Abstract
Context:
A limiting factor for reducing anterior cruciate ligament injury risk is ensuring that the movement adaptions made during the prevention program transfer to sport-specific activity. Virtual reality provides a mechanism to assess transferability, and neuroimaging provides a means to assay the neural processes allowing for such skill transfer.
Objective:
To determine the neural mechanisms for injury risk–reducing biomechanics transfer to sport after anterior cruciate ligament injury prevention training.
Design:
Cohort study.
Setting:
Research laboratory.
Participants:
Four healthy high school soccer athletes.
Interventions:
Participants completed augmented neuromuscular training utilizing real-time visual feedback. An unloaded knee extension task and a loaded leg press task were completed with neuroimaging before and after training. A virtual reality soccer-specific landing task was also competed following training to assess transfer of movement mechanics.
Main Outcome Measures:
Landing mechanics during the virtual reality soccer task and blood oxygen level–dependent signal change during neuroimaging.
Results:
Increased motor planning, sensory and visual region activity during unloaded knee extension and decreased motor cortex activity during loaded leg press were highly correlated with improvements in landing mechanics (decreased hip adduction and knee rotation).
Conclusion:
Changes in brain activity may underlie adaptation and transfer of injury risk–reducing movement mechanics to sport activity. Clinicians may be able to target these specific brain processes with adjunctive therapy to facilitate intervention improvements transferring to sport.
Keywords: anterior cruciate ligament, motor control, motor learning, injury prevention
Successful strategies aimed at the reduction of anterior cruciate ligament (ACL) injury risk hinge on adaptations to the neuromuscular control system that modulates movement patterns and ultimately transfers desirable biomechanics to sport.1 Full optimization of neuromuscular training aimed at injury prevention must target the cognitive, perceptual, and motor processes that synergize to allow athletes to respond to sport-specific demands with resilient, low injury risk movement strategies.2 The inability to ensure motor pattern transfer from the prevention program to the athletic field is a primary limiting factor for reducing ACL injury risk beyond the laboratory or clinic.3 Quantifying movement mechanics during athletic activity could confirm if injury risk–reducing biomechanics transfer from the prevention program, but it is difficult to measure joint mechanics during real-time athletic activity and nearly impossible to impose any experimental standardization. On the other hand, laboratory-based motion capture has exceptional validity and reliability, but it is limited as a real-world substitute.
Some of the laboratory limitations can be ameliorated by incorporating sport-specific tests to assess the transfer of specific movement patterns; examples include utilizing an in-air target, sport-specific equipment or movements that mimic gameplay (such as run to cut as opposed to dropping from a box).4,5 However, those efforts to improve ecological validity are limited in reproducing the perceptual-motor and neurocognitive challenges of interacting with a dynamic athletic environment. The advent of virtual reality (VR) technologies provides a means to overcome this limitation to assess motor pattern transfer to sport. VR allows athletes to be immersed in environments that mimic their respective athletic settings, visually engage with sport-specific objects (eg, a ball, goal, or net) or other simulated athletes, and respond to dynamic, but experimentally controlled, simulations. Therefore, VR allows for the evaluation of real-time athletic activity in close to real-world sport scenarios while permitting the precise quantification of movement mechanics achievable in a laboratory setting.6,7
Despite these advances to improve the ecological validity of the laboratory setting, the field still lacks an understanding of the mechanisms supporting motor performance transfer. Current techniques quantify primarily observable movement adaptations; however, quantification of the neural mechanisms that underlie motor adaptation and transfer is relatively sparse. Changes within the nervous system (ie, neuroplasticity) are required for the effective transfer of learned motor mechanics; however, the vast majority of the research has been completed with relatively simple motor paradigms that are not easily applied to sports medicine. To our knowledge, Powers and Fisher8 have published the only investigation evaluating the relation between neural and biomechanical changes following an ACL injury prevention program. After a 10-week landing skill training program, participants’ motor cortex (M1) were stimulated using transcranial magnetic stimulation and, relative to strength training, landing training decreased corticomotor excitability in the gluteus maximus (indicating motor learning as gluteus maximus activation becomes more automatic and controlled less by the cortex and more by subcortical regions).8 However, these novel findings do not reveal whether those changes were associated with improved landing mechanics or training transference to sport. Further, these prior data were based on the external stimulation of the M1 using transcranial magnetic stimulation. As a result, it is unknown what role other brain regions may have had or how these neural adaptations influence intrinsically driven motor control. Previous neuroimaging work has suggested that motor learning and skill transfer require activation of perceptual processing regions and reduced or more efficient motor cortex activity.9 An improved understanding of the neural mechanisms of injury risk–reducing motor pattern transfer may provide mechanistic targets for clinicians to intervene and improve program development. Therefore, the purpose of this study was to assess neuroplasticity associated with injury prevention training and the subsequent transfer of training adaptations to sport. We hypothesized that decreased motor cortex activity (improved neural efficiency) during loaded and unloaded leg movements would correlate with reduced injury risk biomechanics in a sport-specific VR scenario.
Methods
To understand the mechanisms of adaptation from neuromuscular training, we implemented a 6-week program of augmented neuromuscular training (aNMT) in high school female soccer players with pre- and post-VR sport-specific landing biomechanics testing to assess motor pattern transfer and functional magnetic resonance imaging (fMRI) to assess neural mechanisms.
Augmented Neuromuscular Training
The aNMT implemented a visual stimulus (a rectangle) that deformed in real time as a function of key injury risk biomechanical variables (ie, knee abduction moment of force, knee-to-hip joint moment of force ratio, lateral trunk flexion, and vertical ground reaction forces), which was projected in front of the participants during specific exercises (ie, squat, single-leg Romanian deadlift, tuck jumps, etc.). Participants controlled this visual stimulus in real time via a motion capture to induce implicit learning while eliciting reduced injury risk–movement mechanics. From the participants’ perspective, the goal was to maintain a perfect rectangle (which corresponded to low-risk movement mechanics) as they performed different lower body, closed kinetic chain exercises. For example, if a participant’s knee collapsed into valgus during an exercise, a pattern known to increase ACL load, the box would deform. The participants had to discover a way to move so as to avoid such deformations without any explicit knowledge about the particular mapping between their movement patterns and the stimulus shape. The real-time biofeedback was integrated into a standardized neuromuscular training program adapted from the existing literature and consisted of 6-weeks training 3 times a week.10
Biomechanically Instrumented VR Transfer Task
Sport-specific VR assessments were taken prior to and immediately after the completion of the intervention. Although being fully instrumented for 3D motion analysis (see Hewett et al.11 for more specifics on training and biomechanical methods), participants wore a custom-built, wireless, high-definition head-mounted display and completed a defensive run to cut maneuver in the VR scenario.6
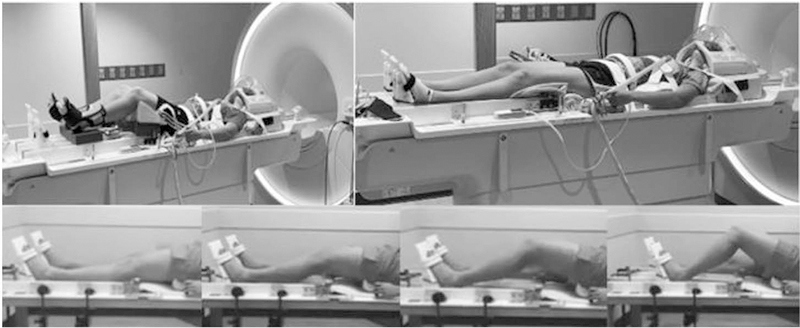
The VR-based biomechanical assessments were displayed on a custom-built, wireless, full high-definition head-mounted display at 60 frames per second using Unity 3D Pro (Unity Technologies, San Francisco, CA) via a high-end Windows 7 gaming PC. Athletes’ head positions and lower-limb angular movement trajectories were tracked with 39 motion capture cameras (Motion Analysis Corporation, Santa Rosa, CA). For lower-limb kinematic measurement, athletes were instrumented with 31 retro-reflective markers on the sacrum, sternum, and bilaterally on the anterior superior iliac spine, greater trochanter, mid-thigh, medial and lateral knee, tibial tubercle, mid-shank, distal shank, medial and lateral ankle, heel, dorsal surface of the midfoot, lateral foot (fifth metatarsal), and toe (between second and third metatarsals). The 3D motion data were postprocessed with Cortex (version 6.2; Motion Analysis Corporation), Visual3D (C-Motion, Inc, German-town, MD), and custom MATLAB (MathWorks, Inc, Natick, MA) software. All athletes entered VR and performed 4 acclimation tasks within the soccer-specific virtual environment: (1) walk 10 m to a floating target; (2) jog 10 m to a floating target; (3) sprint 10 m to a floating target; and (4) jump vertically to perform a soccer header on a floating soccer ball (Figure 1). Prior to the tasks, the athletes were made aware of the task space boundary via 4 orange virtual cones that bounded the virtual movement space and, thus, the real world, ~5 feet from the actual room perimeter (Figure 2). The acclimation period took ~7 minutes to complete. Following acclimation, the cutting scenario began. All athletes performed the cutting scenario at week 1 (pretraining) and week 8 (posttraining). The unanticipated cutting involved a 1-on-1 soccer defensive maneuver, whereby the athlete started with her back to a virtual goal and is instructed to move and cut to prevent a virtual player from reaching the goal; this movement required a quick defensive cut that incorporates both rapid decelerating and reactive lateral movements. Two practice trials were completed prior to data collection trials.
Figure 1.
fMRI participant setup for leg extension (top left) and leg press (top right), and completing the leg press (bottom). fMRI indicates functional magnetic resonance imaging.
Figure 2.
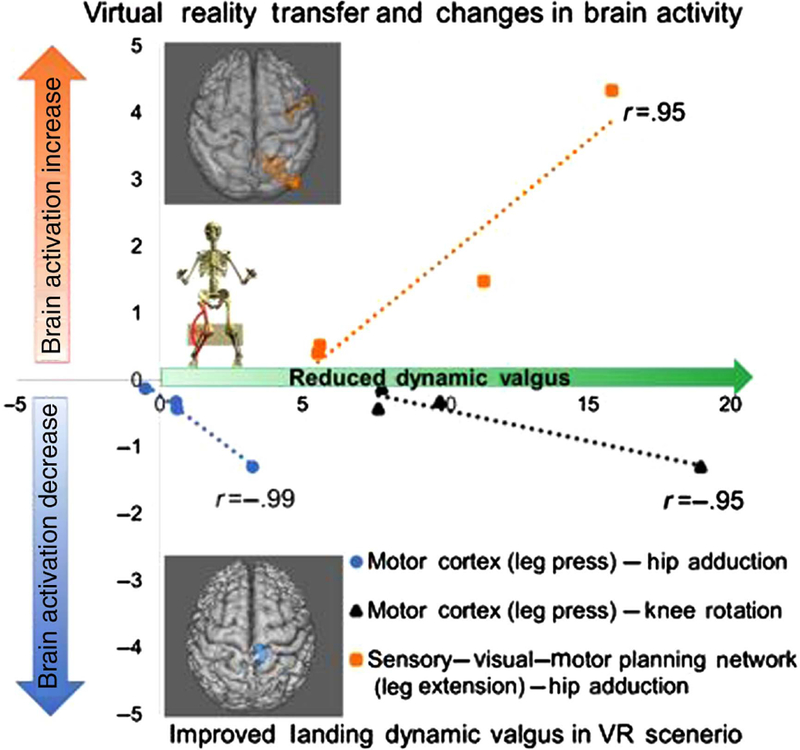
Change pretraining to posttraining in brain activity (vertical axis: % blood oxygen level–dependent signal change pre to post) during neuroimaging of leg motor tasks and landing dynamic valgus (horizontal axis: degree collapse of knee toward midline due to hip and knee rotation indicating higher injury risk). Increases in the x-axis indicate reduced injury risk. Note the high correlation between brain activity changes (increase in sensory–visual–motor planning and decrease in motor cortex activity) and improvements in landing kinematics. Also of note for interpretation is that the correlation between increased sensory–visual–motor planning activity to support decreased motor cortex activity was high (r = .87, P = .001). n = 4 – see online version for color brain image. VR indicates virtual reality.
Neuroimaging
To assess training-induced neuroplasticity, fMRI was also performed using a previously described unloaded knee extension protocol12 and a novel loaded leg press protocol before and after training to determine adaptive changes in brain activity from the aNMT (Figure 1; for more information on neuroimaging collection and analysis, see Grooms et al.13). During the fMRI leg press protocol, participants were instructed to perform a unilateral resisted leg press in coordination with a metronome (1.2 Hz) to standardize the pace of the movements. Subjects completed 4 trials of 30 seconds, yielding 36 repetitions per trial with equal time for flexion and extension on the dominate leg in both the pretraining and the posttraining testing conditions. The fMRI analysis consisted of whole brain analysis at the participant (knee movement > rest) and at the pairwise group level (post > or < pre) at P < .05 cluster corrected to identify regions that changed activity with training.12,13
Pearson and Spearman (due to small sample size) correlations were completed on the change in brain activity pretraining to posttraining and the change in frontal plane knee biomechanics during the VR transfer task.
Results
Following the neuromuscular training protocol, changes in brain activity to execute and control knee motion were highly correlated with the transfer of injury risk–reducing biomechanics during the simulated soccer scenario. Specifically, increased brain activity in participants’ knee sensory (precuneus)–visual–spatial (lingual gyrus) and motor planning (premotor) network during the leg extension task was significantly related to reduced hip adduction during landing in the VR environment (r = .95; P = .04; ρ = 1.0; P < .01; n = 4) (Figure 2). Additionally, we found that decreases in motor cortex activity during the leg press exercise were significantly associated with reduced hip adduction (r = −.95; P = .04; ρ = −0.41; P = .60) and reduced knee rotation (r = −.99; P = .01; ρ = −1.0; P < .01), indicating overall improved dynamic knee valgus during landing (incorporating overall risk profile of combined hip and knee kinematics) in the VR environment.
Discussion
We hypothesize that the increased sensory–visual–spatial and motor planning activity to control knee position in space (leg extension) supported efficiency of the motor cortex to control the loaded leg press.14 This improved efficiency of the motor cortex might enhance learning and retention of complex motor patterns practiced during training and support transfer of such patterns to other sport-specific activities, similar to those performed in the simulated soccer scenario.15 In particular, decreased motor cortex activation during knee movement likely enhances motor cortical capacity to promote more efficient processing during complex environmental demands, such as those encountered in the VR scenario and potentially in actual athletic environments.16 Although a small sample size (n = 4 female athletes), this study provides initial evidence relating to the neural adaptive mechanisms of injury risk–reducing motor pattern transfer to sport.
These novel findings identify the potential neural mechanisms of neuromuscular training adaptation transfer to sport. This integrated multimodal assessment of neuromuscular control from the neural processes to the biomechanical output may support future efforts to optimize injury risk–reducing interventions by targeting specific neural processes that improve the transfer of intervention adaptations to the sport environment. A potential immediate clinical application is to integrate the motor learning principles that guided the development of the aNMT, such as external attentional focus and implicit learning into training.17,18 The specific brain processes regarding sensory–visual–spatial integration for efficient knee motor control provides another target to improve the transfer of injury risk–reducing movement patterns. Specifically, if higher level visual-sensory integration of spatial information supporting motor cortex efficiency is a key neuroplastic factor driving the success of training, then modifications to the training can be made to target these neural processes. Potential clinical examples include optimization of exercise progression not only on classic muscle or motor performance but considering visual–spatial or sensory challenges to add difficulty, this could include stroboscopic training,19 dual tasking, perturbation training, or even isolated neurocognitive training to improve visual–spatial abilities. Additionally, the use of neural stimulation techniques combined with feedback modality differentiation and precision (eg, dosage specifications, adding auditory or tactile feedback to increase sensory region activity) may have future application as well as new yet to be imagined interventions.
Conclusion
The advent of low-cost VR technologies that enable simulated sport environments, on the everyday smartphone combined with knowledge of how specific training can induce neuroplasticity, may introduce a new frontier for injury prevention: The next generation of neuromuscular training programs that will precisely target the “neuro” in neuromuscular training.
Acknowledgments
The authors would like to thank from Seton High School: Ron Quinn, Lisa Larosa, Holly Laiveling, and the entire soccer coaching staff as well as the Seton administration and athletic director Wendy Smith; from Madeira High School soccer head coach Dan Brady, athletic director Joe Kimling, and principal David Kennedy for their support and assistance to conduct this study. Thanks also to the soccer parents and players for participating and supporting the efforts to complete the project. We appreciate their patience with the testing scheduling and follow-up testing. Their enthusiastic support made this study possible. Special acknowledgment goes to the athletic trainers at Seton High School, Cindy Busse and Madeira High School, Glenna Knapp. Without their time, commitment, and passion for the health and well-being of their student-athletes, this study would not have been possible. The authors would also like to acknowledge the University of Cincinnati Simulation and Virtual Environments team for all VR implementation and development, as well as Matt Batie for help in developing the head-mounted display used for all VR-related assessments. This work was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases 1U01AR067997, and Cincinnati Children’s Hospital Research Innovation Funding (IA# 31–41165-587000).
Contributor Information
Dustin R. Grooms, Ohio Musculoskeletal & Neurological Institute, Ohio University, Athens, OH, USA; and the Division of Athletic Training, School of Applied Health Sciences and Wellness, College of Health Sciences and Professions, Ohio University, Athens, OH, USA.
Adam W. Kiefer, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA., University of Cincinnati College of Medicine, Cincinnati, OH, USA.; Center for Cognition, Action, & Perception, Department of Psychology, University of Cincinnati, Cincinnati, OH.
Michael A. Riley, Center for Cognition, Action, & Perception, Department of Psychology, University of Cincinnati, Cincinnati, OH.
Jonathan D. Ellis, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.; University of Cincinnati College of Medicine, Cincinnati, OH, USA
Staci Thomas, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA..
Katie Kitchen, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA..
Christopher A. DiCesare, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
Scott Bonnette, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA..
Brooke Gadd, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA..
Kim D. Barber Foss, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA..
Weihong Yuan, University of Cincinnati College of Medicine, Cincinnati, OH, USA; Pediatric Neuroimaging Research Consortium, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA..
Paula Silva, Center for Cognition, Action, & Perception, Department of Psychology, University of Cincinnati, Cincinnati, OH..
Ryan Galloway, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Duke University School of Medicine, Durham, NC..
Jed A. Diekfuss, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
James Leach, Division of Radiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA..
Kate Berz, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA..
Gregory D. Myer, The SPORT Center, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; University of Cincinnati College of Medicine, Cincinnati, OH, USA; Department of Pediatrics and Orthopaedic Surgery, University of Cincinnati, Cincinnati, OH, USA; the Micheli Center for Sports Injury Prevention, Waltham, MA, USA; and the Department of Orthopaedics, University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.Sugimoto D, Myer GD, Barber Foss KD, Hewett TE. Specific exercise effects of preventive neuromuscular training intervention on anterior cruciate ligament injury risk reduction in young females: meta-analysis and subgroup analysis. Br J Sports Med 2015;49(5): 282–289. doi: 10.1136/bjsports-2014-093461 [DOI] [PubMed] [Google Scholar]
- 2.Kiefer AW, Myer GD. Training the antifragile athlete: a preliminary analysis of neuromuscular training effects on muscle activation dynamics. Nonlinear Dynamics Psychol Life Sci 2015;19(4): 489–510. [PubMed] [Google Scholar]
- 3.Myer GD, Stroube BW, DiCesare CA, et al. Augmented feedback supports skill transfer and reduces high-risk injury landing mechanics: a double-blind, randomized controlled laboratory study. Am J Sports Med 2013;41(3):669–677. doi: 10.1177/0363546512472977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kristianslund E, Krosshaug T. Comparison of drop jumps and sport-specific sidestep cutting: implications for anterior cruciate ligament injury risk screening. Am J Sports Med 2013;41(3):684–688. doi: 10.1177/0363546512472043 [DOI] [PubMed] [Google Scholar]
- 5.Chaudhari AM, Hearn BK, Andriacchi TP. Sport-dependent variations in arm position during single-limb landing influence knee loading: implications for anterior cruciate ligament injury. Am J Sports Med 2005;33(6):824–830. doi: 10.1177/0363546504270455 [DOI] [PubMed] [Google Scholar]
- 6.Kiefer AW, DiCesare C, Bonnette S, et al. Sport-specific virtual reality to identify profiles of anterior cruciate ligament injury risk curing unanticipated cutting. Paper presented at: International Conference on Virtual Rehabilitation; 2017. Montreal, CD. [Google Scholar]
- 7.Rose FD, Attree EA, Brooks BM, Parslow DM, Penn PR, Ambihaipahan N. Training in virtual environments: transfer to real world tasks and equivalence to real task training. Ergonomics 2000; 43(4):494–511. doi: 10.1080/001401300184378 [DOI] [PubMed] [Google Scholar]
- 8.Powers CM, Fisher B. Mechanisms underlying ACL injury-prevention training: the brain-behavior relationship. J Athl Train 2010; 45(5):513–515. doi: 10.4085/1062-6050-45.5.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidler RD, Noll DC. Neuroanatomical correlates of motor acquisition and motor transfer. J Neurophysiol 2008;99(4):1836–1845. doi: 10.1152/jn.01187.2007 [DOI] [PubMed] [Google Scholar]
- 10.Myer GD, Paterno MV, Ford KR, Hewett TE. Neuromuscular training techniques to target deficits before return to sport after anterior cruciate ligament reconstruction. J Strength Cond Res 2008;22(3):987–1014. doi: 10.1519/JSC.0b013e31816a86cd [DOI] [PubMed] [Google Scholar]
- 11.Hewett TE, Ford KR, Xu YY, Khoury J, Myer GD. Effectiveness of neuromuscular training based on the neuromuscular risk profile. Am J Sports Med 2017;45(9):2142–2147. doi: 10.1177/0363546517700128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grooms DR, Page SJ, Nichols-Larsen DS, Chaudhair AM, White SE, Onate JA. Neuroplasticity associated with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2017;47(3):180–189. doi: 10.2519/jospt.2017.7003 [DOI] [PubMed] [Google Scholar]
- 13.Grooms DR, Page SJ, Onate JA. Brain activation for knee movement measured days before second anterior cruciate ligament injury: neuroimaging in musculoskeletal medicine. J Athl Train 2015;50(10): 1005–1010. doi: 10.4085/1062-6050-50.10.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Percio C, Babiloni C, Marzano N, et al. “Neural efficiency” of athletes’ brain for upright standing: a high-resolution EEG study. Brain Res Bull 2009;79(3–4):193–200. doi: 10.1016/j.brainresbull.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Picard N, Matsuzaka Y, Strick PL. Extended practice of a motor skill is associated with reduced metabolic activity in M1. Nat Neurosci 2013;16(9):1340–1347. doi: 10.1038/nn.3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunst B, Benedek M, Jauk E, et al. Neural efficiency as a function of task demands. Intelligence 2014;42(100):22–30. doi: 10.1016/j.intell.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wulf G, Lewthwaite R. Optimizing performance through intrinsic motivation and attention for learning: the OPTIMAL theory of motor learning. Psychon Bull Rev 2016;23(5):1382–1414. doi: 10.3758/s13423-015-0999-9 [DOI] [PubMed] [Google Scholar]
- 18.Gokeler A, Benjaminse A, Hewett TE, et al. Feedback techniques to target functional deficits following anterior cruciate ligament reconstruction: implications for motor control and reduction of second injury risk. Sports Med 2013;43(11):1065–1074. doi: 10.1007/s40279-013-0095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K-M, Kim J-S, Grooms DR. Stroboscopic vision to induce sensory reweighting during postural control. J Sport Rehabil 2017; 12:1–11. doi: 10.1123/jsr.2017-0035 [DOI] [PubMed] [Google Scholar]