Abstract
Objective:
To evaluate if an elevated progesterone (P) level on the day of human chorionic gonadotropin (hCG) administration is associated with a decrease in live-birth rate in patients with a good prognosis.
Design:
Retrospective cohort study.
Setting:
Large, private, assisted reproductive technology (ART) practice.
Patient(s):
One thousand six hundred twenty fresh autologous ART cycles.
Intervention(s):
None.
Main Outcome Measure(s):
Live-birth rate.
Result(s):
A total of 934 blastocyst and 686 cleavage-stage embryo transfer (ET) cycles were evaluated. Serum P levels were not associated with markers of oocyte or embryo quality, including fertilization, embryo stage at transfer, and embryos available for cryopreservation. Patient age, stage of ET, embryo quality, the number of embryos transferred, and P level on the day of hCG administration were all significantly associated with live birth. Higher P levels were associated with decreased odds of live birth for cleavage- and blastocyst-stage embryos, poor-fair and good-quality embryos, and poor- and high-responder patients. The nonsignificance of interaction tests of P levels with embryo stage, embryo quality, patient age, and ovarian response indicated that the relationship between P level and live birth was similar regardless of these factors.
Conclusion(s):
An elevated serum P level on the day of hCG administration was negatively associated with live birth, even in ETs with a good prognosis.
Keywords: Elevated progesterone, premature luteinization, implantation, IVF
Progesterone (P) elevation on the day of human chorionic gonadotropin (hCG) administration refers to rising P levels in the absence of either premature luteinization or a luteinizing hormone (LH) surge. Before the advent of gonadotropin releasing hormone (GnRH) analogues, the lack of pituitary suppression allowed for a premature rise in LH, leading to premature luteinization of the developing follicles and elevated serum P levels. With the implementation of GnRH analogues for pituitary suppression, premature LH surges became less common, and studies have demonstrated profound suppression of LH with GnRH analogues (1–4).
Despite the effective suppression of premature luteinization by GnRH analogues, early rises in P levels continued to occur in 5%–38% of all down-regulated assisted reproductive technology (ART) cycles (1, 5–7). Large recent studies have defined elevated P as serum levels >1.5–2.0 ng/ml on the day of hCG trigger (5, 8). Factors associated with premature elevations in P include larger doses of exogenous follicle- stimulating hormone (FSH), higher serum estradiol (E2) on the day of hCG trigger, and more developing follicles (5, 9).
The effect of premature P elevation on ART-cycle outcomes has historically been a matter of some debate (1, 9). Concerns that premature P elevation is associated with lower pregnancy rates were raised in 1991, by both Silverberg et al. (7) and Schoolcraft et al. (10). Several subsequent studies showed conflicting data with regard to ART outcomes (6, 7, 11–13), and a meta-analysis by Venetisi et al. (14) in 2007 failed to show that P elevation on the day of hCG administration was associated with a decrease in clinical pregnancy. However, in recent years, several large trials using newer P assays, and more-rigorous P threshold values, have documented a negative impact of elevated P on ART outcomes (5, 8, 9, 15). A recent meta-analysis demonstrated that elevated P decreased pregnancy rates in GnRH- antagonist cycles (16).
Most published data examining the effect of elevated P on ART outcomes have focused on cleavage-stage embryo transfers (ETs). In a prospective, observational, cohort study, Papanikolaou et al. (9) evaluated the effect of elevated P on the day of hCG administration, in patients stimulated with recombinant FSH and a GnRH-antagonist down-regulation protocol, in both cleavage and blastocyst transfers (9). Day-3 ETs with a P value of >1.5 ng/ml led to a >50% relative reduction in clinical pregnancy (9). However, day-5 ETs led to similar clinical pregnancy rates, regardless of whether P level was elevated or normal (9).
The authors concluded that elevated P had a detrimental effect on day-3, but not day-5, ETs. The authors suggested that the elevated P caused the endometrium to become asynchronous with embryo development, decreasing implantation on day 3. However, on the fifth luteal day, the endometrium had sufficiently recovered to allow for normal “embryo-endometrial cross-dialog” and implantation potential (9). To our knowledge, that is the only published study that has directly examined the effect of elevated P in both cleavage and blastocyst transfers.
Additional protective factors from the negative effects of premature P elevation have been proposed to include high-responder patients and patients with good-quality embryos. A biologically plausible possibility is that premature P elevation advances the endometrium and leads to embryo-endometrial asynchrony. Another possibility is that good-quality patient or embryo characteristics are not immune to this effect, if the endometrium has been advanced beyond the implantation window in relation to the embryo’s development. Our hypothesis was that premature P elevation negatively affects live birth in ART cycles, regardless of patient or embryo characteristics. The objective of the current study was to compare live-birth outcomes, across P values, in patients with cleavage- and blastocyst-stage embryos, poor-fair and good-quality embryos, various ages, and poor and high response.
MATERIALS AND METHODS
Study Design
This study was a retrospective, cohort analysis of 1,620 fresh autologous ET cycles in which serum P level was measured on the day of hCG administration. The study was performed at the Shady Grove Fertility Reproductive Science Center in Rockville, Maryland. Before 2012, serum P levels were obtained from patients on the day of hCG trigger, but the P levels were not utilized to make clinical decisions. During this time we were assessing the performance of the P assay the the effect of P on ART outcomes. This dataset was ideal for analyzing the effect of P on ART outcomes, as the P values obtained were not utilized to make clinical decisions during this time frame. This study was approved by an institutional review board.
Patients
All patients who underwent a fresh autologous ET during the periods in which serum P levels were measured on the day of hCG administration were included in the analysis. Exclusion criteria were use of frozen-thaw ET and donor oocyte cycles.
Stimulation Protocol
Ovarian stimulation occurred primarily with mixed FSH/LH protocols under GnRH-antagonist or GnRH-agonist (GnRH-a) pituitary suppression, as described elsewhere (17). In general, oral contraceptive treatment was initiated 21 days before stimulation. For GnRH-antagonist cycles, the antagonist (Ganirelix, Merck) was initiated when the lead follicle was 14 mm in size. For GnRH-a cycles, administration of 20 units of leuprolide acetate (LA; Lupron, TAP Pharmaceuticals) was initiated during the last 3 days of oral contraceptives. The LA dose was decreased to 5 units when ovarian suppression was confirmed.
Ovarian stimulation was typically achieved by employing recombinant FSH and human menopausal gonadotropin. When the lead follicle was ≥ 18 mm, final oocyte maturation was triggered with 10,000 international units (IU) of hCG, or with 4 mg of GnRH-a in some of the GnRH-antagonist cycles, as indicated. Serum P levels were obtained on the day of trigger with either hCG or GnRH-a administration. Oocyte retrieval occurred 36 hours later, and insemination was achieved with conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), as clinically indicated. Ultrasound-guided ET was performed on day 3 or on day 5, if an adequate number of high-quality embryos were available. Serum hCG levels were assessed 2 weeks after the hCG-trigger injection, and ultrasonographic confirmation of pregnancy was obtained in all pregnant patients.
Serum P levels were measured using a solid-phase, competitive chemiluminescent enzyme immunoassay (Immulite 2000 Progesterone assay, Siemens Medical Solutions Diagnostic). The lower limit of detection for the assay was 0.2 ng/ml, and the analytic sensitivity of the assay was 0.1 ng/ml. The intraassay and interassay coefficients of variance were 6.7% and 7.2%, respectively.
Statistical Analysis
The primary outcome of the study was live-birth rate. Secondary outcomes included serum E2 on the day of the hCG trigger, number of oocytes retrieved, fertilization rate, implantation rate, and embryo stage at transfer. Implantation rate was calculated with the patient as the unit of analysis. Univariate and multivariate logistic regression were used to correlate serum P on the day of the hCG trigger, with patient baseline characteristics and stimulation parameters.
Generalized estimating equations (GEEs) were used to evaluate the association of live birth with clinical variables. Adjusted GEE models were used, to account for all significant confounders in predicting the effect of P on live birth, and for multiple ART cycles from a single patient, allowing the unit of analysis for the models to be patients, not cycles. Further evaluations of live birth, with P value × ET stage interaction, P value × embryo quality interaction, P value and woman’s age, P value × GnRH-a or GnRH-antagonist protocol, and P value × ovarian response interaction were performed. The interaction evaluations directly tested whether the relationship between P and live birth was different for blastocyst vs. cleavage-stage transfers, and poor-fair vs. good ET, by patient age, and by ovarian response.
Results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). For graphical purposes, arbitrary P levels and ovarian response levels were used, to visually illustrate the effect of P across the range of data. The P levels included for analysis were >1.5 ng/ml and >2.0 ng/ml, because these levels have been previously reported in the literature as having a detrimental effect on ART outcomes (5). Linear-trend line graphs were generated to visually demonstrate the effect of P level, across its range, on live birth. The ovarian response levels included ≤3 oocytes and ≥ oocytes, as these have been used previously to define poor and high ovarian response (18, 19). For all statistical analysis, the regression and GEE models were used, to examine the entire spectrum of data without artificial or arbitrary stratifications. Statistical analysis was performed using SPSS (SPSS, Inc).
RESULTS
A total of 1,530 patients underwent 1,620 fresh autologous ART cycles, of which 934 blastocyst and 686 cleavage-stage ET cycles were performed. The distribution of P levels on the day of hCG administration are shown in Supplemental Figure 1 (available online). In 40 cycles (2.4%), P levels were ≥2.0 ng/ml, and in 114 cycles (7%), P levels were ≥ 1.5 ng/ml.
Linear regression analysis demonstrated no significant association of P levels on the day of hCG trigger with: patient age (P=.48); percentage of oocytes retrieved that were fertilized (P=.15); stage of embryo at transfer (P=.14); embryos available per ET (P=.68); or number of embryos available for cryopreservation (P=.24).
Multivariate linear regression analysis demonstrated that higher P levels were positively associated with greater total gonadotropin dosage, higher E2 on the day of the hCG trigger, longer duration of gonadotropin stimulation, more retrieved oocytes, and the use of an antagonist protocol (P<.0001 for all) (Supplemental Fig. 2, available online). The GnRH-antagonist cycles were associated with a higher median P level (antagonist: 0.84 ng/ml vs. agonist: 0.69 ng/ml, P<.001), and they were twice as likely to have a P level >2 ng/ml (antagonist: 3.2% vs. agonist: 1.6%, P=.04) and >1.5 ng/ml (antagonist: 12.0% vs. agonist: 5.8%, P<.001).
As in previous studies, a positive correlation was found between P levels and numbers of oocytes retrieved. However, after adjusting for age and serum E2 levels, the independent association between P and oocyte numbers was not statistically significant. Likewise, after adjusting for confounding variables, P concentration was not significantly associated with fertilization rate, stage of ET, number of embryos transferred, or the availability of good-quality surplus embryos for cryopreservation.
Unadjusted GEE analysis showed a significant negative association of serum P levels and patient age with live birth (OR 0.55, 95% CI 0.44–0.66) (Table 1). Live birth was positively associated with the stage of ETs, GnRH-a protocol, E2 on the day of hCG administration, number of oocytes retrieved, blastocyst transfer, and embryo quality. The number of embryos transferred was negatively associated with live birth in unadjusted models (OR 0.88, 95% CI 0.78–0.99), because patients with a poor prognosis received more embryos. In adjusted GEE models controlling for confounding variables, the number of embryos transferred was positively associated with live birth (OR 1.33, 95% CI 1.13–1.56). Patient age, blastocyst transfer, and blastocyst quality remained associated with live birth in adjusted models (Table 1). When accounting for confounders, P on the day of hCG administration remained highly negatively associated with live birth (OR 0.45, 95% CI 0.31–0.64).
TABLE 1.
Unadjusted and adjusted GEE models for associations with live birth.
| Variable | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| Age (y) | 0.91 (0.89–0.93) | <.00001 | 0.92 (0.89–0.94) | <.00001 |
| GnRH-agonist protocol | 1.55 (1.26–1.89) | <.001 | 1.51 (1.16–1.96) | .002 |
| Estradiol on day of hCG trigger (pg/ml) (per 1,000 pg) | 1.10 (1.01–1.20) | .0002 | 1.00 (0.99–1.01) | .69 |
| Progesterone on day of hCG trigger (ng/ml) | 0.55 (0.44–0.66) | <.00001 | 0.53 (0.40–0.69) | <.00001 |
| No. of oocytes retrieved | 1.02 (1.01–1.04) | .00006 | 1.00 (0.98–1.02) | .74 |
| No. of embryos transferred | 0.88 (0.78–0.99) | .045 | 1.30 (1.11–1.52) | .001 |
| Embryo stage | 2.75 (2.21–3.41) | <.00001 | 3.07 (2.30–4.10) | <.00001 |
| Embryo quality | 2.05 (1.38–3.04) | .0003 | 2.03 (1.32–3.12) | .001 |
Note: CI = confidence interval; GEE = generalized estimating equation; GnRH = gonadotropin releasing hormone; hCG = human chorionic gonadotropin; OR = odds ratio.
To evaluate whether the type of medication used for oocyte maturation affected live birth, or modulated the effect of P on live birth, the 216 GnRH-a triggers were compared with 1,404 hCG triggers. While the GnRH-a trigger was associated with live birth (P=.002), the interaction between P and the type of trigger with live birth was not significant (P=.71). Progesterone was similarly and negatively associated with live birth in both hCG trigger cycles (OR 0.56, 95% CI 0.44–0.70) and in GnRH-a trigger cycles (OR 0.53, 95% CI 0.40–0.71).
To further evaluate the effect of P on live birth, interaction testing was performed using GEE models. The interaction between P for cleavage and blastocyst transfers, with live birth, was not significant (P=.61). The interaction between P for poor-fair and good ET cycles, with live birth, was not significant (P=.88). The interaction between P and agonist or antagonist protocol, with live birth, was not significant (P=.79). Receiver operating characteristics curves demonstrated similar areas under the curve for P and live birth in both agonist (0.54, 95% CI 0.51–0.58) and antagonist cycles (0.55, 95% CI 0.51–0.60). The interaction between P and patient age, with live birth, was not significant (P=.78). The interaction between P and oocyte yield, with live birth, was not significant (P=.14).
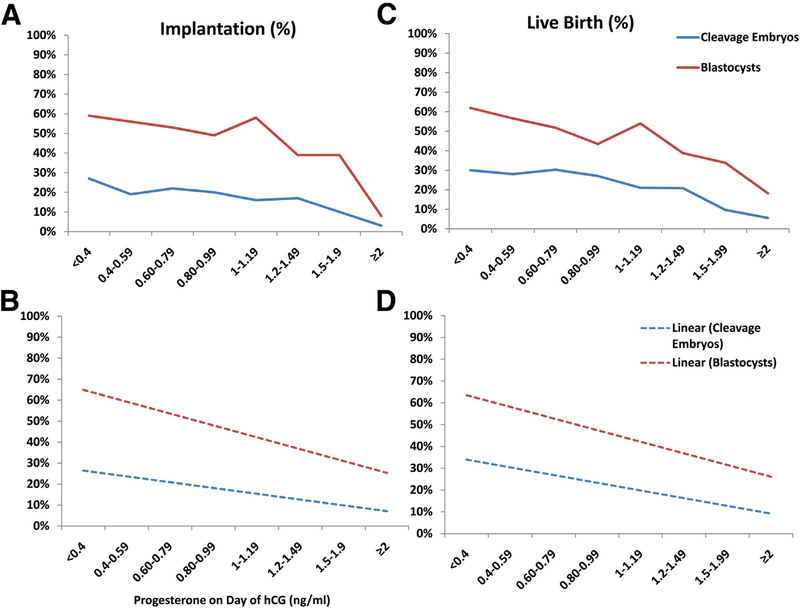
The nonsignificance of the interaction tests demonstrates that the P elevation had a similar effect on live birth, regardless of embryo stage, embryo quality, patient age, or ovarian response. The association of P level with both implantation and live birth was demonstrated graphically across embryo stage, embryo quality, patient age, oocyte yield, and agonist and antagonist protocols (Figs. 1–3; Supplemental Figs. 3–5, available online). These figures demonstrate graphically the consistent negative effect of P across patient and embryo characteristics.
FIGURE 1.
Graphic representation of the effect of serum P values on implantation and live birth in cleavage and blastocyst embryo transfers. Progesterone values (ng/ml) are plotted on the x-axis, and implantation (A and B) and live birth (C and D) are on the y-axis. Actual implantation (A) and live birth (C) are shown per serum P value. Linear trend lines for implantation (B) and live birth (D) are shown per serum P value. hCG = human chorionic gonadotropin; P = progesterone.
FIGURE 3.
Graphic representation of the effect of serum P values on implantation and live birth based on ovarian response. Progesterone values (ng/ml) are plotted on the x-axis, and implantation (A and B) and live birth (C and D) are indicated on the y-axis. Actual implantation (A) and live birth (C) are shown per serum P value. Linear trend lines for implantation (B) and live birth (D) are shown per serum P value. hCG = human chorionic gonadotropin; P = progesterone.
DISCUSSION
These data confirm the recent publications demonstrating a negative impact of elevated serum P levels, on the day of hCG administration, with live birth (5, 9). In addition, these data demonstrated that this negative impact on live birth occurred in both cleavage- and blastocyst-stage ETs, poor-fair and good embryos, and across the spectrum of ovarian response and women’s age. This result is contrary to a prior report from Papanikolaou et al. (9), who demonstrated the negative effect in cleavage-stage ETs, but not in blastocyst transfers. That study demonstrated a decline in pregnancy, from 37.5% to 15.7%, in cleavage-stage ETs, as P changed from <0.7 to >1.53 ng/ml (9).
Our data demonstrated a similar decline from 37.7% to 5.3% in cleavage-stage ETs, as the P level changed from <0.4 to >2 ng/ml. However, the Papanikolaou et al. study had similar blastocyst pregnancy rates of 42.9% and 41.5%, in patient groups with a P level of <0.7 and >1.53 ng/ml respectively. In the current study, blastocyst pregnancy rates decreased, from 70% in patients with a P level of <0.4 ng/ml, to <10% in patients with a P level of >2 ng/ml. Additional GEE models designed to test the interaction between the stage of ET and P levels, with live birth, was nonsignificant.
The current data clearly demonstrate that elevated P levels on the day of the hCG trigger were associated with a decline in live birth, for both cleavage- and blastocyst-stage embryos in our patient population. Possible explanations for the discrepency between these 2 studies include: the smaller sample size in the Papanikolaou et al. (9) study (482 patients); differences in ovarian stimulation protocols; differences in laboratory culture conditions; and differences between the 2 P assays used. In a recent study of >2,500 blastocyst transfers, a similar reduction in pregnancy rates were seen with P levels >2 ng/ml; however, this study did not have a cohort of cleavage embryos to use for direct comparison (8).
Griesinger et al. (20) recently concluded that elevated P levels were detrimental to low- and normal-responding patients, but not to high-responder patients. Patients with >18 oocytes had similar ongoing pregnancy rates; those with P levels <1.5 ng/ml had a rate of 39%, vs. 43% for those with P levels >1.5 ng/ml. However, in all other lower–oocyte-yield groups, an elevated P level was associated with decreased pregnancy rates. The interpretation of this study has been challenged, and Bosch (21) published a letter to the editor suggesting that elevated P levels still have a negative effect on high responders.
In our dataset, 355 cycles had >18 oocytes retrieved. In 306 of these high-responder cycles, the P level was <1.5 ng/ml; in 49, the P level was ≥1.5 ng/ml. A significantly higher live-birth rate occurred when the P level was <1.5 ng/ml, compared with ≥1.5 ng/ml (49.7% vs. 24.5%, P=.001). Further, the interaction testing and GEE modeling in the current study did not demonstrate a protective effect of high ovarian response to P elevation.
Histologic endometrial advancement has been demonstrated to occur in ART patients on the day of oocyte retrieval (22). The advancement of the endometrium in response to early elevations in P level has been proposed to lead to an asynchronous development of the endometrium, compared with the embryo, ultimately decreasing the likelihood of implantation (9). Ultrasonographic evaluation of the endometrium has demonstrated a hastened transition to an echogenic, secretory endometrial pattern in patients with elevated P levels (23). Two recent studies have demonstrated a difference in endometrial gene expression on the day of hCG administration in patients exposed to a premature elevation in serum P level, compared with those not exposed (24, 25). Further, Labarta et al. (26) performed biopsies on oocyte donors during the implantation window 7 days after oocyte retrieval. Patients with high serum P levels at the end of ovarian stimulation had significant differences in the gene expression profile of the endometrium, compared with donors with normal P levels.
Taken together, these data suggest that premature rises in P level have an impact on endometrial development that may lead to a decreased implantation potential. This observation is further supported by numerous studies, including the current one, showing that premature rises in P level are not associated with a detriment to oocyte or embryo quality (5, 9, 27–29). In addition, premature rises in P level in oocyte donors have not been shown to decrease the pregnancy rates in recipients (30). On the whole, these data strongly suggest that the negative impact of premature P elevation on pregnancy rates is an endometrial effect, as opposed to an oocyte effect.
Weaknesses of this study include its retrospective design and the inherent differences in response to ovarian stimulation among the patients. The choice of stimulation protocol and the adjustments in gonadotropin dosage were based on clinician preference and clinical indications. As these data and those of others have shown, ovarian stimulation is correlated with premature P elevation, and this increase explains the wide variation in incidence (5%–38%) reported in the literature (1, 5–7).
Another potential weakness is the assay itself, with a sensitivity of 0.1 ng/ml, which could lead to misclassification of patients at each cutoff point. Differences in P assays may explain the fact that various cutoffs between 1.5 and 2.0 ng/ml have a negative effect on P level, which has been reported. Modeling using GEEs was chosen, as a means to analyze the data across their entire spectrum, account for confounding variables, and account for multiple cycles from a single patient, in an effort to minimize the impacts of these sources of potential bias.
Another significant factor to consider in assessing any study on P level is the accuracy and precision of the assay. A recent study from Patton et al. (31) compared 4 commercial immunoassasys, and liquid-chromatography-tandem mass spectrometry measures of P level (31). The P immunoassays were highly correlated with values from the spectrometry; however, only 2 assays had intrassay coefficients of variation <10%, and only 2 assays had interassay coefficients of variation of <10%. These data suggest that clinicians should use caution in interpreting the values of P assays in clinical decision making, and highlight the importance of a validated P standard being incorporated into daily immunoassays. The assay used in our study was not evaluated by Patton et al., and the manufacturer-reported intra-assay and interassay coefficients of variance were 6.7% and 7.2%, respectively.
As evidence for a negative effect of premature P elevations continues to increase, the salient question becomes how to manage patients in this situation. Preventive measures are certainly warranted and may include mild stimulation, LH-based stimulation protocols, or an early hCG trigger in high responders (1). Given that the duration and intensity of ovarian stimulation is directly related to the rise in P level, mild ovarian stimulation may decrease the risk of premature P elevations. When prevention fails, vitrification of all embryos for a subsequent thaw cycle has been suggested by others as a valid option. In a matched, retrospective analysis, Shapiro et al. (32) demonstrated a higher pregnancy rate when embryos were vitrified for a subsequent cycle, as opposed to being transferred in the current cycle with the elevated P level.
In conclusion, these data clearly demonstrate a negative effect of elevated P levels, on the day of hCG trigger, on live-birth rate, regardless of the stage of ET, the quality of the embryo, the age of the woman, or the level of ovarian response. These data demonstrate that elevated P levels before the hCG trigger were consistently detrimental. Although good-quality embryos and patients with a good prognosis still had higher pregnancy rates, their rates were significantly reduced when the P level was elevated. We believe that embryo vitrification and subsequent frozen-thawed ET should be offered to these patients; however, no randomized controlled trials have yet been performed in this scenario. Ultimately, large randomized controlled trials are needed to determine if patients with elevated P levels on the day of hCG trigger are better served by proceeding to blastocyst transfer or freezing all embryos for transfer in a subsequent cycle.
Supplementary Material
FIGURE 2.
Graphic representation of the effect of serum P values on implantation and live birth in good blastocyst and fair or poor blastocyst embryo transfers. Progesterone values (ng/ml) are plotted on the x-axis, and implantation (A and B) and live birth (C and D) are indicated on the y-axis. Actual implantation (A) and live birth (C) are shown per serum P value. Linear trend lines for implantation (B) and live birth (D) are shown per serum P value. hCG = human chorionic gonadotropin; P = progesterone.
Acknowledgments
Supported, in part, by the Intramural Research Program of the Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland.
Footnotes
M.J.H. has nothing to disclose. G.D.R. has nothing to disclose. M.W.H. has nothing to disclose. K.S.R. has nothing to disclose. G.L. has nothing to disclose. A.H.D. has nothing to disclose. E.D.L. has nothing to disclose. G.S. has nothing to disclose. E.W. has nothing to disclose. M.J.L. has nothing to disclose.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Department of the Army, US Department of Defense, or the US government.
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/hillm-elevated-progesterone-premature-luteinization/
REFERENCES
- 1.Al-Azemi M, Kyrou D, Kolibianakis EM, Humaidan P, Van Vaerenberg I, Devroey P, et al. Elevated progesterone during ovarian stimulation for IVF. Reprod Biomed Online 2012;24:381–8. [DOI] [PubMed] [Google Scholar]
- 2.Olivennes F, Belaisch-Allart J, Emperaire JC, Dechaud H, Alvarez S, Moreau L, et al. Prospective, randomized, controlled study of in vitro fertilization-embryo transfer with a single dose of a luteinizing hormone-releasing hormone (LH-RH) antagonist (cetrorelix) or a depot formula of an LH-RH agonist (triptorelin). Fertil Steril 2000;73:314–20. [DOI] [PubMed] [Google Scholar]
- 3.Westergaard LG, Erb K, Laursen SB, Rex S, Rasmussen PE. Human menopausal gonadotropin versus recombinant follicle-stimulating hormone in normogonadotropic women down-regulated with a gonadotropin- releasing hormone agonist who were undergoing in vitro fertilization and intracytoplasmic sperm injection: a prospective randomized study. Fertil Steril 2001;76:543–9. [DOI] [PubMed] [Google Scholar]
- 4.Younis JS. “Premature luteinization” in the era of GnRH analogue protocols: time to reconsider. J Assist Reprod Genet 2011;28:689–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod 2010;25:2092–100. [DOI] [PubMed] [Google Scholar]
- 6.Edelstein MC, Seltman HJ, Cox BJ, Robinson SM, Shaw RA, Muasher SJ. Progesterone levels on the day of human chorionic-gonadotropin administration in cycles with gonadotropin-releasing-hormone agonist suppression are not predictive of pregnancy outcome. Fertil Steril 1990;54:853–7. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg KM, Martin M, Olive DL, Burns WN, Schenken RS. Elevated serum progesterone levels on the day of human chorionic-gonadotropin administration in in-vitro fertilization cycles do not adversely affect embryo quality. Fertil Steril 1994;61:508–13. [DOI] [PubMed] [Google Scholar]
- 8.Ochsenkuhn R, Arzberger A, von Schonfeldt V, Gallwas J, Rogenhofer N, Crispin A, et al. Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: a retrospective study with 2,555 fresh embryo transfers. Fertil Steril 2012;98:347–54. [DOI] [PubMed] [Google Scholar]
- 9.Papanikolaou EG, Kolibianakis EM, Pozzobon C, Tank P, Tournaye H, Bourgain C, et al. Progesterone rise on the day of human chorionic gonadotropin administration impairs pregnancy outcome in day 3 single-embryo transfer, while has no effect on day 5 single blastocyst transfer. Fertil Steril 2009;91:949–52. [DOI] [PubMed] [Google Scholar]
- 10.Schoolcraft W, Sinton E, Schlenker T, Huynh D, Hamilton F, Meldrum DR. Lower pregnancy rate with premature luteinization during pituitary suppression with leuprolide acetate. Fertil Steril 1991;55:563–6. [PubMed] [Google Scholar]
- 11.Bosch E, Valencia W, Escudero E, Crespo J, Simon C, Remohi J, et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril 2003;80: 1444–9. [DOI] [PubMed] [Google Scholar]
- 12.Ubaldi F, Smitz J, Wisanto A, Joris H, Schiettecatte J, Derde MP, et al. Oocyte and embryo quality as well as pregnancy rate in intracytoplasmic sperm injection are not affected by high follicular phase serum progesterone. Hum Reprod 1995;10:3091–6. [DOI] [PubMed] [Google Scholar]
- 13.Martinez F, Coroleu B, Clua E, Tur R, Buxaderas R, Parera N, et al. Serum progesterone concentrations on the day of HCG administration cannot predict pregnancy in assisted reproduction cycles. Reprod Biomed Online 2004;8: 183–90. [DOI] [PubMed] [Google Scholar]
- 14.Venetisi CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update 2007;13:343–55. [DOI] [PubMed] [Google Scholar]
- 15.Lahoud R, Kwik M, Ryan J, Al-Jefout M, Foley J, Illingworth P. Elevated progesterone in GnRH agonist down regulated in vitro fertilisation (IVFICSI) cycles reduces live birth rates but not embryo quality. Arch Gynecol Obstet 2012;285:535–40. [DOI] [PubMed] [Google Scholar]
- 16.Kolibianakis EM, Venetis CA, Bontis J, Tarlatzis BC. Significantly lower pregnancy rates in the presence of progesterone elevation in patients treated with GnRH antagonists and gonadotrophins: a systematic review and meta-analysis. Curr Pharm Biotechnol 2011;13:464–70. [DOI] [PubMed] [Google Scholar]
- 17.Stillman RJ, Richter KS, Banks NK, Graham JR. Elective single embryo transfer: a 6-year progressive implementation of 784 single blastocyst transfers and the influence of payment method on patient choice. Fertil Steril 2009; 92:1895–906. [DOI] [PubMed] [Google Scholar]
- 18.Ferratti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011;26: 1616–24. [DOI] [PubMed] [Google Scholar]
- 19.Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Godlfarb JM, Muasher SJ. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril 2014;101:967–73. [DOI] [PubMed] [Google Scholar]
- 20.Griesinger G, Mannaerts B, Andersen CY, Witjes H, Kolibianakis EM, Gordon K. Progesterone elevation does not compromise pregnancy rates in high responders: a pooled analysis of in vitro fertilization patients treated with recombinant follicle-stimulating hormone/gonadotropin-releasing hormone antagonist in six trials. Fertil Steril 2013;100:1622–8. [DOI] [PubMed] [Google Scholar]
- 21.Bosch E Does progesterone elevation compromise pregnancy rates in high responders? Insufficient evidence to draw a conclusion. Fertil Steril 2013; 101:e3–4. [DOI] [PubMed] [Google Scholar]
- 22.Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril 2002;78:1025–9. [DOI] [PubMed] [Google Scholar]
- 23.Fanchin R, Righini C, Olivennes F, Taieb J, de Ziegler D, Frydman R. Computerized assessment of endometrial echogenicity: clues to the endometrial effects of premature progesterone elevation. Fertil Steril 1999;71:174–81. [DOI] [PubMed] [Google Scholar]
- 24.Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In’t Veld P, Schuit F, et al. Progesterone rise on HCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online 2011;22:263–71. [DOI] [PubMed] [Google Scholar]
- 25.Li R, Qiao J, Wang LN, Li L, Zhen XM, Liu P, et al. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol 2011;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labarta E, Martinez-Conejero JA, Alama P, Horcajadas JA, Pellicer A, Simon C, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod 2011;26:1813–25. [DOI] [PubMed] [Google Scholar]
- 27.Fanchin R, Hourvitz A, Olivennes F, Taieb J, Hazout A, Frydman R. Premature progesterone elevation spares blastulation but not pregnancy rates in in vitro fertilization with coculture. Fertil Steril 1997;68:648–52. [DOI] [PubMed] [Google Scholar]
- 28.Fanchin R, Righini C, Olivennes F, deZiegler D, Selva J, Frydman R. Premature progesterone elevation does not alter oocyte quality in in vitro fertilization. Fertil Steril 1996;65:1178–83. [DOI] [PubMed] [Google Scholar]
- 29.Fanchin R, Righini C, Olivennes F, Ferreira AL, deZiegler D, Frydman R. Consequences of premature progesterone elevation on the outcome of in vitro fertilization: insights into a controversy. Fertil Steril 1997;68: 799–805. [DOI] [PubMed] [Google Scholar]
- 30.Melo MA, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohi J. The significance of premature luteinization in an oocyte-donation programme. Hum Reprod 2006;21:1503–7. [DOI] [PubMed] [Google Scholar]
- 31.Patton PE, Lim JY, Hickok LR, Kettel M, Larson JM, Pau KY. Precision of progesterone measurements with the use of automated immunoassay analyzers and the impact on clinical decisions for in vitro fertilization. Fertil Steril 2014;101:1629–36. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Embryo cryopreservation rescues cycles with premature luteinization. Fertil Steril 2010;93:636–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.