Significance
Survival from malignant mesothelioma, particularly pleural mesothelioma, is very poor. For patients with some cancers, overall survival following platinum chemotherapy is better for patients with germline mutations in DNA repair and related genes than for patients without such mutations. We evaluated this relationship for patients with malignant mesothelioma. Following platinum chemotherapy, overall survival was significantly longer for patients with loss-of-function mutations in BAP1 and DNA repair genes compared with patients with no such mutations. The effect of genotype was highly significant for patients with pleural mesothelioma, but not for patients with peritoneal mesothelioma, and remained significant after adjusting for gender and age at diagnosis. These patients may benefit from DNA repair targeted therapies such as poly-ADP ribose polymerase inhibitors.
Keywords: mesothelioma, survival, inherited genetics, BAP1, DNA repair genes
Abstract
Survival from malignant mesothelioma, particularly pleural mesothelioma, is very poor. For patients with breast, ovarian, or prostate cancers, overall survival is associated with increased sensitivity to platinum chemotherapy due to loss-of-function mutations in DNA repair genes. The goal of this project was to evaluate, in patients with malignant mesothelioma, the relationship between inherited loss-of-function mutations in DNA repair and other tumor suppressor genes and overall survival following platinum chemotherapy. Patients with histologically confirmed malignant mesothelioma were evaluated for inherited mutations in tumor suppressor genes. Survival was evaluated with respect to genotype and site of mesothelioma. Among 385 patients treated with platinum chemotherapy, median overall survival was significantly longer for patients with loss-of-function mutations in any of the targeted genes compared with patients with no such mutation (P = 0.0006). The effect of genotype was highly significant for patients with pleural mesothelioma (median survival 7.9 y versus 2.4 y, P = 0.0012), but not for patients with peritoneal mesothelioma (median survival 8.2 y versus 5.4 y, P = 0.47). Effect of patient genotype on overall survival, measured at 3 y, remained independently significant after adjusting for gender and age at diagnosis, two other known prognostic factors. Patients with pleural mesothelioma with inherited mutations in DNA repair and other tumor suppressor genes appear to particularly benefit from platinum chemotherapy compared with patients without inherited mutations. These patients may also benefit from other DNA repair targeted therapies such as poly-ADP ribose polymerase (PARP) inhibitors.
Malignant mesothelioma is an aggressive tumor arising from the mesothelial cells lining the pleura, peritoneum, pericardium, or tunica vaginalis, with ∼3,000 cases diagnosed each year in the United States (1). Asbestos is the principal carcinogen associated with mesothelioma development, with exposure to other minerals such as erionite also causally implicated (1, 2). Mesothelioma can also develop in patients who have received radiation therapy for another cancer (3).
Inherited loss-of-function mutations in BAP1 (BRCA1-associated protein) predispose to mesothelioma (4–7), as well as to other conditions (8) including uveal melanoma (9), cutaneous melanoma (10), meningioma (11), basal cell carcinoma (12), and renal cell carcinoma (13). BAP1 encodes a deubiquitinase that binds to BRCA1 and BARD1 and enhances their tumor suppressor function (14). It has been suggested that BAP1 is involved in homologous recombination DNA repair by cleaving ubiquitin from histone 2A at a critical step in the process (15). Inherited loss-of-function mutations in other genes may also predispose to mesothelioma (16–18) with lifetime risks expected to be much lower than risks to carriers of mutations in BAP1.
Median overall survival of patients with pleural mesothelioma is especially poor: ∼12–16 mo following treatment with cisplatin plus pemetrexed (19, 20), the first-line standard of care chemotherapy for this disease. Of potential importance to mesothelioma, ovarian and breast cancers that develop in patients with inherited mutations in BRCA1 and BRCA2 are particularly sensitive to cisplatin chemotherapy and, for ovarian cancer, these patients have better overall survival (21–24). BRCA1 and BRCA2 are integral to DNA repair by homologous recombination. Breast, ovarian, and prostate cancers in patients with mutations in BRCA1 or BRCA2 are also more sensitive to poly-ADP ribose polymerase (PARP) inhibitors, which cause cell death by inducing synthetic lethality of alternate DNA repair pathways (25–27). Cell lines with loss of function of BAP1 are also sensitive to PARP inhibitors (28–30).
The goal of the present project was to evaluate inherited loss-of function mutations in patients with malignant mesothelioma, not selected for family history or age at diagnosis, then to assess whether inherited mutations in BAP1 and in DNA repair genes impact overall survival following platinum-based chemotherapy. If so, patients’ genotypes may impact front-line chemotherapy decision-making and increase the likelihood that PARP inhibitor treatment may benefit these patients as well.
Results
Patients and Clinical Characteristics.
Patients were enrolled from two centers. All patients with malignant mesothelioma attending the Thoracic Medical Oncology Clinic of the National Cancer Institute (NCI) between September 2013 and December 2016 were offered participation in a study of the natural history of malignant mesothelioma (ClinicalTrials.gov no. NCT01950572), and 241 consecutive patients were enrolled. The NCI Laboratory of Pathology confirmed all diagnoses of mesothelioma and characterized their origin as pleural, peritoneal, pericardial, or tunica vaginalis. All patients with malignant mesothelioma attending The University of Chicago (UC) Medicine Mesothelioma Clinic between April 2016 and September 2017 were offered participation in a similar study of the role of inherited genetics in solid tumors. Of 250 eligible patients, 198 unrelated patients had adequate DNA for testing and were enrolled (15). Five patients were seen at both clinics, yielding the final combined study sample of 434 patients. At both clinics, patients were enrolled regardless of asbestos exposure, age at diagnosis, or personal or family history of cancer.
Features of patients and their tumors are indicated in Table 1. Mesotheliomas most frequently originated in the pleura (66%) and second most frequently in the peritoneum (31%). Most tumors had epithelial histology (83%). Average age at diagnosis was 60.3 y (range 12–83 y); 67% of patients were male. Patients with pleural mesothelioma were diagnosed at older ages than patients with peritoneal mesothelioma (64.6 ± 10.9 y vs. 51.4 ± 14.4 y, P < 0.0001) and were more likely to be male (70% vs. 59%, P = 0.02). Approximately one in five patients had a prior history of another cancer, primarily cutaneous melanoma, ocular melanoma, urothelial cancer, or breast cancer. Most patients (66%) had at least one first-degree relative with cancer. In the NCI cohort, cancers of relatives were most frequently mesothelioma, ocular or cutaneous melanoma, breast cancer, or bladder cancer. Of patients providing information on asbestos exposure, 74% reported a history of exposure, either occupational or paraoccupational. Patients reporting asbestos exposure were more likely to have developed pleural disease (P = 0.005).
Table 1.
Characteristics of mesothelioma patients and tumors
| NCI | UC | Total* | |
| n [%] | n [%] | n [%] | |
| Patient characteristics | 241 [100] | 198 [100] | 434 [100] |
| Gender | |||
| Male | 158 [66] | 136 [69] | 292 [67] |
| Female | 83 [34] | 62 [31] | 142 [33] |
| Age at diagnosis, y | |||
| <20 | 3 [1] | 0 0 | 3 [1] |
| 20–29 | 12 [5] | 4 [2] | 15 [3] |
| 30–39 | 18 [7] | 5 [3] | 21 [5] |
| 40–49 | 29 [12] | 5 [3] | 33 [8] |
| 50–59 | 61 [25] | 44 [22] | 105 [24] |
| 60–69 | 71 [29] | 77 [39] | 147 [34] |
| 70–79 | 43 [18] | 57 [29] | 100 [23] |
| ≥80 | 4 [2] | 8 [4] | 12 [3] |
| Ethnicity | |||
| White, non-Hispanic | 223 [93] | 192 [97] | 410 [94] |
| White, Hispanic | 11 [5] | 0 [0] | 11 [3] |
| Black | 4 [2] | 3 [2] | 7 [2] |
| Asian | 3 [1] | 3 [2] | 5 [1] |
| Asbestos exposure† | |||
| Yes | 135 [56] | 126 [64] | 258 [59] |
| No | 59 [24] | 35 [18] | 92 [21] |
| Unknown | 47 [20] | 37 [19] | 84 [19] |
| Mesothelioma site | |||
| Pleura | 140 [58] | 148 [75] | 286 [66] |
| Peritoneum | 92 [38] | 44 [22] | 133 [31] |
| Pericardium | 2 [1] | 0 [0] | 2 [0] |
| Pleura and peritoneum | 0 [0] | 3 [2] | 3 [1] |
| Tunica vaginalis | 7 [3] | 3 [2] | 10 [2] |
| Tumor histology | |||
| Epithelial | 207 [86] | 157 [80] | 359 [83] |
| Sarcomatoid/biphasic | 17 [7] | 36 [18] | 53 [12] |
| Unknown | 17 [7] | 5 [3] | 22 [5] |
| Personal history of other cancer | |||
| Yes | 58 [24] | 27 [14] | 84 [19] |
| No | 183 [76] | 171 [86] | 350 [81] |
| Cancer in first-degree relative | |||
| Yes | 147 [61] | 142 [72] | 286 [66] |
| No | 94 [39] | 54 [28] | 146 [34] |
| Unknown | 0 | 2 | 2 |
NCI, National Cancer Institute; UC, University of Chicago.
Five patients were treated at both clinics; duplicate records were excluded.
Asbestos exposure by self-report.
Genetics of Patients and Tumors in the NCI Cohort.
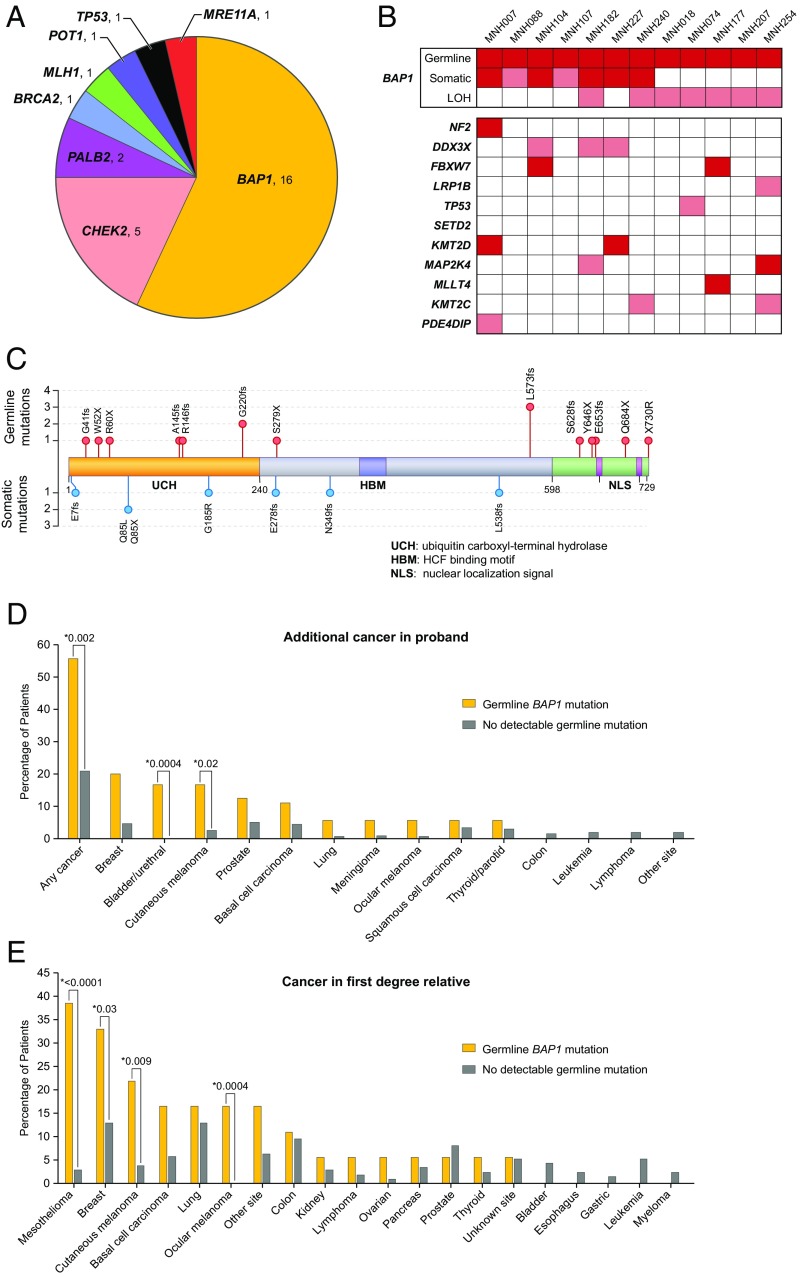
Genetic profiles of patients in the UC cohort have been reported (15). This section provides comparable information for the patients in the NCI cohort. Overall, 28 of 239 unrelated patients (12%) from the NCI cohort carried an unambiguously damaging mutation in a targeted gene: 16 in BAP1 and 12 distributed among CHEK2, PALB2, BRCA2, MLH1, POT1, TP53, and MRE11A (Fig. 1 and SI Appendix, Table S1). Of these 28 mutations, three were large rearrangements, two in CHEK2 and one in MLH1. Most of the genes harboring mutations are required for DNA repair, either by homologous recombination or by mismatch repair.
Fig. 1.
Inherited mutations in mesothelioma. (A) Genes with inherited damaging mutations in patients with malignant mesothelioma from the NCI series. (B) Somatic mutations in tumors from mesothelioma patients with inherited mutations in BAP1. LOH is loss of heterozygosity at BAP1. Dark red boxes represent truncating mutations; pink boxes represent missense mutations, in-frame deletions, or LOH. (C) BAP1 protein, indicating functional domains and sites of mutations in mesothelioma patients and their tumors. (D) History of cancer other than mesothelioma in patients with inherited BAP1 mutations versus patients with no inherited mutation. (E) History of cancer in parents, siblings, or children of patients with inherited BAP1 mutations versus patients with no inherited mutation.
Inherited mutations in all genes except POT1 and MLH1 have been reported in patients with mesothelioma (15, 31). Patient MNH153, diagnosed with peritoneal mesothelioma at age 54, carried a nonsense mutation in POT1 (SI Appendix, Table S1). He reported 6-y asbestos exposure while working in engine rooms during naval service in the 1970s. Inherited mutations in POT1, a critical telomere maintenance gene (32), predispose to melanoma (33) and to glioma (34), but have not been suggested previously to predispose to mesothelioma. Patient MNH145, diagnosed with peritoneal mesothelioma, inherited a genomic deletion of at least 4.6 kb at MLH1 leading to loss of exons 16–19 and 3′UTR. His mother had developed primary cancers of the colon and ovary, and eight other maternal relatives developed MLH1-associated cancers. Patient MNH041, diagnosed with pleural mesothelioma at age 27 with no family history of cancer, carried a loss-of-function mutation in TP53. Her mutation occurred de novo; it was not present in DNA from either parent; familial relationships were verified by multiple genetic markers.
Inherited mutations were approximately equally frequent among patients with pleural mesothelioma (14/140, 10%) and patients with peritoneal mesothelioma (15/92, 16%, P = 0.22) but were significantly more frequent among female patients (16/83, 19%) than among male patients (14/158, 9%, P = 0.02) and among patients diagnosed younger than age 60 (21/123, 17%) versus those diagnosed age 60 or older (9/118, 8%, P = 0.03). Inherited mutations in BAP1 were strongly associated with diagnosis younger than age 60 (16/123, 13%) versus diagnosis at age 60 or older (2/118, 2%, P = 0.001) and modestly associated with gender (10/83, 12% of female patients, versus 8/158, 5% of male patients, P = 0.069).
Mesothelioma tumor tissue was available for 12 of the 18 NCI patients with inherited mutations in BAP1. All 12 tumors carried a second, somatic mutation in BAP1 likely to lead to complete loss of BAP1 function (Fig. 1 B and C). Somatic BAP1 mutations included truncating mutations (n = 5), possible splice or damaging missense mutations (n = 2), and large genomic lesions leading to copy number changes at the BAP1 locus (n = 7) (SI Appendix, Table S2). Most of the copy number changes were deletions; the one copy number gain could be due to endoreduplication of the mutant alleles. All copy number changes led to loss of heterozygosity (LOH) for the wild-type allele at the BAP1 locus. These tumors also harbored somatic point mutations in 10 other genes (Fig. 1B).
Previous studies of sporadic (noninherited) mesothelioma with somatic inactivation of BAP1 revealed tumor mutation profiles enriched for DDX3X (35) or for NF2 and SETD2 (36). Of the 12 mesothelioma tumors in the NCI series with inactivation of BAP1 due to both germline and somatic mutation, one tumor carried a somatic point mutation in NF2, none in SETD2, and three in DDX3X (Fig. 1B). The mutation burden of mesothelioma tumors from patients with a germline BAP1 mutation was compared to reported mutation burden of mesothelioma tumors from The Cancer Genome Atlas (TCGA) (36). For these 12 patients, the average somatic mutation rate was 1.33 (range 0.44–2.88) nonsynonymous mutations per megabase, similar to the somatic mutation rate of <2 nonsynonymous mutations per megabase reported by the TCGA. Although the numbers are small, this observation suggests that there may be no differences in somatic mutation burden of patients with germline BAP1 mutations compared with those without germline mutation. In the future, analysis of all classes of somatic events in tumors of all patients will enable comparison of somatic mutation profiles of patients with inherited versus sporadic disease.
Patients with inherited mutations in BAP1 were more likely than patients with no germline mutation to have a prior personal history of any cancer (P = 0.002), melanoma (P = 0.02), or bladder cancer (P = 0.0004; Fig. 1D), and to have a family history of mesothelioma (P < 0.0001), ocular melanoma (P < 0.01), or breast cancer (P = 0.03; Fig. 1E). It is well established that BAP1 carriers are at increased risk for mesothelioma and melanoma (6, 7, 9–12, 15). A very rough estimate of the magnitude of this risk can be made from the 15 BAP1 families in the NCI cohort with cancer history information available for both parents. Of the 15 obligate BAP1 mutation carrier parents, five developed mesothelioma by the date of the family’s enrollment in the study (SI Appendix, Table S1). Their experience suggests 33% as an estimate of mesothelioma risk by elderly age for BAP1 carriers.
Among carriers of germline mutations in these genes, interaction of genotype and asbestos exposure exacerbates mesothelioma risk (27, 37). In the NCI series, 9 of 12 patients with BAP1 mutations and 8 of 10 patients with mutations in other genes reported asbestos exposure (SI Appendix, Table S1). Six NCI patients had a family history of mesothelioma but no inherited mutation in any sequenced gene. Three of these patients reported personal occupational history of asbestos exposure, and four reported such exposure in their affected relative (SI Appendix, Table S3). For example, MNH125 and his father were both retired insulators who both developed mesothelioma. Shared exposure to asbestos could mimic the effect of shared genetic predisposition.
Genes Enriched for Inherited Loss-of-Function Mutations in the Combined Cohort.
Based on genetic analysis of 432 unrelated patients from both the NCI and UC clinics, loss-of-function mutations in BAP1, BRCA2, CHEK2, PALB2, MLH1, MRE11A, and POT1 were significantly more frequent among cases than among controls (Table 2 and SI Appendix, Table S4). Other than BAP1, each gene harbored mutations in only a few patients, so the associations of these genes with mesothelioma, although significant, remain preliminary. Conversely, mutations in other DNA repair genes may prove associated with mesothelioma as more patients are evaluated.
Table 2.
Genes associated with inherited predisposition to malignant mesothelioma
| Gene | Controls* | OR | (95% CI) | P |
| BAP1 | ExAC | 1,458 | (196, 10,843) | <10e-30 |
| WHI | 393 | (53, 2,923) | <10e-30 | |
| BRCA2 | ExAC | 2.80 | (1.02, 7.66) | 0.036 |
| WHI | 5.67 | (1.82, 17.65) | 0.0007 | |
| CHEK2 | ExAC | 3.29 | (1.61, 6.75) | 0.00004 |
| WHI | 4.28 | (1.96, 9.34) | 0.0006 | |
| MLH1 | ExAC | 20.9 | (2.16, 201.0) | 0.00008 |
| MRE11A | ExAC | 6.98 | (1.62, 30.19) | 0.002 |
| WHI | 6.78 | (1.31, 35.04) | 0.008 | |
| PALB2 | ExAC | 5.24 | (1.24, 22.23) | 0.012 |
| WHI | 4.84 | (1.00, 23.37) | 0.030 | |
| POT1 | ExAC | 8.94 | (1.11, 72.84) | 0.013 |
Control series are ExAC: Exome Aggregation Consortium with exomes from TCGA removed, n = 27,173; and WHI: Women’s Health Initiative participants, age >70 y and cancer free, n = 7,325. All patients with damaging mutations were of European ancestry, so only European ancestry controls were included in analyses.
Association of Genotype with Survival Among Patients Treated with Platinum Chemotherapy.
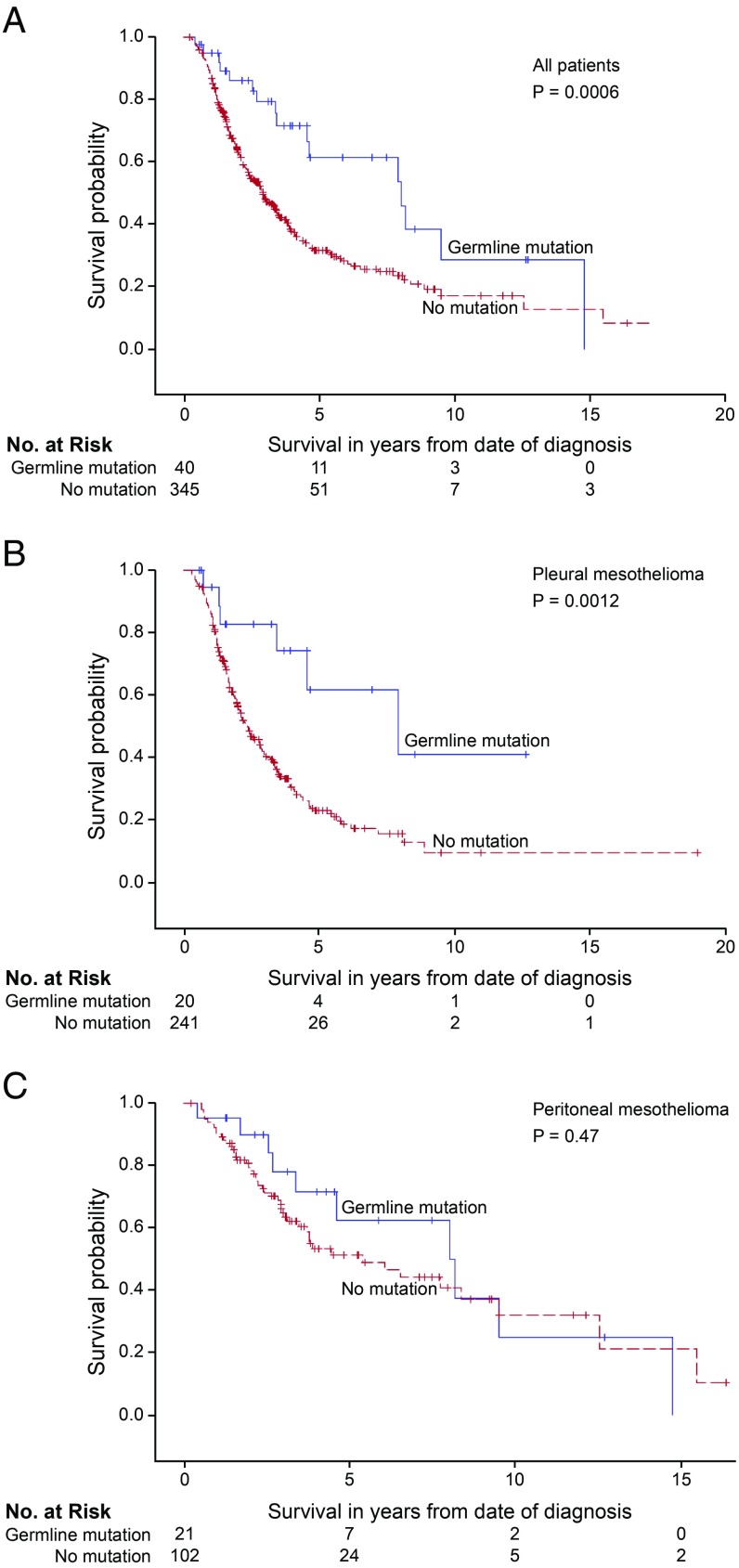
The association of patient genotype with overall survival was evaluated for all 385 patients from the combined cohort who received platinum-based chemotherapy (Fig. 2 and SI Appendix, Table S5). Compared with patients with no germline mutation, overall survival was significantly better for patients with a mutation in BAP1 (P = 0.004) or for patients with a mutation in any of the targeted genes (P = 0.0006). The survival benefit associated with genotype was similar for patients with mutations specifically in BAP1 (SI Appendix, Fig. S1A) and for patients with mutations in any of the targeted genes (Fig. 2A). Multiple genes other than BAP1 contributed to this survival advantage, but there was little power to detect effects of individual genes other than BAP1 (SI Appendix, Table S6). The effect of genotype was highly significant for patients with pleural mesothelioma (Fig. 2B and SI Appendix, Fig. S1B); median survival was 7.9 y, both among patients with mutations in BAP1 and among patients with a mutation in any gene versus 2.4 y for patients without a mutation (P = 0.0021 and P = 0.0012, respectively). In contrast, the effect of genotype was not significant for patients with peritoneal mesothelioma (Fig. 2C and SI Appendix, Fig. S1C); median survival was 8.2 y for mutation carriers versus 5.4 y for those without a mutation (P = 0.47; Fig. 2C). The survival curves for patients with peritoneal mesothelioma suggested possible benefit to mutation carriers in the first years after chemotherapy, but the difference in survival was not significant at any time point in the first 10 y.
Fig. 2.
Survival of patients with mesothelioma treated with platinum chemotherapy, by patient’s genotype and primary site of tumor. Survival of patients with an inherited damaging mutation in any targeted gene is indicated in blue; survival of patients with no inherited mutation is indicated in red. (A) All mesothelioma patients with versus without inherited mutations. Median survival: 8.0 vs. 2.9 y, P = 0.0006. (B) Pleural mesothelioma patients with versus without inherited mutations. Median survival: 7.9 vs. 2.4 y, P = 0.0012. (C) Peritoneal mesothelioma patients with versus without inherited mutation. Median survival: 8.2 vs. 5.4 y, P = 0.47.
Patients with germline mutations were younger at diagnosis and more likely to be female, perhaps because mesothelioma in females is less frequently due to environmental exposure. Because being young and female were both associated with improved survival, we evaluated the effect of genotype on survival for pleural mesothelioma patients adjusted for age and gender. Genotype had an independent and significant effect on survival at 3 y after treatment after adjusting for age at diagnosis and gender (SI Appendix, Table S7). Patients with versus without a germline mutation did not differ with respect to tumor histology (83% versus 88% of tumors were epithelial) or with respect to reported asbestos exposure (57% versus 56% reported exposure). These results suggest that the effect of genotype on overall survival of these patients is independent of histology and asbestos exposure.
Discussion
The proportion of mesothelioma patients from the NCI and UC with germline loss-of-function mutations in any tumor suppressor gene was 11.7% [23/198 from UC (15) and 28/239 from NCI]. In addition to BAP1, loss of function mutations in BRCA2, CHEK2, MLH1, MRE11A, PALB2, and POT1 were more frequent among patients with mesothelioma than among controls. Most significantly for patient care, the data suggests that patients with pleural mesothelioma with inherited mutations in these genes have better overall survival following platinum-based chemotherapy compared with patients with no germline mutation, consistent with previous reports based on small numbers of patients (17, 38).
Cisplatin plus pemetrexed is the current front-line standard of care therapy for pleural mesothelioma (19), and response to chemotherapy is associated with improved survival (39). However, not all patients respond to this therapy, and there have been no biomarkers to identify likely responders. Our results suggest that after adjusting for age at diagnosis and gender, patients with an inherited loss-of-function mutation in one of the targeted genes may have significantly better survival following platinum-based chemotherapy. That is, germline mutation in one of these genes may be a prognostic marker for response to platinum chemotherapy for this disease. Among mutation carriers, survival experience was similar for patients with pleural mesothelioma (median 7.9 y) and for patients with peritoneal mesothelioma (median 8.2 y). For these patients, survival may be driven more by genotype than by site of origin.
In this analysis, survival of patients with germline mutations in tumor suppressor genes was compared with survival of patients with no detectable damaging germline mutation. However, loss of function of BAP1 due to purely somatic events is well established for mesothelioma (31). Loss of function of BAP1, or of other tumor suppressor genes, due to purely somatic events is likely to have the same effect on response to therapy and on survival as loss of function due to germline plus somatic mutation. By assigning patients with undetected, purely somatic inactivating events to the “no mutation” comparison group, we have very likely underestimated the effect of mutation on overall survival. Nonetheless, the effect of mutation on survival for patients with pleural mesothelioma is highly significant. Future analyses should address the impact on survival from mesothelioma of biallelic somatic inactivation of these genes, as well as other tumor markers of homologous recombination deficiency.
The improved overall survival of our patients closely mirrors that of patients with solid tumors due to inherited mutations in BRCA1 or BRCA2. In ovarian cancer, improved survival has been attributed in part to better platinum response rates and longer treatment-free duration after platinum therapy, especially for BRCA2 mutation carriers (21, 22, 40). These clinical observations reflect the underlying deficits in homologous recombination DNA repair seen in BRCA-null cells. These cells are unable to efficiently repair platinum-induced DNA cross-links (41) and, notably, are more susceptible to inhibitors by a synthetic lethal mechanism (42).
Among patients with ovarian, breast, or prostate cancer, the combination of inherited and somatic mutations in DNA repair genes define a subset of tumors that are particularly sensitive to PARP inhibitors. Among the NCI patients with mesothelioma with inherited BAP1 mutations, all tumors harbored additional, somatic mutations likely leading to complete loss of BAP1 function. Cell lines with these features are sensitive to PARP inhibitors (28, 29), suggesting that these primary tumors might also be particularly vulnerable to PARP inhibitors (43). Whether patients with mesothelioma with inherited mutations in BAP1 or related DNA repair genes would benefit from PARP inhibitor therapy is now an open question (15). An enrolling clinical trial of the PARP inhibitor olaparib in patients with mesothelioma will examine the relationship between patient genotype and response to therapy (Clinicaltrials.gov no. NCT03531840). Another trial, of the PARP inhibitor niraparib in patients with various neoplasms including mesothelioma, is evaluating the relationship between patient genotype and response to therapy (Clinicaltrials.gov no. NCT03207347). The observation of better overall survival following platinum therapy in patients with mutations in these genes provides additional rationale for trials of PARP inhibition.
Based on the cancer experience of parents of NCI mesothelioma patients with BAP1 mutations, the risk of mesothelioma was 33% among obligate BAP1 mutation carriers. In contrast, absolute risks of malignant mesothelioma were low for obligate carriers of mutations in other genes. An interaction between genotype and asbestos exposure on mesothelioma risk has been demonstrated in mouse models (44, 45) and is consistent with the reported experience of our patients.
A limitation of this study was that history of asbestos exposure was based on patient report rather than on independent measurement. The interaction of asbestos exposure and genetic predisposition has been established (17), but confirmation in this study was inevitably tentative. A second limitation was that although patients were not selected for clinical characteristics, the NCI cohort included a high proportion of female and young patients, who have better survival from the disease, hence our evaluation of the effect of genotype on overall survival after adjusting for effects of age at diagnosis and gender. Finally, for genes other than BAP1, the number of patients with inherited mutations was small. Evaluation of additional cases will more clearly reveal the role of other genes in predisposition to mesothelioma and of interaction with asbestos exposure in mesothelioma development.
The experience of these patients demonstrates that patient genotype predicts improved overall survival following platinum therapy and that patients with either pleural or peritoneal mesothelioma with a germline mutation survive on average 8 y. We therefore suggest genetic testing at diagnosis for germline mutations in a broad range of DNA repair and related genes for every mesothelioma patient. Results of this testing are important for treatment, prognosis, and surveillance for second cancers for patients, as well as for cancer screening and prevention for their families.
In conclusion, we found that inherited mutations in DNA repair genes among patients with malignant mesothelioma occur at frequencies similar to their frequencies among patients with other solid tumors. Mesothelioma patients with mutations in BAP1 or in DNA repair genes have improved overall survival following platinum-based chemotherapy. These data support exploration of other therapies that exploit synthetic lethality of DNA repair pathways.
Methods
Human subjects committees of the NCI, UC, and the University of Washington approved the studies; all patients provided written informed consent. Methods for genomic and statistical analyses are described in SI Appendix.
Supplementary Material
Acknowledgments
We thank the patients for their enthusiastic participation in the project. This study was supported by National Institutes of Health (NIH) Intramural Award BC010816 (to R.H.); NIH Grant R03 HL145253 and a University of Chicago Cancer Center Award (to J.E.C.); and NIH Grant R35 CA197458 and an American Cancer Society Professorship (to M.-C.K.).
Footnotes
Conflict of interest statement: R.H. has received funding for conduct of clinical trials via a cooperative agreement between NCI and Bayer AG, Aduro BioTech, and Morphotek Inc. H.L.K. has consulting relationships with Aduro Biotech, MedImmune, Bayer, Celgene, GlaxoSmithKline, AstraZeneca, Merck, Bristol-Myers Squibb, Boehringer Ingelheim, Ipsen, Erytech Pharma, Five Prime Therapeutics, and Paredox Therapeutics. J.E.C. received a pilot research grant from the University of Chicago Comprehensive Cancer Center, which was supported with funds from the law firm of Cooney and Conway.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821510116/-/DCSupplemental.
References
- 1.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 2.Carbone M, et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc Natl Acad Sci USA. 2011;108:13618–13623. doi: 10.1073/pnas.1105887108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang ET, Lau EC, Mowat FS, Teta MJ. Therapeutic radiation for lymphoma and risk of second primary malignant mesothelioma. Cancer Causes Control. 2017;28:971–979. doi: 10.1007/s10552-017-0929-4. [DOI] [PubMed] [Google Scholar]
- 4.Testa JR, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bott M, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohar JA, et al. Germline BAP1 mutational landscape of asbestos-exposed malignant mesothelioma patients with family history of cancer. Cancer Res. 2016;76:206–215. doi: 10.1158/0008-5472.CAN-15-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betti M, et al. CDKN2A and BAP1 germline mutations predispose to melanoma and mesothelioma. Cancer Lett. 2016;378:120–130. doi: 10.1016/j.canlet.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Walpole S, et al. Comprehensive study of the clinical phenotype of germline BAP1 variant-carrying families worldwide. J Natl Cancer Inst. 2018;110:1328–1341. doi: 10.1093/jnci/djy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbour JW, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiesner T, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43:1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Rahman MH, et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet. 2011;48:856–859. doi: 10.1136/jmedgenet-2011-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadt KA, et al. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet. 2015;88:267–272. doi: 10.1111/cge.12501. [DOI] [PubMed] [Google Scholar]
- 13.Popova T, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013;92:974–980. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen DE, et al. BAP1: A novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci USA. 2014;111:285–290. doi: 10.1073/pnas.1309085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panou V, et al. Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J Clin Oncol. 2018;36:2863–2871. doi: 10.1200/JCO.2018.78.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betti M, et al. Sensitivity to asbestos is increased in patients with mesothelioma and pathogenic germline variants in BAP1 or other DNA repair genes. Genes Chromosomes Cancer. 2018;57:573–583. doi: 10.1002/gcc.22670. [DOI] [PubMed] [Google Scholar]
- 18.Pastorino S, et al. A subset of mesotheliomas with improved survival occurring in carriers of BAP1 and other germline mutations. J Clin Oncol. October 30, 2018 doi: 10.1200/JCO.2018.79.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogelzang NJ, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 20.Zalcman G, et al. French Cooperative Thoracic Intergroup (IFCT) Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 21.Yang D, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolton KL, et al. EMBRACE; kConFab Investigators; Cancer Genome Atlas Research Network Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isakoff SJ, et al. TBCRC009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norquist BM, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mateo J, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirza MR, et al. ENGOT-OV16/NOVA Investigators Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 27.Robson M, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan G, et al. Synthetic lethality in malignant pleural mesothelioma with PARP1 inhibition. Cancer Chemother Pharmacol. 2017;80:861–867. doi: 10.1007/s00280-017-3401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parrotta R, et al. A novel BRCA1-associated protein-1 isoform affects response of mesothelioma cells to drugs impairing BRCA1-mediated DNA repair. J Thorac Oncol. 2017;12:1309–1319. doi: 10.1016/j.jtho.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Heeke AL, et al. Prevalence of homologous recombination-related gene mutations across multiple cancer types. JCO Precis Oncol. 2018;2018 doi: 10.1200/PO.17.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betti M, et al. Germline mutations in DNA repair genes predispose asbestos-exposed patients to malignant pleural mesothelioma. Cancer Lett. 2017;405:38–45. doi: 10.1016/j.canlet.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Baumann P, Cech TR. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science. 2001;292:1171–1175. doi: 10.1126/science.1060036. [DOI] [PubMed] [Google Scholar]
- 33.Robles-Espinoza CD, et al. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet. 2014;46:478–481. doi: 10.1038/ng.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bainbridge MN, et al. Gliogene Consortium Germline mutations in shelterin complex genes are associated with familial glioma. J Natl Cancer Inst. 2014;107:384. doi: 10.1093/jnci/dju384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bueno R, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–416. doi: 10.1038/ng.3520. [DOI] [PubMed] [Google Scholar]
- 36.Hmeljak J, et al. TCGA Research Network Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018;8:1548–1565. doi: 10.1158/2159-8290.CD-18-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kharazmi E, et al. Familial risk of pleural mesothelioma increased drastically in certain occupations: A nationwide prospective cohort study. Eur J Cancer. 2018;103:1–6. doi: 10.1016/j.ejca.2018.07.139. [DOI] [PubMed] [Google Scholar]
- 38.Baumann F, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis. 2015;36:76–81. doi: 10.1093/carcin/bgu227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blayney JK, et al. Response to chemotherapy is predictive in relation to longer overall survival in an individual patient combined-analysis with pleural mesothelioma. Eur J Cancer. 2012;48:2983–2992. doi: 10.1016/j.ejca.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 42.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 43.Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: Recent advances and future development. J Clin Oncol. 2015;33:1397–1406. doi: 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadariya Y, et al. Bap1 is a bona fide tumor suppressor: Genetic evidence from mouse models carrying heterozygous germline Bap1 mutations. Cancer Res. 2016;76:2836–2844. doi: 10.1158/0008-5472.CAN-15-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Napolitano A, et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene. 2016;35:1996–2002. doi: 10.1038/onc.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.