Abstract
Background
Transposable elements (TEs) make up > 50% of the human genome, and the majority of retrotransposon insertions are truncated and many are located in introns. However, the effects of retrotransposition on the host genes remain incompletely known.
Results
We report here that insertion of a chimeric L1 (cL1), but not IAP solo LTR, into intron 6 of Axin1 using CRIPSR/Cas9 induced the kinky tail phenotype with ~ 80% penetrance in heterozygous AxincL1 mice. Both penetrant (with kinky tails) and silent (without kinky tails) AxincL1 mice, regardless of sex, could transmit the phenotype to subsequent generations with similar penetrance (~ 80%). Further analyses revealed that a longer Axin1 transcript isoform containing partial cL1-targeted intron was present in penetrant, but absent in silent and wild type mice, and the production of this unique Axin1 transcript appeared to correlate with altered levels of an activating histone modification, H3K9ac.
Conclusions
The mechanism for AxincL1 mice is different from those previously identified in mice with spontaneous retrotransposition of IAP, e.g., AxinFu and Avy, both of which have been associated with DNA methylation changes. Our data suggest that Axin1 locus is sensitive to genetic and epigenetic alteration by retrotransposons and thus, ideally suited for studying the effects of new retrotransposition events on target gene function in mice.
Electronic supplementary material
The online version of this article (10.1186/s13100-019-0162-7) contains supplementary material, which is available to authorized users.
Keywords: Retrotransposon, CRISPR/Cas9, LINE-1, IAP, MaLR, Alternative splicing, Histone modification, DNA methylation, Epigenetic inheritance
Background
Transposable elements (TEs) make up > 50% of the human genome [1]. The vast majority of human TEs are retrotransposons, which replicate via a RNA-based process termed retrotransposition [2]. Based on sequence organization, retrotransposons are further classified into LTR (long terminal repeat) and non-LTR retrotransposons. LTR retrotransposons are also called endogenous retroviruses (ERVs), which display ongoing insertional activities in mice but not in humans [3]. Among them, mammalian apparent LTR retrotransposon (MaLR) elements are the most abundant in both human and mouse genomes although they are no longer replicating [3–6]. On the other hand, IAP (intracisternal A-type particle) is one of a few LTR retrotransposon families that remain active in the mouse genome [3–6]. Non-LTR retrotransposons include long interspersed elements (LINEs) and short interspersed elements (SINEs). LINE-1 (L1) sequences are abundant (~ 17% of the human genome) and have been identified as the only active and autonomous mobile element in the human genome [2, 3]. L1 consists of four components: a 5′ untranslated region (UTR) that serves as a promoter, a 3′ UTR containing a polyadenylation signal, an ORF1 (open reading frame 1) encoding an RNA binding protein with nucleic acid chaperone activity, and a conserved ORF2 protein that harbors reverse transcriptase and endonuclease activities [2]. In addition to self-mobilization, L1 proteins can also copy other RNAs into a new locus via several distinct pathways. SINEs, such as human Alu and SVA (SINE-VNTR-Alu) elements, hijack the L1 retrotransposition machinery and have successfully proliferated in the human genome [7–9]. Although not as efficient, non-TE transcripts can also be copied, forming processed pseudogenes [10, 11]. The sequence downstream to a full-length L1 can be mobilized to new locations via 3′ transduction [12–15]. Indeed, a study of 244 cancer patients has revealed that almost 25% of patients have 3′ transductions of L1 sequence [16]. Chimeric or hybrid sequences can be generated when L1 reverse transcriptase switches templates [17–19]. The vast majority of TEs in the genome are truncated or rearranged, leaving behind 3′ fragments of L1 s or single (“solo”) LTRs of ERVs, in which ORFs critical for TE replication are lacking [2, 3, 20, 21]. Moreover, most of the TE insertions described to date in cancers are intronic or intergenic [22, 23]. It remains to be investigated the extent to which TE insertions affect the expression of their host coding genes and genomic activities near the insertion sites.
It is difficult to study retrotransposition and its effects on gene expression because retrotransposon sequences are widespread in the genome and often integral parts of the introns of coding genes [22]. One approach is to follow the fate of de novo insertions that are launched from engineered donor L1 transgenes. In this regard, several cell and mouse models have been generated to study the effects of L1 retrotransposition by tagging human or mouse L1 s with intron-disrupted retrotransposition reporters [24–29]. This approach has indeed provided important insights into L1 retrotransposition activities in various cell lines and tissues [26–31]. In several cell types (e.g. mouse embryonic stem cells, rat neuronal progenitor cells, human embryonic carcinoma and other cancer derived cell lines), the newly integrated L1 s are efficiently silenced by epigenetic marks, such as DNA methylation, histone deacetylation or H3K9me3 (H3 Lys9 trimethylation) [26–29]. In mouse models, when propagated through the germline, the retrotransposed sequences exert a graded influence on the flanking genomic sequences at the level of DNA methylation, creating “sloping shores” around the hypomethylated CpG island in germ cells [32]. A limitation of this approach is that both the site and the length of insertions are unpredictable. So it is impossible to compare the effect of different retrotransposon sequences on flanking genes. A complementary approach is to study the effect of spontaneous insertional mutagenesis by endogenous retrotransposition events in mice [33] and in humans [34]. However, these insertions are fixed, some insertions of retrotransposons in these loci may have no discernable phenotype, and therefore, the effects of these insertions remain to be elucidated. We sought to find some DNA loci that could result in discernable phenotypes to study the effects of L1 retrotransposition. Interestingly, two of most studied mouse models involve spontaneous LTR retrotransposon (e.g. IAP) insertions. The first is AxinFu(AxinFused) mice, in which a 5.1-kb IAP retrotransposon is inserted in antisense orientation into intron 6 of Axin1, causing a kinky tail phenotype [35]. The second case is Avy (agouti viable yellow) mice, which show variable yellow agouti coat color phenotypes and is, similar to AxinFu, caused by a 5.1-kb IAP insertion in antisense orientation into the pseudoexon 1A of agouti (A) locus [36]. In both cases, DNA methylation levels of the IAP retrotransposon appear to inversely correlate with the severity of the phenotype. Additionally, both the DNA methylation patterns and phenotypes can be transmitted to subsequent generations in a metastable manner [37–40]. To study the effects of retrotransposed sequences, we attempted to generate mutant mice carrying either a shorter version of IAP LTR (e.g., a solo LTR) or a chimeric L1 (cL1) at the same sites in Axin1 and A (agouti) loci using the CRISPR/Cas9 technology [41–43]. Surprisingly, we failed to recapitulate the phenotypes when the solo LTR of IAP was inserted into the same two loci as those in the AxinFu and Avy mice. Of interest, we did observe kinky tail phenotype when the cL1 was inserted into intron 6 of Axin1 (termed AxincL1). Moreover, we found that the molecular mechanisms underlying the kinky tail phenotype were different between AxincL1 and AxinFu mice.
Results
Insertion of a chimeric L1, not the IAP solo LTR, into intron 6 of Axin1 induced the kinky tail phenotype
To test whether an IAP solo LTR can induce the kinky tail phenotype, we first inserted a 335 bp IAP solo LTR flanked by two loxP sites in reverse orientation into intron 6 of Axin1 using CRISPR/Cas9 (Additional file 1: Figure S1A and supplemental notes). The insert only contains the LTR of IAP identified in AxinFu mice and has been shown to function as a cryptic promoter in those mice [38]. One founder was obtained, but with no kinky tail phenotype although both PCR-based genotyping and Sanger sequencing results showed that the IAP solo LTR was indeed inserted precisely (Additional file 1: Figure S1A). In AxinFu mice, not all displayed the kinky tail phenotype; some have normal tails because of hypermethylated IAP [38]. When those silent AxinFu mice are bred with wild type (WT) mice, a small proportion of their offspring do display the kinky tail phenotype [38]. Thus, a lack of the kinky tail phenotype in the founder obtained could be due to either that the IAP solo LTR alone could not induce the kinky tail phenotype, or that the insert got silenced in founder mice. To test the two possibilities, we crossed the AxinIAP founder with WT mice, but none of > 20 Axin1+/IAP F1 mice showed the kinky tail phenotype, suggesting that the IAP solo LTR insertion does not disrupt Axin1 gene expression and thus, induces no kinky tail phenotype. Similarly, no variable yellow agouti coat color phenotype was found in either of the founder (F0) or 21 F1 mice when an antisense IAP solo LTR, which is the same as that identified in Avy mice [37, 39, 40], was inserted into the A (agouti) locus (Additional file 1: Figure S1B and supplemental notes).
Next, we tested whether insertion of other repetitive sequences can induce the kinky tail phenotype. We generated a repetitive sequence, called chimeric L1 (cL1) herein, consisting of a partial Orf2 of L1 and an LTR of MaLR, and inserted it into intron 6 of Axin1 using the CRISPR/Cas9 (Fig. 1 a and d and Additional file 1: supplemental notes). To represent a retrotransposed sequence, we also included 6 bp target site duplications (TSDs) and 44 bp 5′ extra nucleotides in the cL1 donor construct (Fig. 1d and Additional file 1: supplemental notes). We chose to use this specific chimeric L1 to mimic retrotransposition in vivo for the following reasons: First, such a chimeric sequence may result from template switching or transduction during retrotransposition, which is a pervasive phenomenon in both human and mouse genomes [17–19]. Second, the 762 bp Orf2 of L1 (Fig. 1d and Additional file 1: supplemental notes), which harbors a partial Z-motif and a partial reverse transcriptase domain, is highly conserved among different L1 families [44–46]. When aligning the Orf2 sequence against the mm10 genome with BLAT [47], one exact match was found on chromosome 3:76892981–76,893,742 and 70 other hits showed > 80% sequence identity (Additional file 2: Table S1). Moreover, the MaLR elements are the most abundant LTR retrotransposon sequences in both human and mouse genomes [3]. When aligning the LTR of the MaLR to the mouse genome mm10, 20 perfect matches were found and over 200 other hits showed > 96% sequence identity (Additional file 3: Table S2). Therefore, insertion of the two conserved regions (L1 Orf2 and MaLR LTR) of TEs into the genome allows for studying the combined genetic/epigenetic impact at this locus. Finally, both TE fragments have no retrotransposition capability, which is further confirmed by our assays for DNA copy number variation (Additional file 1: Figure S1C).
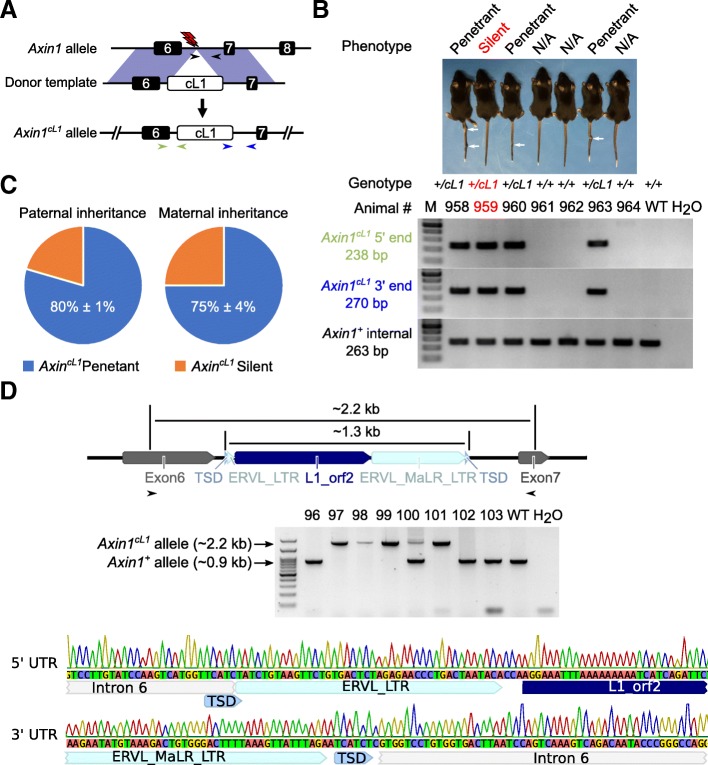
Fig. 1.
Generation of Axin1cL1 mice using CRISPR/Cas9 and phenotypic characterization. a Schematics showing the strategy for generating AxincL1 mice. The red lightning bolt represents the gRNAs used to target the reverse strands of the genomic DNA. The black arrows show the position of internal primers in the Axin1+ allele, whereas the blue and the light green arrows indicate those for amplifying the 5′ and 3’ends of the Axin1cL1 allele, respectively. The expected size of PCR products is indicated in the same color in the lower panels of b. b Image of a representative litter of seven F5 mice derived from a F4 silent AxincL1(Axin1+/cL1) female mouse bred with a WT male, including three penetrant (white arrows pointing to the kinked regions in the tails), one silent AxincL1 mouse and three WT (Axin1+/+) littermates (Upper panel). Genotyping results of these mice are shown in lower panels and the positions of the three sets of primers used are marked in a. c Pie charts showing the distribution of penetrant (with kinky tails) vs. silent (without kinky tails) AxincL1 (Axin1+/cL1) mice in an outbreeding scheme (Axin1+/cL1 × WT) across three generations. Data are represented as means ± SEM (n = 353 for paternal transgenerational inheritance, and n = 425 for maternal transgenerational inheritance). d Confirmation of cL1 insertion into intron 6 of Axin1. Long-range PCR was used to amplify fragments derived from AxincL1 (~ 2.2 kb) and WT (~ 0.9 kb) alleles (upper and middle panels), and the PCR products were sequenced to confirm the successful cL1 insertion in intron 6 of Axin1(lower panels). TSD, target site duplications; ERVL_LTR, LTR of MT2_Mm in ERVL family (5′ extra nucleotide); L1_orf2, orf2 of Lx2 in L1 family; ERVL_MaLR_LTR, LTR of MT_int in ERVL_MaLR family, serving as the 5′ extra nucleotides
We obtained 3 founders (F0) carrying the cL1 insertion, which was confirmed by Sanger sequencing of the long-range PCR products containing the full-length cL1 (Fig. 1d). One of the three founders showed a strong kinky tail phenotype, while the other two had normal tails, despite the same genotype (Axin1+/cL1). By further breeding the F0 s with WT mice, we obtained F1 heterozygous mice. Intercrossing F1 heterozygous mice produced WT, heterozygous and homozygous F2 mice at the Mendelian ratio (Fig. 1d). All homozygous (Axin1cL1/cL1) mice showed kinky tails and also displayed neuronal abnormalities characterized by motor discordances (e.g., spinning with shaky heads and imbalance), whereas ~ 80% of the heterozygous (Axin1+/cL1) mice showed the kinky tail phenotype and the remaining heterozygous mice had normal tails (Fig. 1 b and c). These results suggest that a chimeric L1/MaLR sequence, rather than IAP solo LTR, can cause the kinky tail phenotype once inserted into intron 6 of Axin1 in mice.
Stable transmission of the kinky tail phenotype with a fixed penetrance across multiple generations
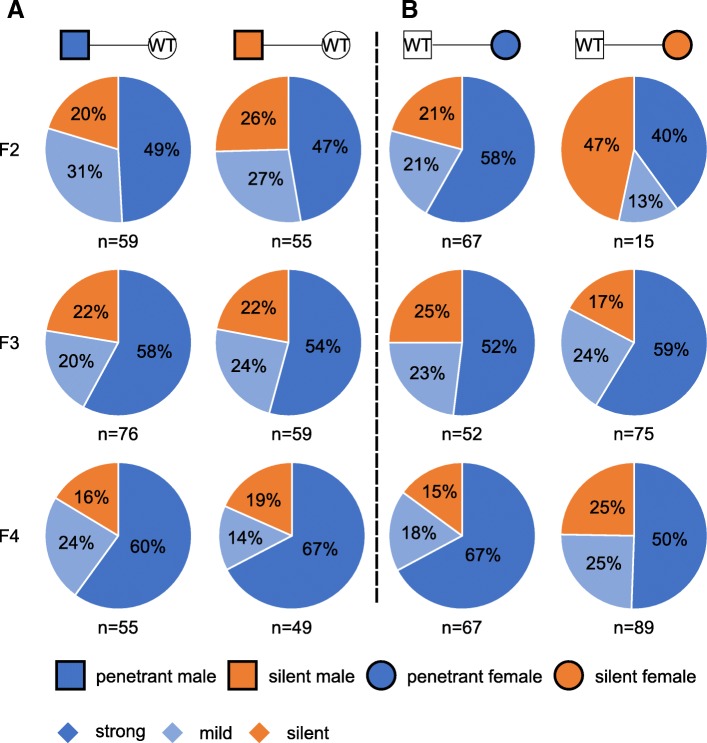
AxinFu (Axin1+/Fu) mice showed kinky tails with highly variable severity, and the penetrant AxinFu mice (with strong or mild kinky tails) produced more penetrant offspring compared to those silent ones (without kinky tails) [38]. This phenotype can be transmitted to the next generation in a metastable manner, and the phenotypic variability correlates with the methylation status of the IAP retrotransposed into intron 6 in the offspring [38]. To examine whether the kinky tail phenotype induced by cL1 also displays a similar variability in phenotypic severity, we conducted breeding experiments to test the transmission of the phenotype through either paternal or maternal germline across three generations. Heterozygous AxincL1 (Axin1+/cL1) penetrant and silent male F2 s were bred with WT females, and ~ 80% of the AxincL1 offspring (F3 s) were penetrant mice (Fig. 2a). Further breeding of the penetrant and silent F3 and F4 AxincL1 males and females with WT controls led to F4 and F5 AxincL1 offspring displaying the kinky tail phenotype with similar penetrance (~ 80%) (Fig. 2a). The phenotypic penetrance stayed the same (at ~ 80%) across all three generations when the cL1 insertion was propagated through either the paternal or the maternal germline (Fig. 1c and Fig. 2). Among the penetrant AxincL1 mice, the ones with stronger kinky tails accounted for ~ 70% across all three generations, regardless of paternal or maternal inheritance (Fig. 2a and b), suggesting the kinky tail phenotype can be stably inherited transgenerationally as long as the cL1 insertion exists.
Fig. 2.
Transgenerational inheritance of the kinky tail phenotype in AxincL1 (Axin1+/cL1) mice. a Paternal transgenerational inheritance of the kinky tail phenotype among AxincL1 mice. Male penetrant (left panel) and silent (right panel) AxincL1 mice were bred with wild type (WT) females, and the percentage of strong (dark blue) or mild (light blue) kinky and silent (orange) AxincL1 offspring, as well as the number of mice counted are indicated (Note that WT pups were excluded from the analyses). b Maternal transgenerational inheritance of the kinky tail phenotype among AxincL1 mice. Female penetrant (left panel) and silent (right panel) AxincL1 mice were bred WT females, and the percentage of strong (dark blue) or mild (light blue) kinky and silent (orange) AxincL1 offspring, as well as the number of mice counted are indicated (Note that WT pups were excluded from the analyses)
The kinky tail phenotype in AxincL1 mice is caused by an aberrantly spliced Axin1 transcript
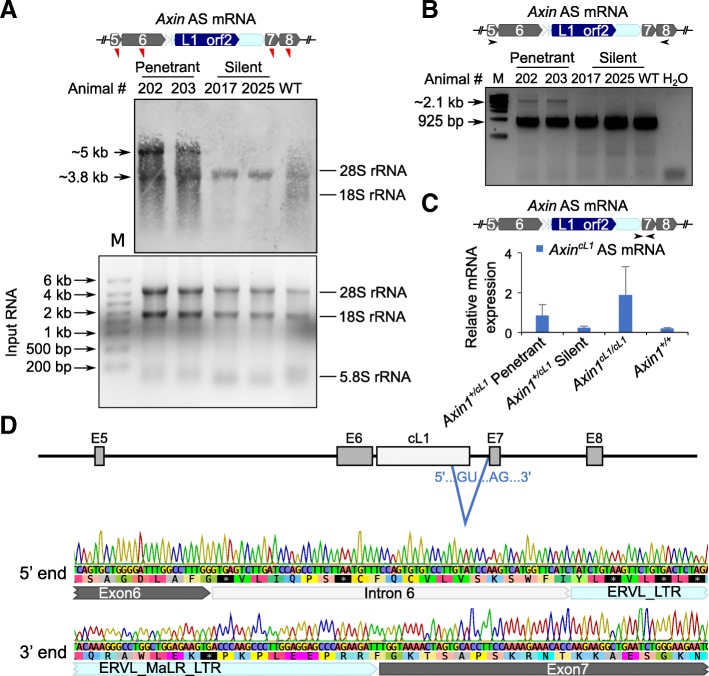
Retrotransposons inserted into the genome have been shown to act as a cryptic promoter, a terminator, and/or to induce alternative splicing [4, 29, 38, 48–53]. First, we performed dual luciferase reporter assays to test whether cL1 could function as a promoter (Additional file 1: Figure S2). Different parts of cL1 were amplified and used to replace the SV40 early promoter of Renilla luciferase reporter (Rluc) in the psiCHECK-2 vector. Promoter activity was detected in the antisense MaLR solo LTR, but not in other fragments, including the cL1 that we used to generate AxincL1 mice (Additional file 1: Figure S2), indicating that cL1 does not function as a cryptic promoter in the context of the reporter construct. To test whether aberrant transcripts are produced, we performed Northern blot analyses with probes specific to Axin1 exons 5, 6, 7 and 8 (Fig. 3a). Indeed, we found that a longer transcript was detected exclusively in penetrant mice (Fig. 3a). Consistent with Northern blot results, our RT-PCR analyses using primers specific to exons 5 and 8 also found an alternative splicing event in penetrant, but not in silent AxincL1 mice (Fig. 3b). Sanger sequencing of the longer isoform revealed that part of the cL1 (L1-MaLR) sequence was included in the aberrant Axin1 transcript, which was spliced at the canonical GU-AG splicing site, in penetrant mice (Fig. 3d and Additional file 1: supplemental notes). We further designed specific primers for the alternatively spliced Axin1 transcript. qPCR confirmed that the alternative spliced Axin1 transcript is exclusively expressed in penetrant AxincL1 mice (Fig. 3c). Taken together, these data strongly suggest that the kinky tail phenotype in the AxincL1 mice is induced by an aberrantly spliced Axin1 transcript due to intron retention of cL1.
Fig. 3.
A longer Axin1 transcript isoform containing partial cL1 is exclusively expressed in the penetrant AxincL1 mice. a A representative Northern blot showing that the wild type transcript (~ 3.8 kb) was detected in AxincL1 mice, both penetrant and silent, as well as wild type mice, whereas a longer transcript (~ 5 kb) was present only in penetrant AxincL1 mice (middle panel). Red triangles indicate the relative positions of the probes used in the upper panel, and the total RNA inputs are shown in the lower panel. b RT-PCR detection of the longer transcript isoform unique to penetrant AxincL1 mice. A pair of primers encompassing exons 5 and 8 (arrows in the upper panel) was used for PCR detection of WT (925 bp) and cL1-containing (~ 2.1 kb) transcripts (lower panel). c qPCR quantification of the longer transcript isoform unique to penetrant AxincL1 and homozygous Axin1cL1/cL1 mice. Data are presented as means ± SEM, n = 3. d Schematic illustration of the alternative splicing event leading to the production of a longer transcript isoform unique to penetrant AxincL1 mice (upper panel), as supported by the Sanger sequencing results (lower panel)
Altered H3K9ac modification, rather than DNA methylation changes, correlates with the aberrantly spliced Axin1 mRNA
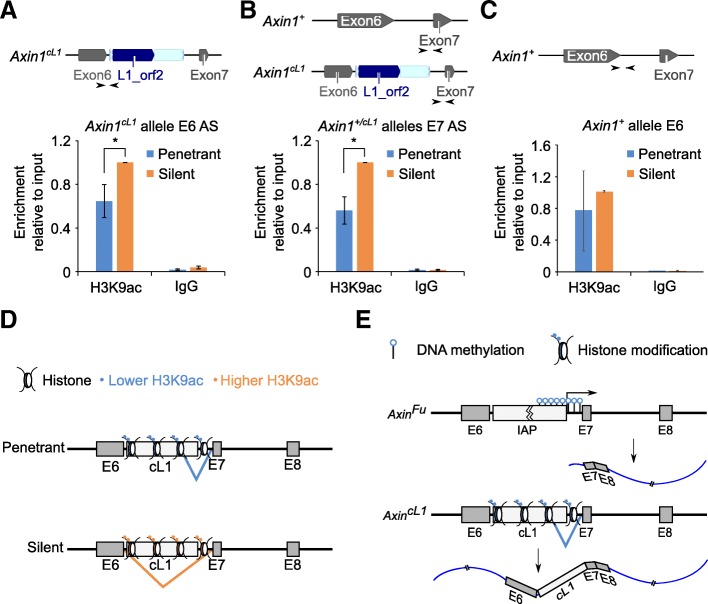
Despite the same genotype (Axin1+/cL1), only ~ 80% of AxincL1 mice express the aberrant transcripts with partial cL1 retention. Therefore, epigenetic mechanisms are likely involved. Given that DNA methylation and histone modifications of the IAP LTR sequences in AxinFu mice have been correlated with the variable phenotypic severity [38, 54], we first examined DNA methylation of cL1 and its flanking regions in both penetrant and silent AxincL1 mice. Surprisingly, bisulfite sequencing showed that DNA methylation patterns were not significantly altered between penetrant and silent AxincL1 mice (Additional file 1: Figure S3 A and B). Additionally, no major changes in DNA methylation were found between the two groups by both methylated DNA immunoprecipitation (MeDIP) of 5-methylcytosine (5mC) and HhaI restriction enzyme (RE) digestion, which cleaves unmethylated GCGC site specifically, followed by qPCR (MeDIP-qPCR and RE-qPCR) (Additional file 1: Figure S3C). Taken together, these results suggest that the aberrant alternative splicing of Axin1 transcript is not due to altered DNA methylation. Given that histone modifications (e.g. H3K9ac) affect alternative splicing [55–57], and H3K9ac and H4K20me3 marks have been associated with proper splicing of intron 6 of Axin1 [54], we performed chromatin immunoprecipitation followed by qPCR (ChIP-qPCR) to examine H3K9ac levels (Fig. 4 a-c) at the AxincL1 locus. Levels of H3K9ac, a histone mark for open chromatin structure, were much higher at the cL1 insertion site in the silent than in the penetrant mice (Fig. 4 a and b). These data suggest that a reduction in H3K9ac levels on the cL1 insertion site and its neighboring regions may affect splicing, leading to the production of a longer transcript containing cL1 (Fig. 4 d).
Fig. 4.
Reduced H3K9ac levels at the cL1 insertion site in penetrant AxincL1 mice. a ChIP-qPCR analyses of H3K9ac levels using primers specific to the exon 6 splicing site of the Axin1cL1 allele. Arrows indicate relative locations of the primers used for ChIP-qPCR analyses (upper panel). Data are presented as means ± SEM, n = 3, *p < 0.05. b ChIP-qPCR analyses of H3K9ac levels using primers specific to the exon 7 splicing site of the Axin1cL1 and Axin1+ alleles. Arrows indicate relative locations of the primers used for ChIP-qPCR analyses (upper panel). Data are presented as means ± SEM, n = 3, *p < 0.05. c ChIP-qPCR analyses of H3K9ac levels using primers specific to the exon 6 splicing site of the Axin1+ allele. Arrows indicate relative locations of the primers used for ChIP-qPCR analyses (upper panel). Data are presented as means ± SEM, n = 3. d Schematic illustration showing the effect of reduced H3K9ac levels on splicing. Briefly, higher H3K9ac levels ensure correct splicing, which excludes cL1 from the transcript, whereas with lower H3K9ac levels, the cL1 tends to be retained and included in the transcript. e Comparison of the molecular mechanisms underlying the kinky tail phenotype between AxinFu and AxincL1 mice. The kinky tail phenotype in AxinFu mice results from a shorter transcript isoform initiated from intron 6, and the phenotypic severity is inversely correlated with DNA methylation status, whereas the kinky tail phenotype in AxincL1 mice is caused by a longer transcript isoform with cL1 intron retention, and the penetrance of the phenotype is fixed at 70–80%, and inversely correlated with H3K9ac levels at the cL1 insertion site
Discussion
Mutant mice with variable yellow agouti coat color and kinky tail phenotypes were first reported 82 and 57 years ago, respectively [58, 59]. It was not until ~ 20 years ago that these phenotypes were correlated with spontaneous retrotransposition of IAPs in the mouse A(agouti) and Axin1 loci, respectively [35, 36]. However, validation by inserting the IAP into these loci to recapitulate the phenotypes in different strains of mice has not been reported. Moreover, identification of a locus that is sensitive to retrotransposition, and tends to produce a visually discernable phenotype (e.g., kinky tails, coat color changes, etc.) as a result of functional disruptions would be ideal for investigating the effects of retrotransposition in vivo. To this end, we generated a number of mouse lines by inserting various repetitive sequences into exactly the same genomic location in either A(agouti) or Axin1 locus as that reported in Avy or AxinFu mice [37–40]. Interestingly, we found that insertion of IAP solo LTR induced no phenotypes, whereas insertion of a composite cL1 sequence into Axin1 locus caused the kinky tail phenotype, which can be transmitted faithfully across multiple generations. These findings indicate that intronic retrotransposition events do not necessarily cause disruptions in the host genes leading to discernable phenotypes and that the effects of retrotransposition depend on sequence context and organization. Indeed, previous studies have shown that heterozygotes of 3 spontaneous mutations in Axin1 gene, including AxinFu (AxinFused), AxinKi (AxinKinky) and AxinKb (AxinKnobby), all display the kinked tail phenotype, yet heterozygotes of a transgenic line called AxinTg1 showed no phenotype [35]. Furthermore, AxinFu homozygotes are viable, whereas AxinKi, AxinKb and AxinTg1 homozygotes die around embryonic days 8–10 [35]. It is highly likely that the variable phenotypes among these strains reflect the positional effects of different insertions, e.g., the AxinTg1 mice contain a ~ 600 bp transgene replacing exon 2, whereas AxinFu and AxinKb contain an IAP insertion in intron 6 and exon 7, respectively [35]. The lack of phenotype in mice carrying an insertion of IAP solo LTR into intron 6 of Axin1 or pseudo exon 1A of agouti (A) loci is consistent with a recent report [21] showing that IAP LTR rarely displays promoter activity in vivo. Given that the IAP solo LTR sequence used was a part of the full-length IAP identified in Avy and AxinFu mice, the negative finding hints that other parts of the full-length IAP sequences may contain certain hidden features (e.g., subtle sequence variations and/or RNA modifications), which are required for functional disruption of the host genes and consequently the induction of the kinky tail or variable yellow coat color phenotypes.
Although insertions of full-length IAP or cL1 into intron 6 of Axin1 locus all induced the kinky tail phenotype, the underlying mechanisms appear to be different. In AxinFu, IAP insertion into intron 6 compromises Axin1 gene expression by producing a truncated transcript, which is inversely correlated to DNA methylation status [38]. In contrast, in our AxincL1 mice, while the inserted cL1 sequence displays neither promoter activities in vitro nor DNA methylation changes in vivo, production of the aberrant transcript resulting from the retention of cL1 appears to correlate with significantly reduced levels of H3K9ac. Supporting our findings, reduced H3K9ac has been shown to cause alternative exon retention in Ncam (Neural cell adhesion molecule) due to decreased RNA polymerase processivity [56, 57]. Moreover, H3K9ac is also significantly more enriched in the IAP LTR of the AxinFu locus in embryos sired by penetrant males than those by silent males [54]. A recent study [53] reports that MaLR LTRs function as splicing donors rather than splicing acceptors, which is consistent with our data showing that the MaLR LTR in the cL1 serves as a splicing donor. While associations between H3K9ac levels and aberrant splicing have been established [55–57], the underlying mechanism remains elusive. In Axin1cL1 mice, the longer splicing variant containing partial chimeric L1 sequence possesses several premature termination codons (PTCs), which are well known to cause transcript degradation via the nonsense mRNA decay (NMD) pathway [60–62]. However, our Northern blot results revealed that the longer transcript, which is unique to the penetrant AxincL1 mice, was nearly as abundant as the shorter wild-type one, suggesting that the splicing variant does not undergo NMD-mediated degradation. Therefore, it is highly likely that the longer splicing variant is translated into a mutant form of AXIN1 with a truncated C terminus lacking DIX domain, as compared to wild-type AXIN1. Unfortunately, we have not been able to identify a commercial antibody that could detect wild-type AXIN1 correctly (~92kD protein). Production of good AXIN1 antibodies and generation of a mouse model over-expressing the splicing variant/mutant AXIN1 lacking DIX domain would provide the ultimate evidence supporting the cause-effect relationship between the splicing variant/mutant AXIN1 without DIX domain and the kinky tail phenotype in the future. Together, our data suggest that the Axin1 locus is sensitive to genetic and epigenetic alterations caused by retrotransposition and thus, can serve as an ideal genomic location for studying the effects of retrotransposition on host gene expression and activities of nearby genome. With advancement of the CRISPR/Cas9 technology, TEs of interest can easily be inserted into the Axin1 locus and the effects of various TEs on Axin1 and nearby genome can be analyzed in vivo.
Transgenerational epigenetic inheritance of the variable yellow agouti coat color and kinky tail phenotypes in Avy and AxinFu mice is of great interest although the underlying mechanism remains elusive. In AxinFu mice, the variable DNA methylation levels of IAP inversely correlate with the severities of the kinky tail phenotype, and penetrant mice tend to produce more penetrant offspring [38]. DNA methylation undergoes two waves of reprogramming during fertilization and germ line specification [63] and IAP seems to be resistant to these reprogramming events [38], which may explain the transgenerational inheritance of the phenotypes in Avy and AxinFu mice. However, in AxincL1 mice, the kinky tail phenotype occurs as long as the cL1 insertion is present, and the penetrance is fixed at ~ 70–80%. Therefore, the kinky phenotype most likely represent a genetic phenomenon at first glance, and the stable inheritance of this phenotype across multiple generations in AxincL1 mice appears to be a simple genetic, rather than an epigenetic, transmission, i.e., a cL1 insertional mutation causes the phenotype in each generation. However, partial penetrance (70–80%) of the phenotype can only be explained by an epigenetic mechanism. Our data have linked H3K9ac to the aberrant splicing events, but it remains unknown how such an alteration in histone modifications causes aberrant splicing at a rate of 70–80% rather than 100%.
In summary, we show that insertion of a chimeric L1 into intron 6 of Axin1 affects histone modification patterns on cL1 and its neighboring regions, leading to the production of an aberrant Axin1 transcript correlated with the kinky tail phenotype. This mechanism is different from that previously identified in mice with spontaneous IAP retrotransposition (e.g., AxinFu and Avy mice), which results from DNA methylation changes. Axin1 locus may serve as an ideal genomic location for studying the effects of new retrotransposition events on target gene function in mice in vivo.
Conclusions
Despite their widespread distribution in the human genome, effects of retrotransposons on their host genes and nearby genome have not been exhaustively investigated in vivo. Here, we show that insertion of a chimeric L1 into intron 6 of Axin1 locus in mice could induce the kinky tail phenotype due to the production of an aberrantly spliced transcript isoform, which is associated with altered histone modifications rather than DNA methylation changes. Together with previous reports, our data strongly suggest that Axin1 is an ideal locus for studying the effects of retrotransposition on host gene expression and nearby genome activities in vivo.
Methods
Animal use and care
All the mice used in this study were on C57Bl/6 J background, and housed under specific pathogen-free conditions in a temperature- and humidity- controlled animal facility at the University of Nevada, Reno.
Generation of knock-in mice and breeding scheme
gRNAs were designed using the MIT website (https://zlab.bio/guide-design-resources) and cloned into pX330 plasmid as previously described [42, 43]. The gRNAs were in vitro transcribed using HiScribe™ T7 High Yield RNA Synthesis Kit (E2040S, NEB) and purified using RNA Clean & Concentrator™-5 (R1013, Zymo Research). Cas9 mRNA was purchased from TriLink BioTechnologies (L7606). The IAP LTR and the chimeric L1 were synthesized by IDT, and two homology arms (~ 1 kb) flanking the gRNA cutting sites of A or Axin1 locus were amplified by Q5® Hot Start High-Fidelity 2X Master Mix (M0494S, NEB) from mouse tail genomic DNA. Donor DNA templates that contain homology arms and the IAP LTR or the chimeric L1 were generated with NEBuilder® HiFi DNA Assembly Master Mix (E2621L, NEB). The gRNAs, Cas9 mRNA and donor DNA template were microinjected into mice zygotes of FVB/NJ × C57BL/6 J background for Axin1 locus knock-in and C57BL/6 J for A locus knock-in. The genomic DNA of founder mice from tail tips or ear snips were extracted for PCR-based genotyping. Founder mice were outcrossed with C57BL/6 J WT to obtain heterozygous F1 (Axin+/IAP or Axin+/cL1). For AIAP, F1 s were outcrossed with C57BL/6 J WT to obtain heterozygous F2 s, and coat color was recorded. For AxinIAP, F1 s were outcrossed with C57BL/6 J WT to obtain heterozygous F2 s, and tail phenotype was examined. For AxincL1, F1 s were outcrossed with C57BL/6 J WT to obtain heterozygous F2 s, and tail phenotype was recorded. Penetrant and silent heterozygous F2 s, F3 s, and F4 s were further outcrossed with C57BL/6 J WT to obtain the breeding data across multiple generations. Primers used for all the constructs are listed in Additional file 4: Table S3.
Mouse genotyping
Mouse tail or ear snip samples were lysed in a lysis buffer containing 40 mM NaOH (221465, Sigma Aldrich) and 0.2 mM EDTA (46–034-CI, Corning) for 1 h at 95 °C, followed by neutralization with the same volume of the neutralizing buffer containing 40 mM Tris-HCl (15567027, Thermo Fisher Scientific). PCR-based genotyping of the AxincL1, AxinIAP and AIAP was conducted using the GoTaq Green master mix (M7123, Promega) or Platinum™ SuperFi™ Green PCR Master Mix (12359010, Thermo Fisher Scientific). Primers used for genotyping are listed in Additional file 4: Table S3.
Dual luciferase assay
Different fragments of the repetitive sequences were amplified from donor templates and then used to replace the SV40 early promotor that drives the expression of the Renilla luciferase-coding sequence in the psiCHECK-2 plasmid (C8021, Promega). HEK293 cells were transfected with psiCHECK-2 containing the different fragments from the repetitive sequences using Lipofectamine 2000 (11668019, Thermo Fisher Scientific) in a 24-well cell culture plate (CLS3527-100EA, Corning). After 24 h, cells were lysed and used for the Dual Luciferase Assay (E1910, Promega) according to the manufacturer’s instructions. The psiCHECK-2 and psiCHECK-2 vectors with deletion of the SV40 early promotor of the Renilla luciferase-coding sequence were used as positive and negative controls, respectively. Renilla luciferase signals were normalized to Firefly luciferase signals to correct the transfection efficiency. Primers used for all the constructs are listed in Additional file 4: Table S3.
DNA, RNA extraction and cDNA synthesis
DNA and RNA were extracted from kidneys and tail snips from penetrant and silent mice using the Quick-DNA Plus Kits (D4074, Zymo Research) and mirVana miRNA Isolation Kit (AM1560, Thermo Fisher Scientific), respectively, according to the manufacturer’s instructions. Briefly, kidney or tail samples were homogenized in 600 μL of Lysis/Binding Buffer with homogenizer (D1000, Benchmark), followed by centrifugation to remove cell debris. The supernatant was passed through a column, in which the genomic DNA was retained, whereas the RNA got eluted. For genomic DNA extraction, the column containing genomic DNA was treated with a genomic lysis buffer at room temperature for 10 min, followed by washing with a DNA Pre-Wash Buffer once and a g-DNA Wash Buffer twice. The genomic DNA was eluted with nuclease-free water and stored at − 80 °C for further use. For RNA extraction, 60 μL of miRNA Homogenate Additive was added into the flow-through followed by incubation on ice for 10 min. The mixture was subjected to Phenol: Chloroform RNA extraction, and total RNA was isolated according to the manufacturer’s instructions. cDNA synthesis was performed using SuperScript II Reverse Transcriptase (18064014, Thermo Fisher Scientific) with random primers. qPCR and long-range PCR were performed using the Fast SYBR Green Master Mix (4385612, Thermo Fisher Scientific) and PrimeSTAR GXL DNA Polymerase (R050B, TaKaRa), respectively. Primers used for qPCR and long-range PCR are listed in Additional file 4: Table S3.
Bisulfite sequencing
Genomic DNA samples were bisulfite-converted using the EZ DNA Methylation-Gold™ Kit (D5005, Zymo Research). PCR was performed using the TaKaRa EpiTaq™ HS enzyme (for bisulfite-treated DNA) (R110B, TaKaRa), which is more tolerant to dUTP-containing templates, with Tm at 55 °C for 40 cycles. PCR products were ligated into the pGEM®-T Easy Vector (A1360, Promega) for Sanger sequencing. Primers used for bisulfite sequencing are listed in Additional file 4: Table S3.
Methylated DNA immunoprecipitation-qPCR (MeDIP-qPCR)
MeDIP was performed using a Methylated DNA immunoprecipitation kit (ab117133, Abcam) according to instructions of the manufacturer. In brief, 100 μL of the antibody buffer and 1 μL anti-5-methylcytosine or mouse IgG antibody were added into strip wells and incubated at room temperature for 1 h. During the incubation, tail genomic DNA was sheared by a focused-ultrasonicator (M220, Covaris) in the reaction buffer. The sheared DNA ranged between 200 and 1000 bp in size and was denatured at 95 °C for 2 min followed by incubation on ice. An aliquot of 5 μL of the denatured DNA was used as input DNA. The strip wells bound with antibody were washed with 150 μL of the antibody buffer once and 150 μL of the wash buffer once, followed by incubation with the sheared DNA at room temperature for 2 h. Then the strip wells were washed with the wash buffer three times. The antibody-enriched DNA was eluted with a DNA release buffer containing proteinase K and purified with columns. qPCR was performed to identify DNA methylation levels. Primers used for MeDIP-qPCR are listed in Additional file 4: Table S3.
Northern blot
Northern blot analyses were performed using a NorthernMax® Kit (AM1940, Thermo Fisher Scientific) and a Biotin Chromogenic Detection Kit (K0662, Thermo Fisher Scientific) following the manufacturer’s instructions. Briefly, RNA extracted from kidney was mixed with 3 volumes of a formaldehyde-containing loading dye followed by denaturation at 65 °C for 15 min. The denatured RNA was the fractionated through 1 × MOPS Gel Running Buffer (1% denaturing gel with a voltage of 140 V for 30 min). Then the RNA was transferred onto a BrightStar®-Plus Positively Charged Nylon Membrane (AM10100, Thermo Fisher Scientific) using Novex™ Semi-Dry Blotter (SD1000, Thermo Fisher Scientific) in 1× TBE buffer with a voltage of 20 V for 30 min. After transfer, the membrane was rinsed 1× Gel Running Buffer, then crosslinked in Spectrolinker™ XL-1500 UV crosslinker (Spectronics Corporation) followed by baking at 80 °C for 15 min. The crosslinked membrane was prehybridized at 65 °C in a preheated ULTRAhyb Buffer in a roller bottle in a hybridization oven at 42 °C for 30 min, followed by incubation with 10pM biotinylated probe (IDT) in the ULTRAhyb Buffer at 42 °C overnight. After rinsing with 1 × Blocking/Washing Buffer for 5 min three times at room temperature, the membrane was blocked with 1 × Blocking Buffer for 30 min in a shaker at room temperature. Following blocking, the membrane was incubated with Streptavidin-AP conjugate for 1 h at room temperature, then washed with 1 × Blocking/Washing Buffer for 5 min three times and 1× detection buffer for 10 min. Then the membrane was incubated with freshly prepared NBT/BCIP Substrate Solution at room temperature in the dark. 2 h later, the reaction was stopped by rinsing with double deionized water. Probes used for Northern blot are listed in Additional file 4: Table S3.
Chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR)
ChIP-qPCR was performed as previously described [64]. Briefly, tail snips were lysed on ice for 30 min in 600 μl of buffer 1 plus detergents [15 mM Tris-HCl (pH 7.5) (15567027, Thermo Fisher Scientific), 60 mM KCl (P217–500, Fisher Scientific), 5 mM MgCl2 (BP214–500, Fisher Scientific) and 0.1 mM EGTA (O2783–100, Fisher Scientific), 0.3 M sucrose (freshly added) (0335-5KG, Amresco), 10 mM DTT (freshly added) (GE17–1318-02, GE Healthcare), 0.25% (volume/volume) NP-40 (NP40S-500ML, Sigma Aldrich) and 0.5% (weight/volume) sodium deoxycholate (freshly prepared) (D6750-100G, Sigma Aldrich)]. Then 600 μl of MNase buffer [85 mM Tris-HCl, pH 7.5, 3 mM MgCl2, 2 mM CaCl2 (C7902-500G, Sigma Aldrich) and 0.3 M sucrose (freshly added)] was added into the lysed solution. The mixture was aliquoted into 200 μl per tube to obtain sufficient digestion, followed by Micrococcal Nuclease (M0247S, NEB) digestion at 37 °C in a thermomixer for 5 min and then terminated by adding 2ul of 0.5 M EDTA (46–034-CI, Corning) and incubation on ice for 5 min. The digested sample was then centrifuged at 15,000×g for 10 min at room temperature to remove cell debris, followed by adding protease inhibitors to the chromatin. 200 μl of the mixture was saved as input DNA. After preclearing of the chromatin with blocked protein G beads (10004D, Thermo Fisher Scientific), 3 μl of H3K9ac antibody (ab4441, Abcam) was added into the precleared chromatin and incubated at 4 °C overnight. Then the chromatin was incubated with blocked protein G beads at 4 °C for 4 h, followed by washing with wash buffer A (50 mM Tris-HCl (pH 7.5), 10 mM EDTA and 75 mM NaCl (BP358–10, Fisher Scientific)) once and wash buffer B (50 mM Tris-HCl (pH 7.5), 10 mM EDTA and 125 mM NaCl) twice. Then the chromatin was eluted by resuspending in 150 μl of elution buffer (1% (weight/volume) SDS (L4509-500G, Sigma Aldrich) in TE) at 25 °C in a thermomixer twice. The eluted chromatin was then subjected to RNase A (EN0531, Thermo Fisher Scientific) and proteinase K (P8107S, NEB) digestion followed by phenol/chloroform extraction of DNA. The pull-down DNA and input DNA were used for qPCR using the Fast SYBR Green Master Mix (4385612, Thermo Fisher Scientific). Primers used for ChIP-qPCR are listed in Additional file 4: Table S3.
Statistical analysis
All data were presented as mean ± SEM, and statistical differences were assessed by the Two-sample t test unless stated otherwise. p < 0.05 was considered as significant differences.
Additional files
Figure S1, S2, S3 and Supplemental notes. Generation of AxinIAP and AIAP founder mice and copy number variation assays for AxincL1 mice (Figure S1); promoter activity analyses (Figure S2); DNA methylation levels around cL1 in penetrant and silent AxincL1 mice (Figure S3); and annotated sequences of retrotransposons used in this study (Supplemental notes). (PDF 5204 kb)
Table S1. Orf2 BLAT results. (XLSX 52 kb)
Table S2. MaLR BLAT results. (XLSX 48 kb)
Table S3. Oligos used in this study. (XLSX 11 kb)
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the NIH (P30GM110767, HD071736 and HD085506 to WY; R21HD080143 and P50GM107632 to WA), the Markl Faculty Scholar Fund (to WA) and the Templeton Foundation (PID: 50183 to WY).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- 5mC
5-methylcytosine
- Avy
Agouti viable yellow
- AxincL1
Axin1 +/cL1
- AxinFu
Axin Fused
- AxinKb
Axin Knobby
- AxinKi
Axin Kinky
- BLAT
BLAST-like alignment tool
- Cas9
CRISPR associated protein 9
- ChIP
Chromatin immunoprecipitation
- cL1
Chimeric L1
- CRIPSR
Clustered regularly interspaced short palindromic repeats
- ERV
Endogenous retroviruse
- IAP
Intracisternal A-type particle
- L1
LINE-1
- LINE
Long interspersed element
- LTR
Long terminal repeat
- MaLR
Mammalian apparent LTR retrotransposon
- MeDIP
Methylated DNA immunoprecipitation
- ORF
Open reading frame
- qPCR
Quantitative PCR
- RE
Restriction enzyme
- SINE
Short interspersed elements
- SVA
SINE-VNTR-Alu
- TE
Transposable element
- TSDs
Target site duplications
- UTR
Untranslated region
- WT
Wild type
Authors’ contributions
WY and ZW designed the research. ZW, HM, SJN, YW, DO, CT, SL, SW, SY, PY and HZ performed the experiments. WA contributed reagents and protocols, and edited the manuscript; all analyzed the data; WY and ZW wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Nevada, Reno (protocol number 00494).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhuqing Wang, Email: zhuqingw@nevada.unr.edu.
Hayden McSwiggin, Email: hmcswiggin@nevada.unr.edu.
Simon J. Newkirk, Email: Simon.Newkirk@sdstate.edu
Yue Wang, Email: ywang2@nevada.unr.edu.
Daniel Oliver, Email: danielkoliver@gmail.com.
Chong Tang, Email: leochong718@icloud.com.
Sandy Lee, Email: sandylee@nevada.unr.edu.
Shawn Wang, Email: shawnwang7@nevada.unr.edu.
Shuiqiao Yuan, Email: yuanshuiqiao@126.com.
Huili Zheng, Email: hzheng@med.unr.edu.
Ping Ye, Email: Ye@sdstate.edu.
Wenfeng An, Email: Wenfeng.An@sdstate.edu.
Wei Yan, Phone: 775 784 7765, Email: wyan@med.unr.edu.
References
- 1.de Koning AP, Gu W, Castoe TA, Batzer MA, Pollock DD. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011;7(12):e1002384. doi: 10.1371/journal.pgen.1002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazazian HH, Jr, Moran JV. Mobile DNA in health and disease. N Engl J Med. 2017;377(4):361–370. doi: 10.1056/NEJMra1510092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mager DL, Stoye JP. Mammalian endogenous retroviruses. Microbiol Spectr. 2015;3(1):MDNA3-0009-2014. doi: 10.1128/microbiolspec.MDNA3-0009-2014. [DOI] [PubMed] [Google Scholar]
- 4.Akagi K, Li J, Stephens RM, Volfovsky N, Symer DE. Extensive variation between inbred mouse strains due to endogenous L1 retrotransposition. Genome Res. 2008;18(6):869–880. doi: 10.1101/gr.075770.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nellaker C, Keane TM, Yalcin B, Wong K, Agam A, Belgard TG, Flint J, Adams DJ, Frankel WN, Ponting CP. The genomic landscape shaped by selection on transposable elements across 18 mouse strains. Genome Biol. 2012;13(6):R45. doi: 10.1186/gb-2012-13-6-r45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouse Genome Sequencing C, Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 7.Dewannieux M, Esnault C, Heidmann T. LINE-mediated retrotransposition of marked Alu sequences. Nat Genet. 2003;35(1):41–48. doi: 10.1038/ng1223. [DOI] [PubMed] [Google Scholar]
- 8.Hancks DC, Goodier JL, Mandal PK, Cheung LE, Kazazian HH., Jr Retrotransposition of marked SVA elements by human L1s in cultured cells. Hum Mol Genet. 2011;20(17):3386–3400. doi: 10.1093/hmg/ddr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raiz J, Damert A, Chira S, Held U, Klawitter S, Hamdorf M, Lower J, Stratling WH, Lower R, Schumann GG. The non-autonomous retrotransposon SVA is trans-mobilized by the human LINE-1 protein machinery. Nucleic Acids Res. 2012;40(4):1666–1683. doi: 10.1093/nar/gkr863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esnault C, Maestre J, Heidmann T. Human LINE retrotransposons generate processed pseudogenes. Nat Genet. 2000;24(4):363–367. doi: 10.1038/74184. [DOI] [PubMed] [Google Scholar]
- 11.Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, Kazazian HH, Boeke JD, Moran JV. Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol. 2001;21(4):1429–1439. doi: 10.1128/MCB.21.4.1429-1439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran JV, DeBerardinis RJ, Kazazian HH., Jr Exon shuffling by L1 retrotransposition. Science. 1999;283(5407):1530–1534. doi: 10.1126/science.283.5407.1530. [DOI] [PubMed] [Google Scholar]
- 13.Pickeral OK, Makalowski W, Boguski MS, Boeke JD. Frequent human genomic DNA transduction driven by LINE-1 retrotransposition. Genome Res. 2000;10(4):411–415. doi: 10.1101/gr.10.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodier JL, Ostertag EM, Kazazian HH., Jr Transduction of 3′-flanking sequences is common in L1 retrotransposition. Hum Mol Genet. 2000;9(4):653–657. doi: 10.1093/hmg/9.4.653. [DOI] [PubMed] [Google Scholar]
- 15.Solyom S, Ewing AD, Hancks DC, Takeshima Y, Awano H, Matsuo M, Kazazian HH., Jr Pathogenic orphan transduction created by a nonreference LINE-1 retrotransposon. Hum Mutat. 2012;33(2):369–371. doi: 10.1002/humu.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tubio JMC, Li Y, Ju YS, Martincorena I, Cooke SL, Tojo M, Gundem G, Pipinikas CP, Zamora J, Raine K, et al. Mobile DNA in cancer. Extensive transduction of nonrepetitive DNA mediated by L1 retrotransposition in cancer genomes. Science. 2014;345(6196):1251343. doi: 10.1126/science.1251343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buzdin A, Ustyugova S, Gogvadze E, Vinogradova T, Lebedev Y, Sverdlov E. A new family of chimeric retrotranscripts formed by a full copy of U6 small nuclear RNA fused to the 3′ terminus of l1. Genomics. 2002;80(4):402–406. doi: 10.1006/geno.2002.6843. [DOI] [PubMed] [Google Scholar]
- 18.Symer DE, Connelly C, Szak ST, Caputo EM, Cost GJ, Parmigiani G, Boeke JD. Human l1 retrotransposition is associated with genetic instability in vivo. Cell. 2002;110(3):327–338. doi: 10.1016/S0092-8674(02)00839-5. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert N, Lutz-Prigge S, Moran JV. Genomic deletions created upon LINE-1 retrotransposition. Cell. 2002;110(3):315–325. doi: 10.1016/S0092-8674(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 20.Thompson PJ, Macfarlan TS, Lorincz MC. Long terminal repeats: from parasitic elements to building blocks of the transcriptional regulatory repertoire. Mol Cell. 2016;62(5):766–776. doi: 10.1016/j.molcel.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazachenka A, Bertozzi TM, Sjoberg-Herrera MK, Walker N, Gardner J, Gunning R, Pahita E, Adams S, Adams D, Ferguson-Smith AC. Identification, characterization, and heritability of murine metastable Epialleles: implications for non-genetic inheritance. Cell. 2018;175(5):1259–71.e13. doi: 10.1016/j.cell.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burns KH. Transposable elements in cancer. Nat Rev Cancer. 2017;17(7):415–424. doi: 10.1038/nrc.2017.35. [DOI] [PubMed] [Google Scholar]
- 23.Elbarbary RA, Lucas BA, Maquat LE. Retrotransposons as regulators of gene expression. Science. 2016;351(6274):aac7247. doi: 10.1126/science.aac7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostertag EM, DeBerardinis RJ, Goodier JL, Zhang Y, Yang N, Gerton GL, Kazazian HH., Jr A mouse model of human L1 retrotransposition. Nat Genet. 2002;32(4):655–660. doi: 10.1038/ng1022. [DOI] [PubMed] [Google Scholar]
- 25.An W, Han JS, Wheelan SJ, Davis ES, Coombes CE, Ye P, Triplett C, Boeke JD. Active retrotransposition by a synthetic L1 element in mice. Proc Natl Acad Sci U S A. 2006;103(49):18662–18667. doi: 10.1073/pnas.0605300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435(7044):903–910. doi: 10.1038/nature03663. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Perez JL, Morell M, Scheys JO, Kulpa DA, Morell S, Carter CC, Hammer GD, Collins KL, O'Shea KS, Menendez P, et al. Epigenetic silencing of engineered L1 retrotransposition events in human embryonic carcinoma cells. Nature. 2010;466(7307):769–773. doi: 10.1038/nature09209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannan M, Li J, Fritz SE, Husarek KE, Sanford JC, Sullivan TL, Tiwary PK, An W, Boeke JD, Symer DE. Dynamic silencing of somatic L1 retrotransposon insertions reflects the developmental and cellular contexts of their genomic integration. Mob DNA. 2017;8:8. doi: 10.1186/s13100-017-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu N, Lee CH, Swigut T, Grow E, Gu B, Bassik MC, Wysocka J. Selective silencing of euchromatic L1s revealed by genome-wide screens for L1 regulators. Nature. 2018;553(7687):228–232. doi: 10.1038/nature25179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM, Kazazian HH., Jr L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev. 2009;23(11):1303–1312. doi: 10.1101/gad.1803909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newkirk SJ, Lee S, Grandi FC, Gaysinskaya V, Rosser JM, Vanden Berg N, Hogarth CA, Marchetto MCN, Muotri AR, Griswold MD, et al. Intact piRNA pathway prevents L1 mobilization in male meiosis. Proc Natl Acad Sci U S A. 2017;114(28):E5635–E5E44. doi: 10.1073/pnas.1701069114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandi FC, Rosser JM, Newkirk SJ, Yin J, Jiang X, Xing Z, Whitmore L, Bashir S, Ivics Z, Izsvak Z, et al. Retrotransposition creates sloping shores: a graded influence of hypomethylated CpG islands on flanking CpG sites. Genome Res. 2015;25(8):1135–1146. doi: 10.1101/gr.185132.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maksakova IA, Romanish MT, Gagnier L, Dunn CA, van de Lagemaat LN, Mager DL. Retroviral elements and their hosts: insertional mutagenesis in the mouse germ line. PLoS Genet. 2006;2(1):e2. doi: 10.1371/journal.pgen.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hancks DC, Kazazian HH., Jr Roles for retrotransposon insertions in human disease. Mob DNA. 2016;7:9. doi: 10.1186/s13100-016-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasicek TJ, Zeng L, Guan XJ, Zhang T, Costantini F, Tilghman SM. Two dominant mutations in the mouse fused gene are the result of transposon insertions. Genetics. 1997;147(2):777–786. doi: 10.1093/genetics/147.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nat Genet. 1994;8(1):59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- 37.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23(3):314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 38.Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, Whitelaw E. Transgenerational inheritance of epigenetic states at the murine Axin (Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci U S A. 2003;100(5):2538–2543. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23(15):5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenfeld CS, Sieli PT, Warzak DA, Ellersieck MR, Pennington KA, Roberts RM. Maternal exposure to bisphenol a and genistein has minimal effect on a (vy)/a offspring coat color but favors birth of agouti over nonagouti mice. Proc Natl Acad Sci U S A. 2013;110(2):537–542. doi: 10.1073/pnas.1220230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, Hsu PD, Wu XB, Jiang WY, Marraffini LA, et al. Multiplex Genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Lee S, Oliver D, Yuan S, Tang C, Wang Y, Zheng H, Yan W. Prps1l1, a testis-specific gene, is dispensable for mouse spermatogenesis. Mol Reprod Dev. 2018;85(10):802–804. doi: 10.1002/mrd.23053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliver D, Yuan S, McSwiggin H, Yan W. Pervasive genotypic mosaicism in founder mice derived from Genome editing through pronuclear injection. PLoS One. 2015;10(6):e0129457. doi: 10.1371/journal.pone.0129457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sookdeo A, Hepp CM, McClure MA, Boissinot S. Revisiting the evolution of mouse LINE-1 in the genomic era. Mob DNA. 2013;4(1):3. doi: 10.1186/1759-8753-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathias SL, Scott AF, Kazazian HH, Jr, Boeke JD, Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991;254(5039):1808–1810. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- 46.Clements AP, Singer MF. The human LINE-1 reverse transcriptase:effect of deletions outside the common reverse transcriptase domain. Nucleic Acids Res. 1998;26(15):3528–3535. doi: 10.1093/nar/26.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kent WJ. BLAT--the BLAST-like alignment tool. Genome Res. 2002;12(4):656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodier JL, Kazazian HH., Jr Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell. 2008;135(1):23–35. doi: 10.1016/j.cell.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Erwin JA, Marchetto MC, Gage FH. Mobile DNA elements in the generation of diversity and complexity in the brain. Nat Rev Neurosci. 2014;15(8):497–506. doi: 10.1038/nrn3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Rattner A, Nathans J. Effects of L1 retrotransposon insertion on transcript processing, localization and accumulation: lessons from the retinal degeneration 7 mouse and implications for the genomic ecology of L1 elements. Hum Mol Genet. 2006;15(13):2146–2156. doi: 10.1093/hmg/ddl138. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Kannan M, Trivett AL, Liao H, Wu X, Akagi K, Symer DE. An antisense promoter in mouse L1 retrotransposon open reading frame-1 initiates expression of diverse fusion transcripts and limits retrotransposition. Nucleic Acids Res. 2014;42(7):4546–4562. doi: 10.1093/nar/gku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Akagi K, Hu Y, Trivett AL, Hlynialuk CJ, Swing DA, Volfovsky N, Morgan TC, Golubeva Y, Stephens RM, et al. Mouse endogenous retroviruses can trigger premature transcriptional termination at a distance. Genome Res. 2012;22(5):870–884. doi: 10.1101/gr.130740.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franke V, Ganesh S, Karlic R, Malik R, Pasulka J, Horvat F, Kuzman M, Fulka H, Cernohorska M, Urbanova J, et al. Long terminal repeats power evolution of genes and gene expression programs in mammalian oocytes and zygotes. Genome Res. 2017;27(8):1384–1394. doi: 10.1101/gr.216150.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez-Gonzalez R, Ramirez MA, Pericuesta E, Calle A, Gutierrez-Adan A. Histone modifications at the blastocyst Axin1(Fu) locus mark the heritability of in vitro culture-induced epigenetic alterations in mice. Biol Reprod. 2010;83(5):720–727. doi: 10.1095/biolreprod.110.084715. [DOI] [PubMed] [Google Scholar]
- 55.Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T. Regulation of alternative splicing by histone modifications. Science. 2010;327(5968):996–1000. doi: 10.1126/science.1184208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naftelberg S, Schor IE, Ast G, Kornblihtt AR. Regulation of alternative splicing through coupling with transcription and chromatin structure. Annu Rev Biochem. 2015;84:165–198. doi: 10.1146/annurev-biochem-060614-034242. [DOI] [PubMed] [Google Scholar]
- 57.Schor IE, Rascovan N, Pelisch F, Allo M, Kornblihtt AR. Neuronal cell depolarization induces intragenic chromatin modifications affecting NCAM alternative splicing. Proc Natl Acad Sci U S A. 2009;106(11):4325–4330. doi: 10.1073/pnas.0810666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed SC. The inheritance and expression of fused, a new mutation in the house Mouse. Genetics. 1937;22(1):1–13. doi: 10.1093/genetics/22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickies MM. A new viable yellow mutation in the house mouse. J Hered. 1962;53:84–86. doi: 10.1093/oxfordjournals.jhered.a107129. [DOI] [PubMed] [Google Scholar]
- 60.Kurosaki T, Maquat LE. Nonsense-mediated mRNA decay in humans at a glance. J Cell Sci. 2016;129(3):461–467. doi: 10.1242/jcs.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conti E, Izaurralde E. Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr Opin Cell Biol. 2005;17(3):316–325. doi: 10.1016/j.ceb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson MF. A new function for nonsense-mediated mRNA-decay factors. Trends Genet. 2005;21(3):143–148. doi: 10.1016/j.tig.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Yan W. Potential roles of noncoding RNAs in environmental epigenetic transgenerational inheritance. Mol Cell Endocrinol. 2014;398(1–2):24–30. doi: 10.1016/j.mce.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hisano M, Erkek S, Dessus-Babus S, Ramos L, Stadler MB, Peters AH. Genome-wide chromatin analysis in mature mouse and human spermatozoa. Nat Protoc. 2013;8(12):2449–2470. doi: 10.1038/nprot.2013.145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, S2, S3 and Supplemental notes. Generation of AxinIAP and AIAP founder mice and copy number variation assays for AxincL1 mice (Figure S1); promoter activity analyses (Figure S2); DNA methylation levels around cL1 in penetrant and silent AxincL1 mice (Figure S3); and annotated sequences of retrotransposons used in this study (Supplemental notes). (PDF 5204 kb)
Table S1. Orf2 BLAT results. (XLSX 52 kb)
Table S2. MaLR BLAT results. (XLSX 48 kb)
Table S3. Oligos used in this study. (XLSX 11 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.