Significance
Across a wide range of animals, it is assumed that leading from the front of a group exposes individuals to greater predation risk, generating a cost that explains variation between individuals in their tendencies to lead and follow. Remarkably, there is scant empirical evidence to support this, and there are no experimental tests. By presenting real predators with a simulation of collective behavior, we were able to exclude any correlation between social behavior and other traits that could confound effects on predation risk. We show that virtual prey leading others were preferentially attacked, but leaders were still safer than solitary prey. Leaders benefit from being followed, and they should act to maintain group cohesion and avoid splitting from their followers.
Keywords: spatial position, followers, predation, collective behavior, virtual prey
Abstract
A long-standing assumption in social behavior is that leadership incurs costs as well as benefits, and this tradeoff can result in diversified social roles in groups. The major cost of leadership in moving animal groups is assumed to be predation, with individuals leading from the front of groups being targeted more often by predators. Nevertheless, empirical evidence for this is limited, and experimental tests are entirely lacking. To avoid confounding effects associated with observational studies, we presented a simulation of virtual prey to real fish predators to directly assess the predation cost of leadership. Prey leading others are at greater risk than those in the middle of groups, confirming that any benefits of leading may be offset by predation costs. Importantly, however, followers confer a net safety benefit to leaders, as prey leading others were less likely to be attacked compared with solitary prey. We also find that the predators preferentially attacked when solitary individuals were more frequent, but this effect was relatively weak compared with the preference for attacking solitary prey during an attack. Using virtual prey, where the appearance and behavior of the prey can be manipulated and controlled exactly, we reveal a hierarchy of risk from solitary to leading to following social strategies. Our results suggest that goal-orientated individuals (i.e., potential leaders) are under selective pressure to maintain group cohesion, favoring effective leadership rather than group fragmentation. Our results have significant implications for understanding the evolution and maintenance of different social roles in groups.
Understanding the origins and maintenance of different social roles is one of the key objectives in the study of animal behavior (1, 2). A particularly important social role is leadership, where a single or few individuals disproportionately determine the timing, movements, and activities of groups (3, 4). In self-organized groups, such as fish shoals and bird flocks, leadership frequently occurs from the front of the group (5, 6). Due to this spatial arrangement, leading individuals often have greater access to encountered resources (7) and can make decisions that benefit themselves at a cost to their followers (8). Followers, however, can benefit from being led by more informed individuals without having to privately sample or detect information in the environment that can be costly to acquire (9, 10).
While there are clear benefits to leading, this strategy is widely assumed to be inherently riskier than following, presumably because the front of moving groups could encounter predators first or because individuals in lead positions are easier to target. This spatial pattern of predation risk within groups has been assumed to apply to groups of animals as diverse as monkeys (11), meerkats (12), coatis (13), muskoxen (14), starlings (15), and guppies (16). However, despite the importance of this assumption for our understanding of the diversity of social strategies, no experimental (i.e., manipulative) tests have been performed to identify whether the risk of leadership can be separated from other confounding factors. For example, although there are observational studies finding that individuals at the front of groups are disproportionally attacked (17, 18), individuals in these groups determined their own positions through self-organization, and leadership and followership covaried with other individual attributes. As the tendency to lead is often associated with goal orientation driven by greater information (19) or greater need (10), individuals that are less risk averse [i.e., bold (20)], less sociable (21), hungrier (22), or larger (7) are more likely to occupy positions at the front of the group. These individual traits can result in an increased risk of predation even when individuals are alone, and leading others can be cognitively demanding (23), which may further increase risk for leaders through reduced vigilance for predators. As such, the limited empirical findings demonstrating a greater predation risk at the front of real prey groups may be due to correlates associated with frontal positions rather than frontal positions themselves being inherently riskier (17, 18). A direct test of whether leadership results in greater predation risk, therefore, is lacking, despite the prominence of this assumption across the literature.
While leadership could be a riskier strategy than following, it remains unknown whether it is a safer strategy than departing the group and acting alone. While there are a number of mechanisms that reduce the individual risk of predation in groups (24), larger groups are also more conspicuous and can be preferentially attacked over smaller groups and solitary individuals (25). If leaders are disproportionately attacked within groups, it may be safer for those individuals to depart the group than remain with it. To do this, individuals could change their reliance on goal-orientated over socially oriented behavior, with individuals departing groups when goal orientation becomes strong enough (19). Alternatively, if followers confer a safety benefit to leaders, leaders may be under pressure to remain with the group, even if their relative predation risk is higher than that of their followers.
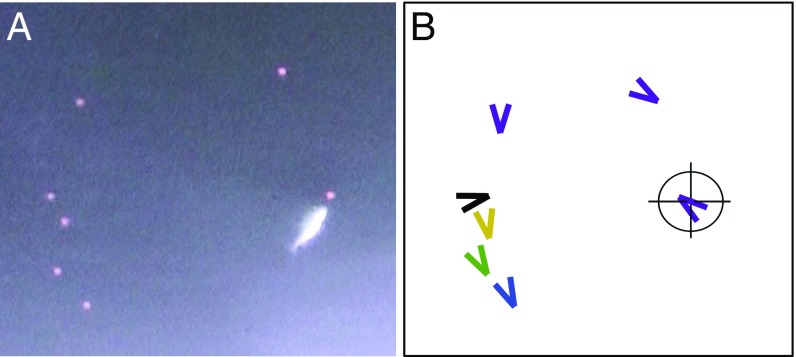
The lack of experimental evidence demonstrating the cost and potentially, the benefit of leadership in terms of predation risk is partly due to the difficulty in manipulating social behavior in real animals. Here, we experimentally test the risk of predation on leaders using virtual prey (26, 27), allowing for precise control of prey behavior and the elimination of confounding factors that could make particular individuals disproportionally targeted. To generate variation in prey social behavior as it appears to the predator, we programmed individuals in a simulation model of collective motion to act as “leaders” (no social tendency but can be followed by others), “followers” (have a social tendency to be attracted to other prey), or “asocials” (no social tendency and cannot be followed by others) (Materials and Methods and Movie S1). At any one time, the simulations frequently included solitary individuals, those leading groups, and those following others (SI Appendix, Fig. S1). We then presented these 2D simulated prey to individual predators [three-spined sticklebacks (Gasterosteus aculeatus)], which subsequently approached and attacked individually selected prey from the third dimension (Fig. 1 and SI Appendix, Fig. S2).
Fig. 1.
The experimental setup. (A) Screenshot of a stickleback attacking a virtual prey in one of the trials. (B) The position and direction of the virtual prey colored by their classifications. Purple indicates solitary (no prey within the threshold distance) (SI Appendix, Fig. S3), black indicates unaligned (neighbors of the focal individual are within the threshold distance but are not heading within of the focal’s heading), blue indicates leading [other neighbors are within the threshold distance from the focal individual and heading in the same direction (within ), but no neighbor has a bearing < (i.e., in front of the focal individual)], green indicates middle (the focal individual has neighbors in front and behind itself within the threshold distance, and neighbors’ headings are within of the focal’s heading), and yellow indicates trailing [the focal individual has neighbors that are within the threshold distance and heading in the same direction (within ), but no neighbor has a bearing > (i.e., behind the focal individual)]. The heading of the prey is indicated by the direction of the arrow. Cross-hairs highlight the prey that was targeted in A.
Results
In total, 133 of 201 fish attacked a virtual prey at least once during the 10-min trials. Only the first attack in each trial was analyzed to minimize behavioral artefacts of the predators not being able to consume the prey (26, 27). The coordinates of each prey at each attack were identified from the corresponding seed and time step in the simulation. These coordinates were first used to classify whether each prey was solitary (classified when no other prey were within a threshold distance) (SI Appendix, Fig. S3) or within a group. Individual prey was also given the positional classifications of (i) solitary, (ii) with the group but unaligned with the group’s direction, (iii) leading the group, (iv) trailing the group, or (v) in the middle of the group (Fig. 1 and SI Appendix, Fig. S4). For each prey, we also determined two social factors that could affect individual predation risk. These were the number of other prey that were within a threshold distance of an individual (as a measure of local neighbor density) and the total size of the group that they were in.
To determine which prey behaviors were important to predation risk, we compared eight binomial generalized linear models that predicted which prey was attacked in each trial as a function of different explanatory variables (Table 1). Based on a difference in the AICc (Akaike information criterion corrected for small sample sizes) of greater than two units [indicating strong support of one model over another (28)], the model with prey type (asocial, leader, or follower) as the explanatory variable was more likely given the data than the null model without any explanatory factors. A simpler model with fewer parameters that classified prey as either having attraction (the follower type) or not (the leader and asocial types) had similar support, suggesting that the leader and asocial types experienced similar levels of predation risk. In contrast, a model that reclassified the leader and follower types together was not as well supported, indicating that per capita predation risk varied significantly between the leader and follower types. However, models that considered the preys’ behavior at the time of attack rather than differences in how prey were programmed (the prey type) were much more likely than the models with prey type as the explanatory variable (Table 1). This confirms our expectation that, although the programmed prey type resulted in behavioral differences, it was the arising behavioral differences that actually determined predation risk.
Table 1.
The AICc for models explaining per capita risk for virtual prey
| Explanatory variable | AICc | df |
| Position | 0 | 5 |
| Number of near neighbors | 6.6 | 2 |
| Whether solitary or not | 13.5 | 2 |
| Total group size | 18.2 | 2 |
| Prey with attraction | 29.6 | 2 |
| Prey type | 31.2 | 3 |
| Prey appears social | 37.2 | 3 |
| Null model (no explanatory variables) | 49.7 | 1 |
Each prey at the time of each attack was included in the data, and the response variable was whether each prey was the attacked prey (one) or not (zero). Different models have different explanatory variables. The null model has no explanatory variable. The prey-type models use which of the three prey types a prey is (asocial, leader, and follower) or reclassifications of these: attraction or not (follower type vs. the asocial and leader types as a single category) and social or not (the follower and leader types as a single category vs. the asocial type). The remaining models consider the behavior of each prey at the moment of attack. The group size model uses the total number of prey in a prey’s group as the explanatory variable, the solitary model uses a binary explanatory variable of whether each prey was solitary or not, and the near-neighbors model uses the number of prey within the threshold distance around each prey as the explanatory. Only the prey position model considers the relative spatial location within a group for each prey based on the heading difference and bearing of other prey in addition to whether prey were solitary.
In agreement with previous studies (24), having near neighbors or being part of a larger overall group reduced the per capita risk of being targeted. However, the model with prey position (solitary, grouped but unaligned, leader, middle, or trailing) as the explanatory variable was more likely than the models that consider only the proximity of neighbors (i.e., models with group size, the number of near neighbors, or whether prey were solitary). This model selection approach demonstrates that the additional behavioral parameters quantifying relative positions of individuals within groups (based on heading and bearing angles to neighbors) is also important for determining predation risk rather than simply proximity to other prey. Interestingly, the next most likely model takes into account only the number of prey around each prey and does not take into account the total size of the group that prey belongs to, suggesting that this local measure of prey density is more important than the total group size (a more global measure) in determining which prey is attacked (25).
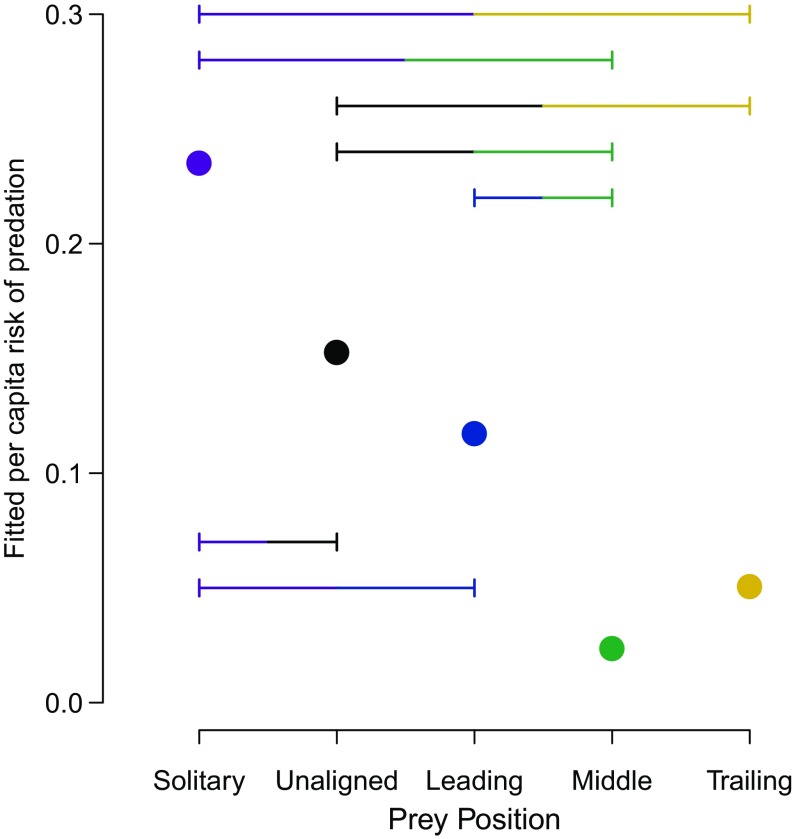
Compared with all other prey positions, prey were most at risk when solitary, supporting previous work that odd prey are at greater risk of being targeted (29, 30). Importantly, this demonstrates that leading others actually reduces risk compared with leaving the group and becoming solitary (Fig. 2). Being in a group in any position, even when unaligned to neighbors (i.e., moving in a different direction), gave some safety relative to being solitary. Within groups of individuals traveling in the same direction (i.e., aligned groups), there was a hierarchy of risk from leading to trailing to the middle positions in the group, confirming that leading is a riskier behavior than other positions within the group. Although there was a tendency for trailing prey to be at greater risk from attack than prey in the middle of groups, this difference was not more than expected by chance in comparison with a randomly targeting predator (Fig. 2).
Fig. 2.
Predation risk of different prey positions. The fitted probability of attack per capita from the observed data is shown by the circles. Horizontal bars indicate which differences in risk between pairs are greater than expected from a predator that attacks prey at random at the same instances in the simulation as the observed attacks (SI Appendix, Table S1).
As with many simulations of collective behavior, the prey simulation was stochastic, causing prey behavior to change over time as they joined and left groups or changed position within a group. There were thus variable numbers of prey performing different behaviors at each attack (e.g., prey in a large vs. small group or solitary vs. leading) (SI Appendix, Fig. S1). To account for this variability and nonindependence (for example, there has to be a leading individual if there is a trailing individual), we developed a randomization test that simulated a random predator that selected a prey in each attack with equal probability (SI Appendix, Table S1). This approach is analogous to that used within social network analysis, where randomization tests are needed to account for the interdependency of individuals’ social network positions (31). Even accounting for such nonindependence, the model with prey position as an explanatory factor remained the most likely model given the data (SI Appendix, Table S1) [the difference in the AICc between the model of prey positions and the next most likely model (difference in AICc = 6.6) is greater than the 97.5% percentile from the randomization (4.1)].
To determine the mechanism by which the predators preferentially selected a solitary and to a lesser extent, leading, prey, we analyzed the risk of being targeted as a function of the distance, heading difference, and bearing to the preys’ nearest neighbor in front (from all prey with a bearing <) and for the nearest neighbor behind (from all prey with a bearing >). Of these six explanatory variables (SI Appendix, Fig. S5), the distance to the nearest neighbor in front of a prey had the greatest explanatory power in predicting predation risk followed by the distance to the nearest neighbor behind a prey and the heading difference to the nearest prey ahead (SI Appendix, Tables S2 and S3). The most likely model given the data included all three of these variables, which outperformed models where each of these variables was included alone. Being targeted by the predators was minimized by having a neighbor close ahead and behind (i.e., being in the middle of a group) and heading in a similar direction to the prey in front (SI Appendix, Fig. S6). The bearing of these neighbors (i.e., whether they were directly ahead and behind or closer to being side by side) did not seem to affect predation risk. Thus, simply having another prey close ahead and behind, regardless of the bearing of these neighbors, is enough to minimize risk for following prey.
These results demonstrate that the predator’s choice of which prey to attack was not random, with the risk faced by prey being determined by the proximity, difference in heading, and bearing of nearby neighbors. However, predators not only may decide which prey to attack based on the available options at a particular moment but also may decide when to attack based on how groups or individuals are configured. To determine whether the timing of attacks was affected by the frequency of the different prey positions or whether attacks effectively occurred at random times, we analyzed whether the different prey positions at the instant of attacks were more or less frequent than expected from the simulation. The simulation was regularly sampled every 100 time steps using the same seeds as in the experiments and over the same range of time steps (yielding 19,324 samples from the simulation). Compared with the other prey positions, there was less variance over time in the number of leading and trailing prey (SI Appendix, Fig. S1) and a close match between the frequencies of these prey positions during the attacks and across the simulation overall (randomization test: leading: P = 0.98; trailing: P = 0.99). Solitary prey were more frequent when attacks were made compared with the simulation (randomization test: P = 0.017). This corresponded to there being fewer prey in the middle and unaligned positions at the instances of the attacks (randomization test: middle: P = 0.12; unaligned: P = 0.19; middle and unaligned positions combined into a single category and the analysis repeated: P = 0.011). Thus, the predators preferentially targeted the prey at instances in the simulation when middle and unaligned prey were less frequent but when solitary prey were more frequent.
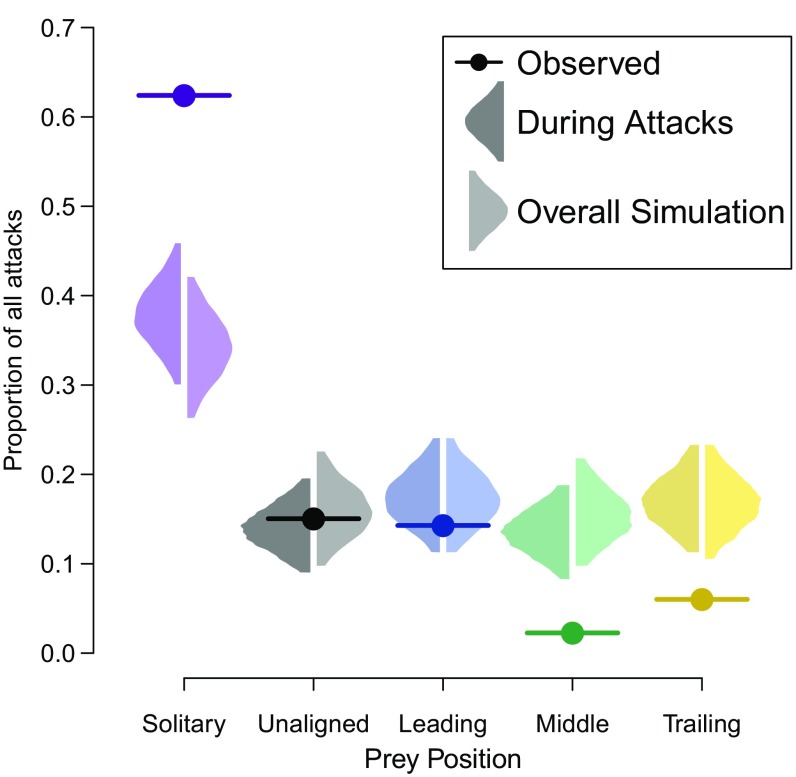
With the predators being more likely to target solitary prey at the time of attack and being more likely to attack when solitary prey were more frequent, these effects together are likely to select against solitary prey behavior. To determine the relative selective strengths of these two mechanisms, the proportion of all observed attacks on each of the prey positions was compared with the proportion expected from a random predator attacking at either the same instances as the observed attacks or randomly chosen sampled times in the simulation. Fig. 3 shows that the relative effect of making attacks when solitary prey are more frequent is relatively weak. The proportion of each prey position being targeted by the randomization test mostly overlaps between randomly selected instances in the simulation and instances of attack in the actual trials. As expected from the previous analysis assessing when attacks were made, solitary prey make up a greater proportion of attacked prey when the randomization is based on the instances of attacks compared with the simulation samples, with unaligned and middle prey being less frequent. However, there is larger variance between the proportions of each prey position actually targeted by the predators than expected from either form of random targeting. The proportion of solitary prey is greater than expected, and the proportion of middle and trailing prey is less than expected (all being outside of the 95% ranges of the randomization tests). This demonstrates that selection between individuals at the instance of attack is of greater importance to the proportion of each prey position attacked rather than when attacks take place.
Fig. 3.
The proportions of all attacks on the different prey positions. The proportions of observed attacks are shown by the circles. The shaded areas show the distributions up to the 95% ranges for the expected proportion of attacks on each prey position if the predator attacked prey randomly at the same instances as the observed attacks (during attacks: darker frequency plots) (SI Appendix, Table S1) or any of the sampled instances in the simulation (overall simulation: lighter frequency plots). To sample the overall simulation, 133 of 19,324 simulation samples were randomly selected, and within each of these sampled instances in the simulation, a single prey was randomly selected to be the “targeted” prey (this process was iterated 10,000 times). If the observed proportion is outside of these 95 ranges, it suggests that targeting by the predator was selective and was not random. Randomly sampling prey at the same instances as the observed attacks or at sampled instances in the simulation gives similar results, while there is much greater variance between the prey positions in the actual observed attacks.
Discussion
We provide experimental evidence in support of a widely assumed hypothesis that individual prey at the front of a moving group are most at risk from predation (11–16). This provides the missing empirical evidence for this central expectation that was limited to potentially confounded observations within a single prey and predator species pair (17, 18). The previous lack of direct examination of the antipredatory costs and benefits of leadership is likely to be due to the rarity of predation events within natural settings and the ethical issues of experimentally exposing vertebrate prey to predation. Furthermore, observations of self-organized groups of real prey animals cannot properly separate the relationship between predation risk and individuals’ social strategies due to various factors that covary with both risk and sociality [e.g., boldness, hunger, and body size (7, 22, 32)], and therefore, these confounding effects impede the ability to confidently assess how individuals’ spatial positions alone shape predation risk. By using a simulation of virtual prey in a laboratory setting, these difficulties can be overcome (26, 27). Additionally, using a 2D prey simulation with the predator attacking from the third dimension minimizes the effect of spatial position being driven by an increased rate of encounter between the leading edge of the group and a predator. As such, although our results are markedly strong, the test procedure used here represents a relatively conservative approach for two reasons. First, in cases where prey groups and predators move in the same dimensional space (i.e., both on a 2D surface or 3D volume), followers are likely to gain an even greater safety benefit compared with leaders. Second, the effect is predicted to be even stronger when predators use sit-and-wait strategies, as encounters will always occur first with the leading edge (17, 18).
Importantly, we also show that, despite the increased risk faced by the prey leading groups compared with followers, leading others was still safer than being isolated as a solitary prey. In laboratory studies where fish have the same level of experience of being trained to associate food with a spatial target, they still show wide variation in their tendency toward the target when tested with untrained individuals (19). At one extreme, trained individuals would leave the group to reach the target quickly, and at the other, their behavior was indistinguishable from untrained individuals, with the fish forming a cohesive group that had no tendency to move toward the target. Thus, individuals with the potential to lead can split from the group and go it alone, lead from the front while maintaining group cohesion, or behave as followers. In our prey simulation, mechanisms, such as risk dilution and the confusion effect caused by close proximity of other prey, outweighed the greater conspicuousness of larger groups as demonstrated by the lower overall risk for prey in groups. Even when taking spatial position within a group into account, prey in the leading position experienced lower risk compared with being solitary. Adapting the simulation to include collective vigilance and escape responses (33) is expected to further increase the safety benefit of being in a group and hence, the benefit of potential leaders remaining with the group rather than going it alone.
Our analysis also demonstrates that, for potential leaders in groups that are led from the front, risk can be minimized by having the nearest follower closer rather than farther away. To achieve this, leaders could use different strategies or interaction rules, such as waiting or slowing down when a neighbor behind them is too far (34–36) or tuning a parameter that balances a potential leader’s goal vs. socially orientated behavior (37). Simulation modeling can now be used to determine which movement rules minimize risk for leading individuals under different scenarios based on the distance to the nearest neighbor behind them. Our results also demonstrate that the bearing of the nearest prey behind an individual has no detectable effect on risk, and therefore, being directly ahead of the nearest follower does not expose a leader to more risk than the follower being almost alongside.
The changing frequencies of prey in different positions (solitary, leading, etc.) arose from the dynamic nature of collective motion (38). Analysis of the predator’s timing of when to attack rather than which prey to attack revealed nonrandom timing, as attacks were more likely when solitary prey were more frequent. In general, studies attempting to understand how prey traits affect predatory behavior have focused on decisions by predators of which prey to attack, the time taken to attack, and whether to attack (17, 18, 39). Very few studies have explored when predators attack based on how prey traits vary over time. By comparing both which prey are attacked and when these attacks are made, we demonstrate, however, that the choice of which prey to attack has the greater effect on how attacks are distributed between different prey positions. This is consistent with other studies, where the effect of prey variation was most evident in the choice of which prey to attack rather than in other predator behaviors, such as the time taken to attack (17, 26, 39). Together, these studies suggest that the choice of which prey to attack is the most sensitive aspect of predator behavior affected by variation in prey. This has implications for selection pressure on these prey traits, as predators attacking prey at a high density are likely to have multiple prey within their visual field to select a target from compared with prey at low density that are encountered individually. Thus, we would predict that selection on prey behavior and appearance is stronger when prey are closer together, as predators are more likely to discern between traits when choosing between multiple prey. Interestingly, any selection that changes the frequencies of different social strategies will inherently feed back into the densities of individuals (e.g., fewer solitary individuals results in more aggregation) and thus, potentially affect the strength of the selection on prey behavior.
Our results imply that, due to the antipredatory benefit of being followed, potential leaders are under selective pressure to achieve effective leadership, directing movement toward their goal while maintaining group cohesion through followership. This may require potential leaders to reduce their goal-orientated tendency to maintain following (19), which may require compromise and turn taking (40), and it would also favor the evolution of traits that encourage following by others. Our study was designed to isolate the effects on predation risk of spatial position in moving groups, and the predation benefit that followers confer on leaders can now be incorporated into theoretical models of how leaders and followers interact and emerge in groups (9, 37, 41). To achieve a full understanding of spatial positions within groups, particularly the relative importance of predation risk, modeling is essential to explore the wide parameter space generated by varying factors known to influence the costs and benefits of different spatial positions. These include individual and collective detection of the predator and subsequent avoidance behavior in prey, the abundance and distribution of the prey’s resources, and any energetic benefits of moving in groups. Our study provides the missing experimental data to inform models about how predators selectively target prey in different spatial positions.
Materials and Methods
Experimental Subjects.
Fish (mean length SD = 36.6 3.60 mm) were caught on September 25, 2014 from the River Cary, Somerset, United Kingdom (grid reference ST 469 303). They were housed in glass tanks (40 × 70 × 35 cm) on a flow-through recirculation system. Each tank housed approximately 40 individuals as well as plants to provide shelter and enrichment. Fish were kept under a 12:12-h light:dark cycle with temperature maintained between 15 °C and 16 °C. Apart from days that they were tested, the fish were fed twice daily with tropical flake food and bloodworms. Fish were not fed on the day that they were tested until after testing.
Prey Simulation.
An agent-based model simulation was projected onto the front wall of a test tank, where fish could be observed attacking individual projected prey types. Netlogo version 5.0.5 (42) was used to adapt an existing model of collective behavior [the Netlogo flocking model (43)] to create our model (Dataset S1). The prey population consisted of one leader, four followers, and two asocial individuals to create variation in prey social behavior. The leader and asocial prey types were both programmed to move without responding to other prey. Followers were attracted to both the leader type and other followers when within a constant distance (6.5 cm as projected on the tank), although they were not attracted to the asocial prey type (they responded to all prey in their repulsion zone when other prey got too close). The blind spot for social attraction was relatively large (182°), which ensured that prey groups moved in a head-to-tail procession (Movie S1). These rules generated a simulation where the leader often led one or more followers [leading by “social indifference” (44)], followers were frequently found in groups (where one would be leading if the leader type was not in the group), and asocial types were frequently alone (SI Appendix, Fig. S4). The behavioral rules and frequencies of the different prey types were designed to generate variation in prey behaviors so that the predatory fish would often have a choice between solitary isolated prey, prey leading others, and prey following others (Fig. 1). Model seeds were generated at random to randomize starting positions and orientations for the prey. Agents in the model were all presented visually on the screen as uniform 2.5-mm red dots when projected, similar to Daphnia in appearance (26).
Experimental Procedure.
Trials were carried out in March and April 2015. After companion fish were moved to the test tank (SI Appendix, Fig. S2), the simulation had reached at least 500 time steps to maintain a steady state, and recording was started, a single fish was transferred from one of the stock tanks to the bottom of the refuge area using a net. Attacks were determined by an accelerated motion by the fish toward a prey with their mouth open and contact with the screen (33). The recordings of attacks from the video were used to attain snapshots of the moment of first attack per trial to the nearest 25th of a second, where the seed and time step of the simulation were recorded as well as which prey was attacked. All procedures were in accordance with institutional guidelines on animal care and were approved by the University of Bristol Ethical Review Group.
Statistical Analysis.
R (version 3.3.3) (45) was used for all analyses, and data used in the analyses are available as Datasets S2 and S3.
Supplementary Material
Acknowledgments
We thank two anonymous reviewers for providing helpful feedback, which improved the manuscript. This work was supported by Natural Environment Research Council Independent Research Fellowship NE/K009370/1 and Leverhulme Trust Grant RPG-2017-041 V (to C.C.I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816323116/-/DCSupplemental.
References
- 1.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. An evolutionary ecology of individual differences. Ecol Lett. 2012;15:1189–1198. doi: 10.1111/j.1461-0248.2012.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeanson R, Fewell JH, Gorelick R, Bertram SM. Emergence of increased division of labor as a function of group size. Behav Ecol Sociobiol. 2007;62:289–298. [Google Scholar]
- 3.Krause J, Hoare D, Krause S, Hemelrijk CK, Rubenstein DI. Leadership in fish shoals. Fish. 2000;1:82–89. [Google Scholar]
- 4.King AJ. Follow me! I’m a leader if you do; I’m a failed initiator if you don’t. Behav Process. 2010;84:671–674. doi: 10.1016/j.beproc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Bumann D, Krause J. Front individuals lead in shoals of three-spined sticklebacks (Gasterosteus aculeatus) and juvenile roach (Rutilus rutilus) Behaviour. 1993;125:189–198. [Google Scholar]
- 6.Nagy M, Akos Z, Biro D, Vicsek T. Hierarchical group dynamics in pigeon flocks. Nature. 2010;464:890–893. doi: 10.1038/nature08891. [DOI] [PubMed] [Google Scholar]
- 7.DeBlois EM, Rose GA. Cross-shoal variability in the feeding habits of migrating atlantic cod (Gadus morhua) Oecologia. 1996;108:192–196. doi: 10.1007/BF00333231. [DOI] [PubMed] [Google Scholar]
- 8.King AJ, Douglas CMS, Huchard E, Isaac NJB, Cowlishaw G. Dominance and affiliation mediate despotism in a social primate. Curr Biol. 2008;18:1833–1838. doi: 10.1016/j.cub.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 9.Guttal V, Couzin ID. Social interactions, information use, and the evolution of collective migration. Proc Natl Acad Sci USA. 2010;107:16172–16177. doi: 10.1073/pnas.1006874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Björnsson B, Karlsson H, Macrander A. Food searching behaviour in adult atlantic cod Gadus morhua during acoustic training: Social learning and leadership within a school. J Fish Biol. 2018;93:814–829. doi: 10.1111/jfb.13783. [DOI] [PubMed] [Google Scholar]
- 11.Teichroeb JA, White MMJ, Chapman CA. Vervet (Chlorocebus pygerythrus) intragroup spatial positioning: Dominants trade-off predation risk for increased food acquisition. Int J Primatology. 2015;36:154–176. [Google Scholar]
- 12.Gall GEC, Manser MB. Spatial structure of foraging meerkat groups is affected by both social and ecological factors. Behav Ecol Sociobiol. 2018;72:1–9. [Google Scholar]
- 13.Blanco YD, Hirsch BT. Determinants of vigilance behavior in the ring-tailed coati (Nasua nasua): The importance of within-group spatial position. Behav Ecol Sociobiol. 2006;61:173–182. [Google Scholar]
- 14.Ihl C, Bowyer RT. Leadership in mixed-sex groups of muskoxen during the snow-free season. J Mammal. 2011;92:819–827. [Google Scholar]
- 15.Attanasi A, et al. Emergence of collective changes in travel direction of starling flocks from individual birds’ fluctuations. J Roy Soc Interface. 2015;12:20150319. doi: 10.1098/rsif.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ioannou CC, Ramnarine IW, Torney CJ. High-predation habitats affect the social dynamics of collective exploration in a shoaling fish. Sci Adv. 2017;3:e1602682. doi: 10.1126/sciadv.1602682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause J, Ruxton GD, Rubenstein D. Is there always an influence of shoal size on predator hunting success? J Fish Biol. 1998;52:494–501. [Google Scholar]
- 18.Bumann D, Krause J, Rubenstein D. Mortality risk of spatial positions in animal groups: The danger of being in the front. Behaviour. 1997;134:1063–1076. [Google Scholar]
- 19.Ioannou CC, Singh M, Couzin ID. Potential leaders trade off goal-oriented and socially oriented behavior in mobile animal groups. Am Nat. 2015;186:284–293. doi: 10.1086/681988. [DOI] [PubMed] [Google Scholar]
- 20.Bevan PA, Gosetto I, Jenkins ER, Barnes I, Ioannou CC. Regulation between personality traits: Individual social tendencies modulate whether boldness and leadership are correlated. Proc R Soc B. 2018;285:20180829. doi: 10.1098/rspb.2018.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A. Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr Biol. 2017;27:2862–2868. doi: 10.1016/j.cub.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClure M, Ralph M, Despland E. Group leadership depends on energetic state in a nomadic collective foraging caterpillar. Behav Ecol Sociobiol. 2011;65:1573–1579. [Google Scholar]
- 23.Piyapong C, et al. A cost of leadership in human groups. Ethology. 2007;113:821–824. [Google Scholar]
- 24.Rieucau G, Fernö A, Ioannou CC, Handegard NO. Towards of a firmer explanation of large shoal formation, maintenance and collective reactions in marine fish. Rev Fish Biol Fish. 2015;25:21–37. [Google Scholar]
- 25.Ioannou CC, Morrell LJ, Ruxton GD, Krause J. The effect of prey density on predators: Conspicuousness and attack success are sensitive to spatial scale. Am Nat. 2009;173:499–506. doi: 10.1086/597219. [DOI] [PubMed] [Google Scholar]
- 26.Ioannou CC, Guttal V, Couzin ID. Predatory fish select for coordinated collective motion in virtual prey. Science. 2012;337:1212–1215. doi: 10.1126/science.1218919. [DOI] [PubMed] [Google Scholar]
- 27.Duffield C, Ioannou CC. Marginal predation: Do encounter or confusion effects explain the targeting of prey group edges? Behav Ecol. 2017;28:1283–1292. doi: 10.1093/beheco/arx090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer Science & Business Media; New York: 2003. [Google Scholar]
- 29.Landeau L, Terborgh J. Oddity and the ‘confusion effect’in predation. Anim Behav. 1986;34:1372–1380. [Google Scholar]
- 30.Milinski M. Experiments on the selection by predators against spatial oddity of their prey 1. Z für Tierpsychologie. 1997;43:311–325. [Google Scholar]
- 31.Firth JA, et al. Personality shapes pair bonding in a wild bird social system. Nat Ecol Evol. 2018;2:1696–1699. doi: 10.1038/s41559-018-0670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald ND, Rands SA, Hill F, Elder C, Ioannou CC. Consensus and experience trump leadership, suppressing individual personality during social foraging. Sci Adv. 2016;2:e1600892. doi: 10.1126/sciadv.1600892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marras S, Batty RS, Domenici P. Information transfer and antipredator maneuvers in schooling herring. Adapt Behav. 2012;20:44–56. [Google Scholar]
- 34.Gueron S, Levin SA, Rubenstein DI. The dynamics of herds: From individuals to aggregations. J Theor Biol. 1996;182:85–98. [Google Scholar]
- 35.Herbert-Read JE, et al. Inferring the rules of interaction of shoaling fish. Proc Natl Acad Sci USA. 2011;108:18726–18731. doi: 10.1073/pnas.1109355108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz Y, Tunstrøm K, Ioannou CC, Huepe C, Couzin ID. Inferring the structure and dynamics of interactions in schooling fish. Proc Natl Acad Sci USA. 2011;108:18720–18725. doi: 10.1073/pnas.1107583108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Couzin ID, Krause J, Franks NR, Levin SA. Effective leadership and decision-making in animal groups on the move. Nature. 2005;433:513–516. doi: 10.1038/nature03236. [DOI] [PubMed] [Google Scholar]
- 38.Calovi DS, et al. Swarming, schooling, milling: Phase diagram of a data-driven fish school model. New J Phys. 2014;16:015026. [Google Scholar]
- 39.Penry-Williams IL, Ioannou CC, Taylor MI. The oddity effect drives prey choice but not necessarily attack time. Ethology. 2018;124:496–503. [Google Scholar]
- 40.Firth JA, Voelkl B, Farine DR, Sheldon BC. Experimental evidence that social relationships determine individual foraging behavior. Curr Biol. 2015;25:3138–3143. doi: 10.1016/j.cub.2015.09.075. [DOI] [PubMed] [Google Scholar]
- 41.Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA. Spontaneous emergence of leaders and followers in foraging pairs. Nature. 2003;423:432–434. doi: 10.1038/nature01630. [DOI] [PubMed] [Google Scholar]
- 42.Wilensky U, et al. 1999. Netlogo (Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL), Version 5.0.5.
- 43.Wilensky U. 1998. Netlogo Flocking Model (Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL), Version 5.0.5.
- 44.Conradt L, Krause J, Couzin ID, Roper TJ. “Leading according to need” in self-organizing groups. Am Nat. 2009;173:304–312. doi: 10.1086/596532. [DOI] [PubMed] [Google Scholar]
- 45.R Core Team 2017. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna), Version 3.3.3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.