Abstract
Background
Gelatinases degrade extracellular matrix (ECM) components to allow for physiological remodeling and contribute to pathological tissue destruction in endometriosis. It is known that the function of gelatinases is resistant to suppression by progesterone in endometriosis. The ability of progesterone to impact gene expression depends on the progesterone receptor-A/-B (PR-A/PR-B) ratio. An imbalanced PR-A/PR-B ratio in endometriotic tissue may be the result of the differential expression of MMP-2 and MMP-9, which could be important in the etiology and pathogenesis of the disease. Hence, we decided to study the association of PR-A/PR-B ratio and gelatinases expression in endometriosis.
Materials and Methods
In this prospective case-control study, we enrolled 40 women, 20 in the case group who were diagnosed with stage III/IV endometriosis and 20 normal subjects without endometriosis (controls) who referred to Royan Institute, Tehran, Iran during 2013-2014. We obtained 60 tissue samples [ectopic (n=20), eutopic (n=20), and normal endometrium (n=20)]. RNA was extracted from the tissue samples in order to analyze PR-A, PR-B, MMP-2, and MMP-9 mRNA levels through real-time polymerase chain reaction (PCR).
Results
There was significantly lower expression of the PR-B isoform in ectopic tissues compared to the control (P=0.002) and eutopic endometrium (P=0.006) tissues. PR-A expression was higher, but not significantly so, in the same ectopic and eutopic endometrium tissues compared to the control tissues (P=0.643). There was significant over- expression of MMP-9 in ectopic samples compared to control (P=0.014) and eutopic endometrium (P=0.012) samples. The PR-A/PR-B ratio was not significantly higher in either eutopic or ectopic samples compared to the control samples (P=0.305).
Conclusion
Our findings support an altered PR-B expression in endometriosis, which may be associated with MMP-9 overexpression. This finding can be important for disease pathogenesis.
Keywords: Endometriosis, Gelatinases, Progesterone, Progesterone Receptor
Introduction
Infertility is a persistent and frustrating problem in women with endometriosis (1). The frequency of endometriosis in females with complaints of pain, infertility, or both symptoms is between 35 and 60% (2). It is suggested that endometriosis affects the follicular microenvironment, oocyte maturity and embryo development (1, 3). Extensive remodeling in the endometrial layer and its extracellular matrix (ECM) is one of the reasons for infertility in endometriosis (1, 4, 5). This remodeling of the ECM is required for the activation of matrix metalloproteinases (MMPs) and their inhibitors (6). The decreased potential for embryo implantation is thought to be one of the critical reason for infertility in women with this disease (1). High concentrations of activated macrophages, prostaglandins, IL-1, TNF, and proteases have been reported in peritoneal fluid of women with endometriosis. These abnormalities may adversely impact oocyte function, embryo development, and implantation (4).
MMPs or Matrixins are calcium/zinc-dependent endoproteinases encoded by 24 distinct genes and expressed as 26 distinct proteins in humans (7). They are secreted in a latent form (pro-MMPs) that require proteolytic activation (8). The biological roles of MMPs are associated with degradation of the ECM to provide normal endometrial remodeling that accompanies menstruation (9), proliferation, angiogenesis, and apoptosis (7). Endogenous tissue inhibitors of MMPs (TIMPs) regulate MMPs under physiological conditions such as tissue repair and menstruation (10-12). Numerous studies have discussed the role of endogenous proteolytic MMPs in the pathogenesis of endometriosis (13) and have reported a significantly different pattern of MMP expression in endometriosis patients compared to healthy women (14, 15). Over-expressions of MMPs alter the MMPs/TIMPs ratio that may underlie the pathogenesis of diseases including tumor invasion, fibrosis, and endometriosis (8, 16-18). MMPs are involved in all steps of endometriotic tissue migration such as degradation, invasion, and implantation to the ECM outside of the uterine cavity (19). Proteolytic enzymes, like gelatinases (MMP-2 and MMP-9), play an important role in the initial development of endometriosis through ECM degradation (20). The role of gelatinases in the development of diseases has been shown through the participation of MMP-2 and MMP-9 in tumor invasion and progression (1, 13).
Under normal conditions progesterone prevents endometrial breakdown by inhibiting MMPs (21) via its nuclear receptors (21, 22). However, in subjects with endometriosis there is a certain degree of resistance to the action of progesterone (23). In women with this condition, the eutopic endometrium is purportedly resistant to the action of progesterone and inhospitable for embryonic implantation (5). The effects of progesterone are controlled by the two progesterone receptor (PR) isoforms, namely PR-A (94 kDa) and PR-B (114 kDa). These isoforms are functionally different. The PR-B isoform is an activator of progesterone target genes, whereas PR-A is an inhibitor of the PR-B isoform (23). In addition, they are members of the superfamily of ligand-activated transcription factors that bind to sequence-specific sites in the promoters of target genes (22).
On the other hand, progesterone represses MMP-2 transcription in cells from the jar choriocarcinoma cell line by reducing PR and specificity protein 4 (SP4) through binding to the MMP-2 promotor (24). Both overexpression and elevated activity of MMP-9 in endometriosis are believed to be regulated by nuclear factor kappa-B (NF-кB) (25). PR can directly interact with one of the subunits of NF-κB, RelA (p65) (26), which is necessary for NF-кB activation. Progesterone efficacy in gene expression depends on the ratio of PR-A to PR-B (27). An altered ratio in ectopic tissue might play an important role in the mechanism that causes progesterone resistance and modifies progesterone activity related to differential regulation of specific progesterone response genes, such as MMPs, which promote endometriosis. Greater understanding of the abnormal genetic mechanisms involved in the etiology and pathogenesis of endometriosis should lead to better diagnostic methods and targeted treatments that counter endometriosis and its symptoms.
Materials and Methods
We conducted this prospective, case-control study in the Department of Genetics at Royan Institute, Tehran, Iran. Approval was achieved from the Institutional Research Ethics Board. The Ethics Committee of Royan Institute approved this study (No: EC/93/1047). All members signed an informed consent form prior to participation.
Subject selection
This study was conducted from 2013 to 2014 at Royan Institute. We obtained 60 tissue samples (ectopic, eutopic, and normal endometrium) from 40 women. The case group comprised 20 patients with stages III and IV endometriosis. The control group consisted of 20 normal healthy women without endometriosis. Endometriotic (ectopic) tissues were collected during laparoscopy from all patients with ovarian endometriosis. The eutopic samples were obtained by pipelle sampling of endometrial tissues obtained from all patients. Endometrial samples from the control women were also obtained by pipelle sampling. The presence or absence of endometriosis was confirmed by laparoscopy and postoperative histology analyses in endometrial tissue samples from all study participants. Patients with confirmed diagnosis of endometriosis were placed in the patient group. Participants without endometriosis (normal tissue results) were assigned to the control group. None of the patients received hormonal treatments for at least 3 months prior to surgery and all reported regular menstrual cycles. Control group participants did not have any visible endometrial hyperplasia or neoplasia, infl ammatory or autoimmune diseases, or endometriosis at the time of the clinical examinations. We also confirmed that women in the control group had given birth to at least one child conceived through natural conception. The menstrual cycle phase at the time of surgery and biopsy was either during the proliferative phase (days 8-14) (80%) or secretory phase (20%) for both patients and controls.
RNA extraction and cDNA preparation
RNA was extracted from snap-frozen tissue samples using TRIzol (Invitrogen, USA) according to the manufacturer’s instructions. Genomic DNA contamination was removed by RNase-free DNase I (#EN0521, Fermentas, Thermo Fisher Scientific, USA) and incubation at 37°C for 30 minutes. DNase I enzyme was inactivated by EDTA (50 mM, Fermentas, Thermo Fisher Scientific, USA) and incubation at 65°C for 7 minutes. cDNA samples were prepared from total RNA for each sample by one-step reverse transcriptase-polymerase chain reaction (RT-PCR) and a First-strand cDNA Synthesis Kit (K1632, Fermentas, Thermo Fisher Scientific, USA). Synthesized cDNA was stored at -20°C until later use.
Quantitative real-time polymerase chain reaction
mRNA expression analysis was performed using SYBR® Pre mix Ex Taq II (Applied Biosystems, USA) on a Lightcycler System, 7500 software version 2.0.1 (Applied Biosystems, USA) as recommended by the manufacturer. We used Primer 3 (version 4.0; http://primer3.ut.ee/), Gene Runner (version 3.05; www.generunner.net), and Perl Primer software (version v1.1.20; perlprimer.sourceforge.net) to design the specific primers used for amplification of MMP-2, MMP-9, PR-A, PR-B, and β-actin (internal control gene). These sequences were analyzed by Nucleotide Blast and Primer Blast in the NCBI database (http://blast.ncbi.nlm.nih.gov/). Table 1 lists the primers used in this current study and their expected product-sizes. Primers were purchased from Pishgam Co., Iran.
Table 1.
Sequences of β-actin, MMP-2, MMP-9, PR-A, and PR-B primers
| Name | Primer sequence (5ˊ-3ˊ) | PCR product (bp) |
|---|---|---|
| β-actin | F: CAAGATCATTGCTCCTCCTG | 90 |
| R: ATCCACATCTGCTGGAAGG | ||
| MMP-2 | F: GCAACCTGTTTGTGCTGAAG | 198 |
| R: GTAGCCAATGATCCTGTATGTG | ||
| MMP-9 | F: TCCAGTACCGAGAGAAAGCCTA | 114 |
| R: GCAGGATGTCATAGGTCACG | ||
| PR-A | F: AATGGAAGGGCAGCACAACT | 192 |
| R: TGTGGGAGAGCAACAGCATC | ||
| PR-B | F: AAGGGGAGTCCAGTCGTCAT | 165 |
| R: CGAAACTTCAGGCAAGGTGT | ||
MMP; Matrix metalloproteinase, PR; Progesterone receptor, and PCR; Polymerase chain reaction.
Each reaction contained 10 μl SYBR® Premix Ex Taq II that consisted of Taq DNA polymerase reaction buffer, dNTP mix, SYBR Green II, MgCl2 and Taq DNA polymerase; 5 pmol of either MMP-9, PR-A, or PR-B primers, or 3 pmol of MMP-2 primer; 25 ng/μl of synthesized cDNA; and water to reach 20 μl. The target gene levels were compared to that of a housekeeping gene, β-actin, from the same cDNA. Each real-time quantitative PCR assay was done in duplicate for each sample to confirm the reproducibility of the results. In this study, both housekeeping genes GAPDH and β-actin were optimized; however, the expression of β-actin appeared to be more stable in our samples. The amplification program contained the following 3 steps. Step 1: a primary heating for 10 minutes at 95°C to denature the cDNA and activate the Taq DNA polymerase. Step 2: DNA amplification for 40 cycles of 15 seconds at 95°C (denaturation) and one minute at 60°C (annealing) for β-actin, MMP-2, MMP-9, PR-A, and PR-B. Step 3: increasing temperature gradually from 60°C to 95°C for 15 seconds and one minute at 60°C for melting curve analysis. After each run, a melting curve analysis was done to confirm the specificity of the PCR reaction. All samples were retested with a cycle threshold coefficient of variation value higher than one degree. To confirm the melting curve results, we assayed representative samples of the real-time PCR products on 2% ultra-pure agarose (Invitrogen, USA) gel electrophoresis (Paya Pazhoh Pars, Iran), and stained them with ethidium bromide (Sigma Aldrich, USA) prior to visualization on a Molecular Imager® Gel Doc™ XR+ (BioRad, USA).
Statistical analysis
We compared the participants’ clinical information between groups (endometriosis and control) using the independent t test. The expression levels of MMP-2, MMP-9, PR-A, and PR-B were compared between tissue extracts of endometriotic or ectopic lesions and eutopic endometrium samples (patient group) to endometrial samples (control group) using one-way analysis of variance (ANOVA) followed by Tukey’s test to conclude significant differences between our groups and pair-wise comparisons. In cases where the data were not distributed normally, we conducted natural logarithmic (Ln) transformation for MMP-2, MMP-9, PR-A, PR-B, and PRA/PR-B before analysis. The relationships between the Ln-transformed expressions of PR-A and PR-B, as well as the PR-A/PR-B ratio with MMP-2 and MMP-9 were assessed by Pearson’s correlation. Statistical analysis was done using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-tailed and a P<0.05 was considered statistically significant.
Results
Table 2 shows the main clinical characteristics of the 40 participants who provided tissue samples. All 20 women with endometriosis were infertile. There were no statistically significant differences in the sample distributions according to the phases of the menstrual cycles, mean age, or body mass index (BMI) in patients with endometriosis compared to the control group.
Table 2.
Clinical characteristics of participants in expression assays
| Groups | Menstrual cycle phase (%) | Disease stage (%) | BMI (kg/m2) | Age (Y) |
|---|---|---|---|---|
| Endometriosis | Proliferative (80) | IV (60) | 25.82 ± 4.91 | 30.03 ± 8.31 |
| n=20 | Secretory (20) | III (40) | ||
| Controls | Proliferative (80) | - | 24.35 ± 4.32 | 29.21 ± 8.72 |
| n=20 | Secretory (20) | |||
| P value | NS | - | NS | NS |
Data are expressed as mean ± SEM and values in parentheses are percentages. BMI; Body mass index and NS; Not significant.
Expression of MMP-2 and MMP-9 in endometriosis
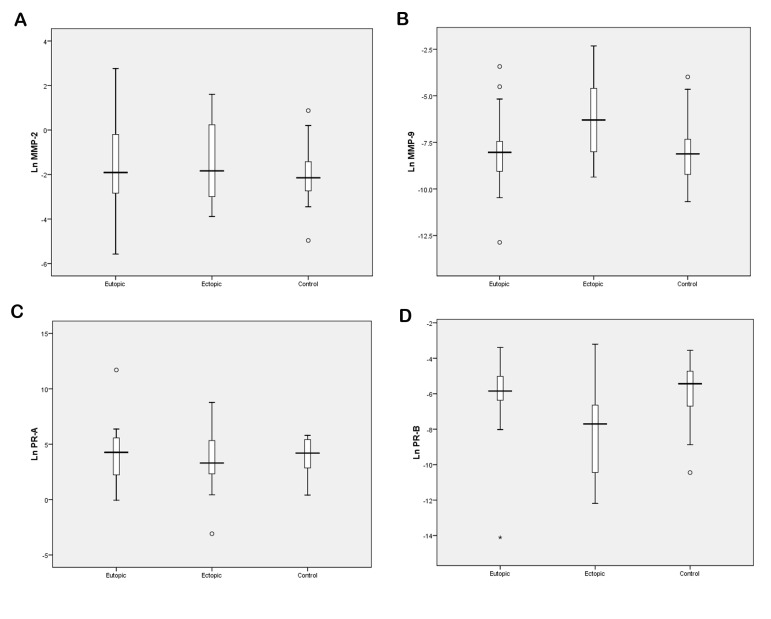
We assessed the differences between the means of mRNA levels in patients and controls with one-way ANOVA. We observed no significant difference in the expression levels of MMP-2 among these groups (P>0.05, Table 3, Fig .1A). Our results showed a significant increase in the expression of MMP-9 in endometriotic tissues compared to eutopic endometrium samples (P=0.012) and the control group (P=0.014, Table 3, Fig .1B).
Table 3.
mRNA expression levels of MMP-2, MMP-9, PR-A, and PR-B in ovarian endometriosis and endometrial tissues obtained from women with and without endometriosis
| Different object | Endometriotic lesions (ectopic) | Eutopic endometrium (endometriosis group) | Endometrium (control group) | P value |
|---|---|---|---|---|
| MMP-2 | 0.16 (0.05, 1.45) | 0.15 (0.05, 0.87) | 0.12 (0.06, 0.29) | 0.512 |
| MMP-9 | 0.02E-1 (0.03E-2, .021)*Δ | 0.03E-2 (0.01E-2, 0.06E-2) | 0.03E-2 (0.09E-3, 0.07E-2) | 0.005 |
| PR-A | 27.20 (9.99, 206.33) | 76.98 (9.00, 268.03) | 67.05 (16.60, 231.78) | 0.643 |
| PR-B | 0.04E-2 (0.03E-3, .01E-1)*Δ | 0.03E-1 (0.01E-1, 0.07E-1) | 0.04E-1 (0.01E-1, 0.09E-1) | 0.001 |
| Ln (PR-A/PR-B) | 11.79 ± 4.82 | 10.28 ± 4.64 | 9.81 ± 2.93 | 0.305 |
Data are expressed as mean ± standard deviation or median (inter-quartile range) when appropriate. ANOVA was performed on the natural-log-transformed values when appropriate. MMP; Matrix metalloproteinase, PR; Progesterone receptor, *; P<0.05 versus endometriotic lesions compared to the controls, and Δ; P<0.05 versus endometriotic lesions compared to the eutopic endometrium.
Fig 1.
Expression levels of matrix metalloProteinases (MMPs) and progesterone receptors (PRs).
A. MMP-2, B. MMP-9, C. PR-A, and D. PR-B in ovarian endometrioma (ectopic) and endometrial tissues from women with (eutopic) and without endometriosis (control). Ln: Logarithmic.
Progesterone receptor isoforms PR-A and PR-B expression in endometriosis
Extracts of endometriotic lesions from women with endometriosis presented a slight decrease in mRNA level of PR-A in comparison to the eutopic endometrium (Table 3, Fig .1C), while the mRNA levels of this isoform were slightly higher in eutopic endometrium samples compared to the control group (Table 3, Fig .1C). However, our data presented no significant differences between these groups (P=0.44). The results generally confirmed that the expression level of PR-B significantly differed between groups (P<0.001, Table 3). As shown in Figure 1D, we found significantly lower expression levels of PR-B in endometriotic tissues compared to the controls (P=0.002) and eutopic endometrium tissues (P=0.006, Table 3). Although eutopic endometrium tissues showed low levels of PR-B expression compared with the control samples, there were no significant differences observed among these two groups (P=0.95).
Association between expression levels of progesterone receptor isoforms PR-A and PR-B in endometriosis
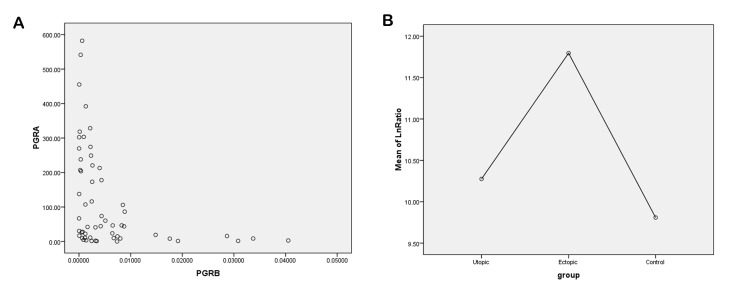
There was a strong negative correlation between PR-A and PR-B isoforms in endometriotic lesions. When the PR-A isoform had increased mRNA levels, we found significantly lower levels of PR-B isoform expression and vice versa (r=-0.789, P<0.001, Fig .2A). Similar results were observed in endometrial tissue from women with (r=-0.844, P<0.001) and without endometriosis (r=-0.579, P=0.008).
Fig 2.
Association of progesterone receptor (PR)-A and PR-B gene expressions in ovarian endometrioma and endometrium tissues from women with and without endometriosis. A. Overexpression of PR-A was associated with a low expression levels of the PR-B isoform in the three different groups and B. There was a higher PR-A/PR-B ratio in endometriutic tissue and eutopic endometrium compared with the control group. Ln; Logarithmic.
PR-A/PR-B ratio and its association with MMP-2 and MMP-9 expressions in endometriosis
We observed higher PR-A/PR-B ratios in both eutopic and endometriutic tissues related to the control group, but this finding was not significant (P>0.05, Table 3, Fig .2B). We were interested to assess the correlation between the mRNA levels of MMP-2 and the PR-A/PR-B ratio in each group. However, our data did not show any significant correlation between overexpression of MMP-2 and an altered PR-A/PR-B ratio in any of the groups (Table 4). We found no significant correlation between the expressions of MMP-2 and PR-A or PR-B (P>0.05, data not shown).
Table 4.
Correlation between mRNA levels of MMP-2, MMP-9, and PR-A/PR-B ratio in ovarian endometrioma and endometrial tissues from women with and without endometriosis
| Different object | LnPR-A/PR-B | ||
|---|---|---|---|
| Endometriotic lesions | Eutopic endometrium | Control endometrium | |
| LnMMP-2 | r=0.09 | r=0.09 | r=-0.19 |
| P=0.701 | P=0.701 | P=0.413 | |
| LnMMP-9 | r=-0.21 | r=-0.62* | r=0.14 |
| P=0.365 | P=0.003 | P=0.542 | |
Pearson test: analysis of correlation between different groups. P values are calculated on logarithmic (Ln)-transformed data. MMP; Matrix metalloproteinase, and PR; Progesterone receptor, and r; Spearman’s rho test.
Our results indicated that the expression level of MMP-9 only had a significant relationship to the mRNA levels of the progesterone receptor ratio (PR-A/PR-B) in eutopic endometrial tissue (P=0.003, Table 4). On the other hand, we found a significant association among the expression level of MMP-9 and the PR-A isoform in eutopic endometrial tissue (P=0.03). There was no significant relation between the expression levels of MMP-9 and the PR-B isoform in the study groups (P>0.05, data not shown).
Discussion
Endometriosis develops as a consequence of ectopic implantation of retrograded menstrual tissue, although the mechanisms that underlie this process are unknown (21). Several studies have underlined a correlation between MMPs and the invasive behavior of endometriotic tissues for establishment of endometrial glands and stromal cells at ectopic sites (7). MMPs coordinate general endometrial remodeling through menstrual cycles, which mediates ECM turnover (21). Upregulation and activation of MMPs related to tumor progression have been found in metastatic activity of tumors dependent on MMP synthesis (28). Hence, the expression of MMP enzymes is tightly regulated in normal tissues, because the delicate balance between MMPs and their inhibitors is crucial to preventing excessive matrix destruction (21).
Follicular fluid surrounds the microenvironment of maturing oocytes and has an important role in this process, affecting fertilization and consequent of embryo development (1). The opposed effect of endometriosis on fertilization has been attributed to its impact on the follicular microenvironment, poor oocyte development, and poor embryo formation (4). Studies indirectly suggest that MMP-2 and MMP-9 in follicular fluids have a direct effect on follicular development and rift of the follicular wall (29). A high level of MMP expression by the endometriotic tissues can be initiated in the pathogenesis of endometriosis (7). It might be responsible for intrafollicular modifications that result in infertility.
Overexpression of different MMPs have been reported in endometriosis and include MMP-1 (30), MMP-2 (18), MMP-9 (20), and MMP-7 (31). The degradation of vascular and epithelial basement membrane components and ECM proteins are mediated by gelatinases (MMP-2 and MMP-9). Gelatinases have been associated with the malignant potential of tumors by increasing tumor invasion and metastasis (32). The role of MMP-2 in endometriosis is debatable. In the current study, we have detected elevated MMP-2 expression in both ectopic and eutopic tissues of endometriosis patients compared to the normal control group. However, no significant difference in MMP-2 expression was observed in our groups. Previous studies have reported higher levels of MMP-2 expression and lower mRNA levels for TIMP-2 in eutopic tissues of endometriosis patients relative to the endometrium from control groups (33, 34).
This highlights potential changes in MMP activity in endometriotic tissues and suggested improved proteolysis activity, which could play an important role in implantation of this tissue in ectopic sites. In addition, our data showed significantly higher expression levels of MMP-9 in the ectopic versus the eutopic and control endometrial tissues. Several researchers have focused on the role of MMP-9 in tumor invasion and metastasis (35, 36). The involvement of this proteolytic enzyme in vascular growth and angiogenesis has been previously reported (20). A higher gelatinase activity was found in endometriotic tissues compared to eutopic endometrium in endometriosis (37). Previous investigations have demonstrated higher expression of MMP-9 in ectopic versus the eutopic endometrium (38). In patients with endometriosis, elevated levels of MMP-9 mRNA in ectopic tissues might play an essential role in endometrial tissue invasion and its ability to be implanted in ectopic sites. High levels of MMP-2 and MMP-9 and low levels of TIMP-1 were related with low production of mature oocytes and subsequent decreased quality of embryos in endometriosis patients who underwent in vitro fertilization (IVF) (1). As a result, MMP-2 and MMP-9 overexpression have adverse effects on the function of the follicular microenvironment, as well as oocyte and embryo quality. These changes might be the cause of infertility due to endometriosis.
Endometriosis is known as a progesterone resistant disease (23). The ability of progesterone to affect gene expression is reliant on the PR-A/PR-B ratio (27). An altered PR-A/PR-B ratio modifies progesterone activity due to differential regulation of specific progesterone response target genes that may lead to the progression of endometriosis. Progesterone reduces the expression of pro-inflammatory genes when the PR-A/PR-B ratio favors PR-B and increases their expression when the ratio tilts towards the PR-A isoform (39, 40). The present study has shown a slightly increased level of PR-A expression in eutopic tissues compared to controls. This increased expression was slightly higher in controls compared to ectopic tissues. On the other hand, PR-B showed a significantly differential expression pattern between the groups. The results clearly showed a decreased expression level for PR-B in endometriotic tissues compared to control and eutopic groups, which can disrupt the PR-A/PR-B ratio in ectopic samples. Eutopic tissues also had decreased PR-B expression. Progesterone resistance might account for the existence of the inhibitory PR isoform, PR-A, and the lack of the stimulatory isoform, PR-B, in endometriotic tissues (23). These results suggested that a decrease in the expression level of PR-B and overexpression of PR-A could alter this ratio in endometriotic tissues. Following this, the imbalanced ratio could alter progesterone activity related to differential regulation of specific progesterone target genes and improve endometriosis. On the other hand, we have demonstrated an association between overexpression of PR-A with low expression of the PR-B isoform, particularly in ectopic tissue and the endometria of women with and without endometriosis.
It has been shown that transcriptional regulation of MMP-2 in the JAr choriocarcinoma cell line is mediated by progesterone treatment with progesterone inhibiting the expression of MMP-2. MMP-2 expression is mediated through the binding of the primary transcription factor SP4 to the MMP-2 proximal promoter. Progesterone inhibits MMP-2 expression by decreasing PR and SP4 binding to the MMP-2 promoter (24). Progesterone also suppresses TGFβ1-induced stimulation of MMP-2 through its nuclear hormone receptors in human endometrial stromal cells (22). Therefore, our data imply that observed alteration in PR-A/PR-B expression ratio may cause overexpression of MMP-2 in endometriotic tissues. However, our analysis did not show any significant correlation between the high level of MMP-2 expression and imbalance in PR-A/PR-B ratio expression in endometriotic tissues.
In contrast, we have shown, for the first time, a significant association between the expression of MMP-9 and altered an PR-A/PR-B ratio in endometrium (eutopic) tissues of women with endometriosis compared to a normal control group. MMP-9, activity in the human endometrium is controlled by estradiol and progesterone (26). This hypothesis can be supported by the fact that progesterone increases the expression level of inhibitor-κBα, a repressor of the NF-кB transcription factor, and inhibits basal and lipopolysaccharide-induced proinflammatory gene expressions via PR-B, which are inhibited by PR-A (27). NF-κB is involved in the regulation of cytokines and MMP transcription (including MMP-9) in the human endometrium. PRs can directly interact with the RelA (p65) subunit of NF-κB, which is necessary to activate NF-кB (26). Thus, an altered PR-A/PR-B ratio may impact the expression level of MMP-9 through the regulation of NF-κB activity, which could be important in the pathogenesis of endometriosis. However, we have not observed any significant correlation between this altered ratio and MMP-9 expression in ectopic tissues in comparison to the control endometrium samples.
Conclusion
We sought to assess the correlation between the expression of MMP-2 and MMP-9 and the PR-A/PR-B ratio in endometriosis. Our data showed a significant negative association between expression levels of MMP-9 and an altered PR-A/PR-B ratio in the eutopic endometrium group compared with the control samples. To our knowledge, there have been few attempts to report these correlations between the MMPs and PR isoforms in endometriosis. It is known that endometriosis affects the follicular microenvironment, oocytes maturity and consequent embryo development. This hypothesis may be correlated further by our observations since overexpression of MMP-9, as a consequence of an imbalanced PR-A/PR-B ratio in endometriosis, may affect the function of the follicular microenvironment, as well as oocyte and embryo quality, which cause infertility in endometriosis.
Acknowledgments
The authors wish to thank Mona Khosravifar and Raha Favaedi for their technical support in addition to Dr. Fariba Ramezanali and Mansoureh Uromiechi of Royan Institute for laparoscopy sample collection (tissue and blood) from patients and controls. We are extremely grateful to all of the women who agreed to participate in this study. The authors dedicate this article to the memory of Dr. Saeid Kazemi Ashtiani, founder of Royan Institute. This study was financially supported by a grant from the Reproductive Biomedicine Research Center, Royan, Institute, Tehran, Iran [91000358]. The authors report no conflicts of interest.
Author’s Contributions
S.M.; Participated in study design, data collection and evaluation, drafting and statistical analysis. A.Gh.; Contributed to all data and statistical analysis and interpretation of data. M.Sh., R.A.; Contributed to conception and design. P.A.; Managed substantially of the design, all experimental work, data and statistical analysis of the study and provided critical revision of the manuscript. All authors performed editing and approving the final version of this manuscript for submission, also participated in the finalization of the manuscript and approved the final draft.
References
- 1.Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Altered circulating levels of matrix metalloproteinases 2 and 9 and their inhibitors and effect of progesterone supplementation in women with endometriosis undergoing in vitro fertilization. Fertil Steril. 2013;100(1):127–134. doi: 10.1016/j.fertnstert.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Signorile PG, Baldi F, Bussani R, D'Armiento M, Falco MD, Baldi A. Ectopic endometrium in human foetuses is a common event and sustains the theory of müllerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J Ex Clin Cancer Res. 2009;28:49–49. doi: 10.1186/1756-9966-28-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansour G, Sharma RK, Agarwal A, Falcone T. Endometriosis-induced alterations in mouse metaphase II oocyte microtubules and chromosomal alignment: a possible cause of infertility. Fertil Steril. 2010;94(5):1894–1899. doi: 10.1016/j.fertnstert.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 4.Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441–447. doi: 10.1007/s10815-010-9436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giudice LC. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannello F, Medda V. Nuclear localization of matrix metalloproteinases. Prog Histochem Cytochem. 2012;47(1):27–58. doi: 10.1016/j.proghi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Gaide Chevronnay HP, Selvais C, Emonard H, Galant C, Marbaix E, Henriet P. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim Biophys Acta. 2012;1824(1):146–156. doi: 10.1016/j.bbapap.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Cox KE, Piva M, Sharpe-Timms KL. Differential regulation of matrix metalloproteinase-3 gene expression in endometriotic lesions compared with endometrium. Biol Reprod. 2001;65(4):1297–1303. doi: 10.1095/biolreprod65.4.1297. [DOI] [PubMed] [Google Scholar]
- 10.Osteen KG, Rodgers WH, Gaire M, Hargrove JT, Gorstein F, Matrisian LM. Stromal-epithelial interaction mediates steroidal regulation of metalloproteinase expression in human endometrium. Proc Natl Acad Sci USA. 1994;91(21):10129–10133. doi: 10.1073/pnas.91.21.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem pharmacol. 2008;75(2):346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada Y, Nagase H, Harris JrE. Matrix metalloproteinases 1, 2, and 3 from rheumatoid synovial cells are sufficient to destroy joints. J Rheumatol. 1987;14:41–42. [PubMed] [Google Scholar]
- 13.Amălinei C, Căruntu I-D, Giuşcă SE, Bălan RA. Matrix metalloproteinases involvement in pathologic conditions. Rom J Morphol Embryol. 2010;51(2):215–228. [PubMed] [Google Scholar]
- 14.Pitsos M, Kanakas N. The role of matrix metalloproteinases in the pathogenesis of endometriosis. Reprod Sci. 2009;16(8):717–726. doi: 10.1177/1933719109333661. [DOI] [PubMed] [Google Scholar]
- 15.Salata IM, Stojanovic N, Cajdler-Luba A, Lewandowski KC, Lewinski A. Gelatinase A (MM-2), gelatinase B (MMP-9) and their inhibitors (TIMP 1, TIMP-2) in serum of women with endometriosis: Significant correlation between MMP-2, MMP-9 and their inhibitors without difference in levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in relation to the severity of endometriosis. Gynecol Endocrinol. 2008;24(6):326–330. doi: 10.1080/09513590802090325. [DOI] [PubMed] [Google Scholar]
- 16.Koks CA, Groothuis PG, Slaats P, Dunselman GA, de Goeij AF, Evers JL. Matrix metalloproteinases and their tissue inhibitors in antegradely shed menstruum and peritoneal fluid. Fertil Steril. 2000;73(3):604–612. doi: 10.1016/s0015-0282(99)00566-x. [DOI] [PubMed] [Google Scholar]
- 17.Osteen KG, Yeaman GR, Bruner-Tran KL. Matrix metalloproteinases and endometriosis. Semin Reprod Med. 2003;21(2):155–164. doi: 10.1055/s-2003-41322. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Dan Yang. Investigation of Matrix Metalloproteinase -1, 2 and tissue inhibitor of matrix metalloproteinases-1 in Endometriosis. Journal of Nanjing Medical University. 2007;21(3):159–164. [Google Scholar]
- 19.Ren Q, Guan Sh, Fu J, Wang A. Spatio-temporal expression of matrix metalloproteinases-2 and -9 in porcine endometrium during implantation. J Anim Vet Adv. 2010;9(15):2074–2081. [Google Scholar]
- 20.Collette T, Bellehumeur C, Kats R, Maheux R, Mailloux J, Villeneuve M, et al. Evidence for an increased release of proteolytic activity by the eutopic endometrial tissue in women with endometriosis and for involvement of matrix metalloproteinase-9. Hum Reprod. 2004;19(6):1257–1264. doi: 10.1093/humrep/deh290. [DOI] [PubMed] [Google Scholar]
- 21.Bruner KL, Eisenberg E, Gorstein F, Osteen KG. Progesterone and transforming growth factor-b coordinately regulate suppression of endometrial matrix metalloproteinases in a model of experimental endometriosis. Steroids. 1999;64(9):648–653. doi: 10.1016/s0039-128x(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 22.Itoh H, Kishore AH, Lindqvist A, Rogers DE, Word RA. Transforming growth factor β1 (TGFβ1) and progesterone regulate matrix metalloproteinases (MMP) in human endometrial stromal cells. J Clin Endocrinol Metab. 2012;97(6):E888–E897. doi: 10.1210/jc.2011-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 24.Goldman S, Lovett DH, Shalev E. Mechanisms of matrix metalloproteinase-2 (mmp-2) transcriptional repression by progesterone in jar choriocarcinoma cells. Reprod Biol Endocrinol. 2009;7:41–41. doi: 10.1186/1477-7827-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng F, Cheng L, Zeng Q, Gao W. Increased expression and activity of MMP-9 in C-reactive protein-induced human THP-1 mononuclear cells is related to activation of nuclear factor kappa-B. J Huazhong Univ Sci Technolog Med Sci. 2009;29(4):399–403. doi: 10.1007/s11596-009-0401-0. [DOI] [PubMed] [Google Scholar]
- 26.Cornet PB, Galant C, Eeckhout Y, Courtoy PJ, Marbaix E, Henriet P. Regulation of matrix metalloproteinase-9/gelatinase B expression and activation by ovarian steroids and LEFTY-A/endometrial bleeding-associated factor in the human endometrium. J Clin Endocrinol Metab. 2005;90(2):1001–1011. doi: 10.1210/jc.2004-1277. [DOI] [PubMed] [Google Scholar]
- 27.Huiqing Tan LY, Neal S. Rote, William W.Hurd, Sam Mesiano.Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97(5):719–730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Ascenzo S, Giusti I, Millimaggi D, Marci R, Tatone C, Colonna RC, et al. Intrafollicular expression of matrix metalloproteinases and their inhibitors in normally ovulating women compared with patients undergoing in vitro fertilization treatment. European J Endocrinol. 2004;151(1):87–91. doi: 10.1530/eje.0.1510087. [DOI] [PubMed] [Google Scholar]
- 30.Kokorine I, Nisolle M, Donnez J, Eeckhout Y, Courtoy PJ, Marbaix E. Expression of interstitial collagenase (matrix metalloproteinase-1) is related to the activity of human endometriotic lesions. Fertil Steril. 1997;68(2):246–251. doi: 10.1016/s0015-0282(97)81510-5. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzaki S, Maleysson E, Darcha C. Analysis of matrix metalloproteinase-7 expression in eutopic and ectopic endometrium samples from patients with different forms of endometriosis. Hum Reprod. 2010;25(3):742–750. doi: 10.1093/humrep/dep435. [DOI] [PubMed] [Google Scholar]
- 32.Planagumà J, Liljeström M, Alameda F, Bützow R, Virtanen I, Reventós J, et al. Matrix metalloproteinase-2 and matrix metalloproteinase-9 codistribute with transcription factors RUNX1/AML1 and ETV5/ERM at the invasive front of endometrial and ovarian carcinoma. Hum Pathol. 2011;42(1):57–67. doi: 10.1016/j.humpath.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Chung HW, Lee JY, Moon HS, Hur SE, Park MH, Wen Y, et al. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil Steril. 2002;78(4):787–795. doi: 10.1016/s0015-0282(02)03322-8. [DOI] [PubMed] [Google Scholar]
- 34.Ning WX, Huang HF, Jin F. The role of VEGF and MMP-2 in the early stage of evolution of endometriosis.International Congress Series. International Congress Series; 2004. pp. 240–243. [Google Scholar]
- 35.Kondraganti S, Mohanam S, Chintala SK, Kin Y, Jasti SL, Nirmala C, et al. Selective suppression of matrix metalloproteinase-9 in human glioblastoma cells by antisense gene transfer impairs glioblastoma cell invasion. Cancer Res. 2000;60(24):6851–6855. [PubMed] [Google Scholar]
- 36.Turner HE, Nagy Z, Esiri MM, Harris AL, Wass JA. Role of matrix metalloproteinase 9 in pituitary tumor behavior. J Clin Endocrinol Metab. 2000;85(8):2931–2935. doi: 10.1210/jcem.85.8.6754. [DOI] [PubMed] [Google Scholar]
- 37.Liu XJ, He YL, Peng DX. Expression of metalloproteinase-9 in ectopic endometrium in women with endometriosis. Di Yi Jun Yi Da Xue Xue Bao. 2002;22(5):467–469. [PubMed] [Google Scholar]
- 38.Chung HW, Wen Y, Chun SH, Nezhat C, Woo BH, Lake Polan M. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: a rationale for endometriotic invasiveness. Fertil Steril. 2001;75(1):152–159. doi: 10.1016/s0015-0282(00)01670-8. [DOI] [PubMed] [Google Scholar]
- 39.Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-κB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20(11):2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 40.Rojas PA, May M, Sequeira GR, Elia A, Alvarez M, Martínez P, et al. Progesterone receptor isoform ratio: a breast cancer prognostic and predictive factor for antiprogestin responsiveness. J Natl Cancer Inst. 2017;109(7) doi: 10.1093/jnci/djw317. [DOI] [PMC free article] [PubMed] [Google Scholar]