Significance
The precise regulation of antibody responses is central to the efficacy of vaccines and to our handling of microbes and infections. Antibody quality is enhanced in germinal center reactions, and this microstructure in tissue has to cope with limited oxygen. This work shows that a pair of hypoxia-inducible factor (HIF) proteins, gene regulators that respond to low oxygen but also are induced by T cell activation, must be expressed in helper T cells in order for immunization to yield a good antibody response. The evidence favors a multifactorial mechanism: HIFs shift the balance between follicular regulatory and helper T cells but also regulate metabolism, numbers of follicular helpers, and molecules they express to promote antibody production.
Keywords: humoral immunigy, HIF, hypoxia, T helper cell, metabolism
Abstract
T cell help in humoral immunity includes interactions of B cells with activated extrafollicular CD4+ and follicular T helper (Tfh) cells. Each can promote antibody responses but Tfh cells play critical roles during germinal center (GC) reactions. After restimulation of their antigen receptor (TCR) by B cells, helper T cells act on B cells via CD40 ligand and secreted cytokines that guide Ig class switching. Hypoxia is a normal feature of GC, raising questions about molecular mechanisms governing the relationship between hypoxia response mechanisms and T cell help to antibody responses. Hypoxia-inducible factors (HIF) are prominent among mechanisms that mediate cellular responses to limited oxygen but also are induced by lymphocyte activation. We now show that loss of HIF-1α or of both HIF-1α and HIF-2α in CD4+ T cells compromised essential functions in help during antibody responses. HIF-1α depletion from CD4+ T cells reduced frequencies of antigen-specific GC B cells, Tfh cells, and overall antigen-specific Ab after immunization with sheep red blood cells. Compound deficiency of HIF-1α and HIF-2α led to humoral defects after hapten-carrier immunization. Further, HIF promoted CD40L expression while restraining the FoxP3-positive CD4+ cells in the CXCR5+ follicular regulatory population. Glycolysis increases T helper cytokine expression, and HIF promoted glycolysis in T helper cells via TCR or cytokine stimulation, as well as their production of cytokines that direct antibody class switching. Indeed, IFN-γ elaboration by HIF-deficient in vivo-generated Tfh cells was impaired. Collectively, the results indicate that HIF transcription factors are vital components of the mechanisms of help during humoral responses.
During humoral immune responses, some antigen (Ag)-activated CD4+ T cells partner with proliferating B cells and provide help to the B cell that directs Ab class switching and guides differentiation of Ab-secreting plasma cells as well as the properties of the B cell Ag receptor (1, 2). Activated T cells may interact with B cells in an extrafollicular environment or enter germinal centers (GCs) (2–4). Differentiation of effector T cell subsets is regulated in part by specific cytokines (5, 6), leading to production of cytokines that then direct Ab class switching to specific isotypes. In addition, after T cell activation via the Ag receptor (TCR), follicular T helper (Tfh) cells can form functionally distinct subsets that migrate deep into B cell follicles and occupy specialized niches in secondary lymphoid organs. Among helpers, sustained interactions of an activated CD4+ T cell with a compatible B cell, along with costimulation via inducible costimulator (ICOS) (4) and cytokines such as IL-6 and IL-21 (7–9) can stably induce and maintain Tfh cells and high levels of the transcription factor BCL6, which directs follicular helper (Tfh) and GC Tfh programs (10–12). To heighten Ab affinity and improve the yield of long-lived plasma cells after immunization, GC B cells undergo iterative cycles of proliferation in the GC dark zone and selection via competition for restimulation by a limiting pool of specialized GC Tfh cells in the light zone (LZ) (2, 13). This process promotes further diversification of Ab specificities through somatic hypermutation (SHM). GC B cells either die or differentiate into memory B cells or short- or long-lived Ab-secreting plasma cells, of which the latter sustain Ag-specific Ig concentrations in the serum (14, 15). However, although increasing SHM can potentiate the affinity of the B cell receptor for the Ag, at the same time it enhances the risk of self-reactivity (16, 17). Since Ab stemming from extrafollicular responses can influence Ag in the GC (18), precisely balanced regulation of both extrafollicular help and Tfh cell function in GC is an important feature of optimal humoral responses. Thus, excesses in Tfh cells unleashed by molecular perturbations intrinsic to the T cell lineage can cause sustained breaches of tolerance and IgG2c-dependent, auto-Ab–mediated pathologies (17, 19–21).
Consistent with the importance of modulating help to B cells, another aspect of follicular CD4 T cells is that both helper and suppressive or inhibitory cells exert influences on Ab output (21–24). GC Tfh cells are defined by particularly high expression of the transcription factor BCL6, and all Tfh express PD1, CXCR5, and ICOS at their plasma membrane (2). Following restimulation of their Ag receptor, helper T cells display CD154, a stimulatory ligand for CD40 on the GC B cells (25). Several cytokines also influence the nature of T cell help and the GC, although papers with different approaches have yielded varied degrees of requirement or potential functional redundancy. Impaired formation and persistence of Tfh in T cell-specific IL-21R–deficient mice along with other findings suggest that an initial capacity to produce IL-21 can lead to an autocrine loop that stabilizes high BCL6 and the Tfh phenotype (7, 9, 26). Alternatively, IL-21 and IL-6 may be functionally redundant in some settings (8). At the same time, functional help to B cells appears to be restrained by T follicular regulatory (Tfr) cells, subsets of FoxP3+ regulatory T cells (Treg) that express CXCR5 (21–24). While the output data support inhibitory functions of regulatory cells, it is less clear whether GC can expand further in their absence (27, 28). Moreover, although IL-21 and/or IL-6 may promote follicular help, at least later after an immune challenge IL-2 restrains Tfh via STAT5 (29–31).
The activation of PI3K by ICOS is required for follicular help (32), whereas strong PI3K signaling is thought to inhibit Treg (33). Multiprotein complexes containing the serine/threonine (S/T) kinase mechanistic target of rapamycin (mTOR) are crucial effectors downstream from PI3K (34). IL-2–induced PI3K can act through mTOR complex 2 (mTORC2) and its S/T kinase target AKT to inhibit regulatory cells (34, 35). Using inactivation of Rictor in postselection T cells we showed that this essential component of mTORC2 is required for optimal Ab responses (35); this effect has been shown to involve Tfh defects (36–38). In parallel, IL-2–induced mTORC1 activity inhibited follicular help (38), whereas complete loss of function for Raptor in T cells decreased Tfh (36). The impact of this signaling branch on T cell help and on the GC is further complicated by findings indicating that mTORC1 promotes the generation and function of Tfr (39) and evidence that in some settings Tfr enhance aspects of GC function (27). As such, each of the mTOR signaling branches downstream from PI3K is well established as an important mediator of follicular help but aspects of the signaling effectors downstream from mTOR, as well as regulators of Tfh–Tfr balance, remain to be elucidated.
Intravital labeling recently revealed that most GCs have an area with hypoxic B cells that localized predominantly to the CD35+ LZ of the GC (40, 41). Pathological states commonly create hypoxia (42, 43), which also can arise during normal physiology and is a central regulator of erythroid mass (44). Accordingly, cells draw on several molecular programs to respond to localized decreases in oxygen availability, among which the hypoxia-inducible factors (HIF) 1 and 2 are prominent and implicated in mitigating risks of excessive inflammation (42, 43, 45). Both HIF-1 and -2 are heterodimeric transcription factors composed of an oxygen-dependent α subunit and a constitutively expressed β subunit (45–47). Under hypoxic conditions, the α subunit accumulates due to a decrease in the rate of proteolytic degradation, and the resulting heterodimerization of α and β subunits promotes transactivation. Of the two HIF α isoforms, HIF-1α and HIF-2α, HIF-1α is ubiquitously expressed whereas HIF-2α is more tissue- and cell type-specific and is especially expressed in lymphocytes and macrophages (47, 48). Importantly, while HIFs were discovered along with the canonical mechanism of their induction through regulation of gene transcription, mRNA translation, and oxygen-sensitive posttranslational stability, it is now recognized that they can be induced and function in oxygen-sufficient conditions—including in lymphocytes (49). Accordingly, we tested if the capacity of activated CD4 T cells to express HIF proteins impacts help to responding B cells and the ultimate qualities of Ab in humoral immunity. These analyses identified effects attributable to HIF-2 as well as HIF-1. As outlined above, it is well established that metabolic flux influences mTOR and thereby impacts the differentiation and function of cytokine-secreting CD4+ T cells, Tfh, and Tfr. Moreover, at least in CD8 T cells HIF-1α and hypoxia each influence IFN-γ production (49). Inasmuch as loss-of-function findings reported herein showed that absence of HIF altered Ab responses we further explored three issues. First, we tested if either HIF might be downstream from mTORC1 or mTORC2 (i.e., could loss of Raptor or Rictor influence steady-state HIF α-subunit stabilization in activated CD4 T cells?). Second, we investigated the relationship among HIF, hypoxia, and metabolism in responding to stimuli. Since T cell help to B cells—and GC biology—involve TCR stimulation we tested if the absence of HIFs altered metabolic output of CD4 T cells and also investigated the effects of cytokines that promote (IL-6 and IL-21) or restrain (IL-2) Tfh. Finally, based on our findings about the alterations in class-switched Ab, we tested how HIFs and hypoxia impacted output of the cytokines that regulate isotype choice by B cells.
Results
mTOR Dependence of HIF Levels in Activated CD4 T Cells.
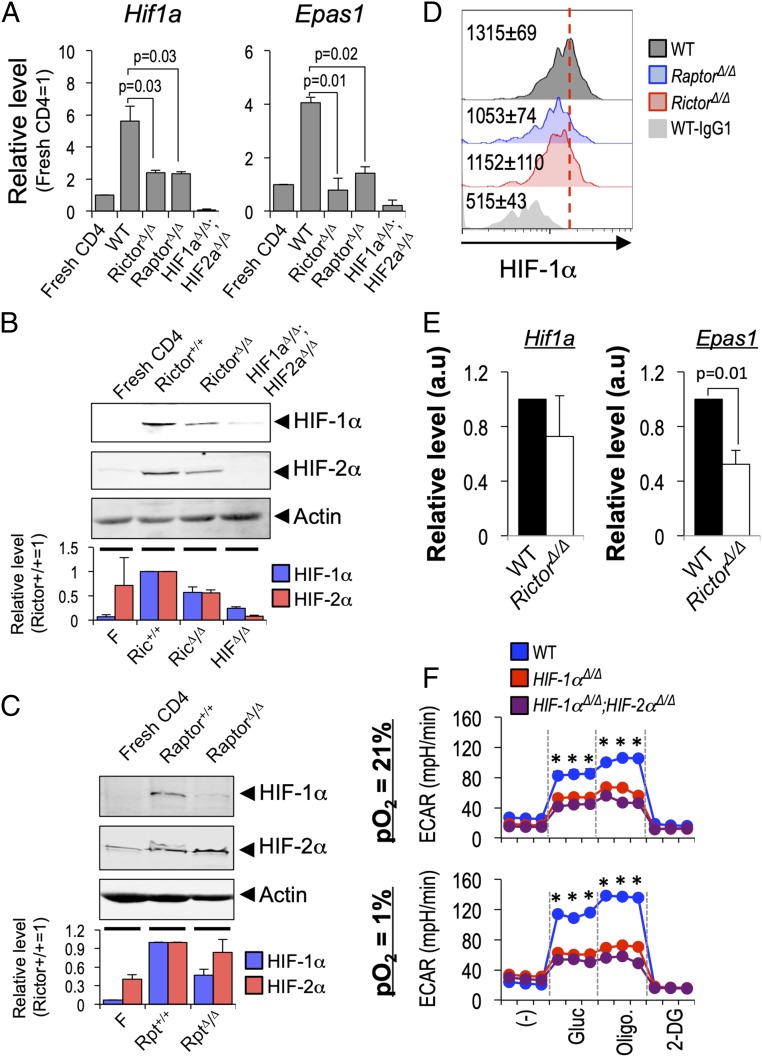
Tfh and Tfr cells are regulated by mTOR complexes 1 and 2 (36, 37, 39), which are downstream from TCR signaling complexes as well as costimulatory molecules such as ICOS, whose capacity to increase PI3K activity is essential for GC reactions (32). Based on evidence that mTORC1 can increase the level of Hif1a mRNA (50), we tested first whether the T cell and mTOR activation that are central to helping B cells might impact HIF transcription factors even in nonhypoxic conditions. To do so, we quantified how loss of Raptor or Rictor, essential subunits of mTORC1 and mTORC2, respectively, from CD4 T cells impacted each HIF α-subunit–encoding mRNA by qPCR. This analysis indicated that Hif1a and Epas1 mRNAs were regulated by mTORC1 and 2 (Fig. 1A). HIF may be induced principally via translation efficiency or posttranslational stabilization, so we tested if either mTOR complex regulates steady-state levels of HIF-1α or HIF-2α protein after activation of CD4+ T cells. Immunoblotting of Rictor- or Raptor-deficient CD4 T cells showed that each of these complexes regulates the level of HIF-1α protein induced by TCR and costimulation of these primary lymphocytes (Fig. 1 B and C). In addition, HIF-2α also was reduced in Rictor-deficient CD4 T cells compared with WT controls (Fig. 1B) but was unaffected by lack of mTORC1 (Fig. 1C).
Fig. 1.
mTOR-dependent regulation of HIF-1α and HIF-2α in CD4 T cells. (A) CD4+ T cells (WT, RictorΔ/Δ, and HIF-1αΔ/Δ; HIF-2αΔ/Δ) purified from spleens of transgenic mice activated (anti-CD3 and anti-C28) and cultured 24 h at 21% pO2 were analyzed by qPCR of their total RNA. Signals for Hif1a (HIF-1α, Left) and Epas1 (HIF-2α, Right) were first normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT), and values for the activated samples in an experiment were then expressed relative to measurements for resting CD4+ T cells (set as relative level of 1). Shown are the mean (± SEM) values for Hif1a and Epas1 for each genotype from three independent replicate samples. (B) As in A, but extracts of CD4+ T cells (WT, RictorΔ/Δ, and HIF-1αΔ/Δ; HIF-2αΔ/Δ) analyzed by immunoblotting with antibodies directed against HIF-1α, HIF-2α, and actin as internal loading control. One result representative of three replicates is shown. The bar graph below shows mean (± SEM) expression of HIF1-α and HIF-2α protein (relative to TCR-activated Rictor+/+ CD4 T cells) of quantified data from three independent experiments. (C) Immunoblots probed for HIF-1α, HIF-2α, and actin after activation of WT and Raptor-deficient CD4+ T cells as in B, with quantified data in the bar graph showing mean (± SEM) expression of HIF1-α and HIF-2α protein (relative to TCR-activated Raptor+/+ CD4 T cells) in three independent experiments. (D) PD1+ CXCR5+ CD4+ Tfh cells (WT, RptorΔ/Δ, or RictorΔ/Δ as indicated) were subjected to intracellular staining for HIF-1α or IgG negative control 1 wk after SRBC immunization. A result from one of three independent replicate experiments is shown. Inset numbers indicate mean (± SEM) geometric MFI of HIF-1α from three independent replicate experiments. Additional data from separate experiments are in SI Appendix, Fig. S1A. (E) Levels of mRNA encoded by the Hif1a1 and Epas1 genes were measured by qRT2-PCR after preparation of total RNA from Tfh cells (WT and RictorΔ/Δ) flow-purified 1 wk after SRBC-immunization. Bar graphs show mean (± SEM) expression (relative to HPRT in the sample) measured in three independent replicate preparations. (F) HIF-dependent regulation of glycolysis in CD4 T cells after TCR stimulation. Purified CD4+ T cells (WT, Hif1aΔ/Δ, and Hif1aΔ/Δ;Epas1Δ/Δ, as indicated) were cultured (2 d) after activation with αCD3 and αCD28, divided between 21% and 1% pO2, then rinsed, and replated at equal numbers at the original oxygen tensions (21% vs. 1% pO2) in the presence of soluble αCD3 (0.5 µg/mL). After 20 h, equal numbers of viable CD4+ T cells were then replated and assayed in a metabolic flux analyzer as detailed in SI Appendix, Materials and Methods. Shown for each analysis point across the time course (1.2 h in 6-min intervals, with injections after three samplings) are the mean (± SD) data for TCR-restimulated cells, as determined in biologically independent experiments, each of whose values was the mean of technical triplicates. *P < 0.05 between WT and HIF-deficient CD4 T cells. Parallel samples cultured at 21% pO2 and analyzed without TCR restimulation confirmed substantial TCR-induced increases in ECAR (SI Appendix, Fig. S1C).
GCs typically have a hypoxic microenvironment, predominantly though not exclusively in the LZ, and Tfh restimulation via display of cognate peptides in MHC-II on GC B cells is an important mechanism in Ab responses. Consistent with the observation that GC B cells have stabilized HIF-1α, intracellular staining with immunized WT mice revealed substantial signal in Tfh cells that was lower in Raptor- and Rictor-depleted samples (Fig. 1D and SI Appendix, Fig. S1 A and B). Intracellular staining of the population of CD44hi CD4+ T cells that lacked Tfh markers suggested that they include cells with HIF-1 stabilized, albeit at a lower level than the Tfh counterparts (SI Appendix, Fig. S1A). Although there were no, or very modest, decreases in Hif1a mRNA between WT and Rictor Δ/Δ Tfh cells, half the level of Epas1 mRNA encoding HIF-2α was detected in Rictor Δ/Δ Tfh (Fig. 1E). Together, these results indicate that HIF stabilization occurs in Tfh cells in vivo, with HIF levels controlled in part by mTORC1- and mTORC2-dependent mechanisms. The finding that Hif1a mRNA was not substantially reduced by Rictor depletion (Fig. 1E) despite a substantial decrease in steady-state protein (Fig. 1D) suggests that the posttranslational (and/or translational) mechanisms may control HIF-1 in Tfh-phenotype cells in vivo. As major components of the response to hypoxia, HIF transcription factors modulate the metabolic programs in a variety of cells (47). As a general rule, hypoxia increases glycolysis though not necessarily rates of extracellular acidification, and HIF-1 appears important for glycolysis in T cells (47, 49–52). The data noted above show that HIF-1 and -2 alpha subunits are stabilized due to activation of CD4+ T cells even at 21% pO2, and TCR stimulation of helper cells is important both in extrafollicular and GC help to B cells. Accordingly, we used both conventional (21%) oxygenation and hypoxic (1% pO2) conditions to culture CD4+ T cells of WT, HIF-1α–deficient, and combined HIF loss-of-function mice and perform metabolic flux analyses (Fig. 1F; also see SI Appendix, Fig. S1C). Consistent with a hypoxia-induced increase in the glycolytic program in these activated cells, the magnitude of glucose-stimulated rate of extracellular acidification (ECAR) after TCR restimulation was greater for WT CD4+ T cells in hypoxic conditions (Fig. 1F, Upper compared with Fig. 1F, Lower). The results further showed that HIF-1α alone could account for the capacity of activated CD4+ T cells to increase their ECAR after TCR restimulation (Fig. 1F), but before that restimulation the defect was more severe with compound depletion of HIF-1 and 2 than with loss only of HIF-1α (SI Appendix, Fig. S1C). Collectively, these results indicate that glucose-stimulated lactate generation after TCR restimulation was HIF-dependent and further induced during hypoxic exposure.
HIF Promotes Germinal Centers and Regulates Ag-Specific Ab Production.
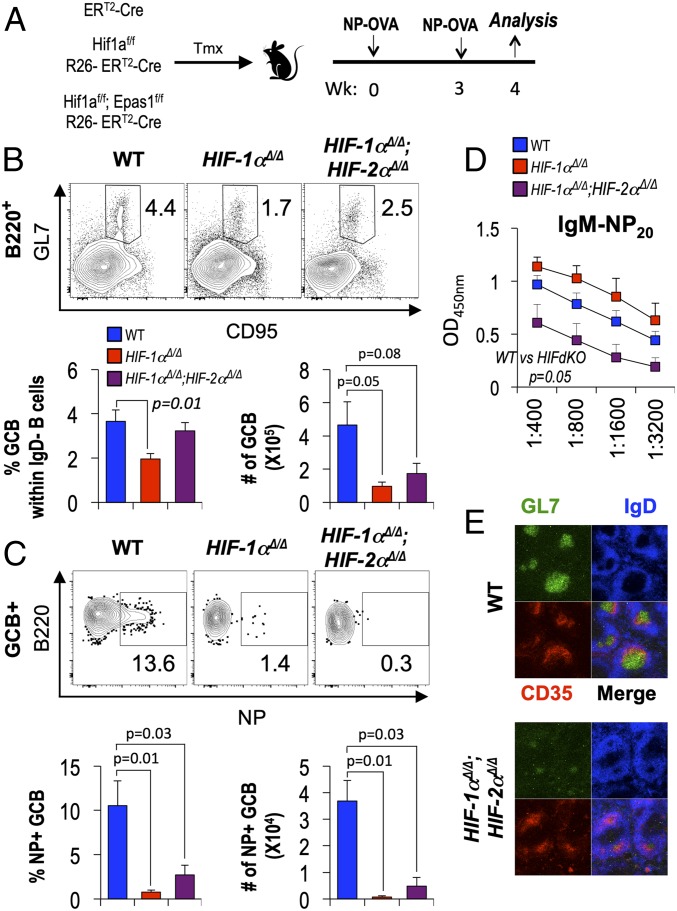
The GC hypoxia, evidence of HIF stabilization in Tfh cells, and HIF-1 dependence of TCR-stimulated metabolism prompted testing of the effect of HIF on Ab responses after immunizations. We first analyzed Ab responses after deletion of Hif1af/f and Epas1f/f conditional alleles using tamoxifen-induced activation of Rosa26-CreERT2, which drives deletion in most hematopoietic cells and is especially potent in lymphocytes. HIF-1α Δ/Δ and HIF-1α Δ/Δ, HIF-2α Δ/Δ mice were compared with controls after immunizing and boosting with hapten-conjugated ovalbumin (NP-OVA) (Fig. 2A). With widespread expression of Cre, the steady-state populations of B and CD4+ T cells B cells after immunization were halved after short-term loss of function for HIF-1α; additional HIF-2α loss either had no additional or a modest compensatory effect (SI Appendix, Fig. S1 D and E). However, the frequency of GC-phenotype B cells (GL7+ CD95+ IgDneg B220+), which depends on the effectiveness of follicular help and on intrinsic programming, was substantially reduced by the loss of HIF-1α compared with WT controls (Fig. 2B). Bystander B cells can be recruited into GC reactions, but analyses of Ag-binding cells of GC phenotype reinforced the finding that absence of HIF from B and T cells caused a substantial reduction in Ag-specific GC function (Fig. 2C). Ab levels are a key functional endpoint in humoral immunity. Recent work in an adoptive transfer model showed that persistent HIF stabilization in B cells led to differential impacts on switched isotypes (IgG2c more repressed by HIF stabilization than IgG1) while IgM was spared (40). Notably, Ag-specific IgM levels were about fourfold lower due to depletion of both HIF-1α and HIF-2α (Fig. 2D) even though loss of HIF-1α from hematopoietic cells (including B and T lymphocytes) yielded increases in hapten-specific IgM in sera after the boost (Fig. 2D). Analyses of GC visualized in the spleens of immunized mice (WT and HIF-depleted) using immunofluorescence and the Rosa26-CreERT2 system revealed that the sizes and activation marker (GL7) intensity were dramatically lower in the absence of HIF-1 and -2 (Fig. 2E and SI Appendix, Fig. S1F). Consistent with data on persistent B cell-intrinsic HIF stabilization (40), Ag-specific IgG2c was increased after impairment of both HIF-1 and -2 while IgG1 was little affected (SI Appendix, Fig. S2). This approach mimicking inhibition of HIF throughout the system does not pinpoint the contributions attributable to expression in CD4+ T cells, but collectively the results indicate that HIF plays a critical role in regulating Ag-specific Ab production and GC responses.
Fig. 2.
HIF in hematopoietic cells regulates GC and Ab responses. (A) Schema of tamoxifen (TMX)-induced, Cre-mediated inactivation of Hif1a, or Hif1a and Epas1, followed by immunization of the HIF-1α, HIF-1α, HIF-2α doubly deficient mice and WT (Rosa26-CreERT2) controls with NP-OVA 11 d after the first TMX injection. Mice were boosted with NP-OVA 3 wk after the primary immunization and analyzed (B–D) 1 wk thereafter. (B and C) HIF required for help yielding Ag-binding GC B cells. (B, Upper) GL7+ CD95+ cells in the gate for viable lymphoid-sized IgDneg B220+ splenocytes of mice of the indicated genotypes in one representative experiment of three. Inset numbers are the % of events in the indicated gate. (B, Lower) Bar graphs display mean (± SEM) fractions (percent) and numbers of GC B cells from spleens of independent samples (n = 9 WT vs. 3 HIF-1α cKO vs. 6 HIF-1α, HIF-2α dKO mice) after immunizations in the three independent experiments. (C) As in B, but representative flow plots quantify hapten (NP)-binding cells in the GL7+ CD95+ B cell gate. Additional data are in SI Appendix, Fig. S1 D and E. (D) HIF regulates Ag-specific Ab production. All-affinity (anti-NP20) IgM in sera of immunized and boosted mice that were either WT or HIF-deficient, as indicated. Shown in the bar graphs are the mean (± SEM) values across serial twofold dilutions. Anti-NP IgG1 and IgG2c responses are shown in SI Appendix, Fig. S2. Welch’s correction of unpaired t tests was used to derive P values. (E) HIF impact on GC response. After TMX injections, HIF-1α, HIF-2α doubly deficient mice (Rosa26-CreERT2) and WT controls (Rosa26-CreERT2) were immunized with SRBC and analyzed 7 d later. Shown are representative images from immunofluorescence staining of spleens with the indicated Ab in two independent experiments (6 WT vs. 5 HIF-1α, HIF-2α dKO). Quantitation of GL7-positive GC size and the intensity of GL7 in GC are shown in SI Appendix, Fig. S1F.
CD4+ Cell-Intrinsic HIF in GC and Ag-Specific Ab Responses.
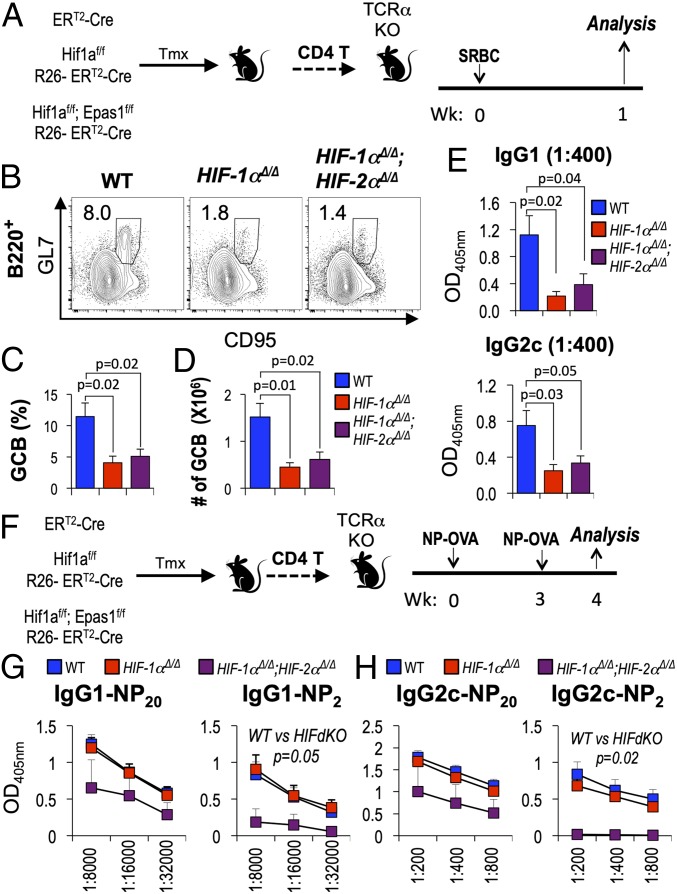
Although the findings in Fig. 2 established that HIF regulates the properties of humoral immunity, the results could not be imputed specifically to an impact on T cell help. Accordingly, we used adoptive transfers to test for CD4+ T cell-intrinsic effects of HIF on T-dependent Ab responses. To do so, we transferred WT, Hif1a Δ/Δ, or Hif1a Δ/Δ, Epas1 Δ/Δ CD4+ T cells into TCRα-deficient recipients (Fig. 3A and SI Appendix, Fig. S3A). In this setting, HIF had no effect on recovery of T or B cells overall, but immunization of the adoptive transfer recipients with sheep red blood cells (SRBC) yielded far fewer GC-phenotype B cells in recipients of Hif1a Δ/Δ, Epas1 Δ/Δ ΧΔ4+ T cells than in littermate controls that received WT CD4+ T cells (Fig. 3 B–D and SI Appendix, Fig. S3 B and C). Of note, the GC B cell population was reduced by lack of HIF-1α only (Fig. 3D), without a further decrease when both HIF-1 and -2 were inactivated. Although we found ELISA measurements of IgM anti-SRBC antibodies to lack specificity, levels of several class-switched SRBC-specific antibodies could be determined reliably. Consistent with a requirement for HIF in help to B cells, anti-SRBC IgG1 and IgG2c were dramatically reduced as a consequence of deleting either both HIF α subunits, or even HIF-1α alone, from CD4+ T cells (Fig. 3E). T cells are essential for GC formation and the development of high-affinity Ab via affinity maturation, which is promoted by repeated immunization. Accordingly, we also tested a T cell-dependent anti-hapten response that allows for determination of the high-affinity component via binding to sparse Ag (low valency of hapten in ELISA). Adoptive transfer recipients were immunized with NP-OVA in alum, rested for 3 wk, and boosted with NP-OVA in alum (Fig. 3F). Levels of NP-specific IgG1 and IgG2c Ab in sera from doubly deficient (Hif1a Δ/Δ, Epas1 Δ/Δ) CD4 T cell recipient mice were lower than those from WT CD4 T cell recipient mice (Fig. 3 G and H). In striking contrast to results with SRBC as the immunogen, lack of HIF-1α alone had no effect on IgG1 or IgG2c anti-NP in the hapten-carrier immunization experiments. Generation of Ab populations with heightened affinity for Ag derives from help by Tfh cells to the B cells before their differentiation to Ab secretion (2, 13). HIF deficiency restricted to CD4+ T cells caused substantially greater reductions of high-affinity NP-specific IgG1 and IgG2c Abs captured on low-valency Ag (NP2) than were observed for the total Ag binding immune globulins (Fig. 3 G and H). Indeed, no high-affinity anti-NP Ab could be detected for the most proinflammatory isotype, IgG2c (Fig. 3H), in the setting of prime-boost immunization with alum adjuvant. These results indicate that, depending on the nature of the immunization, both HIF-1 and HIF-2 may be required in CD4+ T cells to help affinity maturation of class-switched Ab repertoires in normal B cells. This finding also reveals an immune experience in which HIF-2α played an essential role.
Fig. 3.
CD4+ T cell-intrinsic HIF functions in help to B cells. (A) Model of CD4+ T cell adoptive transfers to assess cell-intrinsic function of HIF in humoral responses. After tamoxifen (TMX) injections, HIF-deficient CD4+ T cells or HIF-sufficient controls isolated from Rosa26-CreERT2 mice (+/+; Hif1aΔ/Δ; or Hif1aΔ/Δ, Epas1Δ/Δ as indicated) were transferred into T cell-deficient (Tcra−/−, i.e., TCRα KO) recipients that were then immunized with SRBC and analyzed 7 d later (B–E; data on overall cell numbers are shown in SI Appendix, Fig. S3). (B) HIF-1α, HIF-2α-deficient CD4 T cells provide ineffectual support to GC-phenotype B cells. Shown are plots of GL7+ CD95+ (GC-phenotype) B cells in IgDneg B220+ gate from one experiment analyzing splenocytes from immunized mice (A) as in Fig. 2B [plots from one representative experiment of four independent replications, distributing genotypes (recipients of cells = 22, WT; 17 Hif1aΔ/Δ; 19 Hif1aΔ/Δ, Epas1Δ/Δ CD4+ T cells) evenly in each replication]. Quantified mean (± SEM) data from these recipients are shown as percentages (C) and calculated total numbers (D) of GC B cells recovered from recipients of CD4+ T cells of the indicated genotypes. (E) HIF-1 promotes the isotype-switched anti-SRBC Ab response. Shown are ELISA results with mean (± SEM) signals at a linear point on each titration curve, quantitating relative concentrations of SRBC-specific IgG1 and IgG2c in sera from mice whose CD4 T cells were WT, HIF-1α-deficient, or HIF-1 α, HIF-2 α doubly deficient in four independent experiments (recipients of cells = 11, WT; 9 Hif1aΔ/Δ; 11 Hif1aΔ/Δ, Epas1Δ/Δ CD4+ T cells). (F) Transfer model for measuring CD4+ T cell-intrinsic HIF effects on anti-hapten Ab responses. Purified pools of CD4+ T cells (HIF-sufficient and –deficient as indicated) were transferred into Tcra−/− mice as in A. The recipient mice were immunized with NP-OVA, boosted with NP-OVA after 3 wk, and analyzed 1 wk thereafter. (G and H) High- (NP2) and all-affinity (NP20) anti-hapten IgG1 (G) and IgG2c (H) in sera of immunized and boosted mice that were either WT or HIF-deficient. Shown are the mean (± SEM) values across serial twofold dilutions and P values for t tests comparing deficient to control cells at two different dilutions.
HIF Regulates Tfh Numbers and the Ratio of Tfr to Tfh Cells.
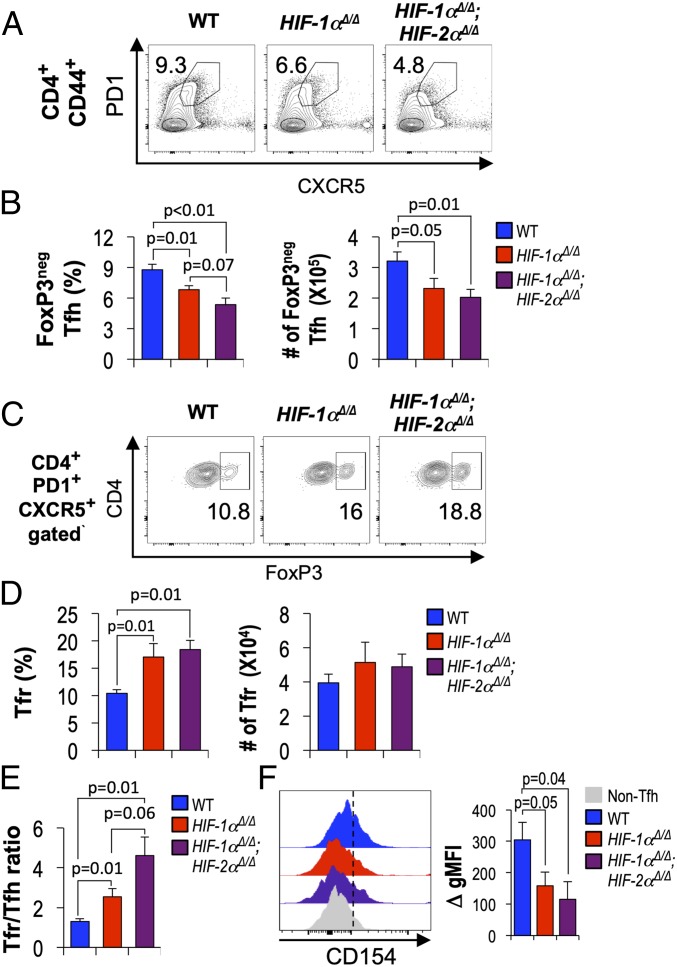
The reductions in GC B cells and affinities of class-switched Ab when CD4+ T cell help was HIF-depleted prompted us to test whether HIF regulates acquisition of the Tfh cell phenotype or the level of Tfr cells. We first investigated if the ineffective help to B cells was due to a failure to generate Tfh-phenotype cells using the recipients of WT, Hif1a Δ/Δ, or Hif1a Δ/Δ, Epas1 Δ/Δ CD4+ T cells after immunization with SRBC. This analysis showed that the prevalence and numbers of FoxP3neg PD1+ CXCR5+ CD44+ CD4+ Tfh cells were reduced by lack of HIF-1α only, with a further decrease when both HIFs were inactivated (Fig. 4 A and B). The cells of this phenotype were positive for ICOS and Bcl6 (SI Appendix, Fig. S3D) at levels higher than CD44+ that were PD1neg CXCR5neg, even in the case of HIF-deficient CD4+ T cells, although the loss of both HIFs led to reproducibly lower Bcl6 levels than those of WT controls. However, the magnitudes of decreases (Tfh numbers and Bcl6 levels) were less than the reductions observed for Ab levels, so additional mechanisms that led to the defects of help to switched and higher-affinity Ab might also be altered. HIF transcription factors inhibit the development or stability of Treg cells in certain microenvironments and conditions (50–52), and the follicular FoxP3+ CD4 T cell population includes peripherally differentiated cells as well as thymus-derived Treg (24, 53) that each can suppress Ab responses. In contrast to Tfh cells, the frequencies of FoxP3+ PD1+ CXCR5+ CD44+ CD4+ Tfr cells were higher when comparing HIF-1α–depleted or HIF-1α, HIF-2α doubly null samples to WT controls and the overall numbers did not decrease (Fig. 4 C and D). The increases, especially for HIF-1α–deficient cells, were not reflections of the donor cell populations (SI Appendix, Fig. S3A). As a result, the Tfr to Tfh cell ratio was substantially higher for the PD1+ CXCR5+ CD44+ CD4+ T cell populations lacking HIF-1α. This change appeared to be augmented further when both HIF-1 and HIF-2 were deficient, so that the average ratio of Tfr to Tfh for donor cells lacking both a subunits was almost double that of Hif1a Δ/Δ samples (Fig. 4E). Tfh cells help GC B cells through CD40 engagement and cytokine signals. Thus, interaction with CD40 ligand (CD40L) is required for the maintenance of GC reactions and the formation of memory B cells and plasma cells (2). Strikingly, analysis of the PD1+ CXCR5+ population compared with CD44+ CD4+ T cells lacking these Tfh markers revealed lower levels and induction of CD154 (CD40 ligand) on HIF-deficient cells relative to controls (Fig. 4F).
Fig. 4.
HIF regulates Tfh and Tfr balance and expression of CD154. WT and HIF-deficient CD4+ T cells were adoptively transferred into Tcra−/− mice, which were then immunized and harvested 1 wk later as in Fig. 3A. (A and B) Decreased Tfh cells in the absence of HIF (-1 α and -2 α). (A) Representative flow cytometry results for PD1+ CXCR5+ Tfh cells among CD4+ CD44hi-gated splenocytes in the viable lymphoid gate, taken from one experiment representative of five independent replications (total recipients of WT T cells = 15; 9 HIF-1α cKO; 13 HIF-1α, HIF-2α double cKO mice). Inset numbers are the percentage of events in the indicated gate. (B) Bar graphs display mean (± SEM) fractions (percent) and numbers of FoxP3neg PD1+ CXCR5+ follicular-phenotype CD4+ T cells aggregated from all recipients in the five independent experiments, along with likelihood that each null hypothesis (no difference between the genotypes being compared) is correct. (C) Representative flow plot of FoxP3+ Tfr in CD4+ PD1+ CXCR5+ cells in the experiments of A and B. Inset numbers are the percentage of events in the indicated gate. (D) Mean (± SEM) frequencies and numbers of Tfr. (E) Mean ratios of Tfr to Tfh frequencies in the five independent experiments. The FoxP3+ Tfr/FoxP3neg Tfh ratio among PD1+ CXCR5+ CD4+ CD44hi splenocytes was calculated for each individual recipient in the five independent experiments, and these results then aggregated. P values provide the likelihood that each null hypothesis (no difference between the genotypes being compared) is correct. Additional data are presented in SI Appendix, Figs. S4 and S5. (F) Representative histograms of surface CD154 on the PD1+ CXCR5+ CD4+ CD44hi (Tfh) gate along with a histogram from CD4+ CD44hi cells negative for PD1 and CXCR5 (Left). (Right) Bar graph showing increases of mean (± SEM) geometric MFI for CD154 on the PD1+ CXCR5+ CD4+ CD44hi (Tfh) gate adjusted for background. Total of nine recipient mice for each genotype of T cells, distributed equally among three biological replication experiments.
Ab responses were decreased when distal Lck-iCre was used to drive loss of T lineage-specific inactivation of Rictor (mTORC2) after the positive selection of T cells (35). Extending work indicating that Rictor is important for follicular help (36, 37) and for normal HIF-1α and HIF-2α levels (Fig. 1 and SI Appendix, Fig. S1 A and B), we found that Rictor-deficient CD4+ T cells yielded a distortion of the Tfr–Tfh ratio and defect of follicular help similar to the HIF-deficient setting including reduced Bcl6 and Il21 and increased Prdm1 and Foxp3 mRNA levels (SI Appendix, Fig. S4). HIF induction and the capacity of T cells to activate NF-κB depend on mTORC2 (35). Moreover, there are many intersections of hypoxia, HIF, and NF-κB, including NF-κB–dependent increases in Hif1a mRNA and cooperation of the two transcription factors in target gene regulation (54). Accordingly, we used CD4+ T cells from mice whose T lineage constitutively blocks both canonical and noncanonical NF-κB/Rel signaling to test if this pathway affects Tfh and Tfr balance in a manner similar to loss of Rictor or of HIF. Consistent with previous work, the recovery of CD4 T cells was dramatically lower after adoptive transfer of IκBα(DN) transgenic (Tg) CD4+ T cells compared with controls (SI Appendix, Fig. S5A). Moreover, the frequency of GC B cells was lower (SI Appendix, Fig. S5B), consistent with the known impact of this T cell type-specific superrepressor transgene on Ab responses (55). However, the frequencies of FoxP3-negative Tfh and FoxP3-positive Tfr populations were comparable in immunized recipients of IκBα(DN) (Tg) vs. WT T cells (SI Appendix, Fig. S5 C and D). These results indicate that unlike loss of mTORC2 or HIF, blockade of NF-κB in CD4+ T cells had little effect on the differentiation or recruitment of follicular T cells. Collectively, although the results indicate that Rictor and HIF in CD4+ T cells promote the GC response by coordinately regulating Tfh cell number, Tfr/Tfh balance, and CD154 expression on Tfh cells, they suggest that these effects are largely by a pathway separate from NF-κB/Rel.
Differential Requirements for HIF in Cytokine-Induced Metabolic Programming.
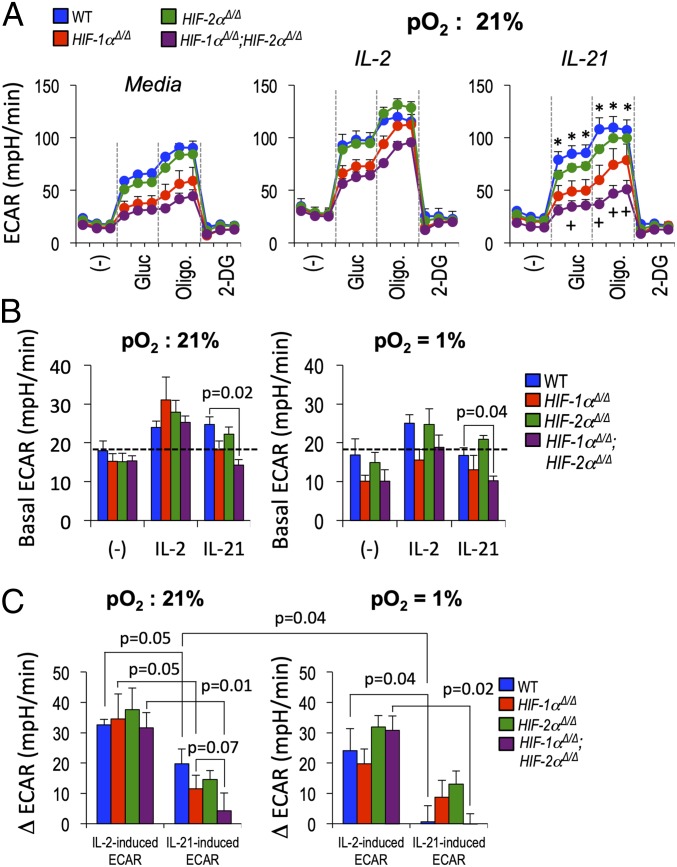
Cytokines are major determinants of T helper and Treg cell development and function. IL-2 can function as a negative regulator, whereas substantial evidence indicates that IL-21, likely with some functional redundancy, promotes optimal Tfh cell differentiation and/or persistence (7, 8, 33, 34, 56). Moreover, recent work suggests that both mTOR and glycolytic metabolism are important mediators of Tfh development (36–38), and that HIF-1 can either promote (57) or restrain (50, 52) Treg differentiation, in part by effects on glycolysis. In light of the relationships among HIF, mTORC1 and 2, and follicular CD4+ T cells revealed by our data, we tested how basic metabolic programming of activated T cells responds to these cytokines, and the impact of hypoxia and HIF on such characteristics. Notably, ECAR by cells that were not restimulated with cytokine depended on HIF-1 but not HIF-2 (Fig. 5 A and B). IL-21 was able to increase glucose-stimulated ECAR of WT activated CD4+ T cells (e.g., ∼20 mpH/min, from ∼65 to ∼85 mpH/min), but to a lesser degree than IL-2 (∼35 mpH/min, for ∼65 to ∼100) (Fig. 5 A and C).
Fig. 5.
Differential interplay of HIF and hypoxia with cytokine-induced metabolism of CD4+ T cells. (A) Purified CD4+ T cells (WT, Hif1aΔ/Δ, Epas1Δ/Δ, and Hif1aΔ/Δ;Epas1Δ/Δ, as indicated) were cultured (2 d) after activation with αCD3 and αCD28, then rinsed, replated in equal numbers, and further cultured for 20 h with and without IL-2 or IL-21 at pO2 of 21% (normoxia) or 1% (hypoxia). Equal numbers of viable CD4+ T cells were then replated and assayed in a metabolic flux analyzer as detailed in SI Appendix, Materials and Methods. Shown for each analysis point across the time course (1.2 h in 6-min intervals, with injections after three samplings) are the mean (± SEM) data determined from biologically independent experiments, each of whose values was the mean of technical triplicates. Assays of IL-6–treated T cells are shown in SI Appendix, Fig. S6C. *P < 0.05 between WT and single or doubly HIF-deficient CD4+ T cells. “+” indicates P ≤ 0.05 in comparing Hif1aΔ/Δ (red) and Hif1aΔ/Δ;Epas1Δ/Δ (purple) CD4+ T cells. (B) For the indicated genotypes and conditions, mean (± SEM) ECAR in glucose-free base medium. (C) Mean (± SEM) cytokine-stimulated changes in ECAR (Δ ECAR, derived by subtracting the mean value for technical triplicates of each of the cytokine-treated samples from the sample’s glucose-stimulated ECAR without added cytokine, and then averaging across the four independent replicate experiments). Shown are the mean (± SEM) values for the cytokine-stimulated ECAR and results of testing null hypotheses as in Fig. 4E. Additional data are given in SI Appendix, Figs. S6 and S7.
Furthermore, glucose-induced ECAR and oligomycin-induced maximum extracellular acidification capacity were reduced in IL-21–treated CD4+ T cells that lacked HIF-1α and decreased even further with depletion of both HIF-1 and HIF-2 (Fig. 5A). In contrast to the ECAR, respiration (oxygen consumption) rates were refractory to IL-21 but almost doubled by IL-2 stimulation in a HIF-dependent manner (SI Appendix, Fig. S6 A and B). Moreover, whereas inactivation of HIF-1 had no effect on IL-2-induced glycolysis, IL-21 required this transcription factor and, strikingly, was completely unable to induce increased glycolysis in the hypoxic state (Fig. 5 A and C). Although in cancer cells and other settings, HIF mediates hypoxia-induced increases in glycolysis (45), in CD8+ T cells hypoxia was recently reported to decrease ECAR (49). Similar to that report, glucose-stimulated increases in ECAR by WT CD4+ T cells were modestly attenuated by reduced oxygen (Fig. 5C). Comparing WT and Epas1 Δ/Δ or Hif1a Δ/Δ, Epas1 Δ/Δ cells also revealed that HIF-2 can contribute to regulation of glycolytic and oxidative performance. Depending on the experimental system, either IL-6 or IL-21 may directly impact Tfh cells and their help to Ab (7, 8, 56). Analyses of IL-6 effects showed again that glucose-stimulated ECAR was strongly dependent on HIF-1 (SI Appendix, Fig. S6C), but although ECAR increased slightly in hypoxic WT cells, this cytokine had even less effect than IL-21 on the metabolism of activated T cells. These in vitro findings suggested the possibility that compared with conventional effectors, activated PD-1hi CD4+ T cells with little IL-2 influence might have lower glucose uptake even if exposed to IL-6 or IL-21. However, in vitro systems that can be used for analyses of loss-of-function mutations of T cells unambiguously developing into functional Tfh are lacking. To approximate glucose uptake for follicular T cells in vivo compared with their activated counterparts lacking the Tfh markers, immunized mice received a fluorescent glucose analog, 2-NBDG. In vivo uptake and labeling with 2-NBDG was lower for Tfh-phenotype cells than the non-Tfh CD44hi population, most notably as a lower frequency of 2-NBDGhi cells (SI Appendix, Fig. S7). Together, these findings show that metabolic changes stimulated by IL-6 and IL-21 differ profoundly from those driven by IL-2, and that hypoxia or loss of HIF each reduced the lactate production but not the oxidative activity of IL-21–stimulated CD4+ lymphoblasts.
HIF Regulates Production of T Helper Cytokine in Tfh Cells.
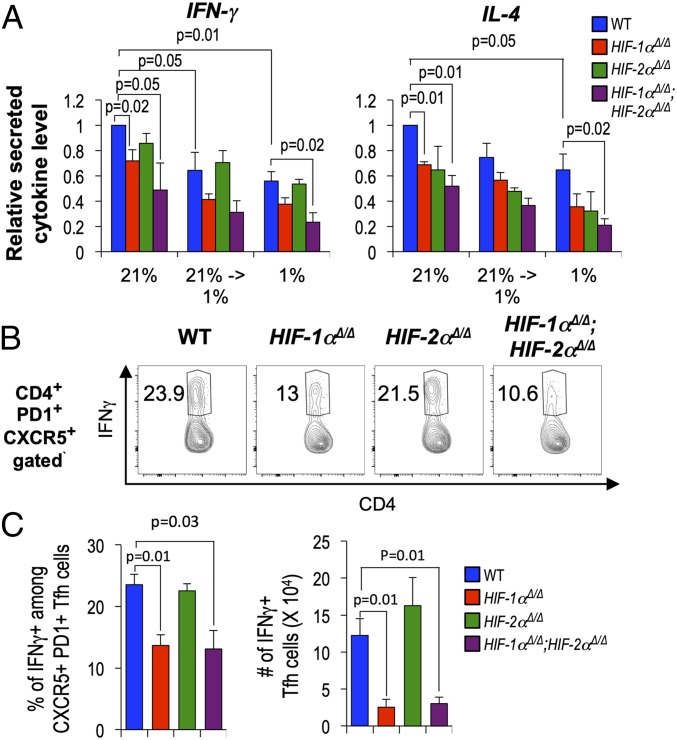
CD4+ T cell-intrinsic HIF promoted Ag-specific IgG1 and IgG2c Ab after immunization (Fig. 3). These isotypes are promoted by T helper cell secretion of the Th2 and Th1 cytokines IL-4 and IFN-γ, respectively, and deprivation of glucose to reduce glycolytic flux reduced IFN-γ production after restimulation of differentiated effector T cells (58, 59). Switching is promoted by cytokines secreted during help before formation of the GC, but Tfh cells can mimic these subset-specific programs of switch cytokine production (60, 61). While hypoxia did not alter the efficiency of Th1 or Th2 differentiation (SI Appendix, Fig. S8), a change to hypoxic conditions (1% pO2) at the point of restimulation to elicit IFN-γ or IL-4 substantially reduced production of these switch cytokines (Fig. 6A). In addition, loss of HIF—which caused reduced glycolysis—also undermined IFN-γ and IL-4 elaboration by CD4+ effectors (Fig. 6A and SI Appendix, Fig. S8). Interestingly, there was a trend in each setting toward lower secretion of these cytokines when comparing loss of both α subunits to depletion of HIF-1α alone. Moreover, intracellular staining of Tfh-phenotype cells ex vivo after immunization revealed that HIF promoted their IFN-γ production (Fig. 6 B and C). These findings indicate that in addition to increasing numbers of Tfh cells, promoting their CD154 expression, and setting the balance between Tfr-phenotype and helper cells, HIF transcription factors act in CD4+ T cells to enhance Ig class switching by activated B cells.
Fig. 6.
Oxygen and HIF promote T helper cytokine production in Tfh. (A) WT, Hif1aΔ/Δ, Epas1Δ/Δ, and Hif1aΔ/Δ, Epas1Δ/Δ CD4+ T cells activated with αCD3 and αCD28 were cultured under Th1 and Th2 differentiating conditions (5 d) at pO2 of 21% (normoxia) or pO2 of 1% (hypoxia). Secreted cytokines in culture supernatants [IFN-γ of Th1 (Left) and IL-4 of Th2 cells (Right)] were measured after restimulation (16 h) of equal numbers of viable cells with αCD3 plus αCD28 at either 21% or 1% pO2. Bar graphs show mean (± SEM) cytokine concentrations from replicates in three independent experiments and results of testing null hypotheses as in Fig. 4E. Data on frequencies of cytokine-expressing CD4+ T cells in each condition are given in SI Appendix, Fig. S8. (B and C) CD4+ T cells (WT and HIF- deficient, as indicated) were adoptively transferred into Tcra−/− recipients followed by immunization with SRBC, as in Fig. 4, and harvested 1 wk later. Splenocytes were stained for CD4, CD44, CXCR5, and PD1, restimulated (3 h) with PMA (50 ng/mL) and ionomycin (1 μg/mL), and subjected to intracellular staining for IFN-γ. Shown are representative fluorescence profiles for IFN-γ in the CD4+ PD1+ CXCR5+ (Tfh) gate (inset numbers represent the percent IFN-γ+) (B) and the mean (± SEM) frequencies and numbers of IFNγ-positive Tfh cells (C) in three independent experiments (recipients of cells = 6 each for WT; Hif1aΔ/Δ; Epas1Δ/Δ; and Hif1aΔ/Δ, Epas1Δ/Δ CD4+ T cells). Welch’s correction of unpaired t test was used to calculate P values.
Discussion
Although there are T-independent types of Ab response, T cell help is crucial for adaptive aspects of humoral immunity. In addition to extrafollicular generation of Ab-secreting plasma cells, major aspects of T cell help including affinity maturation involve the generation, function, and balance of follicular T lymphocytes after initiation of an immune response by Ag, followed after several (∼3.5) days by the organization of GCs in which Tfh and GC B cells interact. The stimulation of GC B cells by Tfh cells occurs predominantly in a hypoxic LZ, in which Tfh cells are restimulated through their surface Ab molecule, the TCR (2, 13). We have shown here that the one major mechanism by which cells adjust to hypoxic conditions—the induction of αβ heterodimers of HIF transcription factors—is essential for normal Ag-specific Ab responses. The findings indicate that in addition to HIF-1, HIF-2α can play a substantial role, for example in a boosted hapten-carrier immunization. Mechanistically, the data indicated that HIF regulates at least three aspects of the CD4+ T cell populations: (i) promotion of CD154 expression on the surface of Tfh cells, which is crucial for stimulation of CD40 on GCB cells (62), (ii) amounts of switch cytokine secreted by differentiated helper cells after restimulation, and (iii) the metabolic programming of activated T cells, in the basal state, or responding to TCR restimulation or cytokines implicated in GC regulation. This latter analysis highlighted striking distinctions between IL-2–, IL-6–, and IL-21–stimulated metabolism.
The data herein show that hypoxia reduced cytokine production upon restimulation of differentiated CD4+ T cells in parallel with the altered metabolic programming and that HIF similarly promoted the capacity of activated CD4 T cells to deliver switch cytokine. Ground-breaking work in Treg cells indicated that HIF-1 promoted Ifng gene expression in the FoxP3+ subset (51). Recent work has presented evidence that although isotype-switched plasmablast production requires CD40–CD40L interaction provided by cognate T cell help, this interaction can be provided by more conventional helper subsets in the absence of GC-Tfh (25). Thus, in our analyses helper cell phenotypes in addition to Tfh cell may also contribute to the isotype-switched Ab data.
Our findings place HIF protein levels downstream from mTOR in activated CD4 T cells in vitro and in vivo and include evidence that TCR-stimulated metabolism is HIF-dependent. Recent work has highlighted that metabolic flux and activity of the glycolytic pathway, along with the activity of mTORC1, are meaningful regulators of Tfh and Tfr (39). Hypoxia can partially inhibit mTORC1 by a HIF-dependent mechanism, but prior work on mTORC1 in Tfh biology poses an apparent conundrum. IL-2–stimulated mTORC1 was implicated as contributing to the IL-2 inhibition of follicular help in the context of virus infection, an effect mitigated by reducing the essential structure component Raptor (38). Conversely, analyses of homozygous Rptor Δ/Δ T cells provided evidence of substantial defects of follicular help when the CD4+ population was completely devoid of mTORC1 (37). The apparent divergence in these reports likely reflects a complex activity–response relationship for mTORC1 in which a decrease can either promote or inhibit net follicular help depending on factors such as the balance between IL-2 and pro-Tfh cytokines in the local microenvironment. Although there is no means to quantitate mTORC1 activity in situ in a way that relates it to the level in other work, collectively the results suggest the possibility that inhibition of this signaling complex by LZ hypoxia may have an effect overridden by direct HIF requirements in follicular CD4+ T cells.
The generation of class-switched antibodies against the diverse antigens of SRBC, or the high-affinity anti-NP class-switched response, was severely impaired when CD4+ T cells lacked HIF-1α, or both HIF-2α and -1α, respectively. One feature of the HIF-based branch among cellular hypoxia-response mechanisms is that stabilization of HIF-1α and -2α can occur despite oxygen tensions of 140 to 150 Torr (pO2 of 21%, i.e., atmospheric) around the responding cell. Our data indicate that the impact of HIF loss in relation to IL-6– or IL-21–induced metabolic programs differs from the effect on IL-2 (Tfh-inhibitory) metabolism, which was refractory to HIF loss at both oxygen tensions in vitro. Although the in vitro systems do not lead to functional Tfh-like T cells, this finding suggests that responses of activated T cells to key cytokines in situ may be HIF-dependent in both hypoxia and normoxia. HIF also was notable in regards to the finding that the prevalence of FoxP3+ follicular T cells (Tfr) increased in the absence of HIF-1α even as Tfh and CD154 were decreasing. Moreover, the ratio of Tfr to Tfh cells appeared to increase even more with depletion of both HIF-1 and HIF-2 than with inactivation only of Hif1a. Substantial work provided evidence that the balance between Tfh and Tfr cells along with their actual numbers can modulate GC output, although acting early but possibly not by changing GC size (27, 63). The selective expansion of the Tfr population in the presence of a stable Tfh pool in vitro resulted in greater B cell suppression (63). Conversely, a Tfh bias over Tfr cells in vivo was associated with a hyperactivity of autoimmune-associated GCs (64). Recent work using Treg-specific inactivation of Bcl6 may insert a caveat as there were conditions in which conversion of Tfr-phenotype cells and Treg-derived IL-10 could promote the GC reaction (27). Notwithstanding this latter point, the weight of evidence is that on balance FoxP3+ CXCR5+ CD4+ T cells reduce GC function and Ab output, so it is likely that the threefold increase in Tfr relative to Tfh deriving from HIF-1– and -2–deficient CD4+ T cells contributed to the lower Ab responses and weakened affinity.
This ratio is also pertinent to consideration of the finding that, in the setting of primary and booster immunization with an alum adjuvant, the lack of HIF-1 in CD4+ T cells had little or no effect whereas Ab outputs that included affinity maturation were substantially decreased by the combined loss of HIF-1 and HIF-2. At least two mutually compatible models may account for this evidence of a role for HIF-2. In one model, HIF-1 still has potent function but cellular programming changed (e.g., different adjuvant, longer time, and secondary immunization) so that HIF-2 is better able to substitute HIF-1. In line with this possibility, comparisons of HIF-1 loss of function to doubly deficient cells included an almost twofold change in Tfr/Tfh ratio, lower frequencies of Tfh cells, greater changes in some metabolic characteristics of activated CD4+ T cells, and even modest trends in the CD154 and BCL6 of Tfh cells. These observations may not provide the full explanation in all circumstances, however, and an alternative model places greater emphasis on HIF-2. Intriguingly, HIF-2α can be induced or stabilized by IL-4 (47, 65, 66). Alum can elicit a burst of IL-4 and prime greater type 2 immunity (67). In addition, there is evidence of a transition from IL-21–producing to IL-4–producing Tfh cells over time in a GC response (68). In this model, HIF-1α may be necessary for proper help in the primary and SRBC immunization, whereas with time and in a secondary immunization model, HIF-2α—perhaps induced by IL-4—may exercise a major function in high-affinity Ab production. While later analyses will be needed to test models such as these, the key findings are that (i) the precise impact of the different HIFs in provision of help to B cells can depend on the exact nature of the immunization and (ii) there are settings in which HIF-2 makes a critical contribution to Ab output.
A cluster of papers investigated how HIF-1 may affect the induction of Foxp3 gene expression in naïve CD4+ T cells to generate peripheral Treg (pTreg or iTreg) cells (50, 52). While reporting that Hif1a deletion either increased or decreased pTreg induction, a key finding in addressing the apparent paradox was that the oxygen tension affected FoxP3 induction, so that the CD4+ T cell response to HIF-1 depended on the environment (50, 52, 57). Moreover, HIF-1α could either enhance FoxP3 expression through direct binding to the Foxp3 promoter region or promote the degradation of FoxP3 protein, depending on the availability of cytokine TGF-β (52). Impacts of persistent HIF stabilization via Vhl deletion in FoxP3-expressing CD4+ cells have also been investigated. Treg-restricted elimination of pVHL caused instability of the regulator phenotype and aggressive inflammation by a HIF-1–dependent mechanism, so that loss of HIF repressed IFN-γ expression and restored the suppressive properties of CD4+ T cells (51). All together, these published results are consistent with a model in which HIF stabilization by LZ hypoxia would attenuate the suppressive potency of follicular regulators, so that Hif1a inactivation would reduce Ag-specific GC B cells and Ab responses, as observed here.
Notably for consideration of our results with ubiquitous versus CD4 T cell-restricted gene inactivation, loss of function for Vhl in B cells decreased IgG2c in a HIF-dependent process while sparing IgM (40). Thus, our results with ubiquitous (Rosa26-CreERT2) HIF depletion suggest that these transcription factors in B cells regulate the properties of the Ab response, including the balance among different isotypes, in a setting where pVHL is present. Our findings also reveal that hypoxia and the presence or absence of HIF in CD4+ T cells, including those of Tfh phenotype in situ, modulate the cytokine production of differentiated T cells. This effect harmonizes with the observed reductions in IgG1 and IgG2c Ab response in mice whose CD4+ T cells were HIF-deficient. Although focusing on FoxP3 and Th17/Treg balance, previous analyses of T helper subsets had touched on Th1 and Th2 subsets but only measured the effect of HIF-1 on differentiation (frequency of cytokine-producing cells) but not the amounts of IFN-γ or IL-4 produced by the cells (50, 52). As such, our findings are in accord with the previous data but extend the observations by finding reduced secreted cytokine upon secondary stimulation and reduced IFN-γ on restimulation of ex vivo Tfh cells. These effects are similar to those recently reported for CD8+ T cells (49). Since glucose-stimulated lactate production of IL-21–treated CD4+ T cells required HIF, this latter effect is similar to work showing that reducing glucose intake lowered the IFN-γ production of CD4 T cells (58). All together, then, our findings provide evidence that HIF and hypoxia influence CD4+ T cell provision of effector cytokines in guidance to class switching, in part through mediation of cytokine-specific metabolic programs in the T cell help to humoral immunity. It is notable that localized hypoxia in tissues is a common feature of inflammatory diseases such as atherosclerosis, rheumatoid arthritis, viral infection, and cancer (69). Accordingly, the findings may have broader implications for Th1 cytokine production in pathophysiology.
Materials and Methods
Reagents; sourcing; mouse strains and usage; flow cytometry; measurements of humoral responses, RNA, and proteins; metabolic flux analyses; and statistical methods are detailed in SI Appendix, Materials and Methods, and all are based on previous published work. All conclusions stated in Results are based on biologically independent replications with appropriate statistical analyses. All animal experiments were completed according to Vanderbilt University Medical Center Institutional Animal Care and Use committee.
Supplementary Material
Acknowledgments
We thank R. Ramiscal and C. Vinuesa for open discussions and suggestions and P. Basso, J. A. Hamilton, and S. K. McLetchie for critical readings of the revised manuscript. V.H.H. holds the Krick-Brooks chair in Nephrology at Vanderbilt University and is supported by NIH Grants DK101791 and DK081646 and Department of Veterans Affairs Merit Award BX002348. This work was supported by NIH Grants R01 AI113292 and R01 HL106812 (to M.R.B.) and benefited from NIH Shared Instrumentation Grant 1S10OD018015, institutional cores, and scholarships from Cancer Center Support Grant CA068485 and Diabetes Research Center Grant DK0205930 to help defray costs of Vanderbilt Cores.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811702116/-/DCSupplemental.
References
- 1.Paus D, et al. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 3.Shulman Z, et al. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345:1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu D, et al. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015;517:214–218. doi: 10.1038/nature13803. [DOI] [PubMed] [Google Scholar]
- 5.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–1711. [PubMed] [Google Scholar]
- 6.Zhu J, Paul WE. CD4 T cells: Fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eto D, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dienz O, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu D, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida T, et al. Memory B and memory plasma cells. Immunol Rev. 2010;237:117–139. doi: 10.1111/j.1600-065X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 15.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 16.Guo W, et al. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boothby M, Rickert RC. Metabolic regulation of the immune humoral response. Immunity. 2017;46:743–755. doi: 10.1016/j.immuni.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SK, et al. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Domeier PP, et al. IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J Exp Med. 2016;213:715–732. doi: 10.1084/jem.20151722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aloulou M, et al. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat Commun. 2016;7:10579. doi: 10.1038/ncomms10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotov JA, Jenkins MK. Cutting edge: T cell-dependent plasmablasts form in the absence of single differentiated CD4+ T cell subsets. J Immunol. 2019;202:401–405. doi: 10.4049/jimmunol.1801349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 27.Xie MM, Dent AL. Unexpected help: Follicular regulatory T cells in the germinal center. Front Immunol. 2018;9:1536. doi: 10.3389/fimmu.2018.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu W, et al. Deficiency in T follicular regulatory cells promotes autoimmunity. J Exp Med. 2018;215:815–825. doi: 10.1084/jem.20170901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Nurieva RI, Dong C. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunol Rev. 2013;252:139–145. doi: 10.1111/imr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballesteros-Tato A, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gigoux M, et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2009;106:20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh PT, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boothby M. Signaling in T cells–Is anything the m(a)TOR with the picture(s)? F1000 Res. 2016;5:191. doi: 10.12688/f1000research.7027.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng H, et al. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity. 2016;45:540–554. doi: 10.1016/j.immuni.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, et al. Critical roles of mTOR complex 1 and 2 for T follicular helper cell differentiation and germinal center responses. eLife. 2016;5:e17936. doi: 10.7554/eLife.17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray JP, et al. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity. 2015;43:690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, et al. The kinase mTORC1 promotes the generation and suppressive function of follicular regulatory T cells. Immunity. 2017;47:538–551.e5. doi: 10.1016/j.immuni.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Cho SH, et al. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature. 2016;537:234–238. doi: 10.1038/nature19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jellusova J, et al. Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol. 2017;18:303–312. doi: 10.1038/ni.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor CT, Doherty G, Fallon PG, Cummins EP. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J Clin Invest. 2016;126:3716–3724. doi: 10.1172/JCI84433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Davidoff O, Niss K, Haase VH. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest. 2012;122:4635–4644. doi: 10.1172/JCI63924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 47.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talks KL, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palazon A, et al. An HIF-1alpha/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell. 2017;32:669–683.e5. doi: 10.1016/j.ccell.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JH, Elly C, Park Y, Liu YC. E3 ubiquitin ligase VHL regulates hypoxia-inducible factor-1α to maintain regulatory T cell stability and suppressive capacity. Immunity. 2015;42:1062–1074. doi: 10.1016/j.immuni.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016;283:413–424. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aronica MA, et al. Preferential role for NF-kappa B/Rel signaling in the type 1 but not type 2 T cell-dependent immune response in vivo. J Immunol. 1999;163:5116–5124. [PubMed] [Google Scholar]
- 56.Zotos D, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clambey ET, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109:E2784–E2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho PC, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang CH, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 62.Han S, et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 63.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim YU, Lim H, Jung HE, Wetsel RA, Chung Y. Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells. PLoS One. 2015;10:e0120294. doi: 10.1371/journal.pone.0120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takeda N, et al. Differential activation and antagonistic function of HIF-alpha isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finlay DK, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brewer JM, et al. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–6454. [PubMed] [Google Scholar]
- 68.Weinstein JS, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17:1197–1205. doi: 10.1038/ni.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.