Abstract
Context
Differentiated thyroid cancer (DTC) is usually treated by thyroidectomy followed by radioiodine ablation and generally has a good prognosis. It may now be possible to limit the amount of treatment without impacting on efficacy. It is not known whether coexistent thyroiditis impacts on radioiodine uptake or on its potential efficacy, but this could provide a rationale for modification to current therapeutic protocols.
Design
This was a retrospective cohort study of radioiodine uptake on imaging after radioiodine ablation for DTC in patients with and without concurrent thyroiditis. All patients with histologically confirmed DTC treated with radioiodine ablation after thyroidectomy in a single centre from 2012 to 2015 were included. The primary outcome assessed was the presence of low or no iodine uptake on post-ablation scan, as reported by a nuclear medicine physician blinded to the presence or absence of thyroiditis.
Results
One hundred thirty patients with available histopathology results were included. Thyroiditis was identified in 42 post-operative specimens and 15 of these patients had low or no iodine uptake on post-ablation scan, compared to only 2 of 88 patients without thyroiditis (P < 0.0001) with further data analysis dividing the groups by ablation activity received (1100 MBq or 3000 MBq).
Conclusions
Concurrent thyroiditis may impair the uptake of radioactive iodine in management of DTC. Given that patients with DTC and thyroiditis already have a good prognosis, adopting a more selective approach to this step in therapy may be indicated. Large, longitudinal studies would be required to determine if omitting radioactive iodine therapy from those patients with concurrent thyroiditis has a measurable impact on mortality from thyroid cancer.
Keywords: differentiated thyroid cancer, radioactive iodine, ablation, thyroiditis
Introduction
Differentiated thyroid cancer (DTC) is the most frequently occurring endocrine cancer and its incidence has been increasing steadily in men and women over the last few decades (1, 2, 3). DTC includes papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC) and although the pathologies and natural history of these subtypes differ, the two share a common treatment pathway. Total thyroidectomy is recommended for nearly all patients with DTC, dependent on TNM staging, either as a single procedure if the DTC is proven by imaging and fine-needle aspiration or as a diagnostic lobectomy followed by a completion thyroidectomy when the histology is confirmed.
Thyroidectomy is followed by replacement of thyroid hormone and in many cases, thyroid remnant ablation with 131I (4, 5, 6). Both total thyroidectomy and ablation contribute to optimal management by allowing the early detection of rising levels of thyroglobulin from a low post-operative/ablation baseline. It is hoped that early detection of persistent or recurrent disease will improve disease-free survival (7). Uptake of 131I into thyroid tissue is a prerequisite for remnant ablation. 131I is predominantly a beta emitter which provides the intracellular therapy, but concurrent gamma emission detected on post-ablation imaging demonstrates the extent and location of residual thyroid remnant or disease.
The exact activity of 131I required for thyroid remnant ablation has been controversial; for example, one study suggested that 1.1 GBq provided an ablation success rate 20% lower than that provided by 3.7 GBq (8). Two large studies – ESTIMABL and HiLo – were devised to elucidate the effect of differing 131I activity and each concluded that 1.1 and 3.7 GBq were equivalent in terms of ablation success rate (6, 9). These studies have led to a change in UK practice over time because they have demonstrated how to minimise radiation exposure and the risk of potential side effects or complications (dry mouth, loss of taste sensation, reduced spermatogenesis and risk of secondary malignancies), while maximising the likelihood of successful remnant ablation. Further to this, the IoN trial is in progress to assess whether omitting radioiodine ablation altogether alters outcomes in low-risk DTC (10).
In keeping with this trend to minimise therapeutic intervention (radiation in particular) without compromising disease-free survival, we have sought to determine whether thyroiditis in patients with DTC is a factor which impacts on the therapeutic benefit of radioactive ablation and which can be used to stratify patients that may or may not benefit from RAI.
Patients with chronic lymphocytic thyroiditis have been shown to have an increased risk of PTC, and the coexistence of the two conditions has been reported in many studies over more than 20 years (11, 12). Although controversial, PTC in association with thyroiditis may have a better outcome and lower rates of recurrence (13, 14, 15), but the cause is not clear. We hypothesise that thyroiditis may have an impact on the ability of DTC to take up 131I and in turn alter the success of attempted remnant ablation. In this study, we analyse the effect of the presence or absence of thyroiditis and different activities of 131I administered to a population with DTC over a period of years, spanning a change in standard clinical practice from a time when 3.7 GBq was administered to current practice of administering 1.1 GBq. We use radioiodine uptake as a surrogate marker for remnant ablation, because, while uptake may not be synonymous with ablation it can be assumed to be a prerequisite.
Materials and methods
Study population
This was a retrospective study of patients with DTC who underwent total thyroidectomy from January 2012 to December 2015 at St. Bartholomew’s Hospital, Barts Health NHS Trust, UK. Permission for this quality improvement project was obtained from the institutional quality improvement review board (project number 7352). As retrospective audit of current standard of clinical practice, consent was not required from the patients.
Treatment protocol
All patients underwent routine preoperative thyroid function tests, ultrasonography of neck and fine-needle aspiration cytology (FNAC) of the primary tumour and were confirmed to have DTC pathologically after thyroid surgery (hemi-thyroidectomy or total thyroidectomy).
The standard ‘traditional’ 131I activity administered for remnant ablation at the start of the study period was 3000 MBq (80 mCi). This was subsequently lowered to 1100 MBq (30 mCi) especially for low-risk patients, based on data from the ESTIMABL and HiLo studies (6, 9). The cohort described contains individuals who received either ‘traditional’ or low-dose ablation. Prior to therapy, patients were advised to eat a diet low in iodine for 1–2 weeks and all doses were administered under conditions of high TSH, predominantly achieved with recombinant TSH. Patients were quarantined upon administration of 131I with specific advice for radiation protection. Patients were discharged when the radiation exposure measured was <30 microsievert/h, at a distance of one metre from the patient. Thyroid function tests and serum thyroglobulin were measured at regular intervals after RAI for surveillance with a further 123I scan if the post-ablation scan showed uptake in the thyroid bed only. The timing of this initial follow-up scan was at 6 months up until mid-2014, after which it occurred at 9 months, due to a change in our institution’s standard practice. Patients were scheduled for 131I therapy if there were iodine-avid metastases on the end-of-post-ablation scan performed during admission for ablation.
Data collection
Patient data were collected and recorded by retrospective review of patient records, searching first for all patients with DTC treated with remnant ablation following thyroidectomy and included for further analysis if histopathology from thyroidectomy was available. Standard patient data collected included patient demographics (age and gender), preoperative investigation findings (thyroid function tests analysed by immunoradiometric assay and ultrasound scan of the thyroid gland) and preoperative cytology, as well as serum thyroglobulin levels from the time of the first post-ablation scan onwards. Tests for anti-thyroid antibodies, when performed, used immunometric assays ECLIA and ELISA. Only the parameters contributing to the subject of our quality review are reported here.
Data on the presence of thyroiditis were collected from evaluation of histopathology reports. All thyroid specimens after thyroidectomy were examined by an endocrine pathologist (the same specialist across more than 70% of cases) and were commented on for gross appearance, microscopic features of the tumour and an assessment of any background inflammatory infiltrate in the non-neoplastic thyroid. Thyroiditis was defined as significant numbers of lymphocytes infiltrating the normal thyroid parenchyma away from the neoplastic process. Where possible, thyroiditis was graded as mild, moderate or severe, based on the intensity of the infiltrate and presence of secondary lymphoid follicles although due to the subjective nature of this grading, it was not included in subsequent data analysis.
Data from post-ablation uptake were obtained via the imaging reports provided by the nuclear medicine department. Intensity of uptake was judged as absent, low or intense by a nuclear medicine physician unaware of the results of the pathology report at the time of reporting.
Statistical analysis
All statistical analyses were performed using SPSS, version 23 and Microsoft Office Excel 2016. Demographic data were analysed using descriptive statistics. A chi-square (χ2) test determined whether distributions of categorical variables differed from one another. Initial data were divided into two groups: ‘DTC with thyroiditis’ and ‘DTC without thyroiditis’ and compared across various parameters including 131I uptake. Data were further analysed based on the 131I activity administered (1100 or 3000 MBq). Continuous variables have been expressed as mean ± s.d. and categorical variables have been expressed as numbers and percentages. Continuous variables were compared by t-test where normally distributed. Categorical variables – the association between thyroiditis and gender and between thyroiditis and low RAI uptake – were assessed using the two-tailed Fisher’s exact test. The statistical level of significance was considered as P < 0.05.
Results
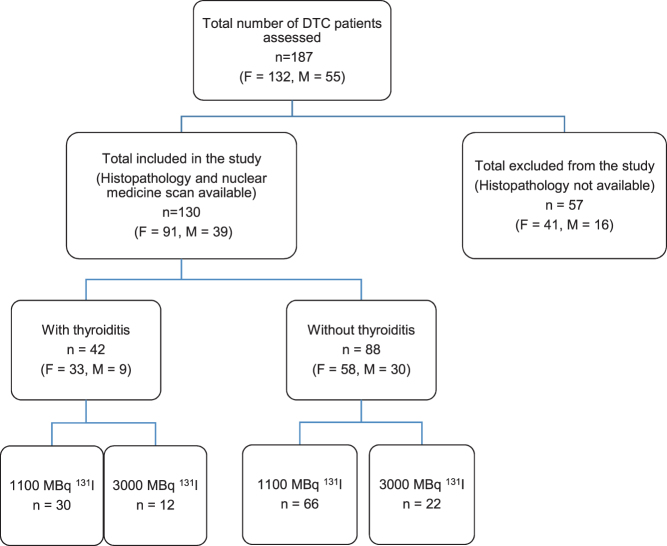
Patients with DTC treated with thyroidectomy and 131I ablation from 2012 to 2015 inclusive numbered 187, consisting of 132 females and 55 males, with mean age 47 ± 12 years. Histopathology reports could not be traced for 57 patients whose thyroidectomy was undertaken in other hospitals and had been referred to our institution for radioiodine therapy and surveillance. Our standard practice is to review the pathology blocks of such patients in our own department where possible but, having confirmed the primary diagnosis of DTC, blocks are returned to the referring hospital and may not have remained onsite for subsequent audit-based analysis such as that reported here. Of the 130 patients with complete data available, 91 (70%) were females and 39 (30%) were males; this suggests that the analysed cohort was reflective of the wider DTC population. PTC with or without metastasis was the most common diagnosis, followed by FTC and Hurthle cell carcinoma. Of the analysed cohort, 96 patients (73.8%) received 1100 MBq and 34 patients (26.1%) received an ablation activity of 3000 MBq (Fig. 1 and Table 1).
Figure 1.
Patient flow chart. Patients with differentiated thyroid carcinoma (DTC) by number, sex (F, female or M, male), presence or absence of thyroiditis and radioactive iodine activity administered (1100 or 3000 MBq).
Table 1.
Demographics of cohort.
| Number | Sex | Age (years) | Abnormal thyroid status prior to thyroidectomy | |||
|---|---|---|---|---|---|---|
| Female | Male | Mean | Median | |||
| All patients with DTC and ablation therapy | 187 | 132 | 55 | 48 | 47 | 26 |
| Total included (histopathology available) | 130 | 91 | 39 | 47 | 46 | 14 |
| With thyroiditis | 42 | 33 | 9 | 43 | 44 | 9 |
| Without thyroiditis | 88 | 58 | 30 | 49 | 50 | 5 |
Of the 130 DTC patients with available histopathology, 42 (32%) had demonstrable thyroiditis on histopathology. Females accounted for 33 of these cases. The mean age for patients with thyroiditis was 43 ± 14 years. There were 88 patients without coexisting thyroiditis (67%) of whom 58 were female. There were more female patients in the cohort overall but no statistically significant difference between the male and female preponderance of thyroiditis. The mean age for the group of patients without thyroiditis was 49.0 ± 16 years. There was no statistically significant difference in age between the two groups (P = 0.053) (Table 1).
For the 42 patients with thyroiditis, 9 were documented as having abnormal thyroid function prior to thyroidectomy (6 hypothyroid or compensated hypothyroid and 3 with suppressed TSH or frankly hyperthyroid), while for the 88 patients without thyroiditis, 5 were documented as having abnormal thyroid function (1 hypothyroid). There was inconsistent practice in measuring thyroid antibody status preoperatively, particularly in patients with normal preoperative thyroid function, so statistical analysis has not been performed for this parameter.
On more detailed review of pathology reports, extra-thyroidal extension of DTC was seen significantly less frequently in patients with thyroiditis: 8 patients compared to 29 patients in the group without thyroiditis (P = 0.028). Patients with thyroiditis were also found to have a lower likelihood of concurrent lymph node metastasis at the time of surgery – 16 patients compared to 31 without thyroiditis – although this finding was not statistically significant (P = 0.7). Additional adverse histological features were not especially apparent in patients with low or no uptake; only one patient with low uptake was noted to have poorly differentiated features, albeit still within the pathological definition of follicular thyroid carcinoma.
Thyroiditis was identified in 42 post-operative specimens and 15 of these patients had low or no iodine uptake on post-ablation scan, compared to only 2 of 88 patients without thyroiditis (P < 0.0001). To exclude the possibility that 131I activity administered was a confounding factor in the uptake of radioactive iodine, in order to examine the effects of the presence of thyroiditis on radioiodine uptake, patients were divided for analysis into two groups: 1100 MBq administered or 3000 MBq administered. The variables of ‘with thyroiditis’ and ‘without thyroiditis’, and the degree of uptake (‘moderate or intense’ and ‘low or no’) were compared within the groups and between the groups. DTC patients who received 1100 MBq numbered 96 in total. Among these, 30 had coexisting thyroiditis. Of the 30 patients with thyroiditis, 12 had low or no radioiodine uptake (three had no uptake and nine had low uptake). These data were compared to the uptake in those patients without thyroiditis who also received 1100 MBq. Of the 66 patients without thyroiditis, only one had low (or no) radioiodine uptake. Patients with thyroiditis showed higher prevalence of low or no radioiodine uptake compared to the patients without thyroiditis (P < 0.001) (Table 2).
Table 2.
Presence of thyroiditis and of no/low uptake on imaging after 131I ablation.
| Ablation activity (MBq) | Total number of patients | Number of patients with low/no uptake | P value | |
|---|---|---|---|---|
| With thyroiditis (n = 42) | 1100 | 30 | 12 | 0.000002 for patients receiving 1100 MBq |
| 3000 | 12 | 3 | ||
| Without thyroiditis (n = 88) |
1100 | 66 | 1 | 0.08 for patients receiving 3000 MBq |
| 3000 | 22 | 1 |
A total of 34 patients received 3000 MBq of 131I for ablation, 12 patients had thyroiditis and 22 did not have thyroiditis. Out of the 12 patients with thyroiditis 3 (25.0%) showed low or no uptake. Out of the 22 patients who were without thyroiditis, one (0.05%) had low or no uptake (P = 0.08) (Table 2).
The relationship between administering 1100 MBq or 3000 MBq and a post-ablation scan recording intense radioactive iodine uptake was not significant in the total cohort (P = 0.79) or within the group with thyroiditis (P = 0.18) (Table 3).
Table 3.
The different activity of RAI administered was not a significant direct factor in yielding no or low uptake in the cohort as a whole or in the group with thyroiditis.
| 1100 MBq | 3000 MBq | P value | ||
|---|---|---|---|---|
| All patients | No/low uptake | 13 | 4 | 0.792 |
| (n = 130) | Intense uptake | 83 | 30 | |
| With thyroiditis | No/low uptake | 12 | 3 | 0.18 |
| (n = 42) | Intense uptake | 18 | 9 |
Thyroglobulin was measured as a tumour marker following ablation in 41 of 42 patients with thyroiditis and was found to be suppressed to an undetectable level in 27 patients. Post-ablation scans demonstrated low or no uptake in 15 of the 42 patients with thyroiditis. The majority had a suppressed thyroglobulin and three patients had detectable thyroglobulin at 6 months. A 9- to 12-month follow-up I-123 scan was performed in 32 of the 42 thyroiditis patients and 29 of these scans demonstrated minimal or no uptake. There was concordant reduction in post-ablation thyroglobulin in all but 2 patients, with 17 patients achieving complete suppression of thyroglobulin.
Post-surgical but pre-ablation thyroglobulin is likewise not measured as standard practice but results are available for 20 of these patients, which limits possible comparison of pre- and post-ablation thyroglobulin as part of retrospective analysis of the utility of radioiodine administration. Thyroglobulin was low prior to ablation in 10 patients and remained so subsequently in all 10; out of the other 10, 3 patients achieved total suppression after ablation and 3 patients had a documented reduction in thyroglobulin, without complete suppression. A rise in thyroglobulin despite apparent ablation was identified in four patients.
Discussion
DTCs originate from thyroid follicular cells. The majority are classified as PTC with a minority of FTC. PTC is the most common thyroid cancer and it carries a good prognosis with a 10-year disease-free survival rate of 93%. Consistent with other studies, our study demonstrated a positive relationship between thyroiditis and certain positive predictors of outcome in patients with DTC. The group of patients ‘with thyroiditis’ had a lower rate of extra-thyroidal extension and a non-significant reduction in lymph node metastasis (12, 13, 14). It is therefore possible that coexisting thyroiditis with DTC may be one of the determinants of reduced morbidity and mortality in patients with DTC. Our specific objective was to assess the immediate impact of thyroiditis on radioiodine uptake, rather than the success of long-term ablation, which is beyond the scope of this paper. Therefore, we did not review the degree of extra-thyroidal uptake seen on the post-ablation or direct detailed analysis towards the available thyroglobulin data, which was used as part of surveillance monitoring from the post-surveillance scan onwards.
While thyroiditis has not been demonstrated in studies to be an independent determinant of prognosis, the finding that outcomes tend to be better in this group means that it is important to consider the potential impact of thyroiditis as well as its potential utility in strategic planning of management. If the remnant is being destroyed by the autoimmune phenomenon, then the cancer should still be vulnerable to destruction by such inflammation even in the absence of 131I administration. Thereafter, research could examine the impact of thyroiditis and omission of RAI on long-term prognosis.
One of the postulated mechanisms by which thyroiditis may improve prognosis has been through the presence of thyroid autoantibodies. These antibodies, through their autoimmune response to thyroid-specific antigens, may cause destruction of cancer cells which further reduces the chances of metastasis or recurrence (1, 16, 17). Separate reports have suggested that DTC, especially PTC with autoimmune thyroiditis, exhibit cytotoxic T-cell-mediated reactions (12, 18). Therefore, Hashimoto’s thyroiditis may facilitate destruction of cancer cells and prevent further tumour growth via both humoral and cytotoxic immune responses. Thyroid antibody production promotes progression to hypothyroidism because higher levels of antibodies against thyroglobulin (Tg) and thyroid peroxidase (TPO) accompany deterioration of thyroid function. Thyroid autoantibody levels differ between goitrous and atrophic thyroiditis, and although high anti-thyroid antibody titres may provide an indication of the likelihood of overt hypothyroidism, no correlation between antibody titre and risk for hypothyroidism has been identified (19, 20). Thyroid autoantibody testing can be useful for diagnosing or monitoring treatment; however, these tests should be employed selectively as adjunctive tests to other diagnostic testing procedures (20). There are limitations with the result of long-term monitoring: titres may remain positive for years and do not always predict abnormalities of thyroid function tests; some patients with well-defined Hashimoto's disease may have undetected antibodies, and conversely, patients with positive TPO antibodies may never develop thyroid disease (20).
Antibody reactions in autoimmune thyroiditis targeted towards thyroid-specific antigens may hasten thyroid destruction (21). It has also been postulated that PTC cells originating from follicular cells may express the antigens that the normal follicular cells produce (21). Accelerating the damage to a thyroid gland infiltrated with cancer cells, thyroiditis reduces morbidity and mortality and improves survival. Various studies have reported ≥ 95% disease-free 10-year survival (11, 21). In our cohort, around 75% of the patients with thyroiditis had positive autoantibodies compared to 45.8% in the patients without thyroiditis; however, uncertainties about the antibody status of a number of patients in whom this was not recorded mean limited conclusions should be drawn.
The frequency of DTC with coexisting thyroiditis varies with age, gender and pre-existing autoimmune thyroid conditions. The variability has been reported to range from 0.5–30% (1).
The ability of DTC to retain the potential to trap iodine is an important avenue to exploit with radioiodine-based diagnostics and therapeutics; the presence of abnormal thyroid function – and indeed the presence of histological thyroiditis – may impact on the retained potential for iodine trapping. In our study, patients with thyroiditis were significantly more likely to have low or no radioiodine uptake demonstrated on the post-ablation scan, with this seen more frequently after 1100 MBq 131I compared to 3000 MBq 131I. Patients with no uptake or less-intense uptake on the post-ablation scan could very well be true-negative cases following surgery; however, another possible explanation is that the presence of thyroiditis impacts radioiodine uptake. Therefore, without further longitudinal studies of outcomes in this type of patient, it is not possible to conclude with certainty that low uptake in these patients portends therapeutic benefit. The presence of thyroiditis may inhibit uptake to an extent that the activity administered is not sufficient to be visualised at all on the post-ablation scan, and it is also possible that there is little benefit to the patient while still being exposed to radiation.
It is important to note that thyroiditis is not the only potential reason for limited or poor radioiodine uptake. A negative whole-body scan in the presence of known residual or metastatic DTC is described (22). There are a number of well-described causes of false-negative 131I scans including inadequate TSH elevation, and iodine contamination from previous diagnostic use. We are confident that the patients in our study did not have negative scans for these reasons. There are also a number of potential reasons for true negative 131I scans that may relate to thyroid cancer but may also be over-represented in patients with thyroiditis. Any defect in the iodine-trapping mechanism can lead to a change in the amount of radioiodine taken up by the DTC and a change of kinetics of iodine release from those cells, contributing to the negative scans (23). TPO is the key enzyme in the synthesis of thyroid hormones and is involved in two important reactions in the biosynthesis of thyroid hormone: the iodination of tyrosine residues on Tg and the intra-molecular coupling reaction of iodinated tyrosine. Thus, a balance between the sodium-iodide symporter (NIS)-mediated iodine influx and TPO-inhibited efflux determines the intracellular concentration of iodine in thyroid tissue. Any defect in symporter or TPO can cause a loss of iodine-trapping capacity. The loss of radioiodine uptake ability observed in some patients with DTC may be ascribed to the reduced expression of an acquired mutation of the NIS or TPO genes (23).
The question remains whether the impact of thyroiditis on radioiodine uptake is sufficient to lead to change in clinical practice, particularly in this era of low-dose ablation in which the impact of thyroiditis on radioiodine uptake appears significant. It would be helpful to have predictors of low/no radioiodine uptake in addition to the presence of thyroiditis to refine the therapeutic pathway for these patients; however, further evaluation of this would require a larger cohort. There may be a case to be made for pre-testing patients with thyroiditis for radioiodine avidity (perhaps with a 123I scan) prior to considering RAI. The rationale for this relates to minimisation of radiation exposure to the patient as well as the potential minimisation of financial cost. An argument for pre-scanning based on cost-saving alone has insufficient strength at present based on a model using the data presented. This modelling assumes a standard of current practice in which all patients with positive histology undergo ablation with 1100 MBq 131I at a total cost per procedure of approximately £2000, accounting for the cost of thyrogen, nursing time for administration, radionuclide and hospital accommodation. Using our data, 32% of patients with histology positive for thyroiditis would first undergo 123I scanning at a cost of approximately £1100 per scan to assess whether sufficient uptake is seen to warrant ablation. Of these patients, our data suggest that fewer than 30% of this group would have no or low uptake on that scan, thus only ‘saving’ the ablation costs in 9% of the total patient cohort, which would not provide sufficient financial offset. However, this model does not take into account the key important saving of unwarranted radiation exposure to patients.
Conclusion
Our study has demonstrated that concurrent thyroiditis may impair uptake of radioactive iodine in management of DTC, particularly in this era of low-dose ablation. This urges consideration of whether this histological feature can be used to stratify patients in whom RAI is most likely to be of clinical utility. Further studies are required to assess prospectively the relevance of the severity and character of thyroiditis – focal, multifocal or diffuse – and any underlying molecular cause which might account for changes in 131I uptake and the success of remnant ablation. The end goal of determining whether thyroiditis influences morbidity and cancer-associated mortality will require a larger number of patients. Because DTC is overall a good-prognosis cancer and if, as the literature suggests, the patients with thyroiditis have a better prognosis still, then the impact of differential iodine uptake on the success of remnant ablation may result in an impact whose measurement would require a substantial longitudinal study.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. European Journal of Endocrinology 2013. 168 343–349. ( 10.1530/EJE-12-0903) [DOI] [PubMed] [Google Scholar]
- 2.Kitihara CM, Sosa JA. The changing incidence of thyroid cancer. Nature Reviews: Endocrinology 2016. 12 646–653. ( 10.1038/nrendo.2016.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kist JW, de Keizer B, Stokkel MP, Hoekstra OS, Vogel WV. THYROPET study group: recurrent differentiated thyroid cancer: towards personalized treatment based on evaluation of tumor characteristics with PET. BMC Cancer 2014. 14 405 ( 10.1186/1471-2407-14-405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, Gilbert J, Harrison B, Johnson SJ, Giles TE, et al. British Thyroid Association. Guidelines for the management of thyroid cancer. Clinical Endocrinology 2014. 81 (Supplement 1) 1–122. ( 10.1111/cen.12515) [DOI] [PubMed] [Google Scholar]
- 5.Cailleux AF, Baudin E, Travagli JP, Ricard M, Schlumberger M. Is diagnostic iodine-131 scanning useful after total thyroid ablation for differentiated thyroid cancer? Journal of Clinical Endocrinology and Metabolism 2000. 85 175–178. ( 10.1210/jcem.85.1.6310) [DOI] [PubMed] [Google Scholar]
- 6.Mallick U, Harmer C, Hackshaw A. The HiLo Trial: a multicenter randomised trial of high- versus low-dose radioiodine, with or without recombinant human thyroid stimulating hormone, for remnant ablation after surgery for differentiated thyroid cancer. Clinical Oncology 2008. 20 325–326. ( 10.1016/j.clon.2008.03.0106) [DOI] [PubMed] [Google Scholar]
- 7.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016. 26 1–133. ( 10.1089/thy.2015.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busaidy NL, Cabanillas ME. Differentiated thyroid cancer: management of patients with radioiodine nonresponsive disease. Journal of Thyroid Research 2012. 2012 618985 ( 10.1155/2012/618985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, Bardet S, Leenhardt L, Bastie D, Schvartz C, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. New England Journal of Medicine 2012. 366 1663–1673. ( 10.1056/NEJMoa1108586) [DOI] [PubMed] [Google Scholar]
- 10.Mallick U, Harmer C, Hackshaw A, Moss L. & IoN Trial Management Group. Iodine or Not (IoN) for low-risk differentiated thyroid cancer: the next UK national cancer research network randomized trial following HiLo. Clinical Oncology 2012. 24 159–161. ( 10.1016/j.clon.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 11.Jeong JS, Kim HK, Lee CR, Park S, Park JH, Kang SW, Jeong JJ, Nam KH, Chung WY, Park CS. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: clinical manifestation and prognostic outcome. Journal of Korean Medical Science 2012. 27 883–889. ( 10.3346/jkms.2012.27.8.883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang BY, Hseuh C, Chao TC, Lin KJ, Lin JD. Well-differentiated thyroid carcinoma with concomitant Hashimoto’s thyroiditis present with less aggressive clinical stage and low recurrence. Endocrine Pathology 2011. 22 144–149. ( 10.1007/s12022-011-9164-9) [DOI] [PubMed] [Google Scholar]
- 13.Girardi FM, Barra MB, Zettler CG. Papillary thyroid carcinoma: does the association with Hashimoto’s thyroiditis affect the clinicopathological characteristics of the disease? Brazilian Journal of Otorhinolaryngology 2015. 81 283–287. ( 10.1016/j.bjorl.2014.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn D, Heo SJ, Park JH, Kim JH, Sohn JH, Park JY, Park SK, Park J. Clinical relationship between Hashimoto’s thyroiditis and papillary thyroid cancer. Acta Oncologica 2011. 50 1228–1234. ( 10.3109/0284186X.2011.602109) [DOI] [PubMed] [Google Scholar]
- 15.Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z, Wang F, Duan Z, Xin S, Zhang J. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngology: Head and Neck Surgery 2013. 148 396–402. ( 10.1177/0194599812472426) [DOI] [PubMed] [Google Scholar]
- 16.Barbaro D, Boni G, Meucci G, Simi U, Lapi P, Orsini P, Pasquini C, Piazza F, Caciagli M, Mariani G. Radioiodine treatment after recombinant human thyrotropin stimulation in thyroid cancer: effectiveness for postsurgical remnants ablation and possible role of iodine content in l-thyroxine in the outcome of ablation. Journal of Clinical Endocrinology and Metabolism 2003. 88 4110–4115. ( 10.1210/jc.2003-030298) [DOI] [PubMed] [Google Scholar]
- 17.Burns WR, Zeiger MA. Differentiated thyroid cancer. Seminars in Oncology 2010. 37 557–566. ( 10.1053/j.seminoncol.2010.10.008) [DOI] [PubMed] [Google Scholar]
- 18.Lucas SD, Karlsson-Parra A, Nilsson B, Grimelius L, Akerström G, Rastad J, Juhlin C. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Human Pathology 1996. 27 1329–1335. ( 10.1016/S0046-8177(96)90346-9) [DOI] [PubMed] [Google Scholar]
- 19.Froehlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Frontiers in Immunology 2017. 8 521 ( 10.3389/fimmu.2017.00521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer CA. Assay of thyroid hormones and related substances. In Endotext. Eds Feingold KR, Anawalt B, Boyce A, et al South Dartmouth, MA, USA: MDText.com Inc., 2000. (available at: https://www.ncbi.nlm.nih.gov/books/NBK279113/) [PubMed] [Google Scholar]
- 21.Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, Hong SJ, Gong G, Shong YK. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clinical Endocrinology 2009. 93 4683–4689. ( 10.1111/j.1365-2265.2009.03537) [DOI] [PubMed] [Google Scholar]
- 22.Schlumberger M, Mancusi F, Baudin E, Pacini F. 131I Therapy for elevated thyroglobulin levels. Thyroid 1997. 7 273–276. ( 10.1089/thy.1997.7.273) [DOI] [PubMed] [Google Scholar]
- 23.Shimura H, Haraguchi K, Miyazaki A, Endo T, Onaya T. Iodine uptake and experimental 131I therapy in transplanted undifferentiated thyroid cancer cells expressing the Na+/I- symporter gene. Endocrinology 1997. 138 4493–4496. ( 10.1210/endo.138.10.5571) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a