Abstract
Although continuous highly active antiretroviral therapy (HAART) is effective for many HIV-infected patients, it can be toxic and prohibitive in cost. By decreasing the total amount of time patients receive medications, intermittent HAART could reduce toxicity and cost. Therefore, we initiated a pilot study in which 10 HIV-infected individuals receiving effective therapy that resulted in levels of HIV RNA <50 copies per ml of plasma and CD4+ T cell counts >300 cells per mm3 of whole blood received repeated cycles of 7 days on HAART followed by 7 days off of HAART. Patients maintained suppression of plasma viremia for 32–68 weeks. There was no significant increase in HIV proviral DNA or replication-competent HIV in peripheral CD4+ T cells or HIV RNA in peripheral blood or lymph node mononuclear cells. There was no significant change in CD4+ T cell counts, no significant increase in CD4+ or CD8+ T cells expressing activation markers or producing IFN-γ in response to HIV, no increase in CD4+ T cell proliferation to p24 antigen, and no evidence for the development of resistance to HAART medications. There was a significant decrease in serum cholesterol and triglyceride levels. Thus, in this proof-of-concept study, short-cycle intermittent HAART maintained suppression of plasma viremia as well as HIV replication in reservoir sites while preserving CD4+ T cell counts. In addition, there was a decrease in serum cholesterol and triglyceride levels. Intermittent therapy may be an important strategy to reduce cost and toxicity for HIV-infected individuals.
Although highly active antiretroviral therapy (HAART) has reduced HIV mortality significantly (1), it is clear now that prolonged treatment that maintains suppression of plasma viremia is unlikely to eradicate HIV (2–4). Therefore, therapy must be life-long for most HIV-infected individuals. Unfortunately, long-term HAART may lead to a broad array of significant toxicities including increases in serum cholesterol and triglycerides (5–15). As a consequence of toxicity and the requirement for long-term, daily medications, adherence to drug regimens is problematic (16–18). Finally, 95% of HIV-infected individuals in the world do not have access to therapy because the monetary cost of HAART is prohibitive (19). Thus, although HAART provides extraordinary clinical benefit, it is important to explore alternative treatment strategies to reduce cost and toxicity and enhance adherence.
One strategy that has been studied is structured treatment interruptions. The theory supporting structured treatment interruptions in individuals who have been successfully treated with HAART has been to allow short bursts of plasma viremia during interruptions of HAART in patients with a history of virologic control and relatively high CD4+ T cell counts to enhance HIV-specific immune responses (20). It has been suggested that repeated “autoimmunization” cycles ultimately could lead to control of plasma viremia during extended discontinuation of HAART in certain individuals who were treated initially during acute infection (20). Our approach has been to evaluate the possibility that structured intermittent therapy (SIT) with predetermined cyclic periods of time on and off of HAART could maintain suppression of HIV and preserve levels of CD4+ T cell counts in individuals with chronic HIV infection. In this strategy, intermittent therapy is used for the sole purpose of reducing the total amount of antiretroviral drugs that patients receive over a given time period.
Although plasma HIV RNA is detected within 2–3 weeks of interrupting HAART in most individuals whose plasma viremia had been below the limit of detection, rebound plasma viremia is uncommonly detected during the first 7 days after cessation of therapy (21–23). Therefore, we reasoned that cycles of 7 days off of HAART would not result in significant plasma viremia. In addition, because the off-drug period was relatively brief, we hypothesized that the on-drug period also could be abbreviated. Therefore, we initiated a proof-of-concept study of 7 days on HAART followed by 7 days off of HAART in 10 individuals with a history of control of plasma HIV RNA while receiving continuous HAART to determine whether short-cycle SIT could maintain suppression of HIV in the periphery and lymphoid tissue and preserve CD4+ T cell counts. We also evaluated the effect of short-cycle intermittent HAART on HIV-specific immune responses, antiretroviral drug susceptibility, and serum cholesterol and triglyceride levels.
Materials and Methods
Patient Characteristics, Antiretroviral Regimen, and Clinical Assays.
Ten individuals receiving HAART with HIV RNA levels <500 copies per ml of plasma for more than 6 months and <50 copies per ml of plasma at enrollment and CD4+ T cell counts >300 cells per mm3 of whole blood signed informed consent to participate in this protocol, which was approved by the Internal Review Board of the National Institute of Allergy and Infectious Diseases. The virologic and immunologic characteristics of volunteers are shown in Table 1. Seven individuals (patients 301–307) had a history of receiving two to five antiretroviral agents before HAART and had received IL-2 in the past; the last dose was received a median of 22 months before enrollment (range, 17–51). Patients 308–310 had not received IL-2 or antiretroviral agents before HAART. After enrollment all individuals received d4T (30–40 mg twice a day), lamivudine (150 mg twice a day), indinavir (800 mg twice a day) and ritonavir (100 mg twice a day) in an intermittent schedule of 7 days on therapy followed by 7 days off of therapy. The drug regimen was selected to provide potent antiretroviral therapy with a high genetic barrier to the development of drug resistance. The last dose of ritonavir was held at the end of each on-drug period to ensure more rapid clearance of the last dose of indinavir. Failure criteria were a 30% decline in absolute CD4+ T cell counts compared with baseline (mean of three screening measurements) or HIV RNA >500 copies per ml of plasma on two consecutive determinations. Individuals had laboratory and clinical evaluations at least after every other off-HAART period including plasma HIV RNA as determined by branched-chain DNA and lymphocyte subsets as determined by standard flow cytometric analysis.
Table 1.
Patient characteristics
| Patient | Prestudy HAART regimen | Nadir CD4+ T cells per mm3 prior to HAART* | CD4+ T cells per mm3 prior to SIT | Plasma HIV RNA prior to HAART† | Months on HAART prior to study |
|---|---|---|---|---|---|
| 301 | d4T, 3TC, SQV | 262 | 727 | 307,000 | 11 |
| 302 | d4T, ddC, IDV | 383 | 1,286 | 16,900 | 35 |
| 303 | AZT, 3TC, IDV | 426 | 1,196 | 61,000 | 11 |
| 304 | d4T, 3TC, IDV, RTV | 465 | 913 | 87,489 | 7 |
| 305 | AZT, 3TC, ABV, NVP | 464 | 548 | 60,426 | 13 |
| 306 | d4T, 3TC, EFV | 484 | 1,375 | 59,000 | 43 |
| 307 | 3TC, NFV, EFV | 260 | 695 | 30,234 | 9 |
| 308 | d4T, 3TC, IDV, RTV | 386 | 553 | 15,771 | 15 |
| 309 | d4T, 3TC, IDV, RTV | 574 | 813 | 5,370 | 14 |
| 310 | d4T, 3TC, EFV | 254 | 758 | 51,400 | 31 |
| Median | 406 | 786 | 55,200 | 13.5 |
3TC, lamivudine; SQV, saquinavir; ddC, zalcitabine; IDV, indinavir; AZT, zidovudine; RTV, ritonavir; ABV, abacavir; NVP, nevirapine; EFV, efavirenz; d4T, stavudine.
Lowest documented CD4+ T cells per mm3.
Highest documented HIV RNA copies per ml.
Proviral HIV DNA and Cell-Associated HIV RNA.
Proviral HIV DNA was quantified as described (24) with adaptation for real-time PCR technology. Cell-associated HIV RNA was determined by reverse transcription–PCR by Amplicor (Roche Diagnostics) per the manufacturer's instructions.
Quantitative Coculture.
CD4+ T cells were isolated from peripheral blood mononuclear cells by means of a column-based purification technique (StemCell Technologies, Vancouver) as described (25). The purity of the cell suspensions was >95%. To determine the frequency of CD4+ T cells from infected patients carrying replication-competent HIV, we performed standard dilutional and high-input micrococulture assays (25, 26).
Lymph Node Analyses.
Excisional inguinal lymph node biopsies were performed on volunteers after 24 weeks following an off-HAART period (patients 308 and 310), after 25 weeks following an on-HAART period (patients 302 and 307), or after 52 weeks following an off-HAART period (patient 302) of intermittent therapy. Lymph node mononuclear cells were isolated and cryopreserved at −80°C. In situ hybridization was performed by a standard procedure (27).
HIV-Specific Immune Responses.
Standard lymphocyte proliferation assays were performed on cryopreserved peripheral blood mononuclear cells such that the assay could be performed simultaneously at different time points (28).
The frequency of CD4+ T cells specific for HIV was determined by analysis of intracellular IFN-γ-positive cells after stimulation with HIV NY5 p24 antigen (Advanced Biotechnologies, Columbia, MD) as described (29). The frequency of CD8+ T cells specific for HIV was determined by analysis of intracellular IFN-γ-positive cells after stimulation with autologous Epstein–Barr virus-transformed B cells infected with vaccinia recombinant viruses as described (30).
Phenotypic and Genotypic Antiretroviral Drug Susceptibility.
Phenotypic drug susceptibility was performed by a rapid recombinant virus assay (Antivirogram, Virco, Mechelen, Belgium), and genotypic resistance analysis was performed by population sequencing on genomic DNA or replication-competent HIV from purified CD4+ T cells as reported (31, 32).
Statistics.
Means with standard errors or means with 95% confidence intervals are reported for normally distributed variables; paired differences were tested for significance by the Student's t test. Medians are reported for variables that were not distributed normally; paired differences for these variables were tested for significance by the Wilcoxon signed rank test. Adjustments of P values for multiple testing were made by using the Bonferroni method.
Results
Effect of SIT on Plasma Viremia, Cell-Associated HIV RNA, and HIV Proviral DNA.
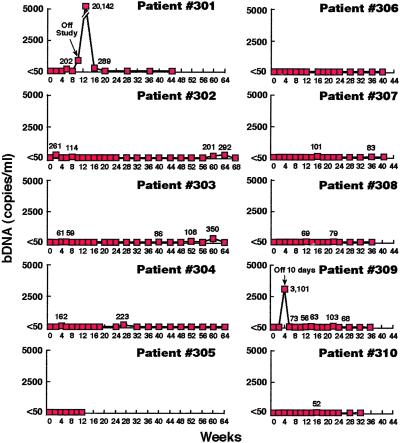
Eight of eight patients who remained on the study regimen maintained suppression of plasma viremia for up to 68 weeks (Fig. 1). It should be emphasized that all determinations were made at the end of the off-HAART period. Certain individuals had infrequent low-level plasma viremia (56–350 copies of HIV RNA per ml of plasma). However, it has been demonstrated that individuals receiving continuous HAART also have infrequent “blips” of plasma HIV RNA (33, **, ‡‡). Patient 305 withdrew from the study at week 12 for personal reasons with plasma HIV RNA <50 copies per milliliter. Patient 301 was removed from the study because of a violation of the protocol by interrupting therapy for 21 days, which resulted in a level of 20,142 copies of HIV RNA per ml of plasma. After resuming continuous HAART, virologic control was rapidly reestablished. Of interest, patient 309 had an interruption of 10 days during the second cycle with a rebound in plasma viremia to 3,101 copies per ml of plasma. After resuming the study regimen he achieved and maintained suppression of plasma HIV RNA for the following 32 weeks.
Figure 1.
Effect of short-cycle intermittent therapy on plasma HIV RNA. Ten patients receiving cycles of 7 days on HAART followed by 7 days off of HAART had measurements of plasma HIV RNA after every other off-HAART period. The results are shown as HIV RNA copies per ml of plasma as determined by branched-chain DNA assays (bDNA); all values >50 copies per ml of plasma are indicated at the designated time points. Patient 301 was removed from the study (arrow) after a 21-day interruption. Patient 309 had a 10-day interruption during the second off-drug cycle (week 4). Patient 305 withdrew from the study at week 12 for personal reasons.
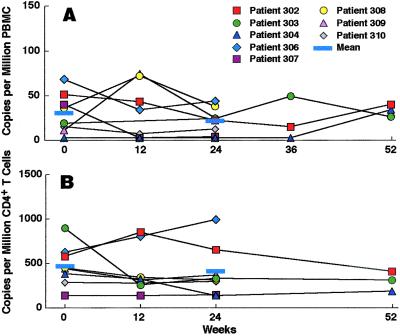
To evaluate the effect of short-cycle intermittent HAART on peripheral HIV burden further, we analyzed cell-associated RNA and proviral DNA. There was no difference in the mean HIV RNA of 30.38 (±7.86) and 21.44 (±5.27) copies per million peripheral blood mononuclear cells at enrollment and after 24 weeks of SIT, respectively (P = 0.42; Fig. 2A). In addition, there was no difference in the mean proviral DNA of 474 (±88) and 404 (±115) copies per million CD4+ T cells at enrollment and after 24 weeks of SIT, respectively (P > 0.5; Fig. 2B). Furthermore, three individuals maintained suppression of cellular HIV RNA and proviral DNA during 52 weeks of short-cycle intermittent therapy (Fig. 2 A and B).
Figure 2.
Effect of short-cycle intermittent therapy on proviral HIV DNA and cell-associated HIV RNA in the peripheral blood. Cell-associated RNA (A) was determined by reverse transcriptase–PCR in 1 million peripheral blood mononuclear cells (PBMC) at enrollment (0 weeks) and after the off-HAART intervals at 12, 24, and 52 weeks of intermittent therapy. Proviral DNA (B) was determined by real-time PCR in 1 million peripheral blood CD4+ T cells at the same time points. The means for weeks 0 and 24 are provided as indicated.
HIV in Reservoir Sites.
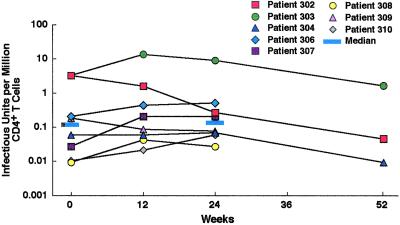
In addition to a lack of increase in HIV RNA or DNA in peripheral blood mononuclear cells during up to 52 weeks of intermittent therapy, there was no change in the pool of CD4+ T cells harboring replication-competent HIV during 24 weeks of intermittent HAART; the median infectious units per million CD4+ T cells of 0.12 (range, 0.009–3.25) at enrollment was no different from the median of 0.14 (range, 0.03–9.14) at 24 weeks of SIT (P = 0.38; Fig. 3). Indeed, several patients with relatively high frequencies of replication-competent HIV in their CD4+ T cell pool at enrollment had evidence for a decrease over a 52-week period of SIT (patients 302 and 304; Fig. 3).
Figure 3.
Effect of short-cycle intermittent therapy on the frequency of replication-competent HIV in peripheral blood CD4+ T cells. Patients who remained on the study regimen were assessed by activation of purified peripheral blood CD4+ T cells and determination of infectious units per million cells before enrollment (week 0) and at 12, 24, and 52 weeks of intermittent therapy. The medians for weeks 0 and 24 are provided as indicated.
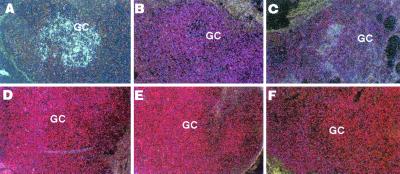
Inguinal lymph nodes were obtained from four volunteers at 24–25 weeks of short-cycle intermittent therapy and from one of these individuals again at 52 weeks. There was no HIV RNA detected by in situ hybridization in any patient (Fig. 4 B–F). Patients 307, 308, and 309 had <3, 62, and 19 copies of HIV RNA per million lymph node mononuclear cells, respectively, in nodes obtained at 24–25 weeks. These low levels of HIV RNA are commensurate with those reported for individuals receiving continuous HAART (27, 34, 35). In addition, patient 302 had no substantial change in HIV RNA from 25 (96 copies per million cells) to 52 weeks (32 copies per million cells) of intermittent therapy.
Figure 4.
Effect of short-cycle intermittent therapy on lymph node HIV burden. As evaluated by in situ hybridization, a lymph node section from an untreated control (A, dark field, original magnification ×125) revealed large amounts of HIV RNA seen as silver grains in germinal centers (GC). Lymph node sections from patient 302 after 25 (B) and 52 (C) weeks of intermittent therapy did not demonstrate HIV RNA in the germinal centers (dark fields, original magnification ×125). The biopsies were performed after the on-HAART and off-HAART periods, respectively. Lymph node biopsy specimens obtained at 24–25 weeks of intermittent HAART after either the on-HAART (patient 307, D) or off-HAART period (patients 308 and 310, E and F, respectively) also showed no evidence of HIV RNA in the germinal centers (dark fields, original magnification ×125).
Immune Responses.
It was possible that a very low-level increase in HIV replication could manifest itself as an increase in cellular markers of immune activation. In this regard, there was no change in absolute CD8+ T cells or markers of cellular activation (CD25, HLA-DR, and CD38) expressed on CD4+ or CD8+ T cells in any individual (data not shown).
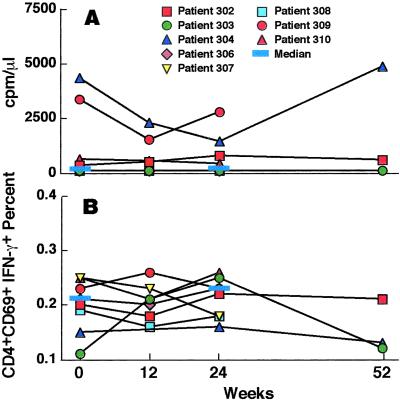
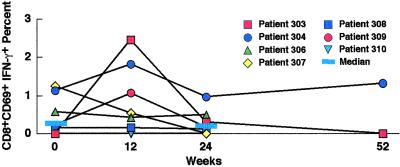
In addition, it was possible that low-level HIV replication could result in autoimmunization, which would lead to an increase in HIV-specific immune responses. In fact, over the course of the study there was no significant change in CD4+ T cell responses to p24 antigen as determined by proliferation or production of IFN-γ (P > 0.5; Fig. 5 A and B); nor was there any change in IFN-γ production by CD4+ T cells in response to staphylococcal antigen B or cytomegalovirus lysate (data not shown). In addition, there was no significant change in the production of IFN-γ by CD8+ T cells in response to the HIV gene products gag, pol, nef, or env presented on autologous Epstein–Barr virus-transformed B cells cumulatively (P > 0.5; Fig. 6) or to any individual gene product (data not shown).
Figure 5.
Effect of short-cycle intermittent therapy on CD4+ HIV-specific immune responses. Proliferation in response to HIV p24 antigen (A) was determined before enrollment (week 0) and at 12, 24, and 52 weeks of intermittent therapy by [3H]thymidine incorporation, and the results are reported with the background proliferation subtracted. Production of intracellular IFN-γ in response to HIV p24 antigen by CD4+ T cells (B) was evaluated at the same time points as determined by flow cytometric analysis and reported as the percentage of CD4+ T lymphocytes that were CD69- and IFN-γ-positive. The medians are shown as indicated.
Figure 6.
Effect of short-cycle intermittent therapy on CD8+ HIV-specific T cell immune responses. The CD8+ T cell response to transformed autologous B cells expressing HIV gag, pol, nef, and env was evaluated. The percentage of CD3+ CD8+ CD69+ T cells producing intracellular IFN-γ was determined by flow cytometric analysis before enrollment (week 0) and at 12, 24, and 52 weeks of intermittent therapy. The percentages shown are cumulative for the four gene products with the background β-galactosidase activity subtracted. The medians are shown as indicated.
Genotypic and Phenotypic Susceptibility to Antiretroviral Drugs.
Because plasma viremia was too low for standard drug susceptibility testing, we evaluated replication-competent HIV or, where that was not detected, genomic DNA from purified CD4+ T cells. No individual receiving short-cycle intermittent HAART developed new resistance-conferring genetic mutations in the protease inhibitor or reverse transcriptase regions during the course of the study, nor did any patient develop phenotypic evidence of decreased susceptibility to the available antiretroviral agents during up to 52 weeks of short-cycle intermittent therapy (Table 2).
Table 2.
Genotypic and phenotypic susceptibility to HAART
| Patient no. | Genotype
|
Phenotype
|
||||||
|---|---|---|---|---|---|---|---|---|
| Pre-SIT | Week 12 | Week 24 | Week 52 | Pre-SIT | Week 12 | Week 24 | Week 52 | |
| 302 | WT | WT | (WT) | WT | <4 | <4 | (<4) | <4 |
| 303 | WT | WT | WT | WT | <4 | <4 | <4 | <4 |
| 304 | WT | (WT) | WT | (WT) | <4 | (<4) | <4 | (<4) |
| 306 | WT | (WT) | (WT) | <4 | (<4) | (<4) | ||
| 307 | (K70R) | (K70R) | (K70R) | (<4) | (<4) | (<4) | ||
| 308 | WT | WT | (WT) | <4 | <4 | (<4) | ||
| 309 | WT | WT | (WT) | <4 | <4 | (<4) | ||
| 310 | (WT) | (WT) | (WT) | (<4) | (<4) | (<4) | ||
WT, wild type for genetic mutations that are known to confer decreased susceptibility to inhibitors of reverse transcription and protease; <4, fold decrease in susceptibility compared to control for all antiretrovirals tested; ( ), replication-competent HIV had insufficient material for amplification, assays were performed on DNA isolated from CD4+ T cells; no ( ), assays were performed on replication-competent HIV from activated CD4+ T cells.
CD4+ T Cell Counts.
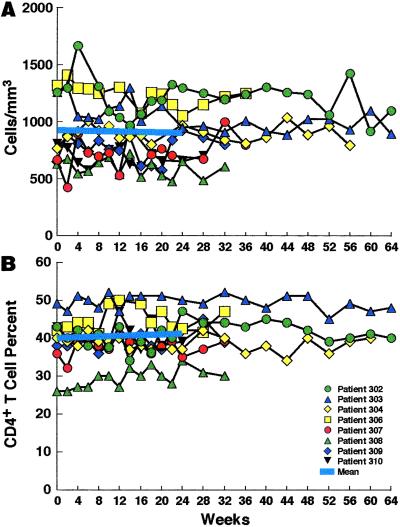
The was no change in mean CD4+ T cell counts at enrollment versus 24 weeks of SIT (921 and 900 cells per cubic millimeter, respectively; P > 0.5; Fig. 7A). In addition, there was no change in mean CD4+ T cell percentage at enrollment (40%) versus 24 weeks (41%; P > 0.5; Fig. 7B).
Figure 7.
Effect of short-cycle intermittent therapy on CD4+ T cell counts. The absolute CD4+ T cells counts (A) and the percentage of CD4+ T cells (B) were determined at the end of every other off-HAART period. The mean values before enrollment (week 0) and at 24 weeks of intermittent therapy are shown as indicated.
Toxicity.
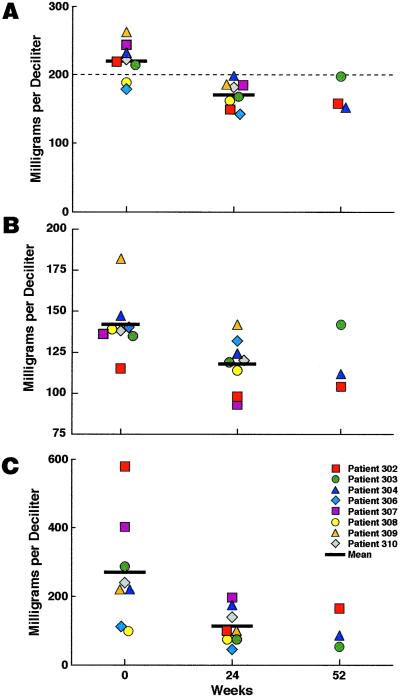
There was a significant decrease in mean level of serum cholesterol of 22% (220–171 mg/dl) during 24 weeks of SIT (95% confidence interval, 17, 26%; P = 0.001; Fig. 8A). In addition, although six of eight individuals had total serum cholesterol >200 mg/dl (Fig. 8A) before short-cycle intermittent therapy, none of eight individuals had a level >200 mg/dl after 24 weeks on the study (Fig. 8A). Lower serum cholesterol levels were maintained up to 52 weeks in three individuals. Although patient 302 began receiving Provachol at week 20, he had already experienced a reduction in total cholesterol from 222 mg/dl at enrollment to 140 mg/dl at week 16 of SIT. In addition, there was a significant decrease in the mean level of serum low density lipoprotein cholesterol from a mean of 142 mg/dl at enrollment to a mean of 118 mg/dl after 24 weeks of SIT (95% confidence interval, 11, 22%; P = 0.001; Fig. 8B). Finally, there was a significant decrease in mean serum triglycerides of 51% (270–113 mg/dl) after 24 weeks of intermittent therapy (95% confidence interval, 36, 66%; P = 0.001) with a below-baseline level maintained out to 52 weeks in three individuals (Fig. 8C).
Figure 8.
Effect of short-cycle intermittent therapy on serum cholesterol and triglyceride levels. Serum total cholesterol (A), low density lipoprotein cholesterol (B), and serum triglyceride levels (C) were determined before enrollment (week 0) and at 24 and 52 weeks of intermittent therapy. The mean levels are shown as indicated.
Discussion
The present pilot study has demonstrated that short-cycle SIT maintained suppression of HIV in peripheral blood and lymphoid tissue and preserved CD4+ T cell counts for 32–68 weeks in all eight individuals who continued on the study. The importance of strict adherence to the prescribed regimen was clear as suggested by the fact that a “drug holiday” for 21 days (patient 301) or a relatively brief interruption of HAART for 10 days rather than 7 days (patient 309) resulted in a significant increase in plasma viremia (Fig. 1). However, with up to 34 cycles of 7 days on HAART followed by 7 days off of HAART, there was no clear increase in HIV replication as determined by plasma viremia and as suggested by the stable levels of HIV proviral DNA, RNA, and replication-competent virus in the peripheral blood and HIV RNA in lymph nodes. In addition, the lack of detectable increases in cellular activation markers or in HIV-specific CD4+ and CD8+ T cell immune responses also suggested that there was not a significant increase in HIV replication during short-cycle SIT.
It has been reported that frequent blips of plasma viremia can result in the development of genotypic and phenotypic drug resistance in vitro in patients receiving continuous HAART (36). However, these blips are not associated with an increased risk of failing HAART regimens in vivo (33, **). We did not detect the prior development of genotypic resistance or decreased phenotypic susceptibility to antiretroviral agents despite significant suboptimal therapy before HAART in seven patients; in this regard, these patients did not manifest a significant increase in viral replication during repeated periods of 7 days of HAART interruptions. Nonetheless, the lack of antiretroviral drug resistance before SIT in patients with extensive pre-HAART antiretroviral therapy (patients 302, 303, 304, 306, and 307) was somewhat surprising. However, because all the patients had maintained suppression of HIV replication for prolonged periods of time after HAART was begun (Table 1), there may have been a selection for individuals that had not developed drug resistance during suboptimal antiretroviral therapy. The long-term effect of intermittent therapy on the risk of developing antiretroviral resistance requires further investigation.
Finally, we noted a significant decrease in serum cholesterol and triglyceride levels over the course of the study. This finding is of particular interest given the relatively high frequency and potential long-range clinical implications of this side effect of HAART. Three of eight patients (304, 308, and 309) were receiving the study regimen before enrollment (Table 1); thus, the decrease in lipid levels in these individuals may be attributed directly to the intermittent nature of the study regimen. Although patients 302, 303, 306, 307, and 310 changed their HAART regimens after they enrolled in the study, the indinavir-plus-ritonavir regimen within the study protocol may have actually enhanced the risk of developing lipid abnormalities (5, 6, 8, 11, 12, 14, 15). Thus, the reduction in lipid levels in these individuals may have particular importance in terms of evaluating the ability of short-cycle intermittent therapy to have a favorable impact on a patient's HAART-associated toxicity profile. In this regard, further evaluation in a randomized, controlled trial is necessary to determine the clinical impact of SIT on parameters of toxicity.
Currently, short-cycle SIT cannot be recommended in clinical settings. Randomized, controlled clinical trials will be necessary to determine the clinical applicability of this approach for the treatment of HIV infection. It will be necessary to evaluate the relevance of this strategy for patients whose plasma viremia had been controlled for a shorter period on HAART as well as the efficacy of this approach in patients with lower CD4+ T cell counts or in those receiving different HAART regimens. It will also be necessary to evaluate the long-term effects of short-cycle intermittent HAART on toxicity and adherence to therapeutic regimens. We would not anticipate a significant reversal of certain toxicities such as lipoatrophy and lipodystrophy with intermittent therapy. However, to the extent that certain toxicities are cumulative (6, 8, 14), a decrease in total drug intake may slow or prevent the onset of such side effects. In addition, although our patients were adherent to the regimen as indicated by clinical results and monitoring of plasma indinavir levels (data not shown), the practicality of applying this approach to community settings requires further evaluation.
In conclusion, this proof-of-concept study has demonstrated the feasibility of reducing the amount of antiretroviral therapy patients are required to receive by 50% while maintaining suppression of HIV in peripheral blood and lymphoid tissue, preserving CD4+ T cell counts and reducing markers of toxicity. Thus, short-cycle intermittent HAART may contribute significantly to reducing the cost and toxicity of antiretroviral therapy. This approach may have particular applicability in resource-poor settings where access to therapy is limited, in part, by the cost of antiretroviral agents.
Acknowledgments
We thank Dimiter Dimitrov, Ph.D. and Michael Polis, M.D. for assistance in designing the treatment regimen. We acknowledge with great appreciation the contribution of the persons living with HIV who participated in this research as well as the clinical staff, particularly the case managers, who cared for them. Finally, we thank Mary Rust for editorial assistance.
Abbreviations
- HAART
highly active antiretroviral therapy
- SIT
structured intermittent therapy
Footnotes
Grueb, G., CozziLepri, A. & Ledergerber, B. Conference on Retroviruses and Opportunistic Infections, Feb. 4–8, 2001, Chicago, p. 522 (abstr.).
Sklar, P., Ward, D. & Baker, R. Conference on Retroviruses and Opportunistic Infections, Feb. 4–8, 2001, Chicago, p. 431 (abstr.).
References
- 1.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. 2000;12:1–44. [Google Scholar]
- 2.Natarajan V, Bosche M, Metcalf J A, Ward D J, Lane H C, Kovacs J A. Lancet. 1999;353:119–120. doi: 10.1016/s0140-6736(05)76156-0. [DOI] [PubMed] [Google Scholar]
- 3.Furtado M R, Callaway D S, Phair J P, Kunstman K J, Stanton J L, Macken C A, Perelson A S, Wolinsky S M. N Engl J Med. 1999;340:1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, Markowitz M, Ho D D. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 5.Behrens G, Dejam A, Schmidt H, Balks H J, Brabant G, Korner T, Stoll M, Schmidt R E. AIDS. 1999;13:F63–F70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 6.Carr A, Samaras K, Thorisdottir A, Kaufmann G R, Chisholm D J, Cooper D A. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 7.Fortgang I S, Belitsos P C, Chaisson R E, Moore R D. Am J Gastroenterol. 1995;90:1433–1436. [PubMed] [Google Scholar]
- 8.Heath K V, Hogg R S, Chan K J, Harris M, Montessori V, O'Shaughnessy M V, Montanera J S. AIDS. 2001;15:231–239. doi: 10.1097/00002030-200101260-00013. [DOI] [PubMed] [Google Scholar]
- 9.Kopp J B, Miller K D, Mican J A, Feuerstein I M, Vaughan E, Baker C, Pannell L K, Falloon J. Ann Intern Med. 1997;127:119–125. doi: 10.7326/0003-4819-127-2-199707150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kotler D P, Rosenbaum K, Wang J, Pierson R N. J Acquired Immune Defic Syndr Hum Retrovirol. 1999;20:228–237. doi: 10.1097/00042560-199903010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Miller K D, Jones E, Yanovski J A, Shankar R, Feuerstein I, Falloon J. Lancet. 1998;351:871–875. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 12.Mulligan K, Grunfeld C, Tai V W, Algren H, Pang M, Chernoff D N, Lo J C, Schambelan M. J Acquired Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 13.ter Hofstede H J, de Marie S, Foudraine N A, Danner S A, Brinkman K. Int J STD AIDS. 2000;11:611–616. doi: 10.1258/0956462001916498. [DOI] [PubMed] [Google Scholar]
- 14.Thiebaut R, Daucourt V, Mercie P, Ekouevi D K, Malvy D, Morlat P, Dupon M, Neau D, Farbos S, Marimoutou C, Dabis F. Clin Infect Dis. 2000;31:1482–1487. doi: 10.1086/317477. [DOI] [PubMed] [Google Scholar]
- 15.Periard D, Telenti A, Sudre P, Cheseaux J J, Halfon P, Reymond M J, Marcovina S M, Glauser M P, Nicod P, Darioli R, Mooser V. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 16.Deeks S G, Hecht F M, Swanson M, Elbeik T, Loftus R, Cohen P T, Grant R M. AIDS. 1999;13:F35–F43. doi: 10.1097/00002030-199904160-00001. [DOI] [PubMed] [Google Scholar]
- 17.Lucas G M, Chaisson R E, Moore R D. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson J. J Am Med Assoc. 1999;281:1069. [Google Scholar]
- 19.Binswanger H P. Science. 2001;292:221–223. doi: 10.1126/science.1057504. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg E S, Altfeld M, Poon S H, Phillips M N, Wilkes B M, Eldridge R L, Robbins G K, D'Aquila R T, Goulder P J, Walker B D. Nature (London) 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 21.Davey R T, Jr, Bhat N, Yoder C, Chun T W, Metcalf J A, Dewar R, Natarajan V, Lempicki R A, Adelsberger J W, Miller K D, et al. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia F, Plana M, Vidal C, Cruceta A, O'Brien W A, Pantaleo G, Pumarola T, Gallart T, Miro J M, Gatell J M. AIDS. 1999;13:F79–F86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- 23.Neumann A U, Tubiana R, Calvez V, Robert C, Li T S, Agut H, Autran B, Katlama C. AIDS. 1999;13:677–683. doi: 10.1097/00002030-199904160-00008. [DOI] [PubMed] [Google Scholar]
- 24.Chun T W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, et al. Nature (London) 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 25.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun T W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Jr, Dybul M, Kovacs J A, Metcalf J A, et al. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 27.Dybul M, Chun T W, Ward D J, Hertogs K, Larder B, Fox C H, Orenstein J M, Baird B F, Li Y, Green L G, et al. J Infect Dis. 2000;181:1273–1279. doi: 10.1086/315407. [DOI] [PubMed] [Google Scholar]
- 28.Dybul M, Mercier G, Belson M, Hallahan C W, Liu S, Perry C, Herpin B, Ehler L, Davey R T, Metcalf J A, et al. J Immunol. 2000;165:1685–1691. doi: 10.4049/jimmunol.165.3.1685. [DOI] [PubMed] [Google Scholar]
- 29.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 30.Gea-Banacloche J C, Migueles S A, Martino L, Shupert W L, McNeil A C, Sabbaghian M S, Ehler L, Prussin C, Stevens R, Lambert L, et al. J Immunol. 2000;165:1082–1092. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 31.Hertogs K, de Bethune M P, Miller V, Ivens T, Schel P, Van Cauwenberge A, Van Den Eynde C, Van Gerwen V, Azijn H, Van Houtte M, et al. Antimicrob Agents Chemother. 1998;42:269–276. doi: 10.1128/aac.42.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larder B A, Kohli A, Kellam P, Kemp S D, Kronick M, Henfrey R D. Nature (London) 1993;365:671–673. doi: 10.1038/365671a0. [DOI] [PubMed] [Google Scholar]
- 33.Havlir D V, Bassett R, Levitan D, Gilbert P, Tebas P, Collier A C, Hirsch M S, Ignacio C, Condra J, Gunthard H F, Richman D D, Wong J K. J Am Med Assoc. 2001;286:171–179. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- 34.Gunthard H F, Havlir D V, Fiscus S, Zhang Z Q, Eron J, Mellors J, Gulick R, Frost S D, Brown A J, Schleif W, et al. J Infect Dis. 2001;183:1318–1327. doi: 10.1086/319864. [DOI] [PubMed] [Google Scholar]
- 35.Lafeuillade A, Chollet L, Hittinger G, Profizi N, Costes O, Poggi C. J Infect Dis. 1998;177:235–238. doi: 10.1086/517362. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Picado J, DePasquale M P, Kartsonis N, Hanna G J, Wong J, Finzi D, Rosenberg E, Gunthard H F, Sutton L, Savara A, et al. Proc Natl Acad Sci USA. 2000;97:10948–10953. doi: 10.1073/pnas.97.20.10948. [DOI] [PMC free article] [PubMed] [Google Scholar]