .013 |
| SF family | −0.09 | .040 | |||
| SF community | −0.12 | .066 | |||
| SF friends | −0.11 | .032 | |||
| SF work | −0.06 | .095 | |||
| Married | 0.03 | .015 | |||
| Social support | 0.13 | .062 | 0.11 | 0.0002 to 0.21 | .049 |
| SF HCW | −0.06 | .152 | |||
| Loneliness | 0.01 | .506 | |||
| Any education | −0.01 | .605 | |||
| Age | −0.002 | .400 | |||
| Hindu | 0.02 | .254 |
Italics not included in multivariable regression (unadjusted p > .10)
CI confidence interval; HCW health care workers; SF stigma fears
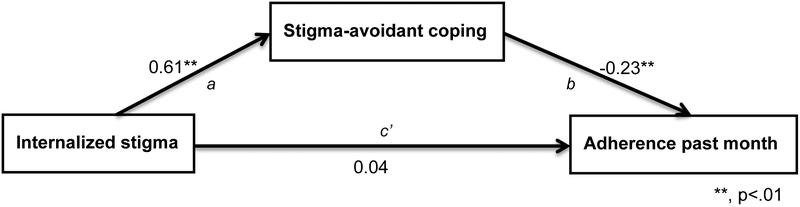
The results of the regression analyses of adherence in the past month (VAS) are presented in Table 5. Again stigma fears related to HCW was the only one of the stigma variables not retained for the initial multivariable regression analysis. In the final regression model, older age (b = 0.01, 95% CI: 0.002 to 0.01, p = .021) and longer time since diagnosis (b = 0.06, 95% CI: 0.04 to 0.09, p = .002) remained significantly positively associated with adherence, while stigma fears related to family (b = −0.07, 95% CI: −0.13 to −0.02, p = .022) and stigma avoidant coping (b = −0.23, 95% CI: −0.36 to −0.10, p = .009) were negatively related. Internalized stigma became non-significant (b = 0.04, 95% CI: −0.02 to 0.10, p = .165) but the coefficient also changed from negative to positive when put in the same model with stigma-avoidant coping, leading us to suspect mediation of the association between internalized stigma and adherence via stigma-avoidant coping (see Figure 1). Further analysis, controlled for age, time since diagnosis and family-related stigma fears, confirmed such a significant indirect effect: ab = −.14, (95% Percentile CI: −.23 to −.06). The total effect of internalized stigma on adherence (ab + c’) was −0.10 (p = .067).
Table 5:
Linear regression analyses for adherence past month outcome
| Predictor | Unadjusted coefficient | p un-adjusted | Adjusted coefficient | 95% CI | p adjusted |
|---|---|---|---|---|---|
| Vicarious stigma | −0.12 | .024 | |||
| Avoidant coping | −0.25 | .026 | −0.23 | −0.36 to −0.10 | .009 |
| Intern. stigma | −0.14 | .018 | 0.04 | −0.02 to 0.10 | .165 |
| SF-family | −0.12 | .004 | −0.07 | −0.13 to −0.02 | .022 |
| SF community | −0.08 | .063 | |||
| SF friends | −0.07 | .084 | |||
| SF work | −0.13 | .005 | |||
| Married | −0.08 | .074 | |||
| # mo. since diagnosis (ln) | 0.08 | .001 | 0.06 | 0.04 to 0.09 | .002 |
| Age | 0.01 | .033 | 0.01 | 0.002 to 0.01 | .021 |
| # Side effects | −0.04 | .075 | |||
| SF HCW | −0.04 | .424 | |||
| Social support | 0.06 | .262 | |||
| # OI | −0.05 | .268 | |||
| # OI quadratic | 0.001 | .743 | |||
| Any education | 0.02 | .411 | |||
| Hindu | 0.07 | .112 |
Italics not included in multivariable regression (unadjusted p > .10)
CI confidence interval; HCW health care workers; OI opportunistic infections; SF stigma fears
Figure 1:
Mediation of association between internalized stigma and adherence by stigma-avoidant coping.
DISCUSSION
The results reveal higher baseline levels of distress among the women in this study than in our previous studies of PLWH (2, 27). The women report having heard of or witnessed multiple acts of discrimination against other PLWH and endorse high levels of internalized stigma. Not surprisingly, they fear stigma and discrimination from multiple sources in their environment. Similar to our first studies among urban PLWH in India (2, 31), these reports are associated with the use of avoidant coping strategies, with loneliness and with a lack of social support. Specifically, women who reported higher levels of internalized stigma messages were also more likely to report a less social support and a lower quality of life, with each 1 point increase in internalized stigma being associated with a 21% drop in quality of life. All forms of stigma were associated with the use of avoidant coping strategies, such as lying to family members about the reason for clinic visits or pill taking. Since such strategies are designed to minimize disclosure, this also seems to have prevented the women from accessing much needed social support and feel lonely. It is not surprising that the use of disclosure avoidant strategies were more likely among women who reported fearing stigma and discrimination from family members and that internalized stigma and a lack of social support were also associated with reporting a lower quality of life.
The association between internalized stigma and adherence was mediated by the use of stigma-avoidant coping strategies among these women, suggesting that keeping one’s diagnosis a secret may have made it more difficult to take their medications on time. Given the very low levels of adherence in this population (41), the high levels of distress and internalized stigma as well as the lack of social support, suggest that these women constitute a very vulnerable population, who need additional services to optimize their health.
These results should be interpreted in light of several study limitations. Firstly, this was a cross-sectional analyses, which precludes us from drawing conclusions in terms of causality between the reported stigma, distress and adherence. Future papers will need to examine temporal relationships among these variables in longitudinal analyses. Secondly, the very low levels of adherence reported by our participants preclude us from generalizing the results to populations that may be optimally adherent in spite of experiencing stigma and discrimination. Finally, this study is restricted to mothers with young children in rural Andhra Pradesh, which may limit our ability to generalize the results to other Indian sub-populations living with HIV.
In spite of these limitations, the results of these analyses have several implications for future research. The fact that both age and time since diagnosis were independently associated with better adherence points to the need to conduct additional studies examining the factors that allow the older women and those who have lived longer with HIV to better manage their regimens despite the challenges they face. Those factors could then be used to develop strategies that could be taught to women who are struggling with their adherence. Since older women and women who have lived with their diagnoses a longer time appear more likely to adhere to their regimens, they may make good peer educators for younger women and those who are newly infected, by sharing their adherence strategies. By using peers to teach those strategies, such interventions may also help decrease the feelings of loneliness and lack of social support that many of these women report. It would also be helpful if future research could identify alternative, less isolating coping strategies that would not get in the way of accessing the social support that these women so sorely need. Finally, since it would not be fair to place the entire burden of change on these vulnerable women, the next generation of stigma research in this region also needs to include the development and evaluation of both community- and family-based stigma reduction interventions to decrease the stigma fears and the perceived need to use disclosure-avoidant stigma strategies by women living with HIV.
CONCLUSION
The very high levels of distress reported by these rural Indian mothers living with HIV are associated with fear and internalization of societal stigma, which in turn may be leading to disclosure avoidance, loneliness, lack of social support and poor medication adherence. Longitudinal research is needed to determine the causal pathways of these relationships as well as the impact of these factors on their children. Supportive interventions targeting this very vulnerable group are urgently needed and should, if possible, also include components to decrease HIV stigma levels among family and community members.
FUNDING
Support for this research was provided by an award R01MH098729 from the National Institute on Mental Health
Footnotes
Conflict of interest: the authors declare that they have no conflict of interests
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards
Informed consent: Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Goffman E Stigma: notes on the management of spoiled identity. New York, USA: Simon and Schuster; 2009. [Google Scholar]
- 2.Steward WT, Herek GM, Ramakrishna J, Bharat S, Chandy S, Wrubel J, et al. HIV-related stigma: adapting a theoretical framework for use in India. Soc Sci Med. 2008;67(8):1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekstrand ML, Ramakrishna J, Bharat S, Heylen E. Prevalence and drivers of HIV stigma among health providers in urban India: implications for interventions. J Int AIDS Soc. 2013;16(3 Suppl 2):18717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kontomanolis EN, Michalopoulos S, Gkasdaris G, Fasoulakis Z. The social stigma of HIV-AIDS: society’s role. HIV AIDS (Auckl). 2017;9:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams K, Haire BG, Nathan S. ‘They say God punishes people with HIV’: experiences of stigma and discrimination among adults with HIV in Dili, Timor-Leste. Cult Health Sex. 2017;19(10):1108–21. [DOI] [PubMed] [Google Scholar]
- 6.Bharat S, Ramakrishna J, Heylen E, Ekstrand ML. Gender-based attitudes, HIV misconceptions and feelings towards marginalized groups are associated with stigmatization in Mumbai, India. J Biosoc Sci. 2014;46(6):717–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekstrand ML, Bharat S, Ramakrishna J, Heylen E. Blame, symbolic stigma and HIV misconceptions are associated with support for coercive measures in urban India. AIDS Behav. 2012;16(3):700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrey AE, Bilsen J, Lacor P, Deschepper R. Perceptions of stigma and discrimination in health care settings towards sub-Saharan African migrant women living with HIV/AIDS in Belgium: a qualitative study. J Biosoc Sci. 2017;49(5):578–96. [DOI] [PubMed] [Google Scholar]
- 9.Nyblade L, Stangl A, Weiss E, Ashburn K. Combating HIV stigma in health care settings: what works? J Int AIDS Soc. 2009;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiriazova T, Lunze K, Raj A, Bushara N, Blokhina E, Krupitsky E, et al. “It is easier for me to shoot up”: stigma, abandonment, and why HIV-positive drug users in Russia fail to link to HIV care. AIDS Care. 2017;29(5):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee C, Fan Y, Starr JR, Dogon IL. Dentists’ and dental students’ attitudes, knowledge, preparedness, and willingness related to treatment of people living with HIV/AIDS in China. J Public Health Dent. 2017;77(1):30–8. [DOI] [PubMed] [Google Scholar]
- 12.Clark KA, Keene DE, Pachankis JE, Fattal O, Rizk N, Khoshnood K. A qualitative analysis of multi-level barriers to HIV testing among women in Lebanon. Cult Health Sex. 2017;19(9):996–1010. [DOI] [PubMed] [Google Scholar]
- 13.Vorasane S, Jimba M, Kikuchi K, Yasuoka J, Nanishi K, Durham J, et al. An investigation of stigmatizing attitudes towards people living with HIV/AIDS by doctors and nurses in Vientiane, Lao PDR. BMC Health Serv Res. 2017;17(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarei N, Joulaei H, Darabi E, Fararouei M. Stigmatized attitude of healthcare providers: a barrier for delivering health services to HIV positive patients. Int J Community Based Nurs Midwifery. 2015;3(4):292–300. [PMC free article] [PubMed] [Google Scholar]
- 15.Arora KS, Wilkinson B. Eliminating perinatal HIV transmission in the United States: the impact of stigma. Matern Child Health J. 2017;21(3):393–7. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Wu Z, Liang LJ, Lin C, Guan J, Jia M, et al. Reducing HIV-related stigma in health care settings: a randomized controlled trial in China. Am J Public Health. 2013;103(2):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varas-Diaz N, Neilands TB, Cintron-Bou F, Marzan-Rodriguez M, Santos-Figueroa A, Santiago-Negron S, et al. Testing the efficacy of an HIV stigma reduction intervention with medical students in Puerto Rico: the SPACES project. J Int AIDS Soc. 2013;16(3 Suppl 2):18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyblade L, Jain A, Benkirane M, Li L, Lohiniva AL, McLean R, et al. A brief, standardized tool for measuring HIV-related stigma among health facility staff: results of field testing in China, Dominica, Egypt, Kenya, Puerto Rico and St. Christopher & Nevis. J Int AIDS Soc. 2013;16(3 Suppl 2):18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah SM, Heylen E, Srinivasan K, Perumpil S, Ekstrand ML. Reducing HIV stigma among nursing students: a brief intervention. West J Nurs Res. 2014;36(10):1323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frain JA. Preparing every nurse to become an HIV nurse. Nurse Educ Today. 2017;48:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stangl AL, Grossman CI. Global action to reduce HIV stigma and discrimination. J Int AIDS Soc. 2013;16(3(Suppl 2)):18934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geibel S, Hossain SM, Pulerwitz J, Sultana N, Hossain T, Roy S, et al. Stigma reduction training improves healthcare provider attitudes toward, and experiences of, young marginalized people in Bangladesh. J Adolesc Health. 2017;60(2S2):S35–S44. [DOI] [PubMed] [Google Scholar]
- 23.Lohiniva AL, Kamal W, Benkirane M, Numair T, Abdelrahman M, Saleh H, et al. HIV stigma toward people living with HIV and health providers associated with their care: qualitative interviews with community members in Egypt. The Journal of the Association of Nurses in AIDS Care : JANAC. 2016;27(2):188–98. [DOI] [PubMed] [Google Scholar]
- 24.Olley BO, Adebayo KO, Ogunde MJ, Ishola A, Ogar AP. Psychosocial factors predicting severity of depression among treatment-seeking HIV/AIDS patients: a multi-site Nigerian study. Niger J Clin Pract. 2017;20(3):296–302. [DOI] [PubMed] [Google Scholar]
- 25.Tesfaw G, Ayano G, Awoke T, Assefa D, Birhanu Z, Miheretie G, et al. Prevalence and correlates of depression and anxiety among patients with HIV on-follow up at Alert Hospital, Addis Ababa, Ethiopia. BMC psychiatry. 2016;16(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitew H, Andargie G, Tadesse A, Belete A, Fekadu W, Mekonen T. Suicidal ideation, attempt, and determining factors among HIV/AIDS patients, Ethiopia. Depress Res Treat. 2016;2016:8913160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steward WT, Chandy S, Singh G, Panicker ST, Osmand TA, Heylen E, et al. Depression is not an inevitable outcome of disclosure avoidance: HIV stigma and mental health in a cohort of HIV-infected individuals from Southern India. Psychol Health Med. 2011;16(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linda P To tell or not to tell: negotiating disclosure for people living with HIV on antiretroviral treatment in a South African setting. SAHARA J. 2013;10 Suppl 1:S17–27. [DOI] [PubMed] [Google Scholar]
- 29.Turan JM, Bukusi EA, Onono M, Holzemer WL, Miller S, Cohen CR. HIV/AIDS stigma and refusal of HIV testing among pregnant women in rural Kenya: results from the MAMAS Study. AIDS Behav. 2011;15(6):1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sen S, Nguyen HD, Kim SY, Aguilar J. HIV knowledge, risk behavior, stigma, and their impact on HIV testing among Asian American and Pacific Islanders: a review of literature. Soc Work Public Health. 2017;32(1):11–29. [DOI] [PubMed] [Google Scholar]
- 31.Steward WT, Bharat S, Ramakrishna J, Heylen E, Ekstrand ML. Stigma is associated with delays in seeking care among HIV-infected people in India. J Int Assoc Provid AIDS Care. 2013;12(2):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rueda S, Mitra S, Chen S, Gogolishvili D, Globerman J, Chambers L, et al. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ open. 2016;6(7):e011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dos Santos MM, Kruger P, Mellors SE, Wolvaardt G, van der Ryst E. An exploratory survey measuring stigma and discrimination experienced by people living with HIV/AIDS in South Africa: the People Living with HIV Stigma Index. BMC Public Health. 2014;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai AC, Bangsberg DR, Kegeles SM, Katz IT, Haberer JE, Muzoora C, et al. Internalized stigma, social distance, and disclosure of HIV seropositivity in rural Uganda. Ann Behav Med. 2013;46(3):285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundberg PC, Doan TT, Dinh TT, Oach NK, Le PH. Caregiving to persons living with HIV/AIDS: experiences of Vietnamese family members. Journal of clinical nursing. 2016;25(5–6):788–98. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Mohanraj R, Rao D, Murray KR, Manhart LE. Positive coping strategies and HIV-related stigma in south India. AIDS Patient Care STDS. 2015;29(3):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charles B, Jeyaseelan L, Pandian AK, Sam AE, Thenmozhi M, Jayaseelan V. Association between stigma, depression and quality of life of people living with HIV/AIDS (PLHA) in South India - a community based cross sectional study. BMC Public Health. 2012;12:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohite VR, Mohite RV, George J. Correlates of perceived stigma and depression among the women with HIV/AIDS infection. Bangladesh J Med Sci. 2015;14(2):151–8. [Google Scholar]
- 39.Mayston R, Lazarus A, Patel V, Abas M, Korgaonkar P, Paranjape R, et al. Pathways to HIV testing and care in Goa, India: exploring psychosocial barriers and facilitators using mixed methods. BMC Public Health. 2016;16(1):765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balasundaram A, Sarkar S, Hamide A, Lakshminarayanan S. Socioepidemiologic profile and treatment-seeking behaviour of HIV/AIDS patients in a tertiary-care hospital in south India. J Health Popul Nutr. 2014;32(4):587–94. [PMC free article] [PubMed] [Google Scholar]
- 41.Nyamathi A, Ekstrand M, Heylen E, Ramakrishna P, Yadav K, Sinha S, et al. Relationships among adherence and physical and mental health among women living with HIV in rural India. AIDS Behav. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahangdale L, Banandur P, Sreenivas A, Turan JM, Washington R, Cohen CR. Stigma as experienced by women accessing prevention of parent-to-child transmission of HIV services in Karnataka, India. AIDS Care. 2010;22(7):836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shet A, Kumarasamy N, Poongulali S, Shastri S, Kumar DS, Rewari BB, et al. Longitudinal analysis of adherence to first-line antiretroviral therapy: evidence of treatment sustainability from an Indian HIV cohort. Curr HIV Res. 2016;14(1):71–9. [DOI] [PubMed] [Google Scholar]
- 44.Kleinman NJ, Manhart LE, Mohanraj R, Kumar S, Jeyaseelan L, Rao D, et al. Antiretroviral therapy adherence measurement in non-clinical settings in South India. AIDS Care. 2015;27(2):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George MS, Lambert H. ‘I am doing fine only because I have not told anyone’: the necessity of concealment in the lives of people living with HIV in India. Cult Health Sex. 2015;17(8):933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madi D, Gupta P, Achappa B, Bhaskaran U, Ramapuram JT, Rao S, et al. HIV status disclosure among people living with HIV in the era of combination antiretroviral therapy (cART). J Clin Diagn Res. 2015;9(8):OC14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mittal D, Babitha GA, Prakash S, Kumar N, Prashant GM. Knowledge and attitude about HIV/AIDS among HIV-positive individuals in Davangere. Soc Work Public Health. 2015;30(5):423–30. [DOI] [PubMed] [Google Scholar]
- 48.Kumar N, Unnikrishnan B, Thapar R, Mithra P, Kulkarni V, Holla R, et al. Stigmatization and discrimination toward people living with HIV/AIDS in a coastal city of South India. J Int Assoc Provid AIDS Care. 2017;16(3):226–32. [DOI] [PubMed] [Google Scholar]
- 49.Ho SS, Holloway A. The impact of HIV-related stigma on the lives of HIV-positive women: an integrated literature review. Journal of clinical nursing. 2016;25(1–2):8–19. [DOI] [PubMed] [Google Scholar]
- 50.Bi S, Klusty T. Forced sterilizations of HIV-positive women: a global ethics and policy failure. AMA J Ethics. 2015;17(10):952–7. [DOI] [PubMed] [Google Scholar]
- 51.Ashaba S, Kaida A, Coleman JN, Burns BF, Dunkley E, O’Neil K, et al. Psychosocial challenges facing women living with HIV during the perinatal period in rural Uganda. PLoS One. 2017;12(5):e0176256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyamathi AM, William RR, Ganguly KK, Sinha S, Heravian A, Albarran CR, et al. Perceptions of women living with AIDS in rural India related to the engagement of HIV-trained accredited social health activists for care and support. J HIV AIDS Soc Serv. 2010;9(4):385–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nyamathi A, Ekstrand M, Srivastava N, Carpenter CL, Salem BE, Al-Harrasi S, et al. ASHA-Life intervention perspectives voiced by rural Indian women living with AIDS. Health Care Women Int. 2016;37(4):412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nyamathi A, Ekstrand M, Zolt-Gilburne J, Ganguly K, Sinha S, Ramakrishnan P, et al. Correlates of stigma among rural Indian women living with HIV/AIDS. AIDS Behav. 2013;17(1):329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malave S, Ramakrishna J, Heylen E, Bharat S, Ekstrand ML. Differences in testing, stigma, and perceived consequences of stigmatization among heterosexual men and women living with HIV in Bengaluru, India. AIDS Care. 2014;26(3):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Hollen C HIV/AIDS and the gendering of stigma in Tamil Nadu, South India. Cult Med Psychiatry. 2010;34(4):633–57. [DOI] [PubMed] [Google Scholar]
- 57.Sinha G, Peters DH, Bollinger RC. Strategies for gender-equitable HIV services in rural India. Health Policy Plan. 2009;24(3):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biradavolu MR, Blankenship KM, Jena A, Dhungana N. Structural stigma, sex work and HIV: contradictions and lessons learnt from a community-led structural intervention in southern India. J Epidemiol Community Health. 2012;66 Suppl 2:ii95–9. [DOI] [PubMed] [Google Scholar]
- 59.Nyamathi AM, Ekstrand M, Yadav K, Ramakrishna P, Heylen E, Carpenter C, et al. Quality of life among women living with HIV in rural India. The Journal of the Association of Nurses in AIDS Care : JANAC. 2017;28(4):575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 61.Ekstrand ML, Chandy S, Heylen E, Steward W, Singh G. Developing useful HAART adherence measures for India: the Prerana study. J Acquir Immune Defic Syndr. 2010;53(3):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ekstrand ML, Shet A, Chandy S, Singh G, Shamsundar R, Madhavan V, et al. Suboptimal adherence associated with virological failure and resistance mutations to first-line highly active antiretroviral therapy (HAART) in Bangalore, India. Int Health. 2011;3(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. [DOI] [PubMed] [Google Scholar]
- 64.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. Hiv Clin Trials. 2004;5(2):74–9. [DOI] [PubMed] [Google Scholar]
- 65.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–77. [DOI] [PubMed] [Google Scholar]
- 66.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–6. [PubMed] [Google Scholar]
- 67.Allison PD. Multiple regression: A primer. California, USA: Pine Forge Press; 1999. [Google Scholar]
- 68.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–91. [DOI] [PubMed] [Google Scholar]
- 69.Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: Does method really matter? Psychol Sci. 2013;24(10):1918–27. [DOI] [PubMed] [Google Scholar]