Abstract
Background
As malaria cases have declined throughout Nepal, imported cases comprise an increasing share of the remaining malaria caseload, yet how to effectively target mobile and migrant populations (MMPs) at greatest risk is not well understood. This formative research aimed to confirm the link between imported and indigenous cases, characterize high-risk MMPs, and identify opportunities to adapt surveillance and intervention strategies to them.
Methods
The study used a mixed-methods approach in three districts in far and mid-western Nepal, including (i) a retrospective analysis of passive surveillance data, (ii) a quantitative health facility-based survey of imported cases and their MMP social contacts recruited by peer-referral, and (iii) focus group (FG) discussions and key informant interviews (KIIs) with a subset of survey participants. Retrospective case data were summarised and the association between monthly indigenous case counts and importation rates in the previous month was investigated using Bayesian spatio-temporal regression models. Quantitative data from structured interviews were summarised to develop profiles of imported cases and MMP contacts, including travel characteristics and malaria knowledge, attitudes and practice. Descriptive statistics of the size of cases’ MMP social networks are presented as a measure of potential programme reach. To explore opportunities and barriers for targeted malaria surveillance, data from FGs and KIIs were formally analysed using a thematic content analysis approach.
Results
More than half (54.1%) of malaria cases between 2013 and 2016 were classified as imported and there was a positive association between monthly indigenous cases (incidence rate ratio (IRR) 1.02 95% CI 1.01–1.03) and the previous month’s case importation rate. High-risk MMPs were identified as predominantly adult male labourers, who travel to malaria endemic areas of India, often lack a basic understanding of malaria transmission and prevention, rarely use ITNs while travelling and tend not to seek treatment when ill or prefer informal private providers. Important obstacles were identified to accessing Nepali MMPs at border crossings and at workplaces within India. However, strong social connectivity during travel and while in India, as well as return to Nepal for large seasonal festivals, provide opportunities for peer-referral-based and venue-based surveillance and intervention approaches, respectively.
Conclusions
Population mobility and imported malaria cases from India may help to drive local transmission in border areas of far and mid-western Nepal. Enhanced surveillance targeting high-risk MMP subgroups would improve early malaria diagnosis and treatment, as well as provide a platform for education and intervention campaigns. A combination of community-based approaches is likely necessary to achieve malaria elimination in Nepal.
Electronic supplementary material
The online version of this article (10.1186/s12936-019-2791-1) contains supplementary material, which is available to authorized users.
Keywords: Imported malaria cases, Mobile migrant populations (MMPs), Focus group discussion, Key informants’ interview, Malaria elimination, Nepal
Background
As a result of sustained control measures, the malaria burden in Nepal has declined steadily since 1985 and malaria transmission has reached a very low level, with an annual parasite incidence (API) of 0.04 cases per 1000 population in 2016 [1]. Although Nepal is moving towards implementing elimination strategies, high rates of importation along its southern border present a major challenge to continued progress. As the number of total cases declined by 92% between 2002 (12,750 cases) and 2016 (1009 cases), the proportion of cases classified as imported increased from approximately 20 to 50% [2]. The majority of imported cases during this period reported a history of travel to malaria-endemic areas of India. Close to two million Nepalese are estimated to work in India as seasonal or long-term labourers, and a large proportion of external labour migration, particularly seasonal migration, is believed to originate in malaria endemic areas such as far- and mid-western Nepal [3–5]. This population movement across Nepal’s southern border, which citizens of both nations may cross freely, is thought to contribute to persistence of local malaria foci in border areas [5–7]. The fact that imported cases have not declined at the same rate as indigenous cases suggests that additional control measures may be needed to effectively prevent, detect and resolve infections among mobile and migrant populations (MMPs).
Free malaria testing and treatment are available to Nepali and Indian nationals through 162 government primary health care (PHC) centres, health posts (HPs) and sub-health posts (SHPs) in 22 border districts of Nepal [8]. The standard of care includes radical cure for Plasmodium vivax consisting of a 14-day course of primaquine in combination with chloroquine [9]. During the eradication era (1960–1970), the National Malaria Control Programme (NMCP) of Nepal operated cross-border screening in posts in Kakarvitta of Jhapa District in east and Gaddha Chowki in Kanchanpur District in far-western region; these posts aided in the early identification and tracking of imported cases. Given sustained malaria importation, the NMCP is once again considering year-round border screening as a surveillance approach for MMPs, yet plans have been delayed due to infrastructure challenges and questions around cost-effectiveness. More generally, the priority that the NMCP should place on reducing importation is unclear due to the lack of evidence of the extent to which imported infections may actually sustain indigenous transmission.
Epidemiological and mathematical modelling studies in numerous eliminating countries bordering higher burden neighbours demonstrate that transmission largely persists due to the continued pressure of imported cases [10–14]. Churcher et al. [12] provide statistical evidence that controlled non-endemic malaria (where the average number of new persons infected by a person with malaria is less than one) has been reached once the proportion of imported cases among detected cases reaches between 32 and 48%, depending on case burden.
Given the low malaria burden in Nepal and high cross-border movement in remaining endemic areas, there is a need to improve understanding of the subgroups of MMPs at greatest risk of importing malaria, their role in sustaining local transmission, and how to access them. There is currently limited information regarding basic characteristics of MMPs that may be necessary to design effective screening and prevention interventions, including operational questions of how they should be timed and targeted, such as the travel patterns of MMPs and the specific conditions that may lead to exposure during travel and while living and working abroad. Potential operational challenges that may limit access of MMPs to routine malaria prevention and treatment and pose a constraint to improving diagnosis and treatment have not been investigated [15, 16].
Relatedly, it is essential to understand whether current surveillance approaches effectively detect cases and accurately assess levels of malaria prevalence among MMPs at the population level. Due to frequent travel, MMPs may be under-represented in routine case data and absent during household visits. Facility-based surveillance approaches may also fail to capture MMPs who face barriers to accessing public health facilities, prefer private facilities, or who do not seek care at all [17, 18]. While several complementary, targeted surveillance strategies are increasingly under investigation for reaching subgroups at elevated risk for malaria, such as border screening, respondent-driven sampling (RDS) and time-location sampling (TLS) [19, 20], it is unclear whether these approaches would be appropriate for high-risk MMPs in Nepal.
This formative research study aimed to address these knowledge gaps in far and mid-western Nepal, specifically to (1) quantify the association between imported and local transmission, (2) document the social determinants of imported malaria, (3) characterize high-risk MMPs, and (4) identify potential surveillance and intervention strategies in MMPs and how they may be optimized through spatial and temporal targeting.
Methods
Study design
This study employed a mixed-methods approach, including (i) a retrospective analysis of passive surveillance data to define temporal and spatial relationships between imported and indigenous malaria cases, (ii) a quantitative health facility-based survey of imported cases and their MMP social contacts to define characteristics and travel patterns of this population, and (iii) focus group (FG) discussions and key informant interviews (KIIs) to explore opportunities and barriers for targeted malaria surveillance in MMPs. Data collection was carried out over a 3-month period between August and November 2016.
Study sites
The study was conducted in three historically high-burden border districts in far and mid-western Nepal with high population movement to India: Kanchanpur, Kailali and Bardiya (Fig. 1). These districts form part of the lowland terai in western Nepal, where malaria transmission is concentrated, and key foci bordering the malaria-endemic, Indian states of Uttar Pradesh and Uttarakhand [5]. Plasmodium vivax predominates malaria transmission, which is highly seasonal with the majority of transmission occurring from June to September during the monsoon season [9]. The primary malaria vectors are Anopheles fluviatilis and Anopheles maculatus (responsible for transmission in the forest fringe, foothills and inner terai) and Anopheles annularis, an inefficient vector found in the outer terai. Historically, Anopheles minimus was also an important and highly efficient vector in forest and forest-fringe areas, but was reported to be eliminated following DDT spraying [9].
Fig. 1.
Study site districts (Kanchanpur, Kailali and Bardiya) and formal border crossings (black squares) in far-western and mid-western Nepal (inset). Green shaded areas represent tree cover
Together, the three study districts have a combined population of 1.9 million and accounted for 34% of the total burden of malaria in Nepal in 2016, with the annual parasite incidence (API) ranging from 0.27 cases per 1000 population in Kailali to 0.14 and 0.07 in Kanchanpur and Bardiya [21]. There is a large private health sector in these districts, but private clinics and pharmacies remain largely unregulated and have low case reporting rates. Based on data from the 2014 Malaria Indicator Cluster Survey, febrile children under the age of 5 years in the far western terai were more likely to seek treatment with a private provider (49.0%) than at a public health facility (26.4%) [22]. An estimated 0.7 million migrants cross annually at four formal border check points on the border with India [23]. Planned implementation of a border malaria check post at the main crossing in Kanchanpur district (Gaddha Chowki) has been delayed since 2013 due to resource limitations [9].
Case definitions and surveillance strategies considered
Following WHO and Nepal national guidelines, all study components defined a malaria case as a person in which the presence of malaria parasites has been confirmed by microscopy or rapid diagnostic test (RDT) [24]. All P. vivax cases were assumed to be incident, based on a lack of identifying information to distinguish potentially relapsing infections. Imported cases were defined according to national guidelines as malaria cases confirmed who moved from their permanent or previous residence within the past 4 weeks to stay or work in a different district or country [25]. This working definition was revised during the pilot stage of prospective data collection due to low case numbers and the predominance of P. vivax. The revised definition extended the period of exposure to include overnight travel within the prior 7–60 days to a destination outside of Nepal or to a “high-risk” district, defined as districts in which indigenous cases have been reported in the past 3 years.
Three possible active malaria surveillance strategies were assessed during the qualitative portion of this study. These approaches are currently or have been under consideration in Nepal to identify and access MMPs for malaria screening and treatment.
Border screening Border screening is the targeted screening of cross-border travellers at or near a border crossing and has had limited use in Nepal. Historically, this approach has had some success in Nepal but is generally implemented either through enforced or voluntary testing, and has had mixed success due to low case yield, limited acceptability and high operational costs [26].
Peer-referral recruitment Peer-referral recruitment is a technique for accessing specific populations where individuals (in this case imported malaria cases) refer acquaintances who meet specified characteristics (e.g. cross-border travel or behaviours that may lead to exposure). Peer-referral can be used to improve the coverage of outreach, education and prevention. The approach can also be applied to case detection, in addition to standard active case detection (ACD) to identify peers at high risk of infection. Peer-referral strategies to support malaria elimination are currently under investigation in several contexts [20].
Venue-based recruitment Venue-based recruitment is a technique to access members of a high risk-population at specific venues or locations (i.e. parks, tea shops, bus stops) where they are known to gather. This method, too, is often used for purposes of outreach, prevention and as a sampling method for surveys [27].
Retrospective study
Study design and population
This was a retrospective analysis using passively detected case-based surveillance data from Kanchanpur, Kailali and Bardiya. In Nepal, confirmed malaria case data are routinely entered into patient registers at all public and private health posts. These data are then reported to the district health office, which collates the information and submits centrally to the Health Management Information System (HMIS). In this study, de-identified case-level data from the three study districts were extracted from the HMIS for the period January 2013 to December 2016.
Data collection and management
Extracted variables included date of diagnosis, age, gender, travel destination (imported cases only), location diagnosed, location of residence, diagnostic test (RDT or microscopy), malaria speciation (if available) and whether the individual was treated. Data were entered into a standardized excel database and linked to the corresponding village development committee (VDC) or municipality (MC) of residence for mapping.
Data analysis
Retrospective case data across the three districts were summarised according to species, month and importation status. Monthly imported and indigenous case numbers were aggregated by village development committee/municipality (VDC/MC) and used to calculate monthly incidence rates using population denominators provided by the NMCP. These data are estimated from the 2011 National Population and Housing Census in Nepal and assume a 1.35 annual growth rate [28]. Annual incidence of imported and indigenous cases were mapped using ArcGIS 10.4 (Redlands, CA) to visualize variation in transmission across the three districts and time period.
The association between monthly indigenous case counts (outcome) and importation rates in the previous month was investigated using Poisson regression models in ‘R-INLA’, which is a widely used software library for approximating Bayesian inference. A non-spatial Poisson regression model was used to initially test univariate associations of importation rates and potential environmental risk factors with monthly indigenous case counts (see Additional file 1). Environmental covariates with a P value of < 0.05 were included in the initial multivariate model with logged importation rate, district and year as fixed effects and evaluated using a process of backwards-stepwise elimination. Covariates were retained where the deviance information criteria (DIC) was lower or changed the primary association of interest more than 10%. Additional details of this covariate selection process are described in Additional file 1, which includes a list of all potential environmental factors, spatiotemporal resolutions and sources and a summary of their association with malaria incidence. The software version used for this study was R version 3.4.
All environmental covariates selected via the preliminary analysis, importation rates in the previous month, reported cases and catchment population were included in a Bayesian spatio-temporal model. Different models were tested and compared using leave-one-out cross-validation based on the conditional predictive ordinate (CPO). The best fit model was a conditional autoregressive (CAR) Poisson model with a periodicity of 12 months, which captures the month-to-month changes in transmission as well as seasonal trends. The details of the model selection process and comparison of candidate models are provided in Additional file 2.
Cross-sectional survey
Study design and population
A cross-sectional survey was conducted amongst imported index cases and a sample of MMPs, selected using snowball sampling. All malaria cases diagnosed at health facilities in the three districts between August 31 and November 15, 2016, were screened according to eligibility criteria in Table 1 and entered into a recruitment registry by health facility staff. Imported cases were asked to provide information for up to five MMP social contacts who met specified eligibility criteria (Table 1), including travel in the prior 60 days. Research assistants then screened MMP social contacts by phone to confirm eligibility and invited eligible individuals to participate in the study.
Table 1.
Eligibility criteria for imported cases, MMP social contacts and locally acquired malaria cases included in prospective surveys and/or focus group (FG)
| Imported case (survey and FG) | MMP social contact (survey and FG)a | Locally acquired malaria case (FG only) |
|---|---|---|
| Confirmed malaria infection by microscopy and/or RDT Aged over 18 years Can speak and understand Nepali First-time participant in the study Spent at least one night outside of study district in the past 7–60 days, either outside of Nepal or in a specified high-risk district in Nepal |
Aged over 18 years Can speak and understand Nepali First-time participant in the study Spent at least one night outside of study district in the past 60 days, in the same destination as referring case |
Confirmed malaria infection by microscopy and/or RDT Aged over 18 years Can speak and understand Nepali First-time participant in the study Has not spent the night outside of study district in the past 7–60 days |
aAt most, only one social contact may be a member of the referring cases’ household
As the cross-sectional survey was conceived of as a pilot study, no formal sample size calculation was conducted.
Data collection and management
Informed consent was obtained by research assistants or a trained health facility staff member prior to administration of a structured questionnaire, which collected information on socio-demographics, occupation, travel histories, malaria prevention and outdoor exposures at night.
MMP social contacts answered additional questions around history of malaria and treatment seeking. All questionnaire data were entered from paper forms into an electronic database using ODK Collect, and regularly uploaded to a central server.
Data analysis
Quantitative data from structured interviews were summarised to develop profiles of imported cases and MMP social contacts. Specific areas of focus included socio-demographic characteristics, travel patterns and malaria attitudes, knowledge and practices. Categorical variables were tabulated and categories combined where levels contained < 5% of responses.
Of 138 specific travel destinations in India, all locations were geolocated to a specific longitude and latitude corresponding to the cited village/city (87%) or greater municipality (13%) using a number of electronic sources of information, including GeoNet Names Server [29], Google Earth [30] and Falling Grain [31]. Similarly, the majority of named transit points (238/246) were successfully geolocated. Locations identified from one source were cross-checked against other sources and to ensure that they fell within the correct administrative area or municipality. Travel routes were condensed and maps created in ArcGIS.
MMP social contacts provided a statistical comparison to cases to identify risk factors for malaria infection within MMPs. Conditional logistic regression was carried out in STATA v14 (College Station, Texas) to identify potential risk factors that may distinguish MMPs known to be infected with malaria (imported cases) from their peers. Age and gender were adjusted for a priori as confounders, and key exposures assessed through this approach included occupation, type of work (indoor vs outdoor), duration of travel, transit times and destination.
Focus group discussions and key informant interviews
Study design and population
Focus groups (FGs) were conducted with a convenience sample of eligible indigenous cases, imported cases, and MMP social contacts. Nine FGs were conducted in-person with 56 participants in two districts, Kailali and Kanchanpur. This included seven FGs with 43 imported cases and MMP referrals in Kailali (4) and Kanchanpur (3) and one FG consisting of 6–7 indigenous cases in each district. A minimum of six FGs is usually considered sufficient to achieve data saturation in a group and allowed us some comparison between MMP and local populations.
Ten key informants were selected in each district by the study coordinator based on their perceived knowledge of MMPs in this area of Nepal. Participants were generally senior-level employees and represented a broad range of individuals with knowledge about MMPs, including non-governmental organizations (NGOs), health officials, political parties, representatives of hotel associations and police/immigration officials.
Data collection and management
FGs and key informant interviews were scheduled through mobile phone communication and the response rate was 100%. FGs and key informant interviews were conducted and transcribed in the Nepali language by a Nepalese research team consisting of a moderator and note-taker. The moderator facilitated discussions based on a thematic guide and sessions were audio recorded with detailed notes captured by the note-taker. All participants were oriented to the study and provided a brief description of why the research was being conducted, prior to being administered written informed consent. FGs were conducted at health facilities in Nepali and lasted around 1 h. Key informant interviews were conducted at the participants’ workplaces.
All FGs and interviews were audio recorded. The research team carried out a debriefing session immediately after completion of the session and a summary form was completed based on the discussion and notes. Thematic guides were pilot tested and revised before the start of data collection. Themes explored in the FGs and in-depth KIIs included travel and behavioural exposures related to malaria, health-seeking behaviours and perceptions of proposed active surveillance and response strategies.
Data analysis
FGs and KIIs were transcribed from audio recordings within 10–30 days. Nepali transcripts were then translated into English and formally analysed using a thematic content analysis approach. Specifically, transcripts were read and an analytic codebook developed around the main themes in the topic guides. Codes and transcripts were organized and analysed in Dedoose [32], including coding segments of textual data and grouping them into discrete units. Additional categories emerging from the data were added to the codebook as necessary and the final codebook was applied by two independent coders, following training to reach a high level of concordance. Themes uniting the categories were identified and relations between and within codes were used to provide additional insight into the categories and qualitative aims of this study.
Thematic analysis identified four main areas of discovery:
Experience with malaria and risk perception (locally and while travelling).
Mobility and travel patterns.
Current treatment-seeking and preventive behaviours.
Opportunities and barriers to accessing MMPs through specific active surveillance strategies.
Results for MMPs were qualitatively compared to indigenous cases to identify any key differences. Qualitative information was used to add detail and perspective to our understanding of the factors that influence treatment seeking and travel behaviours, as well as identify opportunities and barriers to specific active surveillance approaches in MMPs.
Results
Retrospective analysis
There were 1640 individual malaria case records reported through passive health facility based surveillance data from the three districts between 2013 and 2016, including 1418 cases of P. vivax, 184 cases of Plasmodium falciparum, and 38 mixed infections. Data were geo-located to the VDC of residence for the majority (95.8%) of records, and a comparison with unpublished district-level data (health facility based HMIS reporting monthly to the district public health office) showed good correspondence in Bardiya and Kanchanpur, but 24–35% under-reporting in Kailali (see Additional file 3) [12].
Overall, approximately half (54.1%) of malaria cases diagnosed between 2013 and 2016 were classified as imported by district health officers based on their travel history. Both indigenous and imported cases were typically adult males (Table 2), and imported case profiles increasingly aligned with this demographic group over this time period.
Table 2.
Demographic and clinical characteristics of 1640 locally acquired and imported cases from 2013 to 2016
| Local (N = 753) | Imported (N = 887) | P value | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Male | 560 | (74.4) | 756 | (85.2) | < 0.0001 |
| Age category (years) | |||||
| < 5 | 17 | (2.3) | 10 | (1.1) | 0.06 |
| 5–14 | 56 | (7.4) | 49 | (5.5) | |
| 15–29 | 363 | (48.2) | 464 | (52.3) | |
| 30–59 | 267 | (35.5) | 320 | (36.1) | |
| 60+ | 50 | (6.6) | 44 | (5.0) | |
| Plasmodium species | |||||
| P. falciparum | 103 | (13.7) | 81 | (9.1) | < 0.0001 |
| P. vivax | 620 | (82.3) | 798 | (90.0) | |
| Mixed | 30 | (4.0) | 8 | (0.9) | |
| Diagnostic | |||||
| RDT | 285 | (37.9) | 250 | (28.2) | < 0.0001 |
| Microscopy | 444 | (59.0) | 575 | (64.8) | |
| Both | 24 | (3.2) | 62 | (6.7) | |
| District | |||||
| Kailali | 498 | (66.1) | 567 | (63.9) | 0.244 |
| Kanchanpur | 183 | (24.3) | 246 | (27.7) | |
| Bardiya | 72 | (9.6) | 74 | (8.3) | |
| Year | |||||
| 2013 | 338 | (44.9) | 290 | (32.7) | < 0.0001 |
| 2014 | 192 | (25.5) | 265 | (29.9) | |
| 2015 | 94 | (12.5) | 185 | (20.9) | |
| 2016 | 129 | (17.1) | 147 | (16.6) | |
Temporal and spatial trends in transmission
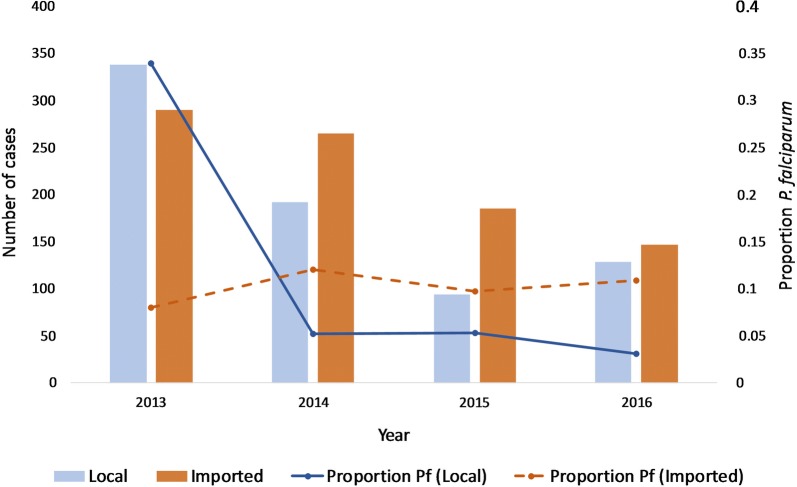
The number of imported cases dropped from 290 to 147 and indigenous cases from 338 to 129 between 2013 and 2016; corresponding to an increase in the proportion of imported cases (46% to 59.8%) (Table 2). Both imported and indigenous malaria transmission in this area of Nepal continues to be dominated by P. vivax, although there has been an overall decline in cases during this period (Fig. 2). In 2014, the number of P. falciparum cases classified as indigenous dropped by 91% (from 88 to 8), which corresponded with a spike in use of RDTs. Although only 10% of imported cases were infected with P. falciparum in 2016, these were the majority (77.8%) of all diagnosed P. falciparum cases.
Fig. 2.
Total number of indigenous and imported cases (bars) and respective proportion of Plasmodium falciparum (Pf) cases (lines) in the three study districts between 2013 and 2016. While the number of indigenous falciparum cases fell in 2014, corresponding with a spike in the use of RDTs, the proportion of imported falciparum cases was stable
Imported cases had distinct bimodal seasonality, with the first peak occurring in April-June and a second peak in August–September (see Additional file 2: Fig. S2). These periods roughly correspond to when travellers commonly return back to Nepal for a month for agricultural activities (harvesting wheat and paddy cultivation) during April–June and return for festivals and harvesting of rice, other grains, and paddies in September–October. In contrast to imported malaria, indigenous malaria transmission in Nepal showed a single, extended peak and greater variation between years (see in Additional file 2: Fig. S2).
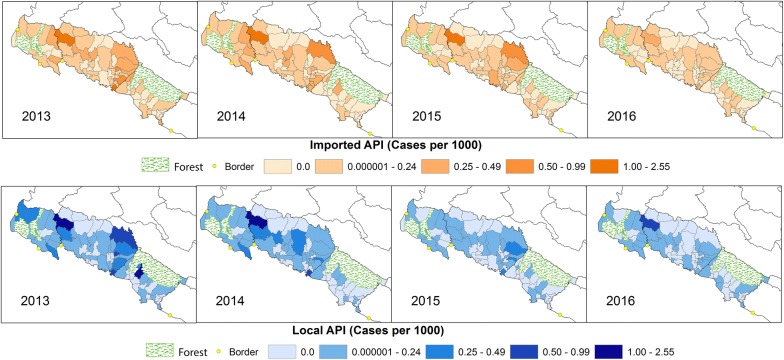
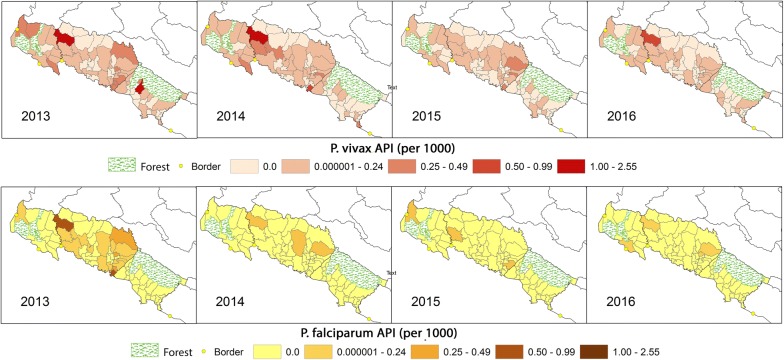
The geographic distribution of imported and indigenous malaria cases between 2013 and 2016 overlapped and foci were predominately observed in northern Kailali, crossing into Kanchanpur (including Godawari VDC and bordering VDCs), as well as southern VDCs in Kailali that border Bardiya (Fig. 3). Foci tended to occur in areas of low to mid population density and the decrease in incidence over time corresponded with smaller geographic foci of indigenous transmission. Species-specific maps of indigenous annual parasite incidence (API) for these years showed similar trends, but highlighted the precipitous decline and highly focal distribution of P. falciparum in comparison to P. vivax in these areas (Fig. 4).
Fig. 3.
All species imported and indigenous annual parasite index (API) at the village development committee (VDC) level in the three study districts between 2013 and 2016. Areas of higher transmission are located in the south of Kailali bordering Bardiya National Park and on the northern border with Kanchanpur. VDCs that are predominantly covered by a national forest with very low populations are shaded green
Fig. 4.
Indigenous annual parasite index (API) of Plasmodium vivax and P. falciparum at the village development committee (VDC) level in the three study districts between 2013 and 2016. National forests are shaded green
The role of imported malaria in local transmission
The number of indigenous malaria cases within a given VDC was directly and strongly related to higher levels of imported infections in the previous month, after adjusting for variation in rainfall, population density and year (Table 3). Each percent increase in the previous month’s importation rate was associated with a two percent increase in the risk of an indigenous infection (IRR 1.02 95% CI 1.01–1.03). After accounting for the effect of environmental covariates, Godawari and Dhansinghapur VDCs in Kailali remained at relatively high risk for unexplained reasons.
Table 3.
Factors associated with monthly indigenous malaria incidence at the VDC level, January 2013–December 2016, in Kailali, Kanchanpur and Bardiya districts
| Posterior mean, (95% Crl) | |||
|---|---|---|---|
| All species | P. vivax | P. falciparum | |
| Fixed effects (IRR) | |||
| Lagged importation ratea | 1.02 (1.009, 1.031) | 1.025 (1.013, 1.037) | 1.011 (0.962, 1.057) |
| LST (°C) | 1.016 (0.978, 1.049) | 1.007 (0.962, 1.05) | 0.924 (0.811, 1.074) |
| Rainfall (mm) | |||
| < 20 | 1 | ||
| 20–119 | 1.117 (0.757, 1.618) | 1.064 (0.684, 1.626) | 0.124 (0.014, 1.633) |
| 120–1450 | 1.334 (0.8, 2.065) | 1.145 (0.642, 1.943) | 0.198 (0.022, 2.642) |
| High population densityb | 0.614 (0.399, 0.947) | 0.616 (0.398, 0.96) | 0.579 (0.293, 1.233) |
| Year | |||
| 2013 | 1 | ||
| 2014 | 0.635 (0.48, 0.838) | 0.921 (0.674, 1.256) | 0.072 (0.03, 0.156) |
| 2015 | 0.442 (0.305, 0.64) | 0.609 (0.408, 0.91) | 0.07 (0.024, 0.174) |
| 2016 | 0.636 (0.436, 0.924) | 0.858 (0.568, 1.289) | 0.09 (0.028, 0.241) |
| District | |||
| Kailali | |||
| Kanchanpur | 0.475 (0.308, 0.736) | 0.603 (0.389, 0.94) | 0.024 (0.006, 0.078) |
| Bardiya | 0.768 (0.486, 1.205) | 1.035 (0.65, 1.634) | 0 (0, 887.532) |
| Random effects (precision) | |||
| Seasonal random effect | 2.949 (0.052, 17.118) | 1.017 (0.016, 6.351) | 0.009 (0, 0.049) |
| VDC effect unstructured | 2.25 (1.464, 3.329) | 1.972 (1.295, 2.905) | 3.527 (0.887, 10.115) |
| VDC effect structured | 3013.549 (745.865, 7326.227) | 2931.545 (708.639, 7408.301) | 3415.982 (835.229, 9202.779) |
Crl, Bayesian credible interval; C, celsius; mm, millimeter; IRR, incidence rate ratio; LST, land surface temperature; VDC, village development committee; DIC, deviance information criteria; CPO, conditional predictive ordinate
aMonthly cases per 1000 population, b defined as the top quartile
In species-specific models, the positive relationship between indigenous cases and lagged imported cases was similar, however only reached statistical significance in the P. vivax model (Table 3), potentially due to the low number of indigenous P. falciparum cases (N = 103), the majority of which were in 2013 (N = 88).
Prospective studies
In total, 60 (98%) imported cases were recruited into the study from 19 health facilities between August 31 and November 15, 2016. Thirty-three (55%) imported cases provided contact details for at least one individual who had travelled outside of the district within the past 60 days, with a total of 100 MMP social contacts referred. Eighty (80%) of these MMP social contacts were successfully contacted and interviewed, reflecting successful recruitment from 27 imported cases. Imported cases who referred at least one contact had information for three individuals on average (ranging from 1 to 5) whom they believed met the criteria for inclusion. The vast majority of MMP contacts were either friends (50%) or family members (48%).
Characteristics of imported cases were similar to their MMP social contacts in terms of residency, gender, age distribution and education level, supporting the theory that they are part of the same source population (Table 4). Amongst imported cases, individuals diagnosed with P. vivax were more likely to be male and reside in Kailali compared to P. falciparum.
Table 4.
Profiles of imported cases and MMP social contacts from three districts, September–November 2016
| Key characteristics | Imported cases | MMP social contacts | P valueb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pf | Pv | P valuea | Total | N = 80 | (%) | |||||
| N = 11 | (%) | N = 49 | (%) | N = 60 | (%) | |||||
| District of diagnosis | ||||||||||
| Bardiya | 4 | (36) | 3 | (6) | 0.01 | 7 | (12) | 2 | (2) | 0.07 |
| Kailali | 4 | (36) | 34 | (69) | 38 | (63) | 51 | (64) | ||
| Kanchanpur | 3 | (27) | 12 | (25) | 15 | (25) | 27 | (34) | ||
| District of residence | ||||||||||
| Bardiya | 3 | (30) | 3 | (6) | 0.22 | 6 | (10) | 2 | (3) | 0.06 |
| Kailali | 4 | (40) | 39 | (61) | 34 | (58) | 41 | (51) | ||
| Kanchanpur | 3 | (30) | 14 | (29) | 17 | (29) | 37 | (46) | ||
| Dadeldhura/Baitadi | 0 | (0) | 2 | (4) | 2 | (4) | 0 | (0) | ||
| Gender | ||||||||||
| Male | 9 | (81) | 48 | (98) | 0.03 | 57 | (95) | 71 | (89) | 0.19 |
| Female | 2 | (18) | 1 | (2) | 3 | (5) | 9 | (11) | ||
| Age category (years) | ||||||||||
| 15–30 | 6 | (54) | 26 | (53) | 0.99 | 32 | (53) | 49 | (61) | 0.42 |
| 30–45 | 4 | (36) | 19 | (39) | 23 | (38) | 28 | (35) | ||
| 45+ | 1 | (9) | 4 | (8) | 5 | (8) | 3 | (4) | ||
| Education | ||||||||||
| None | 2 | (18) | 7 | (14) | 0.47 | 9 | (15) | 27 | (34) | 0.14 |
| Primary | 5 | (46) | 26 | (53) | 31 | (52) | 31 | (39) | ||
| Secondary | 4 | (36) | 10 | (20) | 14 | (23) | 17 | (21) | ||
| Post sec. | 0 | (0.0) | 6 | (12) | 6 | (10) | 5 | (6) | ||
Italic values indicate significance of p value (p < 0.05)
Pf, Plasmodium falciparum; Pv, Plasmodium vivax
aChi squared test of differences in key characteristics between Pf and Pv in imported cases
bChi squared test of difference in key characteristics between imported cases and MMP social contacts
Summary statistics for FG participants are provided in Additional file 4. Ten KIIs were conducted in each of the three districts.
MMP travel profiles
Travel profiles of MMP cases and their social contacts show that the overwhelming majority had travelled to India (91.3%) for work during their last trip (85.0%) (Table 5 and Fig. 4). The two most common destinations were Surat (Gujarat, India) and Mumbai (Maharashtra, India), which made up nearly half (45.0%) of the most recent trips. Due to the respective distance and reason for travel, median transportation time averaged 3 days (IQR 1.5) and trips were of long duration (median of 5 months; IQR 9 months). While only a fifth of trips were reported as seasonal travel, many participants returned home to Nepal in September or October to celebrate festival season, which also coincides with the harvests, as described by the following quote, among others:
“For these destinations I have just described, there is no fixed time. I travel anytime. But we often travel back to harvest crop and to celebrate festival season in Nepal.” (Male, Kailali, FG)
Table 5.
Travel characteristics of 140 MMPs, including both imported cases and MMP social contacts
| Summary trip characteristics | N = 150 | (%) | Characteristics of most recent trip | N = 140 | (%) |
|---|---|---|---|---|---|
| # trips reported in prior 6–60 days | Destination of travel | ||||
| 1st trip | 140 | (93.3) | Surat, Gujarat, India | 30 | (21.4) |
| 2nd trip | 8 | (5.3) | Mumbai, Maharashtra, India | 33 | (23.6) |
| 3rd trip | 2 | (1.3) | Chennai, Tamil Nadu | 12 | (8.6) |
| Country of destination | Other, Gujarat, India | 15 | (10.7) | ||
| India | 137 | (91.3) | Other, Indiaa | 38 | (27.1) |
| Congo, Africa | 12 | (8.0) | Congo, Africa | 12 | (8.6) |
| Nepal | 1 | (0.7) | Transit pointsb | ||
| Frequency of travel to destination | Gaurifanta, Nepal | 58 | (41.4) | ||
| Once per year | 70 | (46.7) | Paliya, UP, India | 85 | (60.7) |
| > Once per year | 18 | (12.0) | Mumbai, India | 32 | (22.9) |
| < Once per year | 60 | (40.0) | Mathura, India | 44 | (31.4) |
| Don’t know/decline | 2 | (1.3) | Delhi, India | 40 | (28.6) |
| Median duration of travel (months) (IQR) | 5 | (9.0) | Worked at destination | 119 | (85.0) |
| Regular/seasonal travel to location | 30 | (20.0) | Type of work (n = 119) | ||
| Return in Sept/Oct/Nov (n = 30) | 23 | (76.7) | Factory | 23 | (19.3) |
| Reason for travel | Security guard | 18 | (15.1) | ||
| Work | 113 | (75.3) | Logging/construction | 15 | (10.7) |
| Family/friends | 17 | (11.3) | Diamond work | 13 | (10.9) |
| Education/holiday | 13 | (8.7) | Hotel service | 17 | (14.3) |
| Decline to answer | 7 | (4.7) | Other | 33 | (22.0) |
| # people travelled with (mean/sd) | 77 | 229 | Work location (n = 119) | ||
| 0 | 15 | (10.9) | Inside | 53 | (44.5) |
| 1–4 | 44 | (31.9) | Outside | 32 | (26.9) |
| 5–14 | 53 | (38.4) | Both | 34 | (28.6) |
| 15–30 | 17 | (12.3) | Mosquitos while working | 107 | (76.4) |
| 30+ | 9 | (6.5) | Mosquitos while sleeping | 130 | (92.9) |
| Median transit time (days) (IQR) | 3 | (1.5) | Residence environment | ||
| Public transit | 150 | (100) | Town | 112 | (80.0) |
| Border crossed | Rural/forest | 15 | (10.7) | ||
| No border | 10 | (6.7) | Other | 13 | (9.3) |
| Gaurifanta, Kailali | 74 | (49.3) | Malaria protection during trip | ||
| Basiha/Parasan | 18 | (12.0) | None | 112 | (80.0) |
| Banbasa/Gadda Chauki | 16 | (10.7) | ITN | 28 | (20.0) |
| Rupaidiya, Nepalganj | 5 | (3.3) | Repellant | 16 | (11.4) |
| Airport | 12 | (8.0) | Chemoprophylaxisc | 12 | (8.6) |
| Jamunaha/Khatima | 4 | (2.7) | Covering clothing | 4 | (2.9) |
| Kamalpur/Belauri | 11 | (7.3) | Other | 2 | (1.4) |
aIncludes locations in Rajasthan, Uttarakhand, Andhra Pradesh, and others in order of frequency
bMultiple transit points possible, those where are least 20% of participants transited are listed
cDrug used was Maploquine
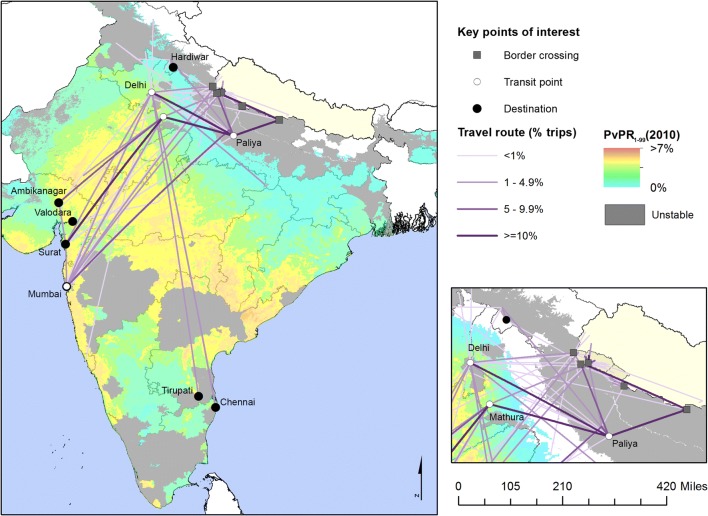
Characteristics that may suggest how to access MMPs for targeted surveillance include membership in specific occupational groups and common transit points along heavily trafficked travel routes. Common occupations included security guards (15.1%), factory work (19.3%), and hotel service (14.3%), but some forms of employment were specific to destination. For instance, Gujarat hosts more factory work (36%) while Maharashtra is common for security guard work (40%) or hotel jobs (24%). Travel routes and transit stops (Fig. 5) were similar among participants as all trips were taken via public bus or train.
Fig. 5.
Destinations, travel routes and key transit points of 138 trips to India reported by 137 imported cases and MMP peers (Table 4), overlaid onto a map of predicted Plasmodium vivax all ages prevalence rate [44]
Potential risk factors during travel
After adjusting for age and gender, MMP participants who worked outside had a sixfold increase in the odds of being an imported case compared with those who worked solely indoors (OR: 6.07, 95% CI 1.15–32.22). Working as a security guard, in particular, was perceived as a high-risk occupation by MMPs in FGs. While no specific destinations were associated with being a case rather than a contact in the survey data, longer transit times (in days) and shorter duration of travel (in months) were weakly associated with greater risk (OR: 13.9 95% CI 1.08–179.0 and OR: 0.74 95% CI 0.54–1.02) after adjusting for age, gender and working outdoors. FGs corroborated the potential risk of infection during travel, citing mosquitoes biting at transit points and within buses, as well as a higher perception of malaria risk at destinations further from Nepal (such as Gujarat or Mumbai).
Malaria knowledge and risk perception
Less than 50% of participants had heard of malaria when surveyed, and there were similar gaps in knowledge during FGs. In general, imported cases had better knowledge of malaria symptoms and availability of free testing compared to their MMP social contacts, which is likely due to their recent illness and subsequent diagnosis at a health facility (Table 6). Three quarters of MMP social contacts (75%) were unable to name one symptom of malaria; those who could were familiar with non-specific symptoms such as fever (20%) or headache (17%).
Table 6.
MMP malaria knowledge, attitudes and practice and treatment seeking
| Imported cases | MMP social contacts | P-valueb | |||
|---|---|---|---|---|---|
| N = 60 | (%) | N = 80 | (%) | ||
| Malaria knowledge, attitudes and practice | |||||
| Not heard of malaria | 32 | (53.3) | 55 | (68.8) | 0.06 |
| Knowledge of transmission | |||||
| Mosquito-borne | 21 | (35.0) | 21 | (26.3) | 0.26 |
| Don’t know | 37 | (61.7) | 59 | (73.8) | 0.13 |
| Othera | 6 | (8.3) | 6 | (3.8) | 025 |
| Knowledge of preventive measures | |||||
| 0 | 40 | (66.7) | 58 | (72.5) | 0.22 |
| 1 | 7 | (11.7) | 13 | (16.3) | |
| 2+ | 13 | (21.7) | 9 | (11.3) | |
| Type of preventive measures known | |||||
| ITNs | 17 | (28.3) | 17 | (21.3) | 0.33 |
| Chemoprophylaxis | 10 | (16.7) | 8 | (10.0) | 0.24 |
| Repellant | 5 | (8.3) | 5 | (6.3) | 0.64 |
| Covering clothing | 3 | (5.0) | 5 | (6.3) | 0.76 |
| Other | 4 | (6.67) | 3 | (3.75) | 0.43 |
| Knowledge of symptoms | |||||
| No symptoms known | 29 | (48.3) | 60 | (75.0) | < 0.0001 |
| 1–3 symptoms known | 4 | (6.7) | 8 | (10.0) | |
| 4+ symptoms known | 27 | (45.0) | 12 | (15.0) | |
| Symptoms known | |||||
| Fever | 31 | (51.7) | 20 | (25.0) | 0.001 |
| Headache | 25 | (41.7) | 17 | (21.3) | 0.009 |
| Other (muscle pain, fatigue, dizzy) | 29 | (48.3) | 12 | (15.0) | < 0.0001 |
| Knowledge of free testing and treatment | |||||
| Knows of free testing | 28 | (46.7) | 18 | (32.9) | 0.005 |
| Believes must pay | 3 | (5.0) | 2 | (2.5) | |
| Does not know | 29 | (48.3) | 60 | (75.0) | |
| History of symptoms and testing (MMP only) | |||||
| Ill with fever in past 6 months | – | – | 16 | (20.0) | – |
| Sought treatment for fever | – | – | 16 | (100) | – |
| Location sought treatment from | – | – | – | ||
| District hospital | – | – | 1 | (6.3) | – |
| Private clinic or hospital | – | – | 5 | (31.3) | – |
| Pharmacy | – | – | 10 | (62.5) | – |
| Diagnostic test used | – | – | 15 | (18.8) | – |
Italic values indicate significance of p value (p < 0.05)
aIncludes water-borne, food-borne and direct transmission
bChi squared test of association
Health-seeking behaviours
There was a low awareness of free, government-provided malaria services at public facilities amongst MMPs (32.9%) (Table 6). Reported treatment-seeking behaviours at these locations were even lower: of the 20% (N = 16) of MMP social contacts reporting a fever in the prior 6 months, the vast majority (N = 15) sought treatment from the private health sector only (pharmacy or private clinic/hospital). Only one participant went to a public hospital for diagnosis. Qualitative findings from FGs and KIIs corroborated these findings and suggested that individuals would preferentially attend private facilities for fever and delay coming to the public health facilities until the fever is non-resolving or more severe. For example, one case stated:
“The private clinic staff suggested that I go to the public health facility, since the government provides free services there. It was only after I had malaria several times that I went straight to the government health facility, because I knew.” (Male, Kailali, FG)
Perceived barriers to treatment according to KIIs were lack of medicines at health posts and increased travel time. A number of key informants suggested that people may also seek treatment from traditional healers or travel to India for medical services, particularly at major transit points like Paliya.
Knowledge and use of malaria prevention methods
Knowledge of malaria prevention was low in MMP participants, with less than a third (21–28%) able to name ITNs as a method to protect against malaria infection (Table 6). Most travellers (80%) did not use any form of malaria prevention on their most recent trip, and ITN and repellant use were both low (20.0% and 11.4%) (Table 6). Despite this, many FG participants reported that they regularly used a net while at home in Nepal if available. While reported use of a net was common, KII and FG participants cited irregular distributions of nets, lack of knowledge around how and where to obtain nets, and low-income financial status as key barriers to ITN access in Nepal. Personal preference to not use the net due to the heat and perceived sensitivity to the chemicals in the net was also reported as a barrier to ITN use.
While travelling, lack of ITN use was generally attributed to lack of availability, affordability, and type of living quarters. Even if workers were in possession of a mosquito net while travelling, some participants would not be able to assemble it in their living quarters in India. A few participants commented on landlord restrictions around hanging nets in rented accommodation as a barrier to use:
“We stay in rented house in India. House owner do not allow us to use nails striking on the wall, so it is impossible for us to use mosquito net. That is why we do not use mosquito net in India.” (Male, Kailali, FG).
Some participants did mention burning wood or mosquito coils in India, however, and one participant reported that his living quarters in Surat were sprayed for mosquitoes.
Opportunities and barriers for malaria screening
Participants were generally positive about targeted malaria surveillance strategies. Two common themes emerged from discussions regarding screening approaches. First, high levels of social connectivity amongst MMPs provide an entry point for surveillance and interventions. Most participants preferred to travel to India within a group, in part due to safety. Corroborating data from Table 5, which shows that MMPs tended to travel in small or moderate sized groups of 2–15 people (70.3%), participants stated:
“Some people have two to three people in a group while some have six to seven people in a group while travelling.” (Male, Kanchanpur, FG)
“We travel in group of ten, twelve, fifteen and sometime twenty of us travel together.” (Male, Kailali, FG)
In India, Nepalese MMPs tend to stay well connected and frequently live together in large communities of varying size (upwards of 400–500 people), with sometimes as many as 20 people from a single village. Imported cases reported a median of 8 MMP social contacts known at the travel destination and engaged in the same activity during the past 60 days with some individuals (n = 7) indicating high numbers of contacts (50 or more) (Table 7). Another potential entry point for venue-based surveillance are Nepalese societies, which organize large cultural gatherings in many common destinations in India, typically on Sundays with several hundred people attending. Finally, there are highly networked MMP individuals (for example, involved in transportation or local leaders) who could be recruited to support peer-driven interventions.
Table 7.
Size and recruitment of peer travel networks of 58 imported cases over 63 trips to India
| Mean (median) [range] | SD | |
|---|---|---|
| Total number of peers in case’s “same destination, same activity” travel network and…a | 16 (21) [0 100] | 21 |
| …Anticipated to be present in study district in next 2 weeks, and… | 3 (0) [0 20] | 4 |
| …Aged over 18 years | 2 (0) [0 20] | 4 |
| Number for whom contact details were providedb | 1 (0) [0 5] | 2 |
Two cases excluded with outliers (850) where travel was to Africa
aNumber of people who live or work in the study district in Nepal, who had travelled to the same destination in India and engaged in the same activity in the past 60 days
bMeeting the above criteria, maximum of 5
Second, optimal timing of screening would correspond with Dashain and Tihar festivals, when people are most likely to return to Nepal for celebrations and agricultural harvesting.
“There are seasonal migrants who come and go in seasonal period of time like during the agriculture season or for the major festivals. Like, they come during Ashadh, stay for a month then goes back again. After that they return in Ashoj for Dashian as well as to harvest the grain.” (Male, Kailali, KII).
Barriers to malaria screening identified during FGs included unawareness of health services, particularly availability of free testing and treatment for malaria, in addition to low literacy, fear of medical visits, and the perception that screening is necessary only if one is ill. One FG referenced a recent polio immunization campaign implemented at the border, in which some people refused to participate based on the belief that they might be drugged or would feel faint.
Peer-referral recruitment
Strong social ties between Nepalese travellers offer an opportunity to conduct malaria screening through social networks. FG participants were confident that MMP social contacts could be recruited by mobile phone and would be willing to participate:
“We have close friend, we can contact them, they will contact another friend and if we inform them, they will contact through mobile.” (Male, Kailali, FG)
Most participants believed individuals referred by peers could be screened at their households or referred to health posts, schools or community meetings. There was variation in perceptions of when MMPs would most likely be free while in Nepal, with participants suggesting Sundays, weekday evenings after work, or mornings.
In the context of this study, however, peer-referral resulted in enrollment of a fraction of the total MMP social contacts reported known by MMP cases involved in the same activity, in large part because few contacts were expected to return from India in the allotted two-week follow-up period (Table 7 and Fig. 6). Lack of contact details was another limitation. Overall, 1.3 (80/60) MMP contacts were successfully recruited per MMP case during the study, and 2.4 (80/33) MMP contacts were recruited, on average, for each of the MMP cases that provided any contact details.
Fig. 6.

MMP peer network statistics of imported cases recruited in Kailali, Kanchanpur and Bardiya and reporting travel to India
Border screening
MMPs expressed mixed views of border screening and highlighted potential operational challenges. First, participants did not perceive any particular MMP subgroup to be at higher risk of fever upon return from India. All participants believed fevers are relatively rare and may appear before, during or after travel. Second, while survey findings indicated most MMPs travel through formal border crossings (Table 5), some FG participants mentioned some people (particularly those involved in illegal activities) may use informal border crossings. Some FG participants believe screening posts would be most successful if located at border stations or nearby roads or markets. Although some favoured mandatory, government-run screening to maximize participation, others cited barriers that included lack of time, concerns about safety at border check posts, and distrust of police:
“When we travel from India then many police threaten us by asking money, asking our passport and citizenship, so people fear to participate in such gatherings. People fear if they will be asked money or get plundered in such programme.” (Male, Kailali, FG)
Venue-based recruitment
Venues where MMPs tended to congregate in transit (in both Nepal and India) included tea shops, bus stations and rail stations. There were more specific locations identified in India, where Nepalese societies organize cultural events on Sundays and during festivals. Groups from a specific village may meet at specific markets in India or cinema halls.
“Like in India, we have different Nepali community, if we contact there then it will be easy to spot all the people because Nepali people organize different cultural programme where most of the Nepalese attend, if you talk about this programme then it will be very easy.” (Male, Kailali, FG)
Both Nepalese communities in Nepal and organized societies abroad have a leader or coordinator (President/VP) who are well networked through formal or informal channels. This person was identified as a point of contact to coordinate screening. Participants also suggested meeting at workplaces rather than recreational venues in India, and in particular, diamond factories in Surat, where they felt many Nepalese worked.
However, few potential venues were identified in Nepal and participants stated that individuals may have privacy concerns and refuse blood tests at public places identified, specifically tea shops or transit points. Differences in the timing of work shifts and availability are also likely to make venue-based recruitment in India more costly.
Discussion
This study sought to address key knowledge gaps around malaria importation in far and mid-western Nepal, including investigating the association between imported and local transmission, better characterizing MMPs and identifying potential opportunities to optimize targeted surveillance and intervention strategies for this population. The analysis of retrospective case data demonstrated an association between (lagged) imported case rate and local rate in this area of Nepal, such that each percent increase in importation the month prior led to a two percent increase in local incidence rate. Recently imported cases and their MMP social contacts were found to be predominantly adult male labourers who travel to malaria endemic areas of India and were characterized by similar risk factors: they lacked a basic understanding of malaria transmission and prevention, rarely used ITNs while travelling, and when ill, tended not to seek treatment or prefer informal private providers. These findings suggest that many cases among these individuals may go undetected and that more targeted strategies are needed to improve coverage of education, testing, and surveillance in this population. To access high-risk MMPs more effectively, the study identified community-based surveillance approaches as the most promising options: strong social connectivity within these groups, concentration of Nepali MMPs in specific communities and workplaces within India; and well-attended seasonal festivals that motivate return to Nepal provide opportunities for peer-referral- and venue-based surveillance and interventions.
The finding that high-risk MMPs tend to be adult male labourers with a history of travel to India has been reported by previous studies in this area [3, 4] and is consistent with a profile of imported cases as adult males in other elimination contexts [2, 33]. The infrequent self-reported net use (20%) during travel contrasted with the high reported programmatic coverage (> 90%) of LLINs in Nepal since 2009 [34]. Even when home in Nepal, many of the MMPs interviewed were unsure how to obtain nets. MMPs may be less likely to receive nets during regular distributions in Nepal due to living in remote areas and absence from home during distributions. Innovative approaches are needed to make nets available to MMPs upon return from India, both for their own protection and to prevent onward transmission.
Given the high proportion of imported cases in this setting and receptivity of the terai region, some ongoing transmission is expected and will be mediated by the malaria species present and vectorial capacity. Past studies have shown that environmental variables are important drivers of vector density and seasonal patterns of malaria [35, 36], however the relationships, metrics and temporal lag periods used vary across settings and vectors [37]. This study demonstrates that receptivity in this area of Nepal is determined by higher levels of rainfall and temperature [38, 39]. Low use of public sector health services by MMPs is a key limitation of routine surveillance data and there is a need to incorporate private sector surveillance alongside campaigns to improve public sector use by these populations. In addition, improving the quality of routinely collected travel histories in case-based surveillance will allow automated analyses and mapping of sources and sinks on an ongoing basis to improve geographical targeting of interventions.
Accessing and achieving high coverage of vector control and surveillance interventions in MMPs is a key challenge to reducing malaria importation [19]. All three strategies examined (border screening, peer-referral, and targeting well-attended venues) were found to be feasible, however would need to be adapted to address the unique operational challenges identified. The finding of strong social connectivity among Nepalese travellers, while in transit and at destinations in India, suggests that peer-supported methods are promising to identify and contact MMPs to disseminate interventions. Improved knowledge of malaria risk avoidance, screening programmes, treatment options, ITNs, and repellants are shown to benefit travellers directly and improve access to and use of existing malaria health services [10].
Decades of research portray migration as a socially embedded process and support a role for utilizing existing social support and network structure to optimize behaviour change interventions [40, 41]. The findings from this study suggest that within Nepal, an obstacle to peer-driven methods is the delay until MMPs return home. This may be addressed by sending periodic follow-up reminders to refer MMP social contacts once they arrive, potentially by SMS as most MMPs were contactable by mobile phones. Peer promotors or navigators (MMP group members who are employed by a malaria programme to provide information or “navigate” health services) could also be employed to reach other MMPs while in transit and at destinations in India with high concentrations of Nepalese from far-western Nepal. Community leaders and established Nepalese societies were also identified that should be enlisted to promote uptake of peer-driven programmes or to directly provide screening or behaviour change communication (BCC) [42] to society members.
Beyond peer-driven approaches, we identified well-attended venues and work sites in India that could provide convenient physical locations to access large numbers of Nepali MMPs through a venue-based approach. Specific occupational groups working outdoors in malaria endemic destinations, such as security guards in Mumbai, could also be targeted upon return from India for voluntary screening. Border screening is resource and labour intensive, making it infeasible in many settings, but targeted approaches are likely to improve the test positivity rate and overall cost-effectiveness [43]. Study participants’ concerns about confidentiality of testing results and distrust of police, suggest that, just as in more stigmatized conditions such as HIV and STIs, malaria screening should be conducted in designated confidential areas within or nearby the venues and/or border check points and with careful selection of staff. Considering the high volume of border crossings during peak times, there is a clear need to use imported case data to construct high-risk profiles (potentially using a more representative MMP control group) to identify age, gender, travel and possibly occupational profiles to target screening to potentially asymptomatic individuals.
This study also found that longer transit times and shorter duration of stay abroad were weakly associated with a greater risk of malaria. Shorter-term migrants may perceive less risk, resulting in poorer malaria preventive behaviours, or have increased occupational exposures compared to longer-term migrants. Alternatively, imported cases may return to Nepal to seek treatment when they fall ill, which could result in a spurious association with shorter duration of travel. The qualitative portion of the study did not substantiate illness as a reason to return to Nepal.
Several methodological limitations should be considered. Radical cure of P. vivax is the standard of care in Nepal, but there is frequently low adherence to these guidelines from both the provider and patient populations. This is believed to be due to concerns around G6PD status and low compliance to the full 14-day course of medication. This study did not investigate these issues in MMPs, but it remains an important barrier to achieving elimination in Nepal. It was not possible to distinguish incident and potentially relapsing P. vivax infections in these analyses. Consequently, local and imported case numbers are likely to be overestimated and may obscure recent geographical trends. Case ascertainment was limited by lack of private sector involvement and, in the case of retrospective data, other reporting gaps. The prospective recruitment period was planned to coincide with the festival season but was a month delayed and subsequently short (3 months) in duration, which limited case and MMP recruitment and may affect generalizability. While a large proportion of imported cases (35%) and MMP contacts (33%) were interviewed on the same day as diagnosis or referral, the remainder were followed up within a week (75% and 39%) or longer. MMP contacts with follow up longer than 4 weeks (12.5%) were due to longer than expected return from travels. The data did not allow calculation of the total number of MMPs within the extended network or the maximum number of potential referrals, as there was no way of accounting for double-counting given the high degree of social connectivity and capped referrals at 5. However, it is likely that one round of peer-referral with a 2 weeks follow up will not be sufficient to recruit a large part of this network. Peer-referral recruitment was further limited by delays in enrollment, indicating that MMPs were not necessarily travelling back to Nepal in groups. In addition, MMP social contacts were sometimes lost to follow up due to incomplete contact information or residing in a remote area of the district, suggesting additional operational challenges for the peer-referral approach. Remote residence and scheduling conflicts were substantial challenges to organizing FGs, which may have influenced findings by restricting our sample to those with better access. The effect of this potential bias was minimized by reimbursing travel costs and working flexible hours to accommodate participants schedules and travel needs. Due to the clustered nature of the data (i.e. MMP social contacts were matched to the imported cases by destination) many potential risk factors are likely to be underestimated in the conditional logistic regression analysis. A more representative MMP control group, recruited through alternative means, would be useful in identifying additional risk factors to guide targeted screening approaches. Findings from the retrospective analysis should be interpreted with caution as it used area level data and observed correlation between imported and local cases could be introduced by regional seasonality or misclassification bias.
A cross-border strategy with India remains key to achieving and maintaining long-term elimination targets in Nepal. This study highlights the routes through which parasites move between Nepal and India and the surveillance gaps that make timely diagnosis and treatment in MMPs so challenging. Key informants also mentioned Indian migrants living in Nepal who may be vulnerable or contributing to local transmission and who are not represented in this study. Ensuring that MMPs from both sides of the border have adequate access to malaria prevention, testing and treatment along key routes and destinations will be critical to achieving regional elimination. A regional initiative in 2010 opened the door towards implementing cross-border activities for the control of three vector-borne diseases, including malaria [5]. Cross-border mechanisms to improve surveillance, information sharing and bilateral support are essential to reduce malaria transmission in MMPs and in highly trafficked destinations on both sides of the border, thereby benefiting both Nepalese and Indian populations.
Conclusion
Population mobility and malaria importation from India are key challenges to malaria elimination in border areas of far and mid-western Nepal. This study has helped to better understand characteristics of MMPs and identify specific ways in which malaria surveillance and response can be adapted to optimize coverage and case detection in high-risk travellers. Specifically, intervention campaigns can tailor their timing, messaging and geographic focus to better target this population, and surveillance and response approaches can leverage strong social connectivity to improve intervention uptake. A combination of community and peer-referral based approaches will likely be needed to achieve malaria elimination in border areas.
Additional files
Additional file 1. Covariate processing and selection methods.
Additional file 2. Model selection criteria and model comparison.
Additional file 3. Comparison of number of cases imported and total number of cases in 2016 reported through case-based surveillance and the World Malaria Report (WMR).
Additional file 4. Characteristics of focus group participants.
Authors’ contributions
JLS and PG drafted the manuscript. KRR, NA, UTS, SH conducted field work in close supervision of JS and PG. JLS conducted all analyses, with contributions from RAP and AM on statistical modelling and analysis of qualitative data. AM, RAP, JOJ, AB reviewed and edited the manuscript. KRR, MRB GDT, PG, BKL led coordination and implementation of the study, and JLS, JOJ, AB led the design and developed methods for this study. All authors read and approved the final manuscript.
Acknowledgements
We would like to acknowledge Epidemiology and Diseases Control Division, Ministry of Health and Population for their strong support of this study. We are grateful to Research Assistants Mr. Samarpan Kafle, Mr. Apil Timilsinia, and Mr. Narendra Chand for their help in data collection. We are thankful to Mr. Hemraj Joshi and Mr. Hari Bhatta, Vector control officers of Kanchanpur and Kailali district respectively for their help during entire study period. We would like to thank all the team members, laboratory technicians of respective health centres and hospitals for their strong and generous support throughout the study period. We are grateful to all the malaria patients and mobile migrant populations who cooperated in the study.
Competing interests
The authors declare that they have no any competing interests.
Availability of data and materials
All data pertaining to this study are within the manuscript.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Written informed consent for questionnaire administration and FG and interview participation was obtained from all respondents. The protocols and consent forms used for this study were reviewed and approved by the University of California, San Francisco (#173046) and the Nepal Health Research Council Ethical Review Board (#132/2016).
Funding
This study was supported by Bill and Melinda Gates Foundation through University of California San Francisco, Global Health Group, USA.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . A framework for malaria elimination. Geneva: World Health Organization; 2017. [Google Scholar]
- 2.Rijal KR, Adhikari B, Ghimire P, Banjara MR, Hanboonkunupakarn B, Imwong M, et al. Epidemiology of Plasmodium vivax malaria infection in Nepal. Am J Trop Med Hyg. 2018;99:680–687. doi: 10.4269/ajtmh.18-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaidya NK, Wu J. HIV epidemic in Far-western Nepal: effect of seasonal labor migration to India. BMC Public Health. 2011;11:310. doi: 10.1186/1471-2458-11-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poudel KC, Jimba M, Okumura J, Sharma M, Poudel Tandukar K, et al. Migration in far western Nepal: a time bomb for a future HIV/AIDS epidemic? Trop Doct. 2004;34:30–31. doi: 10.1177/004947550403400115. [DOI] [PubMed] [Google Scholar]
- 5.Dhimal M, Ahrens B, Kuch U. Malaria control in Nepal 1963–2012: challenges on the path towards elimination. Malar J. 2014;13:241. doi: 10.1186/1475-2875-13-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consortiom of Spatial Information . Elevation data of Nepal 2008. Washington, DC: Consortiom of Spatial Information; 2008. [Google Scholar]
- 7.WHO. Nepal Malaria Programme Review: 7–16 June 2010. World Health Organization; 2011. http://apps.searo.who.int/PDS_DOCS/B4673.pdf. Accessed 20 Jan 2018.
- 8.Epidemiology and Diseases Control Division (EDCD: An assessment of laboratory capacity for malaria Microscopy in Health facilities of malaria endemic districts in Nepal. Government of Nepal, Ministry of Health, Department of Health Services, Epidemiology & Disease Control Division; 2015.
- 9.Epidemiology and Diseases Control Division (EDCD). Ministry of Health and Population. Nepal Malaria Strategic Plan 2014–2025. Kathmandu, Nepal: Government of Nepal, Ministry of Health and Population, Department of Health Services, Epidemiology & Disease Control Division. 2014. http://www.edcd.gov.np/publications.html. Accessed 25 Jan 2018.
- 10.Le Menach A, Tatem AJ, Cohen JM, Hay SI, Randell H, Patil AP, et al. Travel risk, malaria importation and malaria transmission in Zanzibar. Sci Rep. 2011;1:93. doi: 10.1038/srep00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturrock HJW, Novotny JM, Kunene S, Dlamini S, Zulu Z, Cohen JM, et al. Reactive case detection for malaria elimination: real-life experience from an ongoing program in Swaziland. PLoS ONE. 2013;8:e63830. doi: 10.1371/journal.pone.0063830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Churcher TS, Cohen JM, Novotny J, Ntshalintshali N, Kunene S, Cauchemez S. Measuring the path toward malaria elimination. Science. 2014;44:1230–1232. doi: 10.1126/science.1251449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley J, Monti F, Rehman A, Schwabe C, Vargas D, Garcia G, et al. Infection importation: a key challenge to malaria elimination on Bioko Island, Equatorial Guinea. Malar J. 2015;14:46. doi: 10.1186/s12936-015-0579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Routledge I, Chevéz JER, Cucunubá ZM, Rodriguez MG, Guinovart C, Gustafson KB, et al. Estimating spatiotemporally varying malaria reproduction numbers in a near elimination setting. Nat Commun. 2018;9:2476. doi: 10.1038/s41467-018-04577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith C, Whittaker M. Beyond mobile populations: a critical review of the literature on malaria and population mobility and suggestions for future directions. Malar J. 2014;13:307. doi: 10.1186/1475-2875-13-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyant P, Canavati SE, Chea N, Ly P, Whittaker MA, Roca-Feltrer A, et al. Malaria and the mobile and migrant population in Cambodia: a population movement framework to inform strategies for malaria control and elimination. Malar J. 2015;14:252. doi: 10.1186/s12936-015-0773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. Aids. 2005;19(Suppl 2):S67–S72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- 18.Tyldum G, Johnston L. Applying respondent driven sampling to migrant populations: lessons from the field. Palgrave Macmillan UK; 2014.
- 19.Jacobson JO, Cueto C, Smith JL, Hwang J, Gosling R, Bennett A. Surveillance and response for high-risk populations: what can malaria elimination programmes learn from the experience of HIV? Malar J. 2017;16:33. doi: 10.1186/s12936-017-1679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wangroongsarb P, Hwang J, Thwing J, Karuchit S, Kumpetch S, Rand A, et al. Using respondent driven sampling to identify malaria risks and occupational networks among migrant workers in Ranong, Thailand. PLoS ONE. 2016;11:e0168371. doi: 10.1371/journal.pone.0168371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epidemiology and Diseases Control Division (EDCD). Malaria Micro-stratification. Kathmandu: Government of Nepal, Ministry of Health, Department of Health Services, Epidemiology & Disease Control Division. 2016. http://www.edcd.gov.np/resources/download/malaria-microstratification-report-2018. Accessed 20 June 2018.
- 22.Central Bureau of Statistics: Nepal Multiple Indicator Cluster Survey 2014, Final Report. Kathmandu: Central Bureau of Statistics and UNICEF Nepal; 2015. unicef.org.np/uploads/…/597341286609672028-final-report-nmics-2014-english.pdf. Accessed 20 June 2018.
- 23.Epidemiology and Diseases Control Division (EDCD). Situational analysis of malaria in cross boarder settings of Nepal. Kathmandu: Government of Nepal, Ministry of Health, Department of Health Services, Epidemiology & Disease Control Division; 2016.
- 24.WHO . Malaria surveillance, monitoring and evaluation: a reference manual. Geneva: World Health Organization; 2018. [Google Scholar]
- 25.Epidemiology and Diseases Control Division (EDCD). The Internal Assessment of Malaria and Kala-Azar Control Activities 2004, 2005 and 2006. Department of Health services, Ministry of Health and Population, Government of Nepal; 2007.
- 26.Silal SP, Little F, Barnes KI, White LJ. Predicting the impact of border control on malaria transmission: a simulated focal screen and treat campaign. Malar J. 2015;14:268. doi: 10.1186/s12936-015-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semaan S, Lauby J, Liebman J. Street and network sampling in evaluation studies of HIV risk-reduction interventions. AIDS Rev. 2002;4:213–223. [PubMed] [Google Scholar]
- 28.Government of Nepal. National Population and Housing Census 2011: National Report. Kathmandu: Central Bureau of Statistics; 2012. https://www.cbs.gov.np.
- 29.NGA GEOnet Names Server (GNS). http://geonames.nga.mil/namesgaz/.
- 30.Google Earth. https://www.google.com/earth/.
- 31.Falling Grain Global Gazetteer Version 2.3. http://www.fallingrain.com/world/index.html.
- 32.Web application for managing, analyzing, and presenting qualitative and mixed method research data. http://www.dedoose.com.
- 33.Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–911. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hii J, Thakur GD, Marasini BR, Pokhrel YR, Upadhyay MP, Rijal KR, et al. Monitoring the durability of long-lasting insecticidal nets in field conditions in Nepal. WHO South East Asia J Public Health. 2014;3:81–84. doi: 10.4103/2224-3151.206891. [DOI] [PubMed] [Google Scholar]
- 35.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alegana VA, Kigozi SP, Nankabirwa J, Arinaitwe E, Kigozi R, Mawejje H, et al. Spatio-temporal analysis of malaria vector density from baseline through intervention in a high transmission setting. Parasit Vectors. 2016;9:637. doi: 10.1186/s13071-016-1917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss DJ, Mappin B, Dalrymple U, Bhatt S, Cameron E, Hay SI, Gething PW. Re-examining environmental correlates of Plasmodium falciparum malaria endemicity: a data-intensive variable selection approach. Malar J. 2015;14:68. doi: 10.1186/s12936-015-0574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhimal M, O’Hara RB, Karki R, Thakur GD, Kuch U, Ahrens B. Spatio-temporal distribution of malaria and its association with climatic factors and vector–control interventions in two high-risk districts of Nepal. Malar J. 2014;13:457. doi: 10.1186/1475-2875-13-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clements AN, Paterson GD. The analysis of mortality and survival rates in wild populations of mosquitoes. J Appl Ecol. 1981;18:373–399. doi: 10.2307/2402401. [DOI] [Google Scholar]
- 40.Latkin CA, Knowlton AR. Social network assessments and interventions for health behavior change: a critical review. Behav Med. 2015;41:90–97. doi: 10.1080/08964289.2015.1034645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilecen B, Gamper M, Lubbers MJ. The missing link: social network analysis in migration and transnationalism. Soc Netw. 2018;53:1–3. doi: 10.1016/j.socnet.2017.07.001. [DOI] [Google Scholar]
- 42.Sturrock HJW, Roberts KW, Wegbreit J, Ohrt C, Gosling RD. Tackling imported malaria: an elimination endgame. Am J Trop Med Hyg. 2015;93:139–144. doi: 10.4269/ajtmh.14-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tatarsky A, Aboobakar S, Cohen JM, Gopee N, Bheecarry A, Moonasar D, et al. Preventing the reintroduction of malaria in Mauritius: a programmatic and financial assessment. PLoS ONE. 2011;6:e23832. doi: 10.1371/journal.pone.0023832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malaria Atlas Project. Spatial distribution of Plasmodium vivax malaria endemicity globally. 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Covariate processing and selection methods.
Additional file 2. Model selection criteria and model comparison.
Additional file 3. Comparison of number of cases imported and total number of cases in 2016 reported through case-based surveillance and the World Malaria Report (WMR).
Additional file 4. Characteristics of focus group participants.
Data Availability Statement
All data pertaining to this study are within the manuscript.