Abstract
Current chemotherapeutic approaches for cancer are in part limited by the inability of drugs to destroy neoplastic cells within poorly vascularized compartments of tumors. We have here systematically assessed anaerobic bacteria for their capacity to grow expansively within avascular compartments of transplanted tumors. Among 26 different strains tested, one (Clostridium novyi) appeared particularly promising. We created a strain of C. novyi devoid of its lethal toxin (C. novyi-NT) and showed that intravenously injected C. novyi-NT spores germinated within the avascular regions of tumors in mice and destroyed surrounding viable tumor cells. When C. novyi-NT spores were administered together with conventional chemotherapeutic drugs, extensive hemorrhagic necrosis of tumors often developed within 24 h, resulting in significant and prolonged antitumor effects. This strategy, called combination bacteriolytic therapy (COBALT), has the potential to add a new dimension to the treatment of cancer.
Despite enormous progress in understanding the pathophysiology of neoplasia, advanced forms of cancer remain recalcitrant to treatment. Although the basis for this failure is complex, one reason is that most tumors contain large, poorly vascularized areas that limit the efficacy of radiation and chemotherapeutic drugs (1). The poorly vascularized regions are less sensitive to ionizing radiation because its cell-killing effects depend on oxygen; they are less sensitive to chemotherapeutic drugs because drug delivery to these regions is suboptimal. Because a cancer therapeutic agent must not leave significant clusters of viable cells within any lesion to achieve a clinically meaningful effect, the poorly vascularized regions of tumors represent a major obstacle to effective treatment.
One of the most important recent developments in tumor biology is the recognition that neoangiogenesis is essential for the growth of tumors to clinically meaningful sizes (2). What is less well recognized is that this neoangiogenesis often does not keep pace with the growth of the neoplastic cells, resulting in large necrotic areas composed of dead or dying cells. For example, we found that each of 20 randomly selected liver metastases >1 cm3 in size contained relatively large avascular regions, in general constituting 25–75% of the tumor mass (Fig. 1). Importantly, cells adjacent to these necrotic areas are poorly vascularized and likely to be difficult to treat with conventional agents.
Figure 1.
Typical human colorectal metastases. Extensive areas of necrosis, such as one one indicated by arrows, are intermixed with areas of viable tumor cells. Similar large areas of necrosis were observed in each of the metastatic lesions from 20 different patients chosen at random from pathologic archives.
In the work described here we attempted to exploit the fact that necrotic regions exist only within tumors and not in normal tissues. We wished to develop a toxic agent that could be specifically delivered to these areas and, in theory, could kill surrounding viable tumor cells. We chose to investigate anaerobic bacteria for this purpose. It has been recognized for half a century that such bacteria could selectively proliferate in the hypoxic regions of tumors (3–18). Clever strategies for potentially exploiting such bacteria for diagnostic and therapeutic purposes have been devised, although relatively little work in this area has recently taken place. We hoped that a systematic screen for appropriate anaerobic bacteria that could kill tumor cells adjacent to the poorly vascularized regions, rather than just localize to such regions, would rejuvenate interest in this approach. Furthermore, we hoped that chemotherapeutic agents that killed the well vascularized regions of tumors, when administered in conjunction with appropriate bacteria, would result in the destruction of a major proportion of neoplastic cells within the tumors. Our progress toward realizing these goals is described below.
Materials and Methods
Bacterial Strains and Growth.
The bacterial strains tested in this study were purchased from the American Type Culture Collection and are listed in Table 1. All bacteria except Lactobacilli were grown anaerobically in liquid cultures at 37°C in Reinforced Clostridial Medium (RCM) (Difco). Lactobacilli were grown in Lactobacilli MRS broth (Difco). Intravenous injections of bacteria generally included 5 × 107 bacteria suspended in 0.5 ml Dulbecco's PBS (Life Technologies). Mice that received injections of Bifidobacteria were also given i.p. injections of lactulose daily for 5 days to increase bacterial growth (16). Intratumoral injections of bacteria generally included 1 × 107 bacteria suspended in 0.1 ml of PBS.
Table 1.
Bacterial strains tested
| Genus and species | ATCC no. |
|---|---|
| Bifidobacteria | |
| B. adolescentis | 15703 |
| B. animalis | 25527 |
| B. bifidum | 11863, 15696 |
| B. boum | 27917 |
| B. breve | 15700 |
| B. coryneforme | 25911 |
| B. dentium | 15423, 27534 |
| B. indicum | 25912 |
| B. infantis | 15702, 25962 |
| B. longum | 15707 |
| B. magnum | 27540 |
| B. pseudolongum | 25526 |
| Lactobacilli | |
| L. bifidus | 11146 |
| L. delbrueckii | 21815 |
| Clostridia | |
| C. absonum | 27555 |
| C. acetobutylicum | 824 |
| C. bifermentans | 17836 |
| C. difficile | 700057 |
| C. histolyticum | 19401 |
| C. novyi | 19402 |
| C. perfringens | 3624, 13124 |
| C. sordellii | 9714 |
ATCC, American Type Culture Collection.
Drugs.
Dolastatin-10 (D10) was provided by George R. Pettit (Cancer Research Institute, Arizona State University, Tempe, AZ), Gregory P. Kalemkerian (Department of Internal Medicine, Wayne State University, Detroit), and Robert J. Schultz (Drug Synthesis and Chemistry Branch, National Cancer Institute, Bethesda). Combretastatin A-4 was kindly provided by Robert J. Schultz. Cytoxan (CTX), mitomycin C (MMC), vincristine, colchicine, and vinblastine are commercially available chemotherapeutic agents (Sigma).
Cell Lines and Animals.
Female athymic nude and C57BL/6 mice 6–8 weeks of age were purchased from Harlan (Indianapolis). HCT116 colon cancer cells and B16 melanoma cells were grown as monolayers in McCoy 5A medium (Life Technologies, Rockville, MD) supplemented with 5% FBS and 1% penicillin/streptomycin (catalog no. 15140-122).
Sporulation and Generation of a Nontoxigenic C. novyi Strain.
Spores of Clostridium novyi strains were generated by growing the organisms anaerobically at 37°C, pH 7.4 in a medium containing 5 g Na2HPO4, 30 g peptone, 0.5 g L-cysteine, 10 g maltose, and 5% wt/vol dried cooked meat particles (Difco) per 1 liter. After 1 week in this medium, spores settled in the cooked meat particle layer (19). Spores were further purified from contaminating vegetative forms on a discontinuous Percoll gradient. To remove the lethal toxin gene from the wild-type C. novyi strain, C. novyi spores were heated at 70°C for 15 min to inactivate the phage carrying the toxin (20, 21). The spores were then plated on reinforced clostridial medium agar and incubated anaerobically at 37°C for 48 h. Isolated colonies were cultured in liquid RCM for another 24 to 48 h and then tested for the presence of the lethal toxin gene by PCR.
In Vivo Studies.
Six to eight week old female BALB/c athymic nude or C57BL/6 mice were implanted with s.c. tumors through the injection of 2.5 × 106 HCT116 or B16 cells, respectively. After 8–12 days of tumor establishment, treatment was initiated with spores or drugs. Screening of bacterial strains for their ability to populate tumor grafts was done by either intratumoral injection (100 μl volume, 1 × 107 bacteria) or i.v. injection (500 μl volume, 5 × 107 bacteria or spores) into the tail vein. C. novyi-NT spores and D10 were diluted to the appropriate concentration in PBS and then administered by i.v. injection in a volume of 500 μl. CTX and MMC were diluted in PBS and then given by i.p. injection in a volume of 500 μl. Tumor growth was assessed by measuring the size of the major and minor axes of s.c. tumors every 2 and every 4 days for B16 and HCT116 tumors, respectively, using calipers. Tumor volume was then calculated by using the equation length × width2 × 0.5.
Results
Choice Of Bacterial Species.
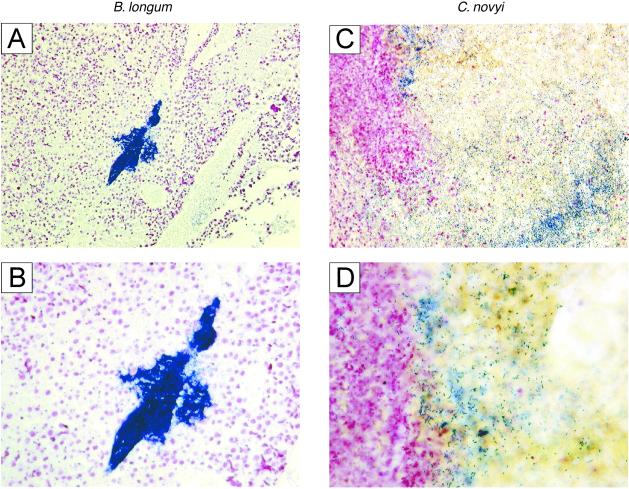
From previous studies it was clear that species of anaerobic bacteria could grow within the hypoxic regions of tumors. An example is provided by Bifidobacterium longum, which, when injected intravenously into mice with s.c. tumors, grew specifically and robustly within the tumors but not within normal tissues (16, 17). Gram stains of sections of the tumors, however, revealed that most bacteria were tightly clustered within colonies rather than distributed throughout the necrotic regions (Fig. 2 A and B). As we considered dispersion of the bacteria essential to achieve the desired effects, numerous anaerobic species of three different genera were tested in an effort to find one(s) exhibiting this phenotype (Table 1). For this purpose, Bifidobacterium and Lactobacillus strains were injected intravenously, whereas Clostridium strains, which are generally highly toxic when injected intravenously, were injected directly into tumors. Among the 26 strains listed in Table 1, only two (C. novyi and C. sordellii) exhibited extensive spreading throughout the poorly vascularized portions of the tumors (data not shown). Although this spread was undoubtedly facilitated by the motile nature of these two species, other motile anaerobic bacteria, including other Clostridium strains, did not exhibit this property when tested under identical conditions.
Figure 2.
Distribution of anaerobic bacteria within tumors. Mice bearing s.c. B16 tumors were intravenously injected through the tail vein with 5 × 107 live B. longum bacteria or wild-type C. novyi spores. Mice that received injections of B. longum were given i.p. injections of lactulose daily for 5 days to increase bacterial growth (16) and then killed for analysis of tumor colonization. Mice with C. novyi were killed the day after injection for analysis. Gram-stains revealed that a large number of B. longum bacteria was concentrated within a few colonies, whereas C. novyi was dispersed throughout the poorly vascularized portions of the tumors. (A and B) High- and low-power views of representative B. longum experiment, showing bacteria (stained deep blue) clustered within a colony. (C) C. novyi experiment, showing dispersion of bacteria throughout the necrotic region of the tumor. (D) High-power view, showing invasion of C. novyi bacteria into surrounding viable tumor cells (stained purple) on the Left.
Infiltration of the Tumor Mass Following i.v. Injection of C. novyi Spores.
For an experimental therapy to represent a potentially viable tool for the treatment of disseminated cancers, it must have the capacity to be delivered systemically rather than through local, intratumoral injection. Although live bacteria are often toxic when injected intravenously, it has been shown that bacterial spores are nontoxic to normal animals. Accordingly, we found that large numbers (up to 108 in a volume of 500 μl) of C. novyi and C. sordellii spores could be injected intravenously into normal mice without causing any noticeable side effects. When intravenously injected into mice with s.c. B16 tumors, however, C. novyi bacteria floridly germinated within the tumors within 16 h (Fig. 2C). In contrast, no germinated bacteria were observed in the liver, spleen, kidney, lung, or brain of these mice (data not shown). Similar results were observed after i.v. injection of C. sordellii spores (data not shown).
Genetic Modification of C. novyi.
Although C. novyi and C. sordellii spores both had the capacity to grow within tumors and kill some surrounding tumor cells, there was at least one “small” problem encountered with this experimental treatment: 16–18 h following the initiation of treatment, all of the mice died. We suspected that the cause of death was the release of potent lethal toxins from the bacteria germinating within the tumors. Indeed, other anaerobic bacterial spores have proved highly toxic to animals and humans following germination within the anaerobic environments present in tumors or wounds, and the resultant mortality was shown to be due to specific secreted toxins (22–26).
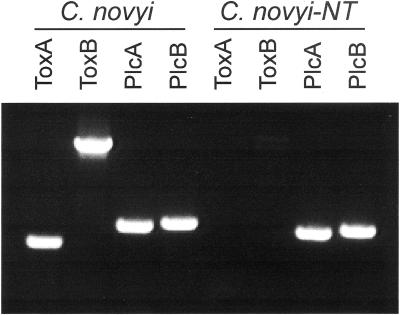
To mitigate systemic toxicity, we attempted to eliminate the lethal toxin gene from C. novyi. We chose C. novyi rather than C. sordellii for this purpose because the latter has two homologous toxin genes (27) rather than one and because the single C. novyi toxin gene is located within a phage episome (20, 21, 28). Bacteria were heat treated to induce loss of the phage and inoculated onto agar plates. Of 400 bacterial colonies screened, three were observed to have lost the toxin gene when assessed by PCR using toxin gene-specific primers (examples in Fig. 3). Phospholipase C, a C. novyi gene contained within the bacterial rather than the phage genome (29), served as control for this PCR experiment. One clone, named C. novyi-NT, that had lost the toxin gene was selected for further analysis.
Figure 3.
Elimination of the lethal toxin gene from C. novyi. Following heat shock, PCR was performed on DNA from colonies to identify those which had lost the lethal toxin gene on the phage episome. Agarose gel electrophoresis of the PCR products with two independent primer sets (ToxA and ToxB) shows results from a C. novyi clone (C. novyi-NT) that had lost the gene and another clone (indicated as “C. novyi”) that retained the gene. Controls were provided by primer sets (PlcA and PlcB) specific for the C. novyi phospholipase C gene demonstrating the integrity of the DNA templates in all reactions.
Destruction of Tumor Cells Following Injection of C. novyi-NT Spores.
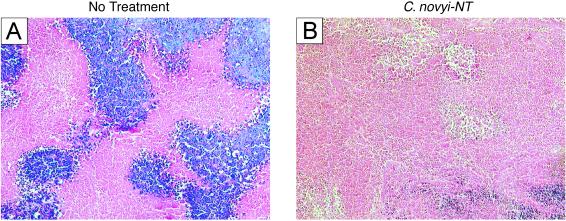
C. novyi-NT spores devoid of the lethal toxin were injected intravenously into mice with tumors. These spores retained their capacity to germinate within tumors and resulted in greatly expanded areas of necrosis (Fig. 4 A vs. B). However, these spores, unlike those of their parents, were nontoxic when injected alone, with no ill effects generally observed after injection of up to 108 spores into mice with tumors. In contrast, all mice died after injection of 5 × 107 parental C. novyi spores into mice with tumors. Growing bacteria could be observed throughout the much-enlarged necrotic regions of tumors after injection of spores (data not shown). The enlargement of the necrotic regions was apparently due to the destruction of viable tumor cells adjacent to the original necrotic regions by the bacteria. Indeed, a bacterial “film” (30) was routinely observed at the interface between the necrotic area and the remaining viable rim of the tumor, as if the bacteria were destroying the viable tumor cells and using its degradation products as nutrients (data not shown). This tumor infiltration effect was similar to that observed with wild-type C. novyi bacteria (Fig. 2D).
Figure 4.
Distribution of C. novyi-NT bacteria after i.v. injection of spores. (A) Hematoxylin/eosin (H&E) stain of a typical HCT116 tumor xenograft from a mouse not injected with bacteria, showing some necrosis. (B) H&E stain of a tumor 24 h after i.v. injection of 5 × 107 C. novyi-NT spores, showing much more extensive necrosis.
Combination Therapy.
As noted in the introduction, we hoped to combine a bacterial agent with more conventional chemotherapeutic agents in an effort to attack the tumors from both the inside and outside, respectively. Following preliminary investigations with several such agents, we concentrated on two classes: (i) DNA damaging agents, such as MMC and CTX, that selectively kill tumor cells, and (ii) agents that appear to partially collapse tumor vasculature, such as flavone acetic acid derivatives and microtubule binding agents (31, 32). The latter class of agents has been shown to be able to interfere with proper circulation through the tumors and thereby trap large molecules, such as antibodies or bacteria, that have gained access to the tumor tissue (33–35). Among flavone acetic acid and the microtubule-binding agents tested (including vinblastine, vincristine, colchicine, combretastatin A-4, and D10), D10 appeared to have the most pronounced effects and was chosen for further experimentation.
Xenografts of the colorectal cancer cell line HCT116 were used to test the effects of this combination therapy in nude mice because the tumors could easily be visualized under the hairless skin. As shown in Fig. 5, sequential treatment with C. novyi-NT spores, D10, and MMC resulted in dramatic effects on large s.c. tumors, easily observable through the skin. Twenty four hours after the injection of C. novyi-NT spores, the tumor mass swelled and became edematous (Fig. 5A). Six hours after receiving D10, a black spot developed near the center of the tumor, representing an area of hemorrhagic necrosis. This spot expanded in size and within 24 h often completely enveloped the tumor (Fig. 5A, 1 day). Hematoxylin/eosin (H&E) staining of sections of these tumors revealed extensive destruction of the tumors, often accompanied by infiltration of inflammatory cells (data not shown). These necrotic masses then shrank over a period of 2–4 weeks (Fig. 5A, 14–30 days). In many mice, these necrotic masses eventually dissolved and disappeared, leaving the animals tumor-free (Fig. 5B; quantified below). Similar, although less dramatic, results were observed following the sequential treatment with C. novyi-NT and D10 (without MMC), but never with D10 and MMC in the absence of C. novyi-NT and rarely with C. novyi-NT alone.
Figure 5.
Hemorrhagic necrosis following COBALT. (A) HCT116 tumor-bearing mouse 24 h after i.v. injection with 5 × 107 C. novyi-NT spores. Slight swelling associated with edema is seen at the tumor site. D10 (0.3 mg/kg) was then given intravenously (time = 0), and followed 24 h later with MMC (4 mg/kg). A black spot indicating hemorrhagic necrosis is evident near the center of the tumor at 0.3 days. The area of hemorrhagic necrosis gradually expanded over the next day (time = day 1). Swelling at the tumor site then resolved and the necrotic tumor mass shrunk and gradually dissolved (time = days 2–30). (B) Selected mice 5 weeks after treatment with a single dose of D10 plus MMC (Upper) or with a single dose of COBALT (Lower). Of the eight mice treated with COBALT in this experiment, four were apparently cured of their tumor, and three of these are shown (see text and Fig. 6A).
The dramatic antineoplastic effects of this combination bacteriolytic therapy (COBALT) were associated with significant toxicity. Approximately 15% of animals with tumors of 350 mm3 in size died within 24–72 h of receiving COBALT. This toxicity was clearly related to the size of the tumors, because ≈45% of animals with larger tumors (≈700 mm3) died. Deaths were not observed after administration of C. novyi-NT spores alone or with chemotherapy alone. Although the reason for the deaths of these animals was not clear, they may have been due to tumor lysis syndrome, a phenomenon previously observed in the clinic when large tumor burdens are rapidly destroyed by antineoplastic agents (see Discussion).
The antineoplastic effects of COBALT were quantified in the experiments shown in Fig. 6. Animals with relatively large s.c. HCT116 tumors (starting tumor volume ≈700 mm3) were treated with drugs alone (D10 plus MMC) or C. novyi-NT spores plus the drugs. As can be seen in Fig. 6A, the drugs alone slowed the growth of the tumors, although the tumors continued to grow and the animals had to be killed at 10–14 days, when tumor weights exceeded 10% of body weight. The addition of C. novyi-NT spores dramatically enhanced the effects of treatment, with tumors actually shrinking rather than simply slowing. In the experiment shown in Fig. 6A, seven of eight animals had dramatic tumor regressions after only one administration of COBALT, and four of the five animals that survived the therapy were completely cured, with no evidence of tumor regrowth after a further three months time. Significant tumor shrinkage was also seen when mice were given sequential treatment with C. novyi-NT spores plus D10 (Fig. 6B). However, there was no long-term tumor-free survival and the treatment had to be repeated once every 2 weeks unless the full combination, including MMC, was included. This repetition was associated with additional toxicity, including deaths of ≈15% of animals with each dose. The full COBALT regimen was therefore preferred on the basis of increased efficacy and reduced overall toxicity.
Figure 6.
Quantification of the effects of COBALT. (A) HCT116 colorectal cancer cells were grown as xenografts in nude mice. When the tumors were ≈700 mm3 in size, the animals were injected intravenously with 5 × 107 C. novyi-NT spores (time 0), followed by i.v. injection with D10 (0.3 mg/kg) at 24 h and i.p. injection with MMC (4 mg/kg) at 48 h. Control groups were given no treatment or treated with D10 plus MMC without spores. Each group consisted of six to ten mice. Animals were killed when their tumors exceeded 10% of body weight. In the experiment shown, seven of eight mice treated with a single dose of COBALT developed a striking hemorrhagic necrosis of their tumors within 24 h after administration of D10. Four of these seven mice were cured, whereas three of the mice died three days after treatment, perhaps from tumor lysis syndrome (see Discussion). One mouse developed less extensive necrosis and its tumor eventually regrew. Only mice that survived treatment were used to obtain the data plotted in the graph. (B) Mice were treated as in A, except that MMC was not used and treatments were given once every 2 weeks. (C) B16 melanoma cells were grown as s.c. syngeneic tumors in C57BL/6 mice. When the tumors were ≈700 mm3 in size, the animals were injected intravenously with 5 × 107 C. novyi-NT spores (time 0), followed by i.p. injection with CTX (100 mg/kg) at 6 h and i.v. injection with D10 (0.3 mg/kg) at 24 h. Other groups were given no treatment, CTX plus D10, or spores plus D10. Each group consisted of at least ten mice and the treatments were repeated at weekly intervals. Four mice died after the first dose of COBALT and only those mice that survived treatment were used to obtain the data plotted in the graph. Animals were killed when their tumors exceeded 10% of body weight.
To determine whether COBALT would affect other tumor types, we treated C57BL/6 mice with large syngeneic B16 tumors. In this case, CTX was substituted for MMC, because B16 tumor cells were more sensitive to CTX than to MMC. The drugs alone had some antitumor effects, as expected, although the tumor continued to grow in size and the animals had to be killed within 1 week after beginning therapy (Fig. 6C). C. novyi-NT spores considerably enhanced these effects: the tumors were observed to shrink rather than simply enlarge at a slower rate (Fig. 6C). D10 plus C. novyi-NT spores (without CTX) had significant antineoplastic effects on B16 tumors, but the addition of the tumor cytotoxic agent (CTX) further enhanced the efficacy of COBALT (Fig. 6C). In the B16 tumor model, maintenance COBALT (once weekly) was required to keep the tumors from regrowing, whereas with HCT116 cells a single treatment cured about half the mice.
Discussion
The results recorded above show that COBALT can result in rapid and dramatic regressions of experimental tumors in mice. Even relatively large tumors could be treated successfully with COBALT, although tumors of the size used in our experiments do not generally respond well to chemotherapeutic agents (Figs. 5 and 6).
It is also clear that many questions remain. For example, the basis for the potent tumor cell killing in the vicinity of the germinating bacteria is not understood. We found that many other bacterial strains could germinate within the necrotic regions of tumors but did not exhibit this potent cytotoxic activity. This killing is clearly not due to the lethal toxin gene of C. novyi, because this gene was deleted in the C. novyi-NT strain used in COBALT. It will be interesting in the future to determine which of the C. novyi-NT genes are responsible for these tumor cytolytic effects.
Another point of interest was that an agent acting on the vasculature (D10) considerably enhanced the ability of C. novyi-NT spores to lyse tumors. Presumably, the vascular collapse further lowered the oxygen tension near the trapped bacteria and thereby increased the potential for bacterial growth. Indeed it has been demonstrated that vascular collapsing agents can increase the germination of bacterial spores in tumors (33). D10 was given after the bacterial spores rather than before because we believed that partial vascular collapse before spore administration might have a deleterious effect on spore delivery. This belief was based on the fact that other vascular collapsing agents, such as 5,6-dimethylxanthenone-4-acetic acid and combretastatin A-4, have been shown to exert their effects in combination with radioactively labeled antibodies only when administered after, and not before, the antibodies (34, 35).
Several obstacles remain before COBALT can be realistically considered for clinical trials. For example, the range of tumors in which COBALT might be successful has not yet been addressed. Although the first two tumor models we investigated (xenografts of HCT116 colorectal cancer cells and syngeneic B16 melanoma cells) were effectively treated, preliminary experiments show that not all tumors are equally susceptible to COBALT. Greater understanding of the basis for C. novyi-NT tumor cell killing should provide insights into this issue. Additionally, different tumor types may require different chemotherapeutic agents to achieve maximal effects in combination with C. Novy-NT. Another issue concerns the size of the tumors to be treated. They must be large enough to have outgrown their blood supply and contain necrotic regions. We do not believe this factor will be limiting for many human tumor situations, as the great majority of clinically apparent human tumors contain large necrotic regions (Fig. 1). However, micrometastatic disease might not be suitable for COBALT. At the other end of the spectrum, COBALT-mediated lysis of large tumors was associated with significant toxicity. Although the basis for this toxicity is not yet known, it could have been due to efflux of toxic bacterial products from the tumors or due to “tumor lysis syndrome.” It has previously been noted that the rapid lysis of very large tumor burdens is associated with systemic toxicity in humans treated with chemotherapy, perhaps due to the sudden efflux of tumor cell metabolites, such as calcium, phosphate, and uric acid, into the circulation (36). Although tumor lysis syndrome can be controlled in humans, it is difficult to control in mice. Any therapy which dramatically shrinks tumors may be subject to this side effect.
One of the encouraging observations made in our study is that a subset of tumors completely regressed after even a single dose of COBALT. Additionally, the C. novyi-NT spores themselves were completely nontoxic to normal mice. The COBALT concept could easily be extended and improved, with different combinations of chemotherapeutic agents to suit particular tumor types. Additionally, it may be possible to genetically manipulate the C. novyi-NT to enhance their potency or selectivity. Further studies along these lines seem warranted.
Acknowledgments
We thank Ching-Tai Huang and Joe Gu for instructions on tail vein injections, Ngoc-Duyen T. Dang for thoughtful discussions, and Leslie Meszler (Cell Imaging Core Facility, The Johns Hopkins Oncology Center, Baltimore) for assistance with microscopy. This work was supported by the Miracle Foundation, the National Colorectal Cancer Research Alliance, the Clayton Fund, and National Institutes of Health Grants CA 43460 and CA 62924.
Abbreviations
- COBALT
combination bacteriolytic therapy
- D10
dolastatin-10
- MMC
mitomycin C
- CTX
cytoxan
Footnotes
See commentary on page 14748.
References
- 1.Jain R K. Adv Drug Delivery Rev. 2001;46:149–168. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. In: Cancer Medicine. Holland J F, Frei E, editors. Hamilton, ON, Canada: B. C. Decker; 2000. pp. 132–152. [Google Scholar]
- 3.Parker R C, Plummer H C, Siebenmann C O, Chapman M G. Proc Soc Exp Biol Med. 1947;66:461–467. doi: 10.3181/00379727-66-16124. [DOI] [PubMed] [Google Scholar]
- 4.Malmgren R A, Flanigan C C. Cancer Res. 1955;15:473–478. [PubMed] [Google Scholar]
- 5.Mose J R, Mose G. Cancer Res. 1963;24:212–216. [PubMed] [Google Scholar]
- 6.Gericke D, Engelbart K. Cancer Res. 1963;24:217–221. [PubMed] [Google Scholar]
- 7.Thiele E H, Arison R N, Boxer G E. Cancer Res. 1963;24:222–231. [PubMed] [Google Scholar]
- 8.Carey R W, Holland J F, Whang H Y, Neter E, Bryant B. Eur J Cancer. 1967;3:37–46. [Google Scholar]
- 9.Kohwi Y, Imai K, Tamura Z, Hashimoto Y. Gann. 1978;69:613–618. [PubMed] [Google Scholar]
- 10.Brown J M, Giaccia A J. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 11.Fox M E, Lemmon M J, Mauchline M L, Davis T O, Giaccia A J, Minton N P, Brown J M. Gene Ther. 1996;3:173–178. [PubMed] [Google Scholar]
- 12.Lemmon M J, van Zijl P, Fox M E, Mauchline M L, Giaccia A J, Minton N P, Brown J M. Gene Ther. 1997;4:791–796. doi: 10.1038/sj.gt.3300468. [DOI] [PubMed] [Google Scholar]
- 13.Sznol M, Lin S L, Bermudes D, Zheng L M, King I. J Clin Invest. 2000;105:1027–1030. doi: 10.1172/JCI9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low K B, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, Ash O, Carmichael E, Chakraborty A, et al. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 15.Clairmont C, Lee K C, Pike J, Ittensohn M, Low K B, Pawelek J, Bermudes D, Brecher S M, Margitich D, Turnier J, et al. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 16.Yazawa K, Fujimori M, Amano J, Kano Y, Taniguchi S. Cancer Gene Ther. 2000;7:269–274. doi: 10.1038/sj.cgt.7700122. [DOI] [PubMed] [Google Scholar]
- 17.Yazawa K, Fujimori M, Nakamura T, Sasaki T, Amano J, Kano Y, Taniguchi S. Breast Cancer Res Treat. 2001;66:165–170. doi: 10.1023/a:1010644217648. [DOI] [PubMed] [Google Scholar]
- 18.Kimura N T, Taniguchi S, Aoki K, Baba T. Cancer Res. 1980;40:2061–2068. [PubMed] [Google Scholar]
- 19.Bagadi H O, Sewell M M. Res Vet Sci. 1973;15:53–61. [PubMed] [Google Scholar]
- 20.Eklund M W, Poysky F T, Meyers J A, Pelroy G A. Science. 1974;186:456–458. doi: 10.1126/science.186.4162.456. [DOI] [PubMed] [Google Scholar]
- 21.Eklund M W, Poysky F T, Peterson M E, Meyers J A. Infect Immun. 1976;14:793–803. doi: 10.1128/iai.14.3.793-803.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd N A, Walker P D, Thomson R O. J Med Microbiol. 1972;5:459–465. doi: 10.1099/00222615-5-4-459. [DOI] [PubMed] [Google Scholar]
- 23.Boyd N A, Thomson R O, Walker P D. J Med Microbiol. 1972;5:467–472. doi: 10.1099/00222615-5-4-467. [DOI] [PubMed] [Google Scholar]
- 24.Bette P, Oksche A, Mauler F, von Eichel-Streiber C, Popoff M R, Habermann E. Toxicon. 1991;29:877–887. doi: 10.1016/0041-0101(91)90224-f. [DOI] [PubMed] [Google Scholar]
- 25.Rood J I, Cole S T. Microbiol Rev. 1991;55:621–648. doi: 10.1128/mr.55.4.621-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryant A E, Chen R Y, Nagata Y, Wang Y, Lee C H, Finegold S, Guth P H, Stevens D L. J Infect Dis. 2000;182:799–807. doi: 10.1086/315756. [DOI] [PubMed] [Google Scholar]
- 27.Martinez R D, Wilkins T D. J Med Microbiol. 1992;36:30–36. doi: 10.1099/00222615-36-1-30. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann F, Herrmann A, Habermann E, von Eichel-Streiber C. Mol Gen Genet. 1995;247:670–679. doi: 10.1007/BF00290398. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsui K, Minami J, Matsushita O, Katayama S, Taniguchi Y, Nakamura S, Nishioka M, Okabe A. J Bacteriol. 1995;177:7164–7170. doi: 10.1128/jb.177.24.7164-7170.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McManus A T, McLeod C G, Jr, Mason A D., Jr Arch Surg (Chicago) 1982;117:187–191. doi: 10.1001/archsurg.1982.01380260057010. [DOI] [PubMed] [Google Scholar]
- 31.Chaplin D J, Pettit G R, Parkins C S, Hill S A. Br J Cancer. 1996;27,Suppl.:S86–S8. [PMC free article] [PubMed] [Google Scholar]
- 32.Sweeney C J, Miller K D, Sissons S E, Nozaki S, Heilman D K, Shen J, Sledge G W., Jr Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- 33.Theys J, Landuyt W, Nuyts S, Van Mellaert L, Bosmans E, Rijnders A, Van Den Bogaert W, van Oosterom A, Anne J, Lambin P. FEMS Immunol Med Microbiol. 2001;30:37–41. doi: 10.1111/j.1574-695X.2001.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 34.Pedley R B, Sharma S K, Boxer G M, Boden R, Stribbling S M, Davies L, Springer C J, Begent R H. Cancer Res. 1999;59:3998–4003. [PubMed] [Google Scholar]
- 35.Pedley R B, Hill S A, Boxer G M, Flynn A A, Boden R, Watson R, Dearling J, Chaplin D J, Begent R H. Cancer Res. 2001;61:4716–4722. [PubMed] [Google Scholar]
- 36.Altman A. Semin Oncol. 2001;28:3–8. doi: 10.1016/s0093-7754(01)90254-4. [DOI] [PubMed] [Google Scholar]